Abstract
Study Objectives:
Recent evidence suggests that certain antidepressants are associated with an increase of periodic leg movements (PLMS) that may disturb sleep. So far, this has been shown in patients clinically treated for depression and in cross-sectional studies for various substances, but not mirtazapine. It is unclear whether antidepressants induce the new onset of PLMS or only increase preexisting PLMS, and whether this is a general property of the antidepressant or only seen in depressed patients. We report here the effect of mirtazapine on PLMS in young healthy men.
Design:
Open-labeled clinical trial (NCT00878540) including a 3-week preparatory phase with standardized food, physical activity, and sleep-wake behavior, and a 10-day experimental inpatient phase with an adaptation day, 2 baseline days, and 7 days with mirtazapine.
Setting:
Research institute.
Participants:
Twelve healthy young (20-25 years) men.
Interventions:
Seven days of nightly intake (22:00) of 30 mg mirtazapine.
Measurements and results:
Sleep was recorded on 2 drug-free baseline nights, the first 2 drug nights, and the last 2 drug nights. Eight of the 12 subjects showed increased PLMS after the first dose of mirtazapine. Frequency of PLMS was highest on the first drug night and attenuated over the course of the next 6 days. Three subjects reported transient restless legs symptoms.
Conclusions:
Mirtazapine provoked PLMS in 67% of young healthy males. The effect was most pronounced in the first days. The possible role of serotonergic, noradrenergic and histaminergic mechanisms in mirtazapine-induced PLMS is discussed.
Citation:
Fulda S; Kloiber S; Dose T; Lucae S. Mirtazapine provokes periodic leg movements during sleep in young healthy men. SLEEP 2013;36(5):661-669.
Keywords: Mirtazapine, periodic leg movements, sleep, restless legs syndrome
INTRODUCTION
Antidepressants profoundly affect sleep in many ways that depend on the specific class of the antidepressant, the dosage, and the duration of treatment.1–3 Antidepressants were shown to influence periodic leg movements during sleep (PLMS), that are stereotyped and repetitive leg movements which arise from sleep, especially during NREM sleep stages 1 and 2.4,5 The contractions last 0.5 to 10 seconds and typically occur every 20 to 40 seconds in series of at least four leg movements in a row,6 and hundreds of PLMS may occur during a single night. The frequency of PLMS is routinely quantified as the number of PLMS per hour of sleep (PLMS index); a PLMS index above 15 is considered abnormal.7 PLMS can be associated with awakenings or cortical arousals, i.e., short awakenings detectable in sleep EEG recordings, and these may contribute to sleep disturbances with frequent PLMS. While sleep disturbances are frequent among depressives and antidepressants are used to improve this condition, PMLS may be an unwanted side effect.4 The clinical significance of PLMS is currently debated,8,9 but recent studies have shown that PLMS are associated with increased heart rate variability and blood pressure increases during sleep.10–14 Specifically, heart rate increases of 12% to 23% shortly after the onset of PLMS have been observed,10–18 and these increases are even larger when EEG arousals are associated with PLMS.12,13,15,16 In addition, continuous blood pressure monitoring has shown that PLMS are associated with a signifi-cant rise in systolic (20 to 30 mm Hg) and diastolic (10 to 12 mm Hg) blood pressure.13,19
Increased PLMS are observed in 20% to 50% of patients with sleep-related central and obstructive breathing disorders,20 narcolepsy,21 or REM sleep behavior disorder.22 In addition, PLMS are also observed in healthy subjects, with the prevalence sharply rising with age.23–26 The closest association with PLMS is found, however, in patients with restless legs syndrome (RLS), where approximately 85% of subjects will show frequent PLMS during nocturnal sleep.27 Nevertheless, most people who suffer from PMLS do not suffer from RLS, and consequently PLMS is a sensitive but not specific marker for RLS. RLS is a neurological sleep-related movement disorder that affects between 3% and 10% of the general population in various degrees of severity. The prevalence of RLS increases with age and across all age groups, and RLS is approximately twice as prevalent in women as in men.28 RLS is characterized by an unpleasant sensation resembling paresthesias and an urge to move, that is most prominent in the evening and at night, occurs only at rest when subjects are lying or sitting, and are at least partially relieved with movement.29 It is associated with disturbed sleep and reduced quality of life. Symptomatic or secondary forms of RLS are found with iron deficiency, end-stage renal disease, and pregnancy.28 The pathophysiology of RLS is unclear, but dopaminergic mechanisms and iron metabolism have been implicated. In the idiopathic form of RLS, a positive family history is often reported. Genome-wide association studies have recently identified genetic polymorphisms with no obvious relationship to dopamine that account for 70% of the population risk for RLS.30,31 A single variant in the BTBD9 gene on chromosome 6 contributed to 50% of the population risk. Sub-analyses revealed, however, that BTBD9 may be a genetic marker for PLMS rather than RLS.31
Previous studies4 and case reports5 have suggested that anti-depressants are associated with increased PLMS. This has been shown for various selective serotonin re-uptake inhibitors (SSRI) but does not seem to occur with bupropion.4,32 In particular, increased PLMS have been observed under treatment with venlafaxine, fluoxetine, citalopram, paroxetine, and sertraline.4,5,33
So far, the occurrence of PLMS with antidepressants has mostly been shown in clinical conditions such as depression, where there is also an increased risk for other sleep disorders that may be associated with PLMS.34 In addition, most studies have been cross-sectional and have observed patients only during antidepressant treatment; therefore, it is unknown whether antidepressants induce the new onset of PLMS or increase preexisting PLMS in these patients. To answer this question, studies are needed that explore drug-induced PLMS in healthy controls. This study aimed to clarify this and administered to healthy subjects mirtazapine, a noradrenergic, specific serotonergic antidepressant (NaSSA), which is, according to clinical observations, the antidepressant with the highest risk for inducing RLS.35
We report here that treatment with mirtazapine induced significant PLMS in eight of twelve young healthy males. The data is part of a larger study on “Short-term Metabolic Effects of Mirtazapine in Healthy Subjects” (NCT00878540) that was designed to explore metabolic alterations induced by mirtazapine under standardized conditions.
METHODS
Participants
Twelve healthy Caucasian, 20- to 25-year-old males were recruited at the Max Planck Institute of Psychiatry, Munich, Germany. Physical disorders were excluded by a complete physical exam, clinical history, routine blood tests (including lipid profile, complete blood count, liver enzymes, renal function, thyroid hormone) and urinalysis, electrocardiogram, electroencephalogram (EEG) and a cranial magnetic resonance imaging scan. Subjects were required to be of normal weight with a body mass index (BMI) between 18.5 and 25 kg/m2. A maximum of 2 caffeinated drinks per day at inclusion was allowed. Exclusion criteria were smoking within the last 6 months, any medication within last 6 months (with the exception of incidental use of pain medication [excluding opioids] until 2 weeks prior to start of the study), prior use of the study medication for any reason, shift work or time zone travel within the last 12 months, positive family history (first-degree relatives) for metabolic diseases (including diabetes mellitus and hereditary hyperlipidemia), and current or former alcohol or drug abuse. Current or former mental axis I disorders according to criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) were excluded using a modified version of the Munich-Composite International Diagnostic Interview (DIA-X/M-CIDI36). The complete list of exclusion criteria can be obtained on http://clinicaltrials.gov/ct2/show/NCT00878540. All subjects were good and regular sleepers without daytime sleepiness or extreme circadian orientation verified with interviews, questionnaires (Pittsburgh Sleep Quality Index,37 Epworth Sleepiness Scale,38 Morningness-Eveningness Questionnaire,39,40 Munich Parasomnia Screening41), sleep diaries, and actimetry.
The study was approved by the competent authorities and the Ethics Committee of the Medical Faculty at the Ludwig Maximilians University, Munich, Germany. Written informed consent was obtained from all subjects, and the study was carried out in accordance with the latest revision of the Declaration of Helsinki.
Protocol
After extensive screening, all subjects entered a 3-week, ambulatory preparatory phase with standardized food based on individual caloric need and provided in total by the experimenters. Regarding drinks, only water was allowed during the entire study period. Regular sleep-wake cycles and moderate exercise was controlled by sleep diary, actimetry, and step counters.
Subsequently, participants spent 10 days as inpatients in the experimental unit, where they were observed 24 h/day. During this period standardized diet, sleep-wake cycles, and physical activity were maintained
Participants arrived on the evening of the first day, which served as an adaptation night. The next 2 days and nights served as baseline nights for the study (BL1 and BL2). At 22:00 of the fourth night (D1) and on the 6 subsequent nights 30 mg mirtazapine (Remergil SolTab, Organon, Oberschleißheim, Germany) was given orally to the participants. The last dosage of mirtazapine was given on day 10 (D7), and participants left the institute 22 h after the last dose of mirtazapine.
Sleep Recording
The first night served as an adaptation night with electrodes attached but without sleep recording. Sleep was recorded on both baseline nights (BL1 and BL2), on the first 2 nights of drug intake (D1 and D2), and the last 2 nights of drug intake (D6 and D7). Time in bed was from 23:00 to 07:00 during the entire study period (preparatory phase and experimental phase).
All polysomnographic recordings were acquired with Brain-lab 3.30 (Schwarzer, Munich, Germany). EEG electrodes were placed according to the international 10-20 system and included F3, F4, C3, C4, Oz, M1, and M2. All electrodes were referenced to a common electrode (CPz) and offline re-referenced to M1 or M2. EEG was recorded with a sampling rate of 500 Hz with low- and high-pass hardware filters set at 0.095 Hz and 70 Hz, respectively. Electrooculography (EOG) and surface electromyography (EMG) of the mentalis and submentalis muscles and right and left anterior tibialis muscles were recorded with a sampling rate of 500 Hz, with electrode placement and filter settings in accordance with international guidelines.6,42 Airflow, oximetry, rib cage, and abdominal movements were recorded to assess respiratory parameters.
Sleep stages, arousals, respiratory events, and leg movements were scored according to the rules of the American Academy of Sleep Medicine.42 All leg movements were verified manually in all recordings. Standard parameters for sleep and leg movements were derived from the scoring.6,42 In addition, we computed the periodicity index for periodic leg movements, which characterizes the periodicity of the leg movements independent from their number.43 For the distribution of leg movement intervals, all leg movements during sleep with a duration of 0.5 to 10 s were considered.
Statistical Analysis
Descriptive statistics were employed to summarize data, and nonparametric between-groups comparisons were applied, as appropriate.
RESULTS
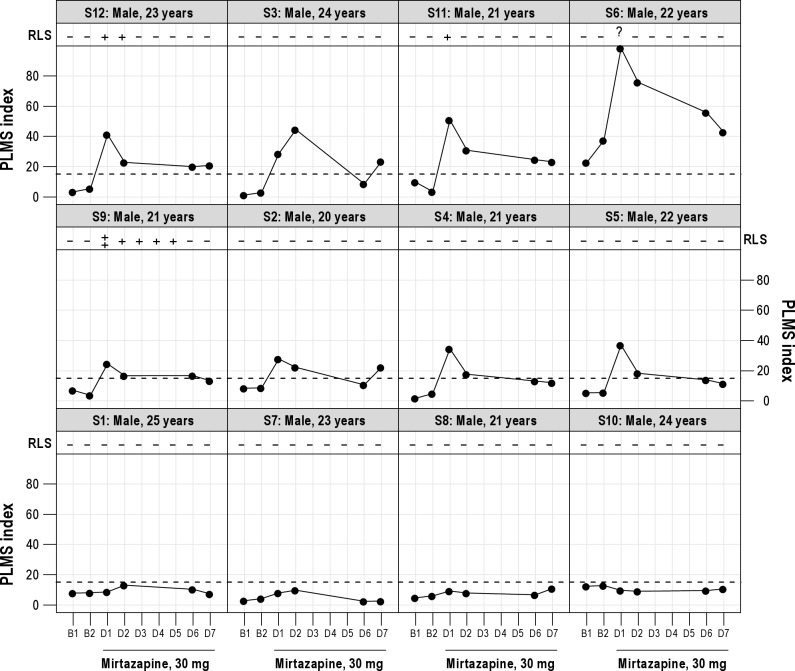
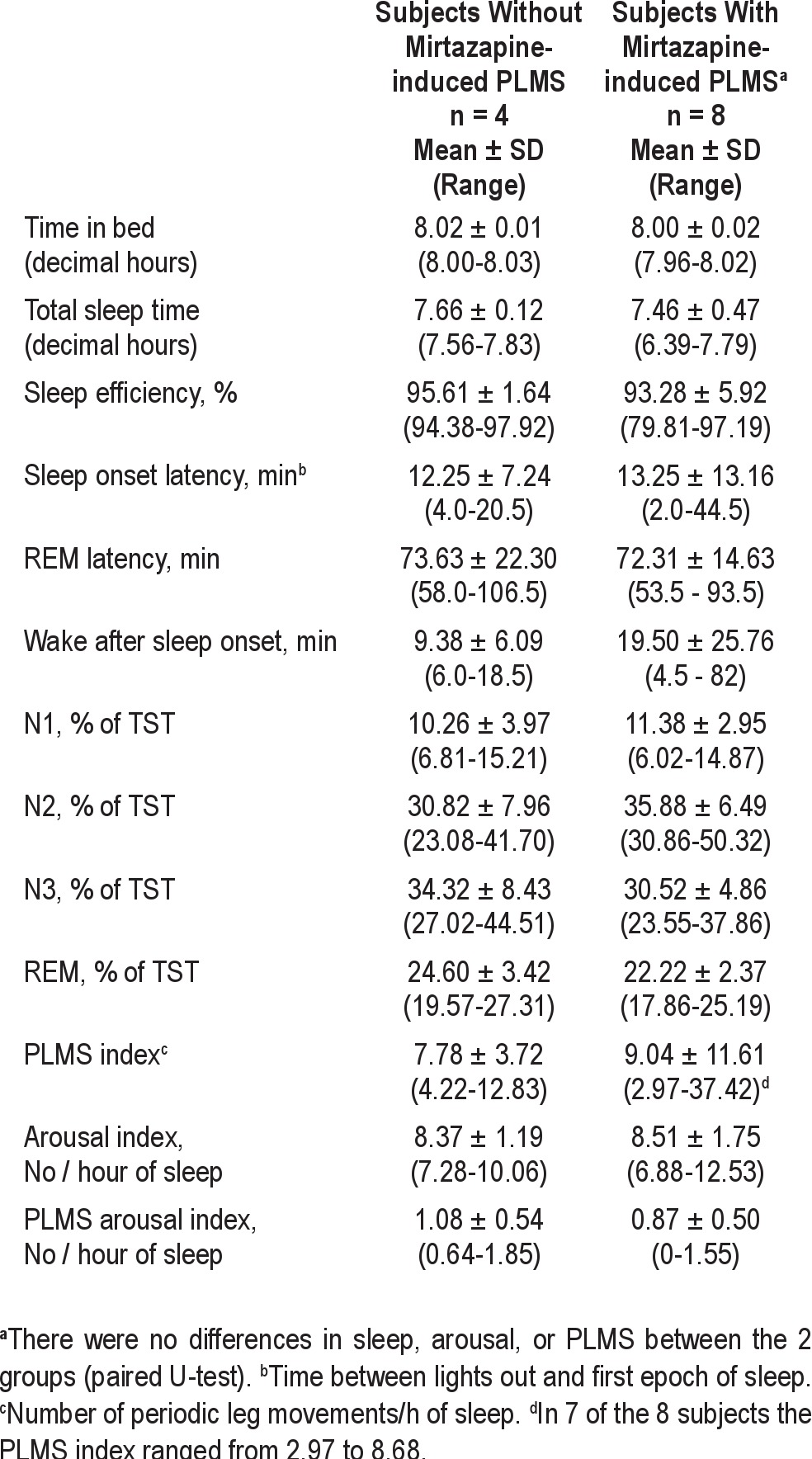
Twelve healthy male subjects, aged 20 to 25 years, participated in the study (Table 1). All subjects had sleep of good quality during the 2 baseline nights. Eleven of the 12 subjects had no evidence for increased periodic leg movements during baseline sleep (PLMS index < 15/h). One subject showed increased PLMS in both baseline nights (PLMS indices: 37 and 21) but without frequent PLMS arousals and with sleep of high quality. None of the subjects had any evidence for clinically significant sleep-related breathing disorders on any baseline or drug night. On the first night (D1) after intake of 30 mg mirtazapine at 22:00 hour, 8 of the 12 subjects showed a significant increase of PLMS (Figure 1), with PLMS indices between 28 and 98 (mean PLMS index: 43 ± 24 [SD]). In these 8 subjects, frequency of PLMS was somewhat attenuated but still increased on the second drug night (D2; PLMS index 31 ± 20 [17-76]). After 6 (D6) and 7 (D7) nights of mirtazapine, PLMS were further decreased (D6: PLMS index 20 ± 15 [11-56]; D7: PLMS index 21 ± 10 [11-43]; D1 vs. D7: t = 3.78, P = 0.007). Increased PLMS (PLMS index > 15) were seen in one subject in both baseline nights, in 8 subjects in the first 2 drug nights, in 4 subjects at D6, and in 5 subjects at D7.
Table 1.
Description of 12 male study participants
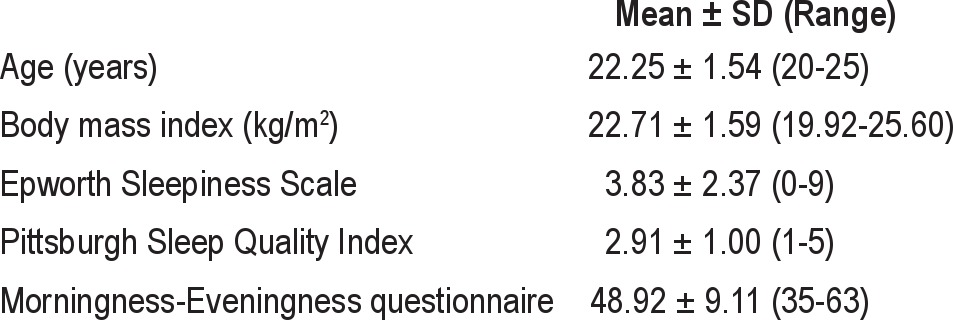
Figure 1.
PLMS index and RLS symptoms before and during intake of mirtazapine. (B1, B2: baseline nights; D1 to D6: drug nights; PLMS index: Number of periodic leg movements per hour of sleep; RLS: Restless legs syndrome symptoms (- no symptoms, + mild symptoms, ++ moderate symptoms, ? questionable symptoms); S: Subject.
The periodicity index quantifies the proportion of periodic leg movements within the total number of leg movements. It is high (> 0.5, typically 0.7 to 0.9) in subjects with RLS and lower in other sleep disorders with increased PLMS but without RLS. For subjects without mirtazapine-induced PLMS (n = 4) the periodicity index was low (< 0.40) for all baseline and drug nights. For subjects with PLMS, excluding the subject with PLMS during baseline nights, the periodicity index was low during the 2 baseline nights (< 0.20) and substantially increased on the first drug night (0.43-0.76). Mean periodicity indices for all subjects with mirtazapine-induced PLMS were 0.17 ± 0.21 and 0.18 ± 0.20 for the 2 baseline nights, 0.63 ± 0.16 and 0.52 ± 0.18 for the first 2 drug nights, and 0.42 ± 0.18 and 0.41 ± 0.20 for the last 2 drug nights. The distribution of leg movement intervals for the 2 baseline nights and the first 2 drug nights is given in Figure 2. In subjects with mirtazapine-induced PLMS, leg movement intervals between 20 and 40 s were specifically enhanced with mirtazapine.
Figure 2.
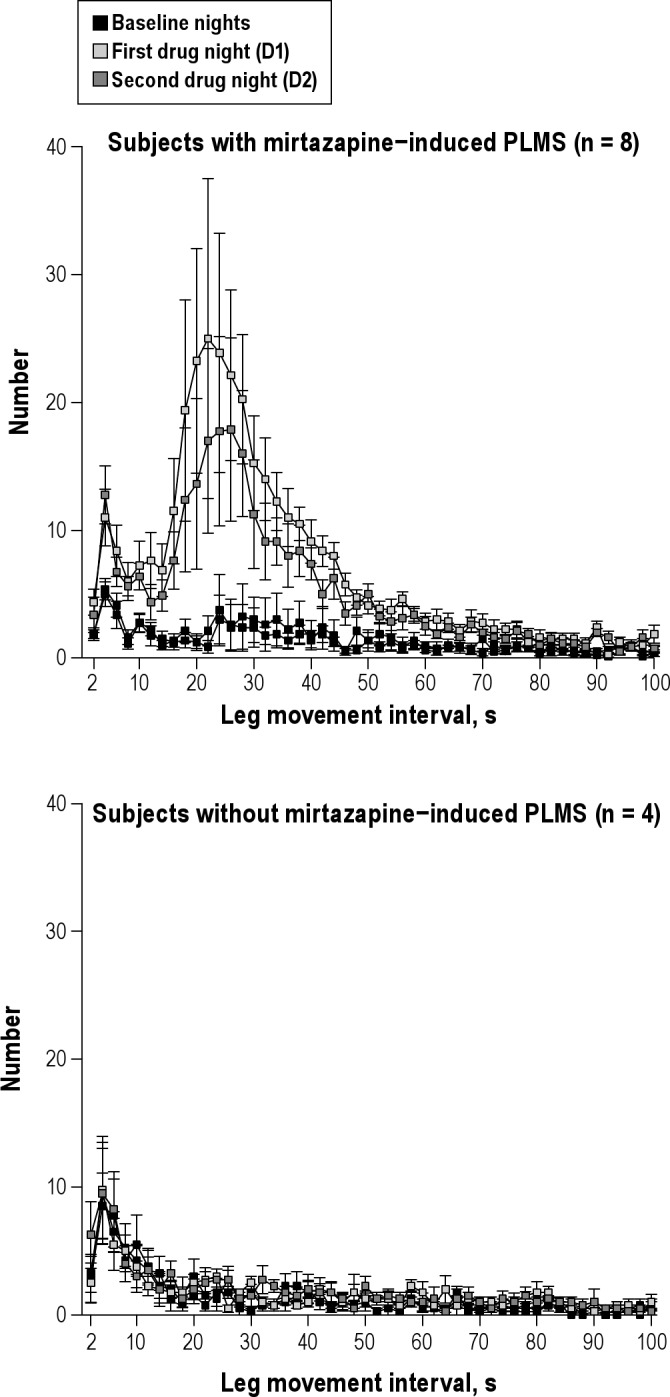
Leg movement intervals for all leg movements (duration 0.5 to 10 s) for subjects with (upper panel) and without (lower panel) mirtazapine-induced PLMS before and during intake of mirtazapine. Values are shown as mean and SEM (whiskers).
All subjects were questioned daily about RLS symptoms (Figure 1). A positive family history for restless legs was denied by all subjects. None of the 4 subjects without drug-induced PLMS reported any RLS symptoms. From the 8 subjects with drug-induced PLMS, 4 did not report any RLS symptoms on any day. One subject reported clear RLS symptoms of moderate intensity on the first drug night, and very mild symptoms on the second to fifth drug nights. Two further subjects reported mild RLS symptoms on the first drug night, and one of them had also very mild symptoms on the second drug night. The subject with the increased PLMS at baseline and the massively increased PLMS during drug intake reported only questionable RLS symptoms on the first drug night (unpleasant sensations and an urge to move in the upper right leg only). RLS symptoms did not interfere with sleep onset in any of the subjects: sleep onset latencies for all subjects with drug-induced PLMS were between 1 and 16 minutes on any drug night.
The 8 subjects with mirtazapine-induced PLMS did not differ in any sleep parameter from the 4 subjects without mirtazapine-induced PLMS at baseline (Table 2). During all drug nights, subjects with mirtazapine-induced PLMS had a significantly increased number of PLMS and PLMS arousals compared to subjects without PLMS (paired U-test, data not shown). On the first drug night, subjects with PLMS had increased N2 sleep, and on the last drug night subjects with PLMS had increased N2 and decreased N3 sleep (data not shown); no other difference was found between the 2 groups.
Table 2.
Baseline sleep characteristics (second baseline night)
DISCUSSION
Here we report that intake of 30 mg mirtazapine provoked PLMS in 8 of 12 young healthy males. The frequency of PLMS was highest after the first drug intake and decreased during the next 6 nights. Both the periodicity indices and the distribution of leg movement intervals, showed that mirtazapine-induced PLMS closely resembled those found in subjects with RLS.43,44 These findings were unexpected for several reasons: First, mirtazapine-induced PLMS have not been previously described in clinical studies, and our study subjects were young healthy males not explicitly at increased risk for PLMS and RLS. Second, although drug-induced PLMS were pronounced in our subjects, symptoms of RLS were either absent or, if present, only very mild. Third, initially the drug-induced PLMS were pronounced but rapidly decreased over time, despite continuing drug treatment, suggestive of rapid tolerance.
Several previous studies have recorded sleep during mirtazapine treatment in healthy subjects,45,46 depressed patients,47–51 and patients with obstructive sleep apnea syndrome52–54 (Table 3). In the majority of studies, PLMS were either not recorded45–48,51 or not reported.49,50,52,54 In two studies, reported in one publication, sleep was assessed after 7.5 mg to 45 mg mirtazapine in patients with obstructive sleep apnea syndrome.53 In these two studies, PLMS were recorded but no detailed results were reported. However, the authors stated that “we found no evidence that mirtazapine at any dose worsened periodic limb movements during sleep.”53 In these studies, sleep was recorded at baseline and after 14 or 28 days. Given that we have observed a significant attenuation of mirtazapine-induced PLMS over the first six days, it can be speculated that the reported negative findings may be due to a loss of effect of mirtazapine on PLMS after longer periods of time. Due to the limited treatment time of seven days, our study cannot answer whether PLMS disappear with longer use, remain at an asymptotic lower but increased level, or will increase again. This would have to be explored in future studies with longer recording times.
Table 3.
Previous findings with regard to PLMS and RLS in polysomnographic studies with mirtazapine

Mirtazapine is the antidepressant with the highest risk for developing RLS, a condition with 85% co-occurrence of PLMS.35 In a carefully conducted study, 271 psychiatric outpatients without antidepressant use for at least three months were prospectively assessed for the occurrence of RLS after initiation of a monotherapy with various antidepressants.35 In 9% of the patients, RLS developed after the antidepressant treatment was begun; the rate was highest for mirtazapine, where 28% (15 of 53) of patients reported RLS. In contrast, RLS was observed in ten percent or fewer patients with paroxetine, sertraline, and escitalopram, and in five percent or fewer with venlafaxine, duloxetine, fluoxetine, and citalopram. No RLS cases were observed with the use of reboxetine, a selective noradrenergic reuptake inhibitor. In 2 cases, RLS appeared after dose escalation of mirtazapine (dosage not reported), but RLS was already observed in 5 other cases at doses as low as 15 mg. Importantly, RLS symptoms appeared very early during the course of treatment, after a median of 2.5 days. Although the time course of antidepressant-induced RLS is not known in healthy subjects, the results of the above mentioned study,35 several case reports,55–59 and a retrospective chart review study60 showed that mirtazapine-induced RLS typically develops within the first week, often within the first 3 days of treatment in the vast majority of patients. These findings argue against the assumption that significant RLS would have developed at a later time with continued long-term use in our subjects. In the present study, 67% of the participants showed increased PLMS with mirtazapine but only 3 (25%) experienced any definite RLS symptoms, which were mostly mild and short-lived. This is in line with a previous study that recorded sleep and PLMS in 8 healthy volunteers during a baseline night and 4 consecutive nights with venlafaxine (75 mg in the first 2 nights; 150 mg in the last 2 nights).33 Six of eight subjects developed PLMS during venlafaxine, but only two of them also reported RLS symptoms.33 Indeed, it is a current debate to which extent PLMS and RLS share common pathophysiological mechanisms or can be differentially influenced by drugs with distinct pharmacological properties.61 Relative differences in the number of subjects experiencing RLS symptoms or PLMS also mirror known population frequencies where PLMS are a distinctly more frequent finding than RLS.20,62 Recent, genome-wide association studies have identified genetic variants that confer the risk for RLS.30,31 Among the identified genes, one SNP in the BTBD9 gene was associated preferentially with PLMS and RLS with PLMS rather than RLS without PLMS.31 Intriguingly, the frequency of this variant is high, about 68%, in the white population. Given that we have observed mirtazapine-induced PLMS in 67% of our subjects, it is tempting to hypothesize that the gene variant conferring the risk for PLMS may be the same that predisposes individuals also towards medication-induced PLMS.
Another unexpected finding was the time course of mirtazapine-induced PLMS with a pronounced peak after the first administration and a rapid decrease over the next 6 days. The time course of PLMS paralleled that of mirtazapine's effects on slow wave sleep (SWS) and REM sleep: In the first night, SWS increased and REM sleep decreased. This change gradually disappeared during the observation period of 6 nights (data not shown). The observed steep increase of SWS and subsequent return near baseline suggests rapid tolerance to mirtazapine in our subjects. Studies in healthy subjects, so far, have only recorded sleep after acute administration,45,46,63 while studies in patients with sleep-related breathing disorders have recorded sleep after 7 or 14 days of mirtazapine but not after acute administration.52–54 In depressed patients, one study recorded sleep in after 2, 9, 16, 30, and 58 days of 30 mg mirtazapine.49 The paper included a graphical display of SWS and REM sleep during the first third of the night that is strongly suggestive of a pronounced effect after 2 nights and a subsequent decrease of this effect to a low at day 16.
Mirtazapine blocks noradrenergic α2-auto and heteroreceptors as well as 5-HT2 and 5-HT3 receptors.64 Mirtazapine also binds to H1-receptors, which accounts for drug-induced sedation and weight gain.65–67 Compared to its antagonistic effects on H1- and α2-receptors, mirtazapine exhibits only minor affinity to α1-adrenergic, cholinergic, or dopaminergic receptors.68 Antagonism of the 5-HT2 receptor, and in particular the 5-HT2C receptor, has been assumed to be responsible for drug-induced increase in SWS,69,70 and antidepressants affecting serotonergic transmission exert acute effects on sleep that are sustained with continued administration.3,71 The action of mirtazapine on serotonergic transmission could therefore explain the effects on SWS and PLMS.
Nevertheless, mirtazapine predominantly blocks α2 auto- and heteroreceptors increasing both serotonergic and noradrenergic neurotransmission.64 Several tricyclic and tetracyclic antidepressants that have been linked to RLS and/or PLMS act also as noradrenaline reuptake inhibitors.5 In addition, venlafaxine, which has been shown to induce PLMS, is a dual-acting antidepressant that inhibits mainly noradrenaline rather than serotonin reuptake at doses equal or lower than 150 mg.72,73 Thus, it can be hypothesized that noradrenaline transmission is involved in mirtazapine-induced PLMS. Noradrenalin is one of the major neurotransmitters of the sympathetic nervous system (SNS), and PLMS has been linked to a possible hyperactivation of the SNS through the observation of increased heart rates associated with PLMS and with an onset that precedes the onset of the leg movement.16,74,75 It has been suggested that the autonomic nervous system plays a primary role in the generation of PLMS,74,76 which leaves the possibility that mirtazapine provokes PLMS through the increase in noradrenergic sympathetic tone. In line with this, phenoxybenzamine, a nonselective postsynaptic α-adrenergic blocker, decreased mild to moderate PLMS in two insomniac patients.76 However, clonidine, a sympathicolytic drug acting as a presynaptic α2-adrenergic agonist, has been reported to decrease RLS symptoms77–79 and in the only study that recorded leg movements during sleep, clonidine failed to reduce PLMS.78 Further, neither noradrenergic nor serotonergic effects of mirtazapine, can be easily linked to the transitory nature of the effect on sleep and PLMS.
Although increased presynaptic dopamine transporter availability80–82 has been discussed as a possible mechanism for PLMS induced by serotonergic drugs,4 mirtazapine has been reported to increase central dopamine neurotransmission and release.83,84 Specifically, increased dopamine may be a secondary effect of mirtazapine via 5-HT1A receptor activation.83,85 Speculatively, the indirect increase of central dopamine may explain why mirtazapine-induced PLMS decrease over time. Similarly, mirtazapine exhibits also significant antinociceptive effects in animal studies, which involves action on μ- and κ-3- opioid receptors.86,87 Similar to the effects on dopamine discussed above, opioid-mediated antinociceptive effects are in the opposite direction of what would be expected from an RLS- and PLMS-inducing drug mechanism, and may therefore explain why mirtazapine-induced PLMS are only short-lasting in the present study.
Mirtazapine also interacts with histaminergic signaling and the transient increase of PLMS and SWS may be related to the strong antihistaminergic properties of mirtazapine, as tolerance to daytime sedative effects of H1 antagonists develops within three days in healthy subjects,88 an effect that was also observed in the present study. In addition, in healthy subjects, promethazine, a first-generation H1 antagonist, showed a pronounced effect on sleep in the first night and a rapid decrease to baseline values by night 7 or night 5.89,90 Further, diphenhydramine, another first-generation H1 antagonist, provoked periodic leg movements during wake in subjects with RLS.91 While larger studies are lacking and the role of histamine in PLMS (and RLS) is mostly unknown, these findings could point to antihistaminergic mechanisms in the induction of PLMS that deserves systematic exploration in future studies.
Our study has several limitations, as it was open-label and uncontrolled. We also used only a single dosage of mirtazapine and therefore cannot generalize our findings to lower or higher dosages. This also precludes us from observing a possible dose-response relationship of mirtazapine and PLMS. Further, since genetic variants (esp. BTBD9) have recently been found to confer a high risk for PLMS in the white population,31 it is tempting to hypothesize that these variants may be the same that predisposes individuals also towards medication-induced PLMS. Finally, although young healthy males can be considered a low-risk population, we can only speculate whether this finding will also generalize to populations at a higher risk such as females and older participants.
In summary, we observed that mirtazapine induced PLMS in a considerable portion of young healthy males. Although the course of PLMS development parallels transient sedative effects of mirtazapine and hints to the antihistaminergic action of the drug, the role of noradrenergic, serotonergic, and secondary neurotransmitter systems, such as dopamine, in drug-induced PLMS is still unclear.
DISCLOSURE STATEMENT
Drs. Dose, Fulda, Kloiber, Lucae, and Schaaf reported no biomedical financial interests or potential conflicts of interest. Dr. Hennings has received travel support to scientific congresses from Elli Lilly and Novartis. Dr. Holsboer reported his patent on “Means and methods for diagnosing predisposition for treatment emergent suicidal ideation (TESI)”, European application number: 08016477.5, International application number: PCT/ EP2009/061575
ACKNOWLEDGMENTS
The authors thank Gabi Kohl, Katharina Mahler, Birte Balzer, Luise Vogl, Christine Zitzmann, Sarah Hehl, Katharina Lechner, and the sleep lab team of the Max Planck Institute of Psychiatry for their valuable help in conducting the study. Work for this study was performed at the Max Planck Institute of Psychiatry, Munich, Germany.
REFERENCES
- 1.Argyropoulos SV, Wilson SJ. Sleep disturbances in depression and the effects of antidepressants. Int Rev Psychiatry. 2005;17:237–45. doi: 10.1080/09540260500104458. [DOI] [PubMed] [Google Scholar]
- 2.Mayers AG, Baldwin DS. Antidepressants and their effect on sleep. Hum Psychopharmacol. 2005;20:53–59. doi: 10.1002/hup.726. [DOI] [PubMed] [Google Scholar]
- 3.Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927–47. doi: 10.2165/00003495-200565070-00003. [DOI] [PubMed] [Google Scholar]
- 4.Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58:510–5. doi: 10.1016/j.biopsych.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Hoque R, Chesson AL. Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010;6:79–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, Illinois: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 8.Högl B. Periodic limb movements are associated with disturbed sleep: Pro. J Clin Sleep Med. 2007;3:12–4. [PubMed] [Google Scholar]
- 9.Mahowald MW. Periodic limb movements are NOT associated with disturbed sleep: Con. J Clin Sleep Med. 2007;3:15–7. [PubMed] [Google Scholar]
- 10.Gosselin N, Lanfranchi P, Michaud M, et al. Age and gender effects on heart rate activation associated with periodic leg movements in patients with restless legs syndrome. Clin Neurophysiol. 2003;114:2188–95. doi: 10.1016/s1388-2457(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 11.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–48. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: Support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 13.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–18. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 14.Manconi M, Ferri R, Zucconi M, et al. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 2011;12:47–55. doi: 10.1016/j.sleep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–80. doi: 10.1093/sleep/22.5.575. [DOI] [PubMed] [Google Scholar]
- 16.Sforza E, Juony C, Ibanez V. Time-dependent variation in cerebral and autonomic activity during periodic leg movements in sleep: implications for arousal mechanisms. Clin Neurophysiol. 2002;113:883–91. doi: 10.1016/s1388-2457(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 17.Fantini M L, Michaud M, Gosselin N, Lavigne G, Montplaisir J. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology. 2002;59:1889–94. doi: 10.1212/01.wnl.0000038348.94399.f6. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie S, De Bilbao F, Haba-Rubio J, Ibanez V, Sforza E. Influence of sleep stage and wakefulness on spectral EEG activity and heart rate variations around periodic leg movements. Clin Neurophysiol. 2004;115:2236–46. doi: 10.1016/j.clinph.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Ali NJ, Davies RJ, Fleetham JA, Stradling JR. Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep. 1991;14:163–5. [PubMed] [Google Scholar]
- 20.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10:169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Dauvilliers Y, Pennestri M-H, Petit D, Dang-Vu T, Lavigne G, Montplaisir J. Periodic leg movements during sleep and wakefulness in narcolepsy. J Sleep Res. 2007;16:333–9. doi: 10.1111/j.1365-2869.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 22.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 23.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991;14:496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- 24.Bixler EO, Kales A, Vela-Bueno A, Jacoby JA, Scarone S, Soldatos CR. Nocturnal myoclonus and nocturnal myoclonic activity in the normal population. Res Commun Chem Pathol Pharmacol. 1982;36:129–40. [PubMed] [Google Scholar]
- 25.Morrish E, King MA, Pilsworth SN, Shneerson JM, Smith IE. Periodic limb movement in a community population detected by a new actigraphy technique. Sleep Med. 2002;3:489–95. doi: 10.1016/s1389-9457(02)00064-3. [DOI] [PubMed] [Google Scholar]
- 26.Scofield H, Roth T, Drake C. Periodic limb movements during sleep: population prevalence, clinical correlates, and racial differences. Sleep. 2008;31:1221–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol. 2010;6:337–46. doi: 10.1038/nrneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Borreguero D, Egatz R, Winkelmann J, Berger K. Epidemiology of restless legs syndrome: the current status. Sleep Med Rev. 2006;10:153–67. doi: 10.1016/j.smrv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 30.Winkelmann J, Schormair B, Lichtner P, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39:1000–6. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 31.Stefansson H, Rye DB, Hicks A, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–47. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 32.Nofzinger EA, Fasiczka A, Berman S, Thase ME. Bupropion SR reduces periodic limb movements associated with arousals from sleep in depressed patients with periodic limb movement disorder. J Clin Psychiatry. 2000;61:858–62. doi: 10.4088/jcp.v61n1108. [DOI] [PubMed] [Google Scholar]
- 33.Salín-Pascual RJ, Galicia-Polo L, Drucker-Colín R. Sleep changes after 4 consecutive days of venlafaxine administration in normal volunteers. J Clin Psychiatry. 1997;58:348–50. doi: 10.4088/jcp.v58n0803. [DOI] [PubMed] [Google Scholar]
- 34.Picchietti D, Winkelman JW. Restless legs syndrome, periodic limb movements in sleep, and depression. Sleep. 2005;28:891–8. [PubMed] [Google Scholar]
- 35.Rottach KG, Schaner BM, Kirch MH, et al. Restless legs syndrome as side effect of second generation antidepressants. J Psychiatr Res. 2009;43:70–5. doi: 10.1016/j.jpsychires.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Wittchen H-U, Pfister H. Frankfurt: Swets & Zeitlinger; 1997. DIA-X-Interviews: Manual für Screening-Verfahren und Interview: (a) Interviewheft Längsschnittuntersuchung (DIA-X-Lifetime); (b) Ergänzungsheft (DIA-X-Lifetime); (c) Interviewheft Querschnittuntersuchung (DIA-X-12 Monate); (d) Ergänzungsheft (DIA-X-12 Monate); (e) PC-Programm zur Durchführung des Interviews (Längs- und Querschnittuntersuchung); (f) Auswertungsprogramm. (Manual) [Google Scholar]
- 37.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 38.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 39.Horne J A, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 40.Griefahn B, Künemund C, Bröde P, Mehnert P. Zur Validität der deutschen Übersetzung des Morningness-Eveningness-Questionnaires von Horne und Östberg. Somnology. 2001;5:71–80. [Google Scholar]
- 41.Fulda S, Hornyak M, Müller K, Cerny L, Beitinger P, Wetter T. Development and validation of the Munich Parasomnia Screening (MUPS) Somnology. 2008;12:56–65. [Google Scholar]
- 42.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 43.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 44.Ferri R, Zucconi M, Manconi M, et al. Different periodicity and time structure of leg movements during sleep in narcolepsy/cataplexy and restless legs syndrome. Sleep. 2006;29:1587–94. doi: 10.1093/sleep/29.12.1587. [DOI] [PubMed] [Google Scholar]
- 45.Aslan S, Isik E, Cosar B. The effects of mirtazapine on sleep: a placebo controlled, double-blind study in young healthy volunteers. Sleep. 2002;25:677–9. [PubMed] [Google Scholar]
- 46.Ruigt GS, Kemp B, Groenhout CM, Kamphuisen HA. Effect of the antidepressant Org 3770 on human sleep. Eur J Clin Pharmacol. 1990;38:551–4. doi: 10.1007/BF00278580. [DOI] [PubMed] [Google Scholar]
- 47.Winokur A, Sateia MJ, Hayes JB, Bayles-Dazet W, MacDonald MM, Gary KA. Acute effects of mirtazapine on sleep continuity and sleep architecture in depressed patients: a pilot study. Biol Psychiatry. 2000;48:75–8. doi: 10.1016/s0006-3223(00)00882-9. [DOI] [PubMed] [Google Scholar]
- 48.Schmid DA, Wichniak A, Uhr M, et al. Changes of sleep architecture, spectral composition of sleep EEG, the nocturnal secretion of cortisol, ACTH, GH, prolactin, melatonin, ghrelin, and leptin, and the DEX-CRH test in depressed patients during treatment with mirtazapine. Neuropsychopharmacology. 2006;31:832–44. doi: 10.1038/sj.npp.1300923. [DOI] [PubMed] [Google Scholar]
- 49.Shen J, Chung SA, Kayumov L, et al. Polysomnographic and symptomatological analyses of major depressive disorder patients treated with mirtazapine. Can J Psychiatry. 2006;51:27–34. doi: 10.1177/070674370605100106. [DOI] [PubMed] [Google Scholar]
- 50.Winokur A, DeMartinis NA, McNally DP, Gary EM, Cormier JL, Gary KA. Comparative effects of mirtazapine and fluoxetine on sleep physiology measures in patients with major depression and insomnia. J Clin Psychiatry. 2003;64:1224–9. doi: 10.4088/jcp.v64n1013. [DOI] [PubMed] [Google Scholar]
- 51.Schittecatte M, Dumont F, Machowski R, Cornil C, Lavergne F, Wilmotte J. Effects of mirtazapine on sleep polygraphic variables in major depression. Neuropsychobiology. 2002;46:197–201. doi: 10.1159/000067812. [DOI] [PubMed] [Google Scholar]
- 52.Carley DW, Olopade C, Ruigt GS, Radulovacki M. Efficacy of mirtazapine in obstructive sleep apnea syndrome. Sleep. 2007;30:35–41. doi: 10.1093/sleep/30.1.35. [DOI] [PubMed] [Google Scholar]
- 53.Marshall NS, Yee BJ, Desai AV, et al. Two randomized placebo-controlled trials to evaluate the efficacy and tolerability of mirtazapine for the treatment of obstructive sleep apnea. Sleep. 2008;31:824–31. doi: 10.1093/sleep/31.6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunner H. Success and failure of mirtazapine as alternative treatment in elderly stroke patients with sleep apnea-a preliminary open trial. Sleep Breath. 2008;12:281–5. doi: 10.1007/s11325-008-0177-7. [DOI] [PubMed] [Google Scholar]
- 55.Chang C-C, Shiah I-S, Chang H-A, Mao W-C. Does domperidone potentiate mirtazapine-associated restless legs syndrome? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:316–8. doi: 10.1016/j.pnpbp.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Pae CU, Kim T-S, Kim J-J, et al. Re-administration of mirtazapine could overcome previous mirtazapine- associated restless legs syndrome? Psychiatry Clin Neurosci. 2004;58:669–70. doi: 10.1111/j.1440-1819.2004.01319.x. [DOI] [PubMed] [Google Scholar]
- 57.Bahk W, Pae C, Chae J, Jun T, Kim K. Mirtazapine may have the propensity for developing a restless legs syndrome? A case report. Psychiatry Clin Neurosci. 2002;56:209–10. doi: 10.1046/j.1440-1819.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 58.Ağargün MY, Kara H, Ozbek H, Tombul T, Ozer OA. Restless legs syndrome induced by mirtazapine. J Clin Psychiatry. 2002;63:1179. doi: 10.4088/jcp.v63n1214a. [DOI] [PubMed] [Google Scholar]
- 59.Bonin B, Vandel P, Kantelip JP. Mirtazapine and restless leg syndrome: a case report. Therapie. 2000;55:655–6. [PubMed] [Google Scholar]
- 60.Kim S-W, Shin I-S, Kim J-M, Park K-H, Youn T, Yoon J-S. Factors potentiating the risk of mirtazapine-associated restless legs syndrome. Hum Psychopharmacol. 2008;23:615–20. doi: 10.1002/hup.965. [DOI] [PubMed] [Google Scholar]
- 61.Manconi M, Ferri R, Zucconi M, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71:834–44. doi: 10.1002/ana.23565. [DOI] [PubMed] [Google Scholar]
- 62.Ohayon MM, O'Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283–95. doi: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sennef C, Heukels A, Van Proosdij J, Ruigt G. Effects of mirtazapine, amitriptyline and their combination on sleep in healthy volunteers measured by an ambulatory digital recording system (VITAPORT II(r)). XXIst Collegium Internationale Neuropsychopharmacologicum; 12th-16th July, 1998; Glasgow, Scotland. [Google Scholar]
- 64.Szegedi A, Schwertfeger N. Mirtazapine: a review of its clinical efficacy and tolerability. Expert Opin Pharmacother. 2005;6:631–41. doi: 10.1517/14656566.6.4.631. [DOI] [PubMed] [Google Scholar]
- 65.Kraus T, Haack M, Schuld A, Hinze-Selch D, Koethe D, Pollmächer T. Body weight, the tumor necrosis factor system, and leptin production during treatment with mirtazapine or venlafaxine. Pharmacopsychiatry. 2002;35:220–5. doi: 10.1055/s-2002-36390. [DOI] [PubMed] [Google Scholar]
- 66.Nicholas LM, Ford AL, Esposito SM, Ekstrom RD, Golden RN. The effects of mirtazapine on plasma lipid profiles in healthy subjects. J Clin Psychiatry. 2003;64:883–9. doi: 10.4088/jcp.v64n0805. [DOI] [PubMed] [Google Scholar]
- 67.Radhakishun FS, Van den Bos J, Van der Heijden BC, Roes KC, O'Hanlon JF. Mirtazapine effects on alertness and sleep in patients as recorded by interactive telecommunication during treatment with different dosing regimens. J Clin Psychopharmacol. 2000;20:531–7. doi: 10.1097/00004714-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 68.De Boer TH, Maura G, Raiteri M, De Vos CJ, Wieringa J, Pinder RM. Neurochemical and autonomic pharmacological profiles of the 6-aza-analogue of mianserin, Org 3770 and its enantiomers. Neuropharmacology. 1988;27:399–408. doi: 10.1016/0028-3908(88)90149-9. [DOI] [PubMed] [Google Scholar]
- 69.Sharpley AL, Elliott JM, Attenburrow MJ, Cowen PJ. Slow wave sleep in humans: role of 5-HT2A and 5-HT2C receptors. Neuropharmacology. 1994;33:467–71. doi: 10.1016/0028-3908(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 70.Sharpley AL, Vassallo CM, Cowen PJ. Olanzapine increases slow-wave sleep: evidence for blockade of central 5-HT(2C) receptors in vivo. Biol Psychiatry. 2000;47:468–70. doi: 10.1016/s0006-3223(99)00273-5. [DOI] [PubMed] [Google Scholar]
- 71.Beitinger M, Fulda S. Long-term effects of antidepressants on sleep. In: Pandi-Perumal SR, Kramer M, editors. Sleep and mental illness. Cambridge: Cambridge University Press; 2010. pp. 183–210. [Google Scholar]
- 72.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–58. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- 73.Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of anti-depressants. CNS Spectr. 2005;10:732–47. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- 74.Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep. 2007;30:755–66. doi: 10.1093/sleep/30.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 76.Ware JC, Blumoff R, Pittard JT. Peripheral vasoconstriction in patients with sleep related periodic leg movements. Sleep. 1988;11:182–6. doi: 10.1093/sleep/11.2.182. [DOI] [PubMed] [Google Scholar]
- 77.Ausserwinkler M, Schmidt P. Erfolgreiche Behandlung des ‘restless legs’-Syndroms bei chronischer Niereninsuffizienz mit Clonidin. Schweiz Med Wochenschr. 1989;119:184–6. [PubMed] [Google Scholar]
- 78.Wagner ML, Walters AS, Coleman RG, Hening WA, Grasing K, Chokroverty S. Randomized, double-blind, placebo-controlled study of clonidine in restless legs syndrome. Sleep. 1996;19:52–8. doi: 10.1093/sleep/19.1.52. [DOI] [PubMed] [Google Scholar]
- 79.Zoe A, Wagner ML, Walters AS. High-dose clonidine in a case of restless legs syndrome. Ann Pharmacother. 1994;28:878–81. doi: 10.1177/106002809402800711. [DOI] [PubMed] [Google Scholar]
- 80.Kugaya A, Seneca NM, Snyder PJ, et al. Changes in human in vivo serotonin and dopamine transporter availabilities during chronic antidepressant administration. Neuropsychopharmacology. 2003;28:413–20. doi: 10.1038/sj.npp.1300036. [DOI] [PubMed] [Google Scholar]
- 81.Warwick JM, Carey PD, Cassimjee N, et al. Dopamine transporter binding in social anxiety disorder: the effect of treatment with escitalopram. Metab Brain Dis. 2012;27:151–8. doi: 10.1007/s11011-012-9280-3. [DOI] [PubMed] [Google Scholar]
- 82.Pogarell O, Poepperl G, Mulert C, et al. SERT and DAT availabilities under citalopram treatment in obsessive-compulsive disorder (OCD) European Neuropsychopharmacology. 2005;15:521–4. doi: 10.1016/j.euroneuro.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Nakayama K, Sakurai T, Katsu H. Mirtazapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Brain Res Bull. 2004;63:237–41. doi: 10.1016/j.brainresbull.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 84.Millan MJ, Gobert A, Rivet JM, et al. Mirtazapine enhances frontocortical dopaminergic and corticolimbic adrenergic, but not serotonergic, transmission by blockade of alpha2-adrenergic and serotonin2C receptors: a comparison with citalopram. Eur J Neurosci. 2000;12:1079–95. doi: 10.1046/j.1460-9568.2000.00982.x. [DOI] [PubMed] [Google Scholar]
- 85.Morita M, Nakayama K. Mirtazapine in combination with perospirone synergistically enhances dopamine release in the rat prefrontal cortex via 5-HT1A receptor activation. Psychiatry Clin Neurosci. 2011;65:246–53. doi: 10.1111/j.1440-1819.2011.02191.x. [DOI] [PubMed] [Google Scholar]
- 86.Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects--a possible opioid involvement in severe depression? J Mol Neurosci. 2002;18:143–9. doi: 10.1385/JMN:18:1-2:143. [DOI] [PubMed] [Google Scholar]
- 87.Schreiber S, Rigai T, Katz Y, Pick CG. The antinociceptive effect of mirtazapine in mice is mediated through serotonergic, noradrenergic and opioid mechanisms. Brain Res Bull. 2002;58:601–5. doi: 10.1016/s0361-9230(02)00825-0. [DOI] [PubMed] [Google Scholar]
- 88.Richardson GS, Roehrs TA, Rosenthal L, Koshorek G, Roth T. Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol. 2002;22:511–5. doi: 10.1097/00004714-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 89.Risberg A, Risberg J, Ingvar DH. Effects of promethazine on nocturnal sleep in normal man. Psychopharmacologia. 1975;43:279–84. doi: 10.1007/BF00429264. [DOI] [PubMed] [Google Scholar]
- 90.Almqvist M, Liljenberg B, Hetta J, Rimon R, Hambert G, Roos BE. Effects of propiomazine on the EEG sleep of normal subjects. Pharmacol Toxicol. 1987;61:278–81. doi: 10.1111/j.1600-0773.1987.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 91.Allen R, Lesage S, Earley C. Anti-histamines and benzodiazepines exacerbate daytime restless legs syndrome (RLS) symptoms. Sleep. 2005;28:A279. [Google Scholar]