Abstract
Study Objectives:
To determine whether short sleep duration alone or combined with obstructive sleep apnea (OSA) is associated with regional body fat including abdominal visceral fat area (VFA) among Korean adults.
Design:
Cross-sectional study.
Setting:
Ansan, South Korea.
Participants:
There were 838 community participants age 40-69 y from the Korean Genome and Epidemiology Study.
Measurements and Results:
Subjective habitual sleep duration and OSA were defined based on a structured sleep questionnaire and a home portable sleep study, respectively. Abdominal VFA and hepatic fat components were assessed by computed tomography. Adjusted mean VFA and hepatic fat were highest in the shortest sleep duration group (< 5 h) and decreased linearly with increasing sleep duration. Individuals with OSA (apnea-hypopnea index ≥ 5) had a higher body mass index, waist circumference, percent body fat, VFA, and hepatic fat than those without OSA after adjusting for age and sex. The adjusted odds ratio (OR) for visceral obesity (VFA ≥ 100 cm2) was 2.05 (95% confidence interval [CI], 1.09-3.86) in individuals sleeping less than 5 h compared with those sleeping longer than 7 h, and 1.57 (95% CI, 1.08-2.26) in individuals with OSA compared with those without OSA, after adjusting for all confounding factors including body mass index. A combination of short sleep duration (< 5 h) and OSA substantially increased the OR for visceral obesity (OR, 4.40, 95% CI, 1.80-10.77) compared with those who slept longer (≥ 7 h) without OSA.
Conclusion:
Short sleep duration and OSA are independently associated with visceral obesity in adults. The association is particularly strong in short sleepers with OSA.
Citation:
Kim NH; Lee SK; Eun CR; Seo JA; Kim SG; Choi KM; Baik SH; Choi DS; Yun CH; Kim NH; Shin C. Short sleep duration combined with obstructive sleep apnea is associated with visceral obesity in Korean adults. SLEEP 2013;36(5):723-729.
Keywords: Body fat composition, obstructive sleep apnea, sleep disordered breathing, short sleep duration, visceral obesity
INTRODUCTION
Modern lifestyles are characterized by partial sleep deprivation and obstructive sleep apnea (OSA). The median sleep time in adults has gradually decreased from 8 h per night to 7 h per night over the past four decades.1 The prevalence of sleep disordered breathing (SDB) in adults in the United States is 24% in men and 9% in women,2 whereas it is 27% and 16% in middle-aged Korean men and women, respectively.3 Interestingly, these trends have paralleled the obesity epidemic.4,5
Previous studies have shown that OSA is closely related to general and abdominal obesity.6 In addition, a number of studies have suggested an association between sleep duration and obesity risks as indicated by a change in body mass index (BMI).7,8 However, visceral obesity is a more reliable predictor for metabolic derangements and cardiovascular diseases than BMI.9 Because short sleep duration was reportedly associated with insulin resistance10 and components of metabolic syndrome,11,12 visceral obesity may provide a link between increased metabolic risk and short sleep duration. However, to the best of our knowledge, only one study showed that extreme sleep duration (≤ 5 h or ≥ 8 h) is related to increases in visceral adipose tissue in African-American and Hispanic-American cohorts, and these findings were only significant in persons younger than 40 y.13 In addition, associations between visceral obesity and OSA have been studied exclusively in individuals of European descent, but not in Asians, who typically have lower BMIs. Despite lower BMI, East Asian men often have more severe OSA than men of European descent.14 This discrepancy may be attributed to the relatively higher visceral fat areas (VFA) in Asians for similar BMI.15 Therefore, the relationship between regional fat distribution, short sleep duration, and OSA may differ in Asians compared with other populations.
Short sleep duration and OSA share similar pathophysiologic mechanisms potentially linking to obesity, such as increased appetite, sympathetic activation, and a proinflammatory state. The presence of OSA in those with short sleep duration may further contribute to visceral obesity when compared with each condition in isolation. However, this combined effect has not yet been studied in Asians or other populations.
Therefore, the goal of this study was to document the distribution of regional body fat including abdominal VFA and subcutaneous fat, hepatic fat, and percent body fat according to subjective habitual sleep duration and the presence or absence of OSA. We also aimed to investigate whether short sleep duration and OSA, separately or jointly, were associated with visceral obesity in Korean adults.
METHODS
Participants
All study participants were drawn from a cohort in the ongoing, prospective, population-based Korean Genome and Epidemiology Study. The Korean Genome and Epidemiology Study was designed to establish a representative adult cohort in the city of Ansan, Korea and to identify the epidemiologic characteristics and the frequency and determinants of chronic diseases in Koreans. From June 2001 to January 2003, a longitudinal cohort was formed consisting of 5,015 participants (2,521 men and 2,494 women age 40-69 y) who participated in a comprehensive health examination and on-site interviews at Korea University Ansan Hospital. Follow-up assessments were conducted biennially with scheduled site visits. At each visit, participants signed an informed consent form, which was approved by the Human Subjects Review Committee at the Korea University Ansan Hospital. Data from the fifth biennial examination from May 2009 to April 2011 were used for the current study because comprehensive body composition measurements using computed tomography (CT) and dual energy X-ray absorptiometry (DXA) were taken at that time. A total of 838 individuals (554 men and 284 women) were recruited. Further details about the protocol and design of the Korean Genome and Epidemiology Study were described previously.16
Anthropometric and Laboratory Measurements
All participants responded to an interviewer-administered questionnaire and underwent a comprehensive physical examination. Sociodemographic characteristics were noted, including age, sex, occupation, marital status, and income. Lifestyle characteristics were also assessed; cigarette smoking and alcohol drinking status were categorized as never, former, and current. Level of exercise was categorized as never, lightly (less than three times/w, ≥ 30 min per session), or regular (three times/w or longer, ≥ 30 min per session) during the previous mo. The presence of disease including diabetes, hypertension, dyslipidemia, and cardiovascular disease was noted as well as medications prescribed to the patients. Diabetes was defined according to the American Diabetes Association criteria using a 75-g oral glucose tolerance test.17 Hypertension was defined according to the Seventh Joint National Committee criteria.18 Diabetes or hypertension was also considered to be present if a previous clinical diagnosis was documented. Individuals with documented events or medical records of myocardial infarction, angina, heart failure, stroke, or peripheral artery disease were considered to have cardiovascular disease.
Blood pressure was measured in a standardized manner by a trained research assistant using a mercury sphygmomanometer. Measurement of seated blood pressure was taken after a 5-min period of rest. At least two blood pressure readings were recorded at 30-sec intervals and the average value was used as a measure of systolic and diastolic blood pressure. Height and body weight were measured to the nearest 0.1 cm or 0.1 kg, respectively. BMI was calculated as weight in kilograms divided by height in meters squared. Waist circumference (WC) was measured at the midpoint between the lower rib margin and the iliac crest in the standing position.
Blood was drawn for biochemical analysis after an overnight fast. Total cholesterol, triglyceride, and high-density lipoprotein cholesterol were measured enzymatically (ADVIA 1650; Bayer, Tarrytown, NY, USA). Enzyme-linked immunosorbent assay was used to measure serum insulin levels.
Sleep Measurements
All participants were asked to answer sleep related questions based on average sleep pattern during the past mo. Subjective average sleep duration per day for the preceding mo was reported in h and min, and classified into five categories; < 5, 5 to 6, 6 to 7, 7 to 8, and ≥ 8 h per day.
The overnight sleep study was performed for each participant at home with a portable device (Embletta® X-100; Embla Systems, San Carlos, CA, USA). Two trained sleep technologists visited the participants' homes in the evening, applied sensors, and instructed them on how to turn the sensors on and off. Participants were also required to record the time they turned the lights on and off and report the times the next morning. Recording channels were as follows: one electroencephalography (C4-A1), one electrooculography (right upper outer canthus-left lower outer canthus), one chin electromyography, one modified lead II electrocardiography, one airflow from nasal airflow pressure transducer, two respiratory effort from chest and abdominal respiratory inductance plethysmography, one pulse oximeter, and one position sensor. For qualified data, sleep status and respiratory events were scored according to standard guidelines.19 Obstructive apnea was defined when airflow dropped ≥ 90% of baseline with ongoing chest and abdominal movement, and hypopnea as a reduction in airflow by ≥ 70% associated with at least 4% oxygen desaturation. The duration threshold for these respiratory events was 10 sec. The apnea-hypopnea index (AHI) was calculated. Depending on the AHI, participants were categorized into three groups: no OSA (AHI < 5), mild OSA (5 ≤ AHI < 15), and moderate to severe OSA (AHI ≥ 15).
Body Composition Measurements
Single-slice CT scanning (Brilliance 64; Philips, Cleveland, OH, USA) was used to quantify intra-abdominal adipose tissue. Scans were conducted at 120 kV with a slice thickness of 5 mm at the level of the L4-L5 vertebral interspace. The total area of intra-abdominal fat was delineated by manual tracing within the muscle wall, and VFA was defined as an area with an attenuation range between -190 and -30 Hounsfield units (HU). Subcutaneous fat area (SFA) was calculated as the total abdominal fat area minus the VFA. Visceral obesity was defined as a VFA of ≥ 100 cm2, which is the proposed cutoff point for obesity in Asians according to the Japan Society for the Study of Obesity.20
Hepatic attenuation was also measured by CT. An experienced radiologist calculated mean hepatic attenuation by randomly selecting three regions of interest on five transverse sections. The mean splenic attenuation was also calculated by averaging the values from two random regions of interest of splenic attenuation measurements. The liver attenuation index (LAI) as a parameter for liver fat accumulation was calculated as the mean hepatic attenuation minus mean splenic attenuation.
Total lean mass, total body fat, and percent body fat (%BF) were estimated by whole body DXA using DPX-MD+ (General Electric, Madison, WI, USA).
Statistical Analyses
Baseline characteristics were compared among groups stratified by sleep duration and three AHI categories, using one-way analysis of variance for numeric variables and a chi-square test for categorical variables. Nonnormally distributed variables such as triglyceride and insulin were presented as the median and interquartile range for each group, and the differences were tested after logarithmic transformation.
Because body fat measures such as BMI, WC, %BF, VFA, SFA, and LAI were strongly influenced by age and sex, subsequent analyses to detect associations of body fat with sleep duration or OSA were conducted after adjusting for age and sex using univariate analysis of covariance.
Multivariate logistic regression analyses were conducted to identify the combined effects of short sleep duration and OSA on visceral obesity. All participants were assigned to six groups based on sleep duration (< 5 h, 5 to 7 h, ≥ 7 h) and presence or absence of OSA. In the analysis, two models were fit for each outcome: model 1 (adjusted for age and sex) and model 2 (adjusted for age, sex, alcohol, smoking, exercise, diabetes mellitus, hypertension, cardiovascular disease, and BMI). Subsequently, the combined effects were tested in participants with BMI ≥ 25 kg/m2 or without obesity (BMI < 25 kg/m2) to evaluate whether the effects were modified by obesity status.
A P value less than 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
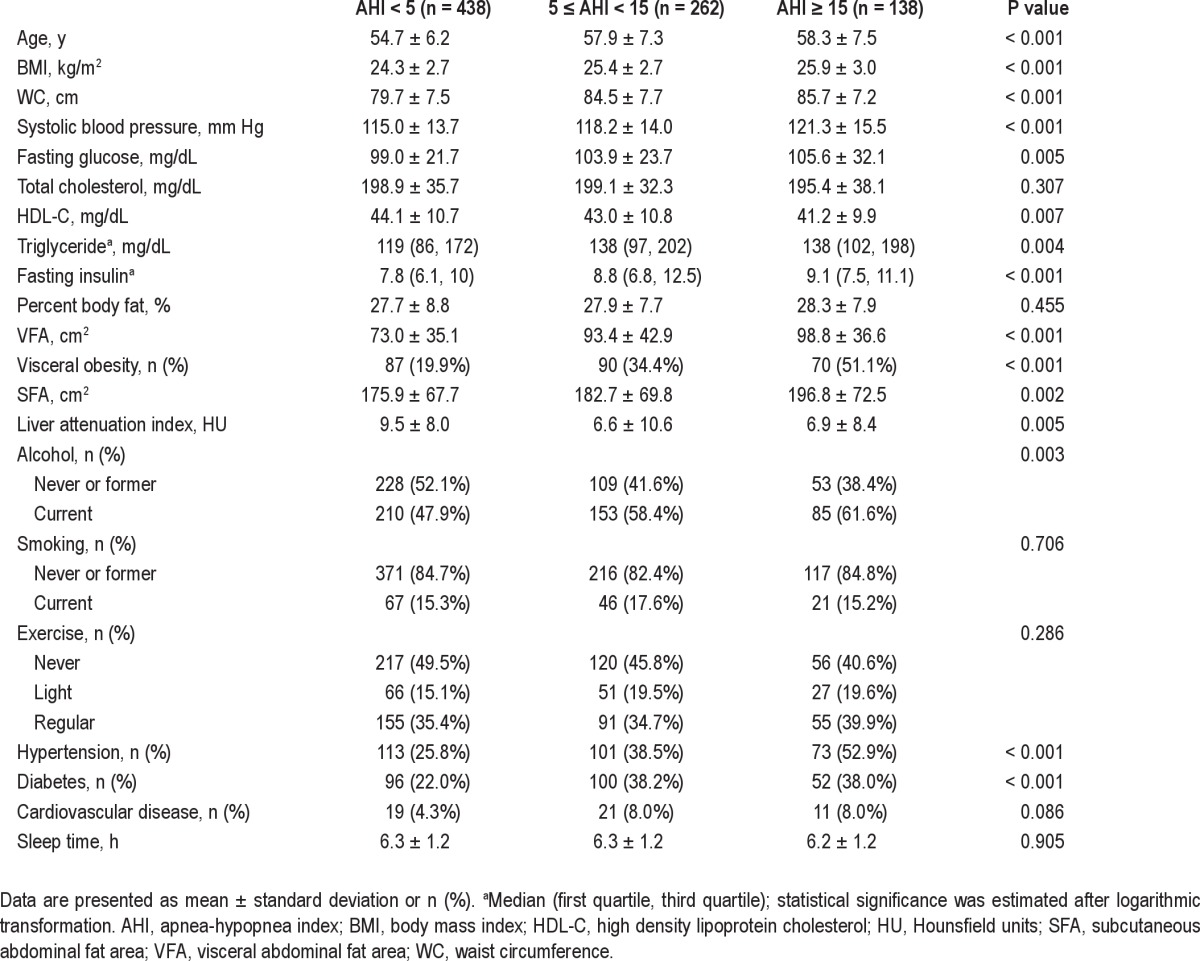
The mean age of all participants was 56.3 ± 6.9 y, and mean sleep duration was 6.3 ± 1.2 h per day. Table 1 shows the characteristics of the study sample stratified by sleep duration. All the body fat measures including BMI, WC, %BF, VFA, and SFA were highest in the shortest sleep duration (< 5 h) group. Mean %BF and VFA decreased linearly with increasing sleep duration (P for trend < 0.001, = 0.007, respectively). LAI was also lowest in those with sleep duration < 5 h and increased linearly with sleep duration, although this trend was not significant. The presence of hypertension, diabetes, cardiovascular disease, and OSA was comparable among the groups. The second baseline analysis according to three categories of AHI (< 5, 5 to 15, and ≥ 15) showed that all body fat composition measurements except % BF were significantly higher in participants with higher AHI values (Table 2). Mean serum fasting glucose, blood pressure, triglycerides, fasting insulin, and the proportions of participants with hypertension and diabetes mellitus were significantly higher in those with high AHI values.
Table 1.
Baseline characteristics according to duration of sleep
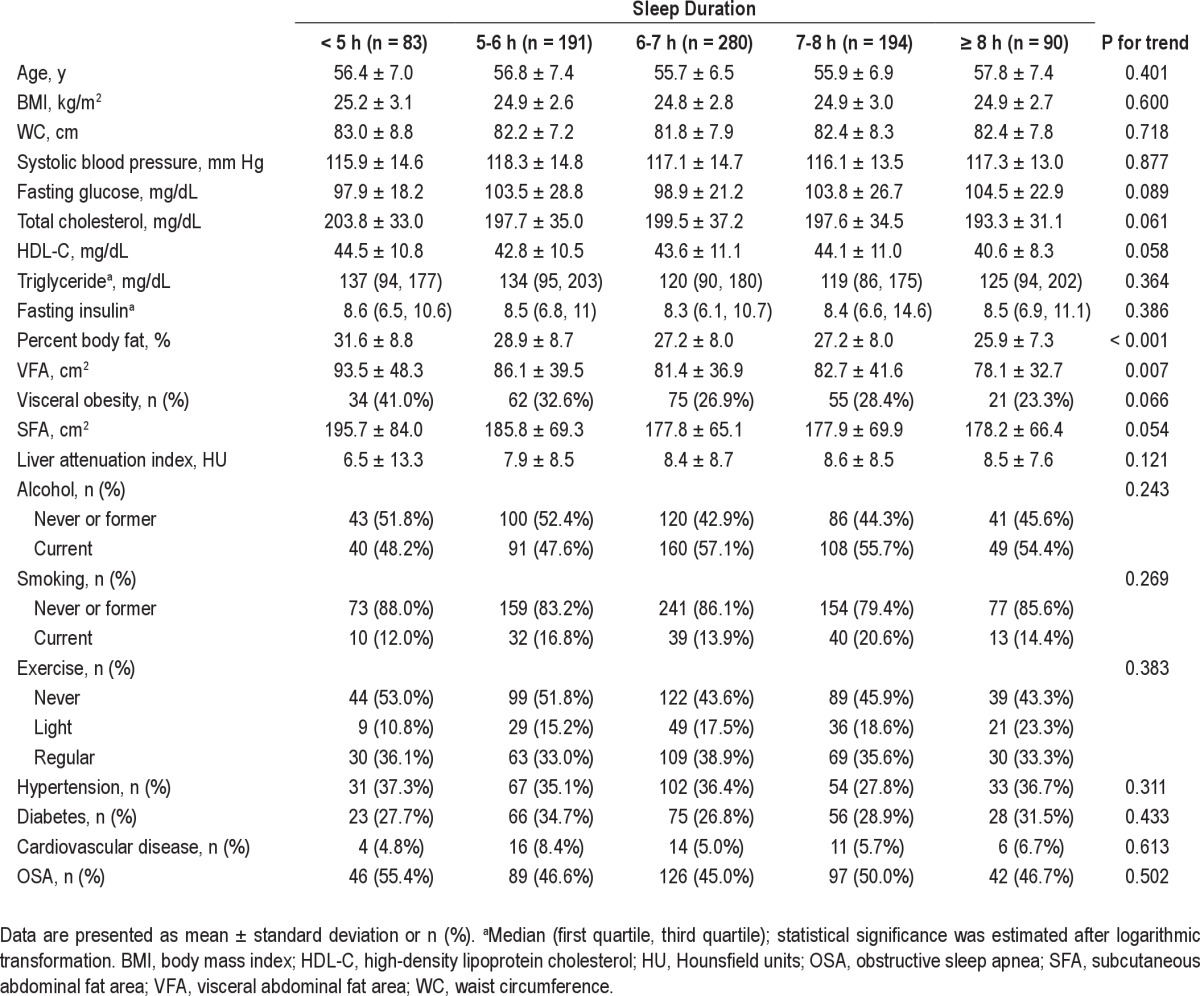
Table 2.
Baseline characteristics according to severity of obstructive sleep apnea
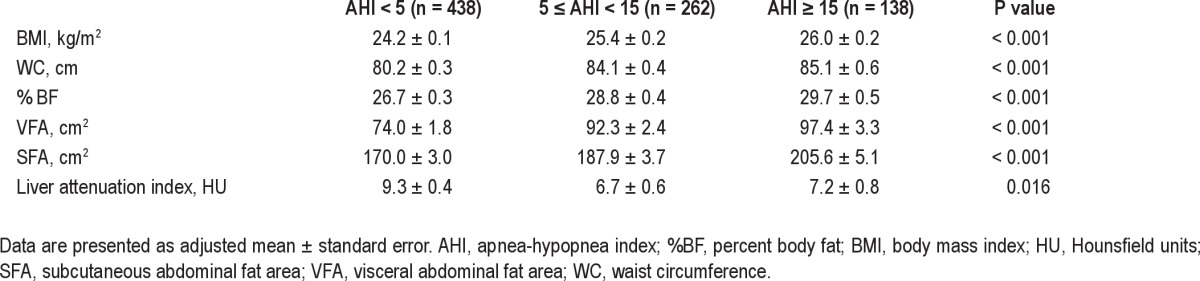
Even after adjusting for age and sex, there was a significant negative association between sleep duration and VFA (Table 3), and all body fat measures including BMI and VFA were positively associated with severity of OSA (Table 4).
Table 3.
Age- and sex-adjusted mean body composition variables according to duration of sleep
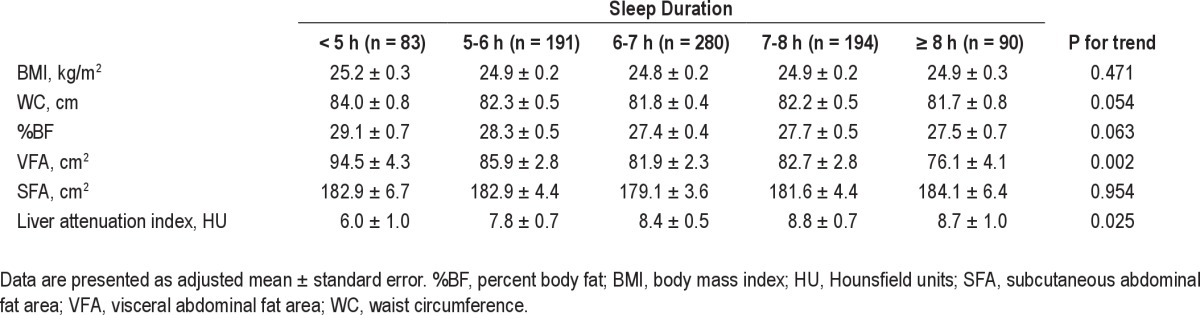
Table 4.
Age- and sex-adjusted mean body composition variables according to severity of obstructive sleep apnea
Multivariate logistic regression analyses were conducted to investigate the effects of sleep duration and OSA on visceral obesity (VFA ≥ 100 cm2) (Table 5). The short sleep duration group (< 5 h) had an increased likelihood of visceral obesity in comparison with the longer sleep duration group (> 7 h), even after adjusting for confounding variables such as BMI (odds ratio [OR] 2.05, 95% confidence ratio [CI], 1.09 - 3.86) (Table 5). Similarly, the presence of OSA (AHI ≥ 5) was associated with a 57% increase in OR for visceral obesity (OR, 1.57, 95% CI, 1.08 - 2.26) (Table 6).
Table 5.
Odds ratios (95% confidence interval) for visceral obesity (visceral abdominal fat area ≥ 100 cm2) according to duration of sleep
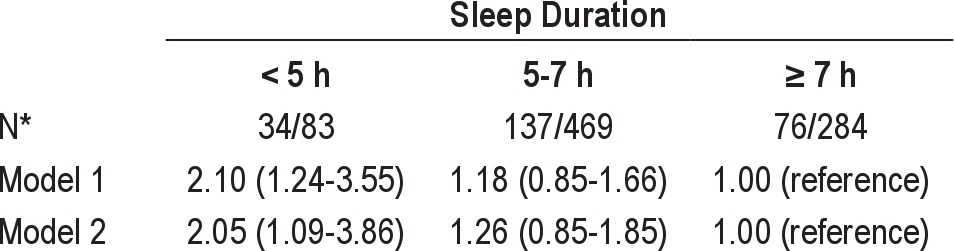
Table 6.
Odds ratios (95% confidence interval) for visceral obesity (visceral abdominal fat area ≥ 100 cm2) according to the presence or absence of obstructive sleep apnea

Interestingly, OSA and short sleep duration differently contributed to visceral obesity when analyzing the data separately in men and women. The influence of OSA on visceral obesity was more prominent in men than women (OR, 2.62 [1.78-3.85] versus 1.64 [0.90-3.00]), whereas short sleep duration (< 5 h) was more closely associated with visceral obesity in women than in men (OR, 3.15 [1.28-7.76] versus 1.88 [0.94-3.76]) after adjusting for age.
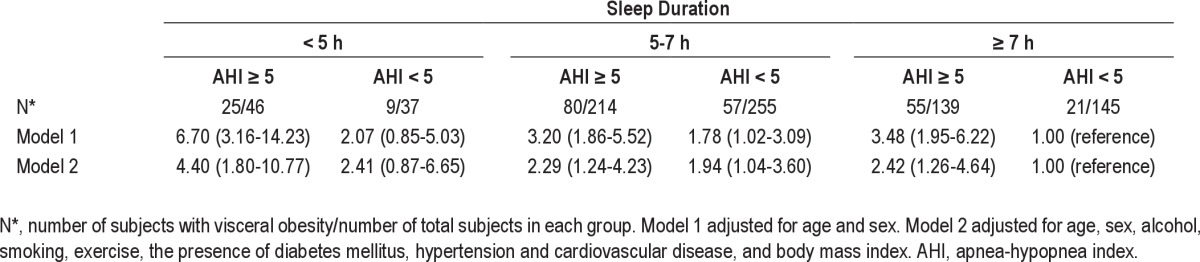
Finally, when the effects of sleep duration and OSA were modeled jointly, shorter sleep duration combined with OSA resulted in a greatly increased likelihood of visceral obesity (Table 7). Participants sleeping less than 5 h per day who also had OSA were more than four times as likely to have visceral obesity, when compared with those sleeping more than 7 h per day without OSA (OR, 4.40, 95% CI, 1.80-10.77), even after adjusting for age, sex, alcohol consumption, smoking, exercise, the presence of diabetes mellitus, hypertension and cardiovascular disease, and BMI (model 2).
Table 7.
Odds ratios (95% confidence interval) for visceral obesity (visceral abdominal fat area ≥ 100 cm2) according to duration of sleep and obstructive sleep apnea
In the subsequent subgroup analysis, the synergistic effect of short sleep duration and OSA on VFA was more remarkable among participants with BMI less than 25 kg/m2 than those with BMI greater than 25 kg/m2 (OR, 9.34 [1.72-50.22] versus 3.81 [1.29-11.27], respectively).
DISCUSSION
To the best of our knowledge, this is the first study investigating the relationships between sleep duration and OSA with various components of body fat composition measured by CT and DXA. In this study, participants with short sleep duration and OSA had higher mean BMI, WC, VFA, and %BF than those with long sleep duration and without OSA. Short sleep duration (< 5 h) combined with OSA was also associated with increased risk for visceral obesity.
In contrast to a previous study13 that reported that ≤ 5 h and ≥ 8 h of sleep were related to a greater accumulation of visceral adipose tissue compared with sleep duration between 6 and 7 h in young individuals, we found that the shorter the sleep duration, the higher the mean VFA in adults older than 40 y. Although metabolic risks associated with short sleep duration have been demonstrated in previous studies, the relationship between long sleep duration and metabolic derangement and obesity is controversial.11,12,21 Furthermore, as described previously, there are definite differences in body composition between populations. That is, East Asians generally have lower BMI but higher VFA compared with individuals of European descent.15,22 Therefore, the effect of short or long sleep duration on visceral adiposity may differ by age, BMI, population, study design, and adjustment for confounding factors.
In addition, OSA is closely associated with general and central obesity. The Wisconsin Sleep Cohort study showed that over 8 y, increased mean AHI was significantly greater in obese (BMI > 30) participants than in nonobese participants.23 Another epidemiologic study showed that VFA was a reliable predictor of AHI after adjusting for confounding factors.24 Obesity is a significant risk factor for the development of OSA via fat accumulation in the cervical region, resulting in upper airway narrowing.25 However, intermittent hypoxia and associated increased inflammation in response to the OSA may be a causal factor for obesity, especially for visceral obesity.26,27 Although the current study did not determine a causal relationship, it showed that participants with OSA had a higher BMI and VFA than those without OSA.
Interestingly, the effect of OSA is more remarkable in individuals who report short sleep duration. In other words, if individuals with OSA sleep for a shorter duration, they may have increased likelihood of visceral obesity. Individuals with OSA are commonly known to have shorter sleep duration compared with those without OSA.28 In addition, short sleep latency, low sleep efficiency, and high arousal index were observed in patients with OSA.29,30 However, several studies have reported that total sleep time was not different in individuals with or without OSA.31,32 We also noticed that sleep time did not differ by OSA severity in this study. In addition, the frequency of OSA according to sleep duration did not differ. These data suggested that short sleep duration and OSA each independently contributed to the presence of visceral obesity, and their combination had synergism in this study.
There was a sex difference in these associations. In men, the presence of OSA rather than sleep duration was a stronger contributor for visceral obesity. On the contrary, short sleep duration was more influenced by visceral obesity than OSA among women. Exact causes about the difference are unknown at this point, but Simpson et al.33 found that abdominal obesity was more clearly associated with OSA severity in men compared with women. The stronger association between short sleep duration and visceral obesity in women may be explained by eating behavior.34,35 However, the mechanism of this heterogeneity should be evaluated in future studies.
The combined effects of short sleep duration and OSA on metabolism and body composition have not yet been studied extensively. Only a few studies indirectly showed the augmented combined effects. For example, combined OSA and short sleep slightly increased the likelihood of gestational diabetes mellitus compared with OSA alone or short sleep alone in pregnant women.36 There is a growing evidence from both clinical and laboratory studies that short sleep duration or sleep deprivation and OSA share common pathophysiologic features of obesity and metabolic dysregulation. Appetite increases via alteration of hormones related to satiety and weight control in patients with OSA as well as in patients who only sleep for a short duration.37,38 Moreover, both OSA and short sleep duration are associated with increased sympathetic activation38,39 and systemic inflammatory state.40,41 Therefore, if short sleep duration is combined with OSA, the associated metabolic risks may be augmented. The most important finding of the current study is that this association was evident in relatively lean individuals, even after adjusting for BMI. The mean BMI of the study sample was 24.9 kg/m2, and 95.6% (801 of 838) of total participants had a BMI less than 30 kg/m2. Although most studies investigating the effects of sleep disorders are performed using obese individuals, there is some evidence of the effect of sleep disorders on obesity and metabolic risk factors in nonobese individuals. For example, OSA was associated with dyslipidemia and hypertension in nonobese Chinese individuals42 and habitual snoring was independently associated with elevated glucose and insulin levels in nonobese Korean individuals.43 This study also showed that the synergistic effect of short sleep duration and OSA on visceral obesity was prominent in nonobese individuals. Short sleep duration and OSA may have a lesser effect on metabolic risks in obese persons than in nonobese persons, perhaps due to preexisting metabolic derangements in obese persons. These results have important therapeutic implications and suggest that normalizing sleep time and treatment of OSA may be efficient in reducing combined metabolic risks even in nonobese persons.
This study has some limitations. First, because of its cross-sectional design, it was difficult to characterize the causal relationship between sleep disorders and obesity measures. Second, sleep duration and snoring were self-reported, not objectively measured. Therefore, bed partners or family members also responded to the questionnaire about sleep habits to strengthen the reliability of answers. Third, when testing the combined effect of sleep duration and OSA on visceral obesity, the number of individuals in several subgroups was relatively small, resulting in large confidence intervals.
In conclusion, we found that short sleep duration and OSA are both positively associated with regional body fat accumulation, and their combination has a synergistic effect on visceral obesity. Visceral obesity was evident in those who slept less than 5 h per night and had OSA, after adjusting for all confounders. This study suggests a possible mechanism of increased cardiovascular disease in those with short sleep duration and OSA, and also provides a treatment strategy that involves identifying high-risk groups and encouraging increased sleep duration and OSA treatment among these individuals to reduce their metabolic risks.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. Nan Hee Kim and Chol Shin contributed equally to this work.
ACKNOWLEDGMENTS
This research was supported by grants (2009-E71002-00, 2010-E71001-00) from the Korean Centers for Disease Control and Prevention.
REFERENCES
- 1.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occur-rence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, In K, You S, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35:561–6. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711–9. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Garcia E, Faubel R, Leon-Munoz L, Zuluaga MC, Banegas JR, Rodriguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87:310–6. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33:161–7. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowers JR. Obesity as a cardiovascular risk factor. Am J Med. 2003;115:37S–41S. doi: 10.1016/j.amjmed.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi KM, Lee JS, Park HS, Baik SH, Choi DS, Kim SM. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes (Lond) 2008;32:1091–7. doi: 10.1038/ijo.2008.62. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Relation between metabolic syndrome and sleep duration in Japan: a large scale cross-sectional study. Intern Med. 2011;50:103–7. doi: 10.2169/internalmedicine.50.4317. [DOI] [PubMed] [Google Scholar]
- 13.Hairston KG, Bryer-Ash M, Norris JM, Haffner S, Bowden DW, Wagenknecht LE. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep. 2010;33:289–95. doi: 10.1093/sleep/33.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li KK, Kushida C, Powell NB, Riley RW, Guilleminault C. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. 2000;110:1689–93. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT) Am J Clin Nutr. 2007;86:353–9. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 16.Shin C, Abbott RD, Lee H, Kim J, Kimm K. Prevalence and correlates of orthostatic hypotension in middle-aged men and women in Korea: the Korean Health and Genome Study. J Hum Hypertens. 2004;18:717–23. doi: 10.1038/sj.jhh.1001732. [DOI] [PubMed] [Google Scholar]
- 17.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 20.Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66:987–92. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 21.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24:731–43. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9:381–7. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 23.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara E, Kihara S, Yamashita S, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241:11–8. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 25.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–6. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 26.Patel SR. Shared genetic risk factors for obstructive sleep apnea and obesity. J Appl Physiol. 2005;99:1600–6. doi: 10.1152/japplphysiol.00501.2005. [DOI] [PubMed] [Google Scholar]
- 27.Alam I, Lewis K, Stephens JW, Baxter JN. Obesity, metabolic syndrome and sleep apnoea: all pro-inflammatory states. Obes Rev. 2007;8:119–27. doi: 10.1111/j.1467-789X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 28.Chin K, Oga T, Takahashi K, et al. Associations between obstructive sleep apnea, metabolic syndrome, and sleep duration, as measured with an actigraph, in an urban male working population in Japan. Sleep. 2010;33:89–95. doi: 10.1093/sleep/33.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y, Ning Y, Huang L, et al. Polysomnographic characteristics of daytime sleepiness in obstructive sleep apnea syndrome. Sleep Breath. 2012;16:375–81. doi: 10.1007/s11325-011-0515-z. [DOI] [PubMed] [Google Scholar]
- 30.Roure N, Gomez S, Mediano O, et al. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med. 2008;9:727–31. doi: 10.1016/j.sleep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Svensson M, Franklin KA, Theorell-Haglow J, Lindberg E. Daytime sleepiness relates to snoring independent of the apnea-hypopnea index in women from the general population. Chest. 2008;134:919–24. doi: 10.1378/chest.08-0847. [DOI] [PubMed] [Google Scholar]
- 32.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 33.Simpson L, Mukherjee S, Cooper MN, et al. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. Sleep. 2010;33:467–74. doi: 10.1093/sleep/33.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trace SE, Thornton LM, Runfola CD, Lichtenstein P, Pedersen NL, Bulik CM. Sleep problems are associated with binge eating in women. Int J Eat Disord. 2012;45:695–703. doi: 10.1002/eat.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbalán-Tutau MD, Madrid JA, Garaulet M. Timing and duration of sleep and meals in obese and normal weight women. Association with increase blood pressure. Appetite. 2012;59:9–16. doi: 10.1016/j.appet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Reutrakul S, Zaidi N, Wroblewski K, et al. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care. 2011;34:2454–7. doi: 10.2337/dc11-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulukavak Ciftci T, Kokturk O, Bukan N, Bilgihan A. Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respiration. 2005;72:395–401. doi: 10.1159/000086254. [DOI] [PubMed] [Google Scholar]
- 38.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 39.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 41.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 42.Lin QC, Zhang XB, Chen GP, Huang DY, Din HB, Tang AZ. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome in nonobese adults. Sleep Breath. 2012;16:571–8. doi: 10.1007/s11325-011-0544-7. [DOI] [PubMed] [Google Scholar]
- 43.Shin C, Kim J, Lee S, et al. Association of habitual snoring with glucose and insulin metabolism in nonobese Korean adult men. Am J Respir Crit Care Med. 2005;171:287–91. doi: 10.1164/rccm.200407-906OC. [DOI] [PubMed] [Google Scholar]


