Key Points
A single exposure to protamine and heparin during CPB is highly sensitizing; 29% of patients develop Abs to PRT/H complexes by day 30 after CPB.
PRT/H Abs share several features with platelet factor 4/heparin Abs, including high titer formation after CPB, heparin dependence, antigen specificity, and platelet activation.
Abstract
Protamine is routinely used to reverse heparin anticoagulation during cardiopulmonary bypass (CPB). Heparin interacts with protamine to form ultralarge complexes that are immunogenic in mice. We hypothesized that patients exposed to protamine and heparin during CPB will develop antibodies (Abs) to protamine/heparin (PRT/H) complexes that are capable of platelet activation. Specimens from a recently completed prospective clinical trial (HIT [for heparin-induced thrombocytopenia] 5801 study; n = 500) of CPB patients were examined for PRT/H Abs at baseline, at time of hospital discharge (between days 3 through 7), and 30 days after CPB. PRT/H antibody features were characterized and correlated with adverse cardiovascular outcomes. We found a high incidence of PRT/H antibody formation (29%) in patients undergoing cardiac surgery. PRT/H Abs were of high titer (mean titer 1:14 744), showed heparin-dependent binding, and activated platelets in the presence of protamine. PRT/H Abs showed no cross-reactivity to platelet factor 4/heparin complexes, but were cross-reactive with protamine-containing insulin preparations. In the absence of circulating antigen at day 30, there were no complications of thrombocytopenia, thrombotic events, or long-term cardiovascular events. These studies show that Abs to PRT/H occur commonly after cardiac bypass surgery, share a number of serologic features with HIT Abs, including platelet activation, and may pose health risks to patients requiring drug reexposure.
Introduction
Protamine sulfate is currently the only therapeutic agent approved by the US Food and Drug Administration for reversal of heparin anticoagulation. It is the mainstay of therapy in cardiopulmonary bypass (CPB), where rapid reversal of heparin anticoagulation is essential for achieving surgical hemostasis. However, protamine use in CPB is associated with the development of a number of adverse effects, ranging from minor hemodynamic instability to life-threatening anaphylactic complications and fatal cardiovascular collapse.1-4 Major adverse reactions related to protamine exposure have been reported to occur in 2.6% of cardiac surgical procedures,3 and these complications of protamine therapy are highly associated with adverse postoperative outcome.5,6
Protamine, a highly basic protein, binds heparin through charge interactions and sequesters heparin from its catalytic effects on antithrombin. Recently, we have shown that heparin-binding proteins such as protamine and lysozyme interact with heparin in a charge-dependent manner to form protamine/heparin (PRT/H) or lysozyme/H ultralarge complexes that are immunogenic in mice.7 In this preliminary clinical study, we show that patients exposed to protamine and heparin in the course of cardiac surgery become sensitized to PRT/H complexes.7 Preliminary findings of PRT/H antibodies (Abs) in CPB patients prompted this study of an expanded cohort of patients. Using data obtained from a recently completed multicenter, prospective, observational National Institutes of Health (NIH) study of cardiac surgery patients (HIT [for heparin-induced thrombocytopenia] 5801 study), we describe the incidence, serologic characteristics, functional characteristics, and clinical outcomes associated with the development of a novel class of heparin-dependent Abs to PRT/H complexes.
Methods
Clinical data
The HIT 580l study was a NIH-sponsored clinical observational trial (Rare Disease Clinical Research Network 5801) to examine the association of platelet factor 4/heparin (PF4/H) antibody seropositivity with thromboembolic events after cardiac surgery and incidence of delayed HIT in patients undergoing cardiac surgery between 2005 and 2009. Plasma was collected from patients prior to surgery (baseline), on days 3 through 7, and 30 days after surgery and stored at −80°C. Platelet counts from the day after surgery were also sought by chart review. With institutional review board approval (Duke University Medical Center Institutional Review Board Pro00010736), patients from a single participating center (Duke) who had samples available in EDTA at all 3 time points and who underwent coronary artery bypass grafting or coronary artery bypass grafting with valve repair and CPB were included (n = 500). Patients gave informed consent in accordance with the Declaration of Helsinki. Control patients for the laboratory-based study were comprised of healthy volunteers (n = 101) without diabetes, prior cardiac surgery, prior heparin exposure, or chronic medications.
Long-term follow-up was available for a median of 765 days. A major adverse cardiac event was defined as death, repeat cardiac surgery, myocardial infarction, or need for myocardial revascularization. Thromboembolic complications, that is, all new arterial or venous thrombosis occurring after surgery, were sought up to 90 days after surgery as part of the HIT 5801 study protocol.
Serologic and platelet activation studies
PF4/H Abs were measured using a commercial immunoglobulin G (IgG)–specific PF4/H enzyme-linked immunosorbent assay (GTI, Waukesha, WI). Serologic characterization of PRT/H Abs was performed using previously published methods.7 Briefly, microtiter plates were coated overnight with an optimized ratio of PRT/H complexes (31 ug/mL:4 U/mL; molar ratio 3:1). After washing unbound antigen, wells were incubated for 1 hour at room temperature with plasma diluted at 1:500 in 10% fetal calf serum/ phosphate-buffered saline (PBS), followed by detection of human Abs with an anti-human IgG γ peroxidase (1:2500 dilution; Sigma, St. Louis, MO). Bound Abs were detected colorimetrically using tetramethylbenzidine peroxide substrate (KPL, Gaithersburg, MD). Absorbance was measured at 450 nm using Spectramax 384 PLUS (Molecular Devices, Sunnyvale, CA), and results were analyzed using SoftMax PRO software (Molecular Devices). Antibody titers were performed using serial dilutions, and final titers were reported as the dilution necessary for absorbance at 450 nm (A450nm) <1.2. Specificity assays were completed at dilutions necessary for 50% maximal binding. Intra- and interassay performance was measured as described in supplemental Figure 1. Reactivity of the control patients in the PRT/H enzyme-linked immunosorbent assay (ELISA) was used to establish the background signal in the assay as well as a positive cutoff value for patients undergoing CPB. Positivity in the PRT/H ELISA was calculated as the mean A450nm of 101 control patients +3 times the standard deviation (SD) of the cohort. The serotonin release assay was performed on representative samples from day 30 and was used to examine the functional effects of PRT/H Abs,8 with modifications as provided in supplemental materials.
Statistical analysis
In vitro antibody binding was compared using Student t test. Correlation of PRT/H and PF4/H antibody levels was tested with Pearson’s correlation coefficient. Patient characteristics were compared by χ2 or Student t test as appropriate.
Spearman’s rank correlation was used to relate platelet counts to PRT/H antibody levels at the three sampling timepoints. Statistical analysis of clinical outcomes is described in Supplemental Methods.
Results
Patient demographics
Demographics of patients undergoing cardiac surgery are shown in supplemental Table 1. Patients undergoing CPB were predominantly male (71.6%) and had known risk factors for heart disease (hypertension, peripheral vascular disease, smoking, and congestive heart failure). In this cohort, 30.8% were diabetic, of whom 45% required insulin. The mean Hannan score, a commonly used perioperative risk score based on patient characteristics and premorbid conditions, was 0.036. This translates into a 3.6% risk for in-hospital mortality after surgery.9
PRT/H antibody formation after CPB
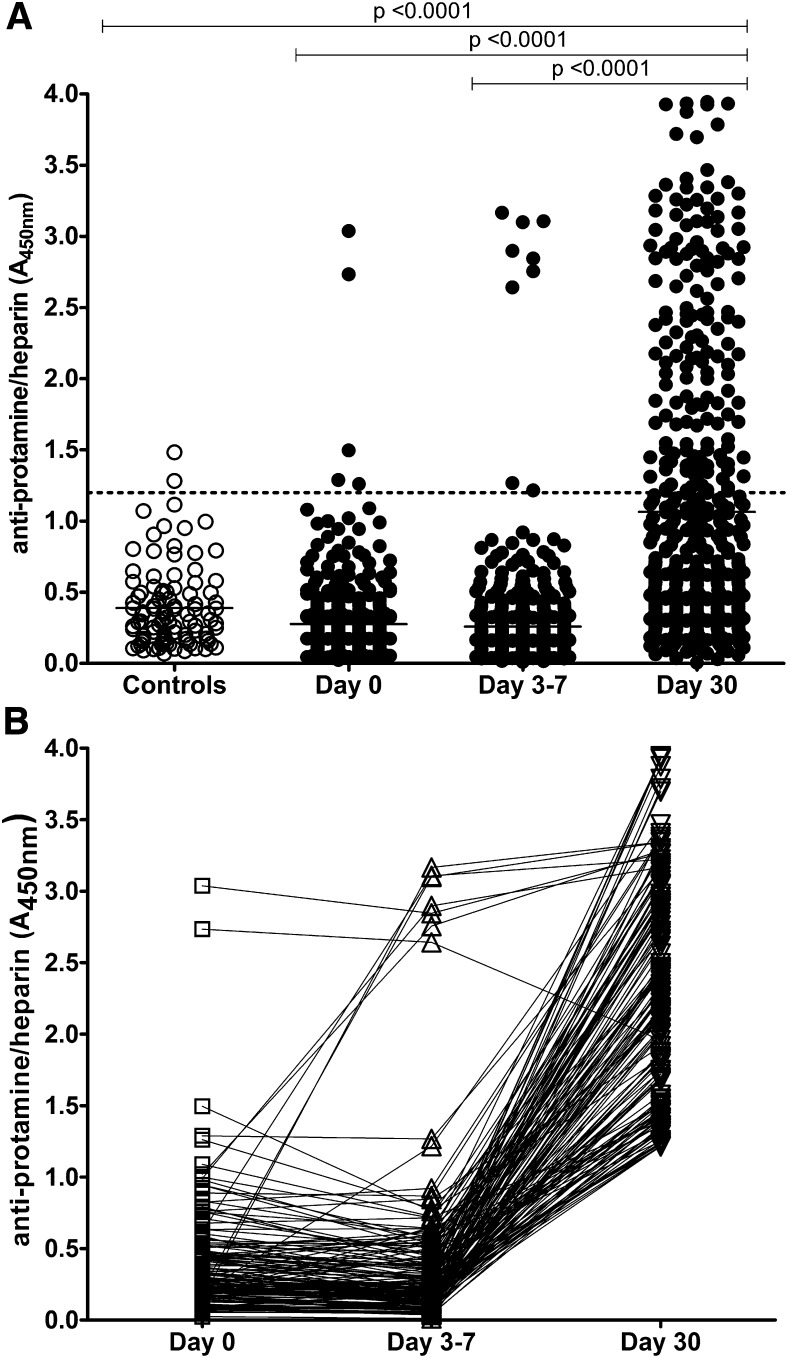
Plasma from 101 healthy volunteers and 500 patients undergoing CPB was screened for PRT/H Abs by ELISA. As shown in Figure 1A, the mean A450nm for control patients was 0.39 + 0.28, yielding a positive cutoff value (≥ mean A450nm +3 SD) for the PRT/H ELISA of 1.2. Using this positive cutoff value, the prevalence of PRT/H Abs was 2/101 (2%) in the control patients, 5/500 (1%) of patients at day 0, 9/500 (2%) of patients at days 3 through 7, and 154/500 (31%) of patients at day 30 after CPB. While most patients who were seropositive at day 0 remained seropositive through day 30, 2/5 patients became transiently seronegative at days 3 through 7 (Figure 1B). New seroconversions were seen in 6/497 (1.2%) patients at days 3 through 7 and in 143/489 (29%) patients at day 30. The mean PRT/H reactivity (mean A450nm + SD) of control patients (0.39 ± 0.28) did not differ from the reactivity of patients at day 0 (0.28 + 0.26) or days 3 through 7 (0.26 + 0.37), but differed significantly from patient plasma at day 30 (1.06 + 0.96, P < .0001). When patients were analyzed for timing of seroconversions, only a few patients seroconverted during the hospital stay (11/500 by days 3 through 7), while the majority of seroconversions occurred between discharge (days 3 through 7) and day 30 (Figure 1B).
Figure 1.
Incidence of PRT/H Abs in healthy patients and patients undergoing CPB. (A) PRT/H antibody reactivity was measured by ELISA in healthy patients (Controls, n = 101) and patients after CPB (n = 500) at baseline, days 3 through 7, and day 30 after CPB. (B) Time course of seroconversion in all seropositive patients (n = 154; A450nm >1.2). Each line represents the seroconversion profile of a patient after CPB.
Serologic characteristics of PRT/H Abs
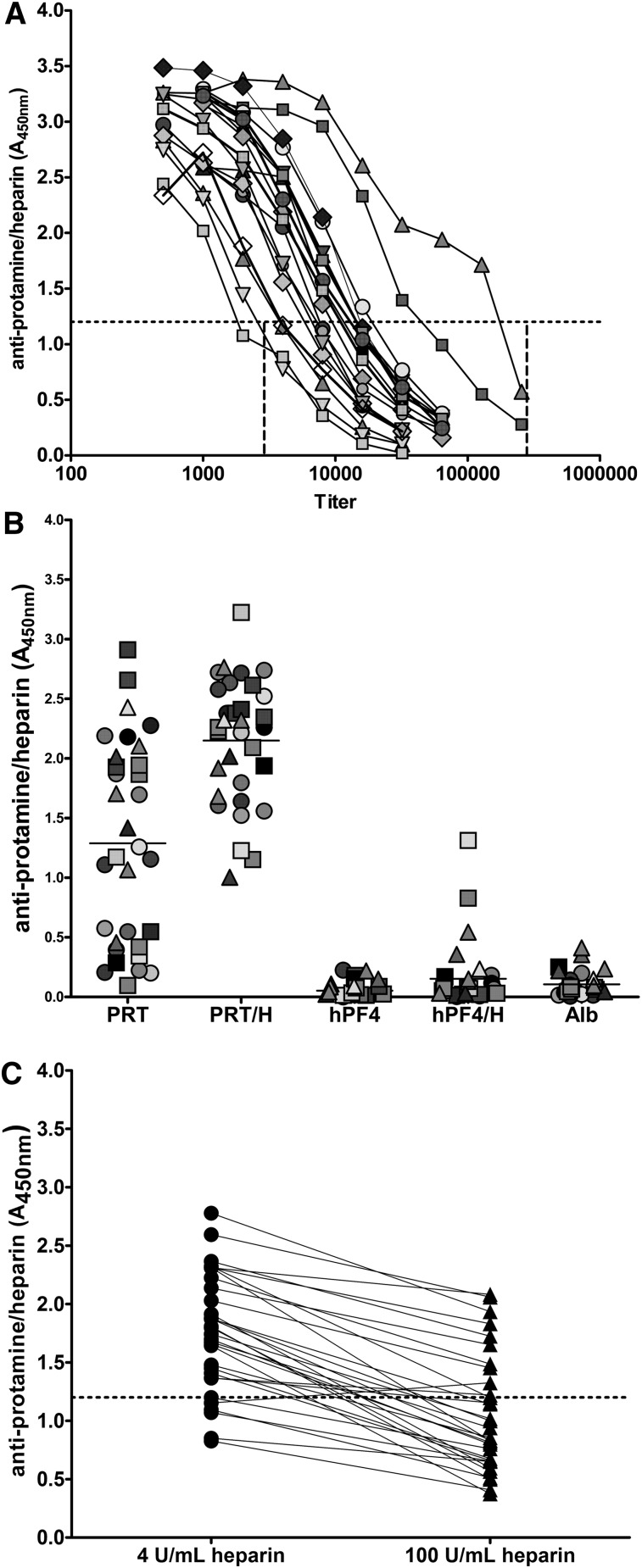
Serologic features of PRT/H Abs, including titer, specificity, and heparin dependence, were examined in patients expressing high-titer Abs (mean A450nm >3.0, n = 32). To overcome the effects of antibody saturation in binding assays and to “normalize” antibody concentrations among samples in the absence of a monoclonal antibody or established standards for PRT/H Abs, titration assays were performed to establish the range of titers for each high-positive patient. As shown in Figure 2A, titers of PRT/H Abs ranged from 1:1800 to 1: 175 000 (mean antibody titer, 1:14 744).
Figure 2.
Serologic characteristics of PRT/H Abs. (A) Representative titration curves of high-titer PRT/H Abs in 19 patients demonstrating antibody titer range of 1:1800 to 1:175 000. (B) Specificity of high-titer PRT/H Abs with PRT/H antibody levels of A450nm >3 (n = 32). Each symbol represents an individual CPB patient. (C) Heparin-dependent binding of PRT/H Abs of patients depicted in B.
As shown in Figure 2B, all high-titer PRT/H Abs bound (mean A450nm ± SD) to PRT/H complexes (2.15 ± 0.49) and 15/32 (47%) recognized protamine alone (1.29 ± 0.84). In contrast, PRT/H Abs showed minimal binding to human platelet factor 4 (hPF4) alone (0.07 ± 0.07), hPF4/H complexes (0.15 ± 0.25), or albumin (0.1 ± 0.1; P < .0001 for PRT/H vs hPF4, PRT/H vs hPF4/H, and PRT/H vs albumin). Because antibody binding was significantly higher with PRT/H complexes than with protamine alone, we examined the effects of excess heparin (100 U/mL) on heparin binding. As seen in Figure 2C, all Abs, with the exception of 1 subject, showed decreased binding in the presence of excess heparin (range: 8% to 76%, mean inhibition = 45.3%).
Functional properties of PRT/H Abs
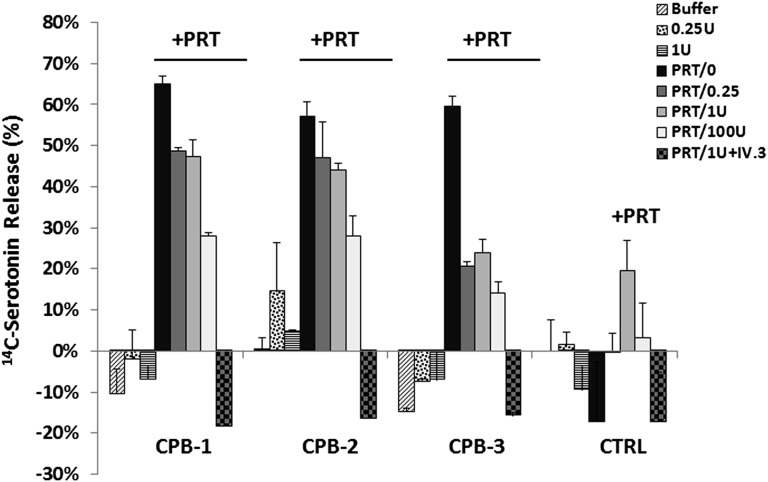
Next, we examined the functional consequences of PRT/H Abs on platelet activation. For these assays, we incubated plasma from 3 representative PRT/H–seropositive, but PF4/H–seronegative, patients, with 14C-serotonin–labeled platelets in the presence or absence of protamine (1.25 µg/mL) and increasing amounts of heparin (0.25, 1, or 100 U/mL). As shown in Figure 3, PRT/H–seropositive plasma did not activate platelets in the presence of buffer or heparin (0.25 or 1 U/mL) but did show robust platelet activation in the presence of protamine (1.25 µg/mL) alone, with decreasing 14C-release in the presence of protamine and increasing amounts of heparin. Platelet activation in the presence of protamine was inhibited by IV.3, a monoclonal antibody to human CD32 that blocks platelet activation via the platelet FcγRIIA.
Figure 3.
PRT/H Abs activate platelets in a protamine-dependent manner. Washed 14C-serotonin–labeled platelets from healthy donors were incubated with or without protamine (PRT; 1.25 ug/mL) in the presence of 3 representative patient samples obtained at day 30 (CPB patients or control patients [CTRL]) with increasing amounts of unfractionated heparin (0, 0.25, 1, 100 U or 1U+ IV.3). 14C releasate was measured by liquid scintillation counting. These results are representative of 4 independent experiments.
Correlation between PF4/H and PRT/H seropositivity
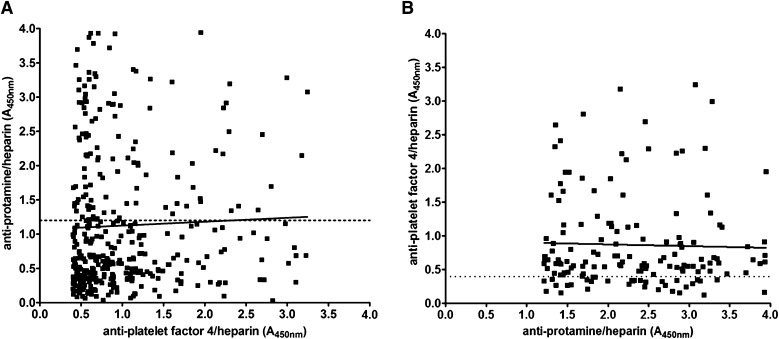
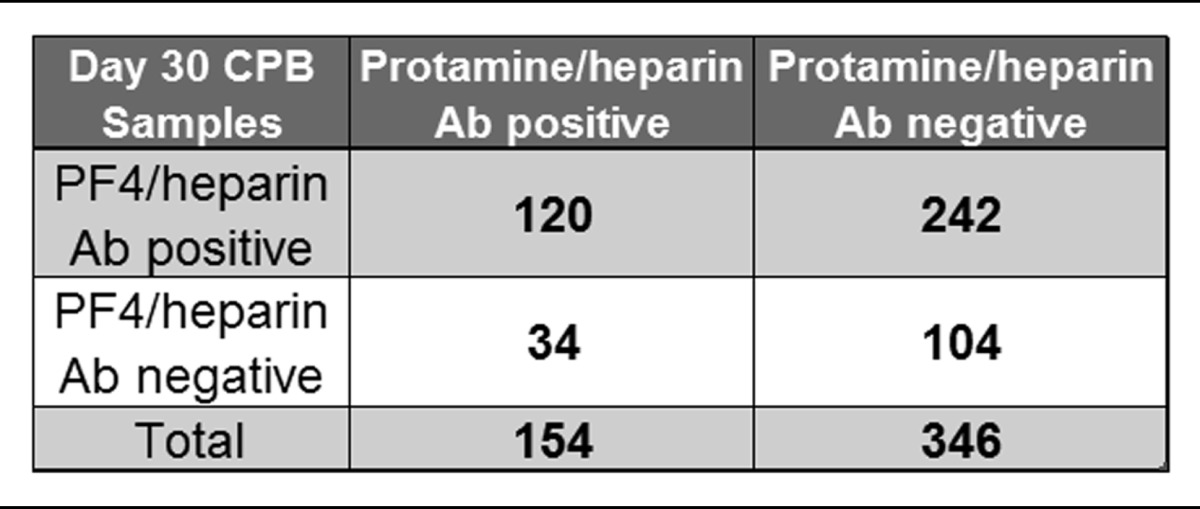
Figure 4 shows the serologic results for PF4/H and PRT/H Abs in CPB patients at day 30. As shown in Figure 4, and consistent with prior published results,10-13 there was a high incidence of PF4/H seroconversion after cardiac surgery. While the majority of PRT/H–seropositive patients (n = 154) were also positive for PF4/H Abs (120/154 or 78%), the converse was not true. Of the 362 PF4/H–seropositive patients, only 120 (33%) were seropositive for PRT/H Abs. A minority of patients in this study were seronegative in both assays (104/500 or 21%) (Table 1). To determine if seropositivity in 1 assay correlated with reactivity in the other assay, day 30 results were plotted for PF4/H–seropositive individuals against their corresponding PRT/H results (Figure 4A) and vice versa (Figure 4B). As shown in Figure 4, results in 1 assay were not predictive of results in the other.
Figure 4.
Correlation of PF4/H and PRT/H seropositivity. (A) PRT/H results at day 30 plotted as a function of PF4/H positivity (A405nm >0.4; R2 = 0.001454). (B) PF4/H results at day 30 plotted as a function of PRT/H positivity (A405nm >1.2; R2 = 0.0008695).
Table 1.
Breakdown of seropositivity at d 30 for all CPB patients
Clinical outcomes associated with PRT/H Abs
A history of peripheral vascular disease (40% vs 10% at baseline; P = .003), previous myocardial infarction (70% vs 36.6% at baseline; P = .03), and diabetes (54.6% vs 27.7% at baseline; P = .05) was more common in patients who were PRT/H seropositive at baseline (supplemental Table 2). Subdividing for diabetes identified a history of insulin-dependent diabetes mellitus as a risk factor for preoperative seropositivity (55.6% vs 11.6% at baseline; P < .001). Younger patients and those with a history of diabetes were more likely to seroconvert by day 30 (Table 2).
Table 2.
Clinical features associated with PRT/H antibody seropositivity at d 30
| D 30 protamine/heparin antibody status | |||
|---|---|---|---|
| Clinical feature | A450nm ≤1.2 | A450 >1.2 | P value |
| Age, y (SD) | 64 (11.5) | 61.45 (11.46) | .02 |
| Female, % | 28 | 31 | .49 |
| Weight, kg (SD) | 86 (19) | 89 (23) | .1 |
| Smoking, % | 22 | 14 | .04 |
| Serum creatinine, preoperative (SD) | 1.16 (0.75) | 1.28 (1.18) | .18 |
| Previous myocardial infarction, % | 35 | 42 | .13 |
| Chronic obstructive pulmonary disease, % | 24 | 25 | .83 |
| Renal disease, % | 6 | 10 | .08 |
| Hypertension, % | 84 | 78 | .1 |
| Congestive heart failure, % | 46 | 40 | .22 |
| Platelet count (SD) | 260 (112) | 294 (101) | .84 |
| Thrombosis, % | 5.1 | 2.7 | .22 |
| Peripheral vascular disease, % | 9.6 | 13.6 | .18 |
| Diabetes, % | 27 | 41 | .002 |
| Hannan sore (SD) | 0.04 (0.03) | 0.04 (0.04) | .11 |
| Pump time (min) | 147.77 (56.4) | 141.85 (56.21) | .28 |
SD, standard deviation.
To determine if PRT/H antibody positivity was predictive of adverse outcome, the mean absorbance values at each time point were logarithmically transformed and used as a dependent variable in a logistic regression model. For our statistical analysis of short-term adverse events, we included only patients with samples at all 3 time points (baseline, days 3 through 7, and day 30) and excluded patients if death occurred before day 30 or if samples were not available at all 3 time points. In analyzing long-term outcomes (>30 days) and after adjusting for age in a Cox proportional hazards model, there was a trend toward an association between PRT/H antibody positivity at baseline (P = .07; hazard ratio [HR] = 1.65, 0.96-2.82) and event-free survival (days to major adverse cardiac event; supplemental Table 3). Seropositivity at other time points was not associated with decreased event-free survival (days 3 through 7 HR = 1.26, 0.82-1.92; P = .28 and day 30 HR = 1.17, 0.96-1.44; P = .13).
To determine which patient characteristics were associated with seropositivity at day 30, a multivariable logistic regression model was created (supplemental Table 4). In this model, age, smoking, and diabetes were significantly associated with seropositivity at day 30. Smoking and diabetes were identified as risk factors for seropositivy (odds ratio [OR], 1.90; 95% CI, 1.11-3.23; and OR, 1.72; 95% CI, 1.13-2.63, respectively); increasing age appears to be protective (OR, 0.97; 95% CI, 0.96-0.99, per year). CHF and the Hannan score were both marginally associated with seropositivy (supplemental Table 4).
For the 454 patients with complete platelet counts, mean values were 227, 152, 202, and 295 × 109/liter at baseline, postoperative day 1, postoperative days 3 through 7, and postoperative day 30, respectively, following a typical pattern of immediate postoperative decrease and recovery (supplemental Figure 2). There was no significant difference between platelet counts by antibody status.
Thromboembolic complications and major adverse cardiovascular outcomes were also examined for patients who were double positive for both PRT/H and PF4/H Abs (PRT/H A450nm ≥1.2 and PF4/H A450nm ≥0.4). In all, thromboembolic complications were observed in 22/500 (4.4%) patients. Of those with a thromboembolic complication, 3/22 (14%) were double positive compared with 115/478 (24%) of those without a thromboembolic complication. Double seropositivity at baseline, days 3 through 7, or day 30 was not associated with short-term or long-term adverse cardiovascular complications (OR [95% CI] = 0.47, 0.10-2.13; P = .33). Being double positive at baseline (HR = 1.15, 0.15-87; P = .89) or double positive at day 30 (HR = 1.04, 0.39-2.82; P = .93) was not associated with major adverse cardiac events during long-term follow-up.
Cross-reactivity with neutral protamine Hagedorn insulin
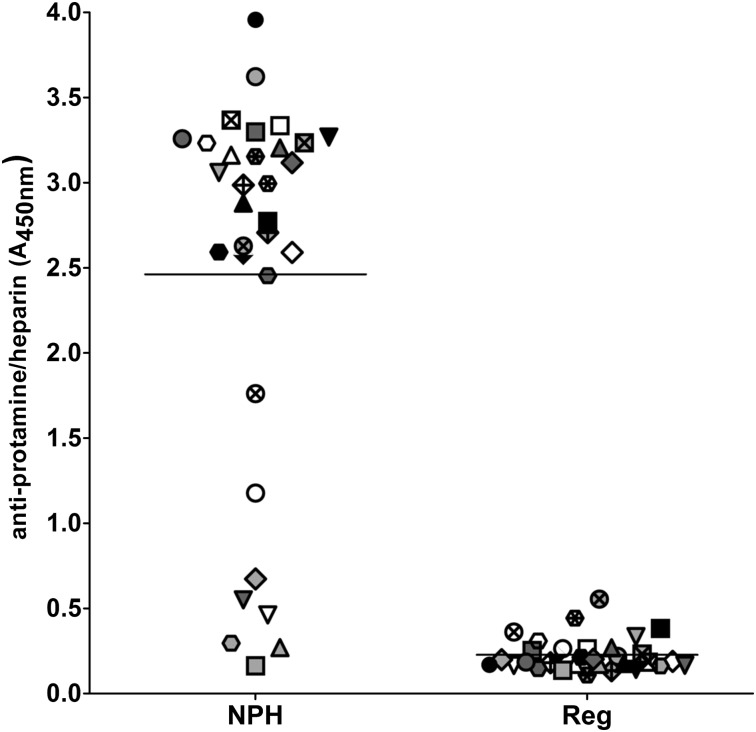
Because a history of diabetes was identified as a risk factor for both preoperative seropositivity and PRT/H antibody seroconversion after CPB, we examined the cross-reactivity of PRT/H Abs with neutral protamine Hagedorn (NPH) insulin, which contains protamine in complex with zinc insulin. As shown in Figure 5, 25/32, or 78%, of PRT/H Abs recognized NPH insulin, with 7/32 (22%) patients showing no cross-reactivity. In contrast, PRT/H Abs did not cross-react with regular insulin (P ≤ .0001).
Figure 5.
PRT/H antibody cross-reactivity with NPH insulin. Plasma from 32 CPB patients with high PRT/H antibody reactivity (A450nm >3.0) was analyzed for reactivity by ELISA on microtiter plates coated with NPH insulin, which contains protamine (diluted 1:160 in PBS), and with regular insulin, which does not contain protamine (diluted 1:160 in PBS; P = < .0001). Each symbol represents an individual CPB patient.
Discussion
Our study characterizes a new class of heparin-dependent Abs that arise from exposure to protamine and heparin during cardiac surgery. We show that Abs to PRT/H complexes share a number of serologic properties with PF4/H Abs that, to date, were the only known human Abs to show heparin-dependent binding. We found that 29% of patients undergoing CPB form high-titer IgG Abs to PRT/H complexes. PRT/H Abs, like PF4/H Abs, bind optimally to antigen in the presence of heparin and activate platelets in a protamine-dependent manner. Our studies also show that PRT/H Abs cross-react with protamine-containing insulin preparations. In the absence of circulating protamine, PRT/H Abs do not appear to be pathogenic, although a trend toward long-term adverse outcomes was noted in seropositive individuals at baseline.
Protamine sulfates belong to a class of small (molecular weight ∼4100–7000 Da), highly cationic proteins found in sperm nuclei of vertebrates and nonvertebrates. In the 1930s, it was recognized that protamine’s affinity for heparin could be used therapeutically to reverse heparin’s anticoagulant effect.14 Around the same time, it was also recognized that protamine–insulin preparations could prolong the absorption of insulin, leading to improved glycemic control.15 Current preparations of long-acting insulin combine protamine with zinc insulin to form stable insoluble complexes for subcutaneous injection (NPH insulin, as found in commercial preparations of Novolog 70/30, Humulin N, and Novolin N).
Commercial protamine preparations are derived from salmon seminal fluid. Previous studies have shown that both PRT and PRT/H complexes can affect cellular toxicity and activate the immune system. PRT triggers mast cell degranulation and histamine release,16 a mechanism attributed to the immediate hypotensive effects of drug administration.17 Studies have also shown that PRT or PRT/H complexes can inhibit the C1 esterase inhibitor,18 activate the classic complement pathway,19 and directly induce granulocytopenia and thrombocytopenia in vivo.20 IgG Abs to PRT have been described in 29% to 38% of NPH insulin–treated patients,21,22 as compared with 8% of diabetic patients treated with protamine-free insulin and 2.5% of healthy patients.22 Protamine reexposure in these highly sensitized individuals is associated with an approximately 50-fold increase in adverse reactions,23,24 ranging from dyspnea and flushing to chest pain and respiratory arrest.24 High-titer IgG as well as IgE protamine Abs have also been described in individuals with fish allergies25-27 and in 22% to 30% of vasectomized men,28-30 who develop auto Abs to protamine after disruption of the blood–testes barrier.
Our previous murine studies7 suggest that the immunogenicity of salmon protamine is further increased by heparin exposure during CPB (Figure 1). Before the release of recent studies,7,31,32 CPB was only rarely implicated in the pathogenesis of protamine antibody formation.2 Several variables contributed to the high rates of seropositivity in our study compared with prior publications. Our study benefited from the availability of clinical specimens at day 30, a time point not routinely used in most clinical studies of CPB, which rely on blood samples collected during a patient’s hospital course.2,33 In our study, of total new seroconversions (n = 149/500), we found that only 4% (6/149) of PRT/H seroconversions occurred during the hospitalization, while the majority occurred between discharge and day 30 (96%; 143/149 were negative at day 0 and days 3 through 7). Our studies were also informed by previous observations documenting the similarity of PRT/H and PF4/H interactions both in vitro and in vivo.7 These earlier studies show that protamine interacts with heparin to form ultralarge complexes that are highly immunogenic in vivo. In vivo, we noted that immunization with PRT/H complexes resulted in increased seroconversions as compared with immunization with PRT alone. Based on these observations, we screened patient samples using a microtiter plate coated with PRT/H complexes, rather than with protamine alone. This approach resulted in identification of additional Abs with enhanced reactivity to conformational-dependent epitopes on PRT. The heightened immunogenicity of PRT/H complexes in cardiac surgery is additionally confirmed in concurrent reports by Bakchoul et al31 and Pouplard et al32 wherein PRT/H seroconversions were documented in 158/591 (27%) and 59/232 (25%) patients undergoing cardiac surgery.34 Distinct from these reports, our study provides additional serologic characterization of PRT/H Abs, including information on titers (Figure 2A), antigen specificity (Figure 2B), and cross-reactivity with protamine-containing insulin preparations (Figure 5).
In agreement with recent reports of PRT/H antibody formation,27,31 our studies establish that the immune response to PRT/H and PF4/H share a number of serologic features. For both antigens, maximal seroconversion occurs after surgery, between discharge and 6 weeks (52% PF4/H seropositivity seen at 6 weeks in other studies35) and Abs show heparin-dependent binding (Figure 2C) and elicit platelet activation in the presence of protamine (Figure 3). Similar to PF4/H Abs,36 we note that a proportion of high-titer PRT/H Abs also bound to antigen in the absence of heparin (Figure 2B) or displayed minimal loss of binding in the presence of excess heparin (Figure 2C).36 Our findings that PRT/H Abs recognize antigenic determinants on PRT itself is also consistent with previous observations of PF4/H Abs binding to PF4 alone.36 Possible explanations for binding of PRT/H Abs to PRT alone include: (1) a polyclonal immune response with a subset of Abs binding to PRT alone; (2) binding of some Abs to non-conformational dependent epitopes on PRT; and (3) generation of subtle conformational changes in PRT binding to the microtiter plate.37 Despite the serologic similarities of PRT/H and PF4/H Abs, our study also makes it clear that the host’s response to each antigen is distinct. While 24% (120/500) of patients were seropositive in both PRT/H and PF4/H ELISAs by day 30 (Figure 4 inset), reactivity in 1 assay was not predictive of reactivity in the other (Figure 4). Finally, the results of our specificity assays and the occurrence of cohorts who are only seropositive for PRT/H (n = 34, 6.8%) or PF4/H alone (n = 242, 48.4%) suggest that the immune response is antigen specific.
We found that seropositivity at baseline is correlated with insulin-dependent diabetes, history of previous myocardial infarction, and peripheral vascular disease (P = .003-.05; supplemental Table 2). Although not statistically significant, there was a trend toward significance (P = .07) in patients with baseline seropositivity and event-free survival (supplemental Table 3). These findings were reinforced by Bakchoul et al,31 who noted that the presence of PRT/H Abs with platelet-activating capability prior to surgery was associated with poor cardiovascular outcomes (3/7 patients developed early arterial thromboembolic complications as compared with 11/584 controls; OR, 39.1; P < .001).
The lack of serious adverse outcomes in our study does not imply that PRT/H Abs are inconsequential. The serological and functional properties of these Abs suggest that the Abs could be pathogenic in the appropriate clinical context of reexposure. Our studies show that seroconversions in some patients are extremely high titer (Figure 2A), ranging from 1:1800 to 1: 175 000, far higher than those reported to occur in patients with HIT.38 Whether titers of PRT/H Abs are predictive of greater biologic activity, as seen with Abs in HIT,39,40 remains to be demonstrated. We also note that PRT/H Abs activated platelets in the presence of only PRT and additional heparin appeared to mitigate platelet activation. These findings suggest that PRT likely binds to platelet glycosaminoglycans and that additional heparin likely removes PRT from the platelet surface. In cardiac surgery, 5 to 9 mg/kg of protamine (associated with circulating levels of 70 to 126 ug/mL in a 70-kg adult) is routinely administered for heparin reversal.41 Based on our studies, we suspect that the functional effects of heparin become evident when PRT is either in excess of heparin and/or when PRT is administered in the absence of heparin (as occurs in diabetic patients). In addition, our findings of a higher incidence of antibody formation in diabetic patients and antibody cross-reactivity with protamine-containing insulin preparations (Figure 5) warrant further investigation, as PRT/H Abs may interfere with absorption and/or the functional activity of protamine-containing insulin preparations. Finally, the risk of protamine reexposure in patients with high-titer PRT/H Abs remains unknown. Although Bakchoul et al31 demonstrate that PRT/H Abs show serologic transience (only a third of seropositive patients remain positive at >120 days), at least some patients have Abs that persist over a period of years. In a recent case report,42 a patient with a history of repetitive protamine exposure (3 cardiac surgeries over a 12-year period) developed profound thrombocytopenia and bleeding after protamine reexposure. The patient was found to have a high-titer PRT/H antibody with serologic features similar to Abs described in the present report.42 Taken together, these observations suggest that PRT/H Abs are likely to be pathogenic in settings of protamine reexposure. Future prospective studies are necessary to define populations at risk and to further characterize the physiologic consequences of protamine reexposure in sensitized patients.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants (HL110860, HL109825, AI101992, G.M.A., and 2T32HL007057-36, G.M.L.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Authorship
Contribution: G.M.L. and G.M.A. conceived and designed the experiment; I.J.W., B.P.-B., and T.L.O. provided the study materials and patients; G.M.L., I.J.W., B.P.-B., T.L.O., and G.M.A. collected, assembled, analyzed, and interpreted data; G.M.L., I.J.W., T.L.O., and G.M.A. wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gowthami M. Arepally, Duke University Health System, Box 3486, Room 304 Sands Building, Durham, NC 27710; e-mail: arepa001@mc.duke.edu.
References
- 1.Weiss ME, Nyhan D, Peng ZK, Horrow JC, Lowenstein E, Hirshman C, Adkinson NF., Jr Association of protamine IgE and IgG antibodies with life-threatening reactions to intravenous protamine. N Engl J Med. 1989;320(14):886–892. doi: 10.1056/NEJM198904063201402. [DOI] [PubMed] [Google Scholar]
- 2.Weiler JM, Gellhaus MA, Carter JG, et al. A prospective study of the risk of an immediate adverse reaction to protamine sulfate during cardiopulmonary bypass surgery. J Allergy Clin Immunol. 1990;85(4):713–719. doi: 10.1016/0091-6749(90)90189-b. [DOI] [PubMed] [Google Scholar]
- 3.Kimmel SE, Sekeres MA, Berlin JA, Goldberg LR, Strom BL. Adverse events after protamine administration in patients undergoing cardiopulmonary bypass: risks and predictors of under-reporting. J Clin Epidemiol. 1998;51(1):1–10. doi: 10.1016/s0895-4356(97)00241-2. [DOI] [PubMed] [Google Scholar]
- 4.Kimmel SE, Sekeres M, Berlin JA, Ellison N. Mortality and adverse events after protamine administration in patients undergoing cardiopulmonary bypass. Anesth Analg. 2002;94(6):1402–1408. doi: 10.1097/00000539-200206000-00005. [table of contents.] [DOI] [PubMed] [Google Scholar]
- 5.Kimmel SE, Sekeres MA, Berlin JA, Ellison N, DiSesa VJ, Strom BL. Risk factors for clinically important adverse events after protamine administration following cardiopulmonary bypass. J Am Coll Cardiol. 1998;32(7):1916–1922. doi: 10.1016/s0735-1097(98)00484-7. [DOI] [PubMed] [Google Scholar]
- 6.Welsby IJ, Newman MF, Phillips-Bute B, Messier RH, Kakkis ED, Stafford-Smith M. Hemodynamic changes after protamine administration: association with mortality after coronary artery bypass surgery. Anesthesiology. 2005;102(2):308–314. doi: 10.1097/00000542-200502000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Chudasama SL, Espinasse B, Hwang F, et al. Heparin modifies the immunogenicity of positively charged proteins. Blood. 2010;116(26):6046–6053. doi: 10.1182/blood-2010-06-292938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warkentin TE, Greinacher A. Laboratory testing for heparin-induced thrombocytopenia. In: Thrombocytopenia H-I, editor. Warkentin TEG, Andreas. 4th ed. New York: Informa Healthcare USA, Inc; 2007. pp. 227–260. [Google Scholar]
- 9.Hannan EL, Wu C, Bennett EV, et al. Risk stratification of in-hospital mortality for coronary artery bypass graft surgery. J Am Coll Cardiol. 2006;47(3):661–668. doi: 10.1016/j.jacc.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 10.Bauer TL, Arepally G, Konkle BA, et al. Prevalence of heparin-associated antibodies without thrombosis in patients undergoing cardiopulmonary bypass surgery. Circulation. 1997;95(5):1242–1246. doi: 10.1161/01.cir.95.5.1242. [DOI] [PubMed] [Google Scholar]
- 11.Pouplard C, May MA, Iochmann S, Amiral J, Vissac AM, Marchand M, Gruel Y. Antibodies to platelet factor 4-heparin after cardiopulmonary bypass in patients anticoagulated with unfractionated heparin or a low-molecular-weight heparin : clinical implications for heparin-induced thrombocytopenia. Circulation. 1999;99(19):2530–2536. doi: 10.1161/01.cir.99.19.2530. [DOI] [PubMed] [Google Scholar]
- 12.Trossaërt M, Gaillard A, Commin PL, Amiral J, Vissac AM, Fressinaud E. High incidence of anti-heparin/platelet factor 4 antibodies after cardiopulmonary bypass surgery. Br J Haematol. 1998;101(4):653–655. doi: 10.1046/j.1365-2141.1998.00750.x. [DOI] [PubMed] [Google Scholar]
- 13.Warkentin TE, Sheppard JA, Horsewood P, Simpson PJ, Moore JC, Kelton JG. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood. 2000;96(5):1703–1708. [PubMed] [Google Scholar]
- 14.Chargaff E, Olson KB. Studies on the Chemistry of Blood Coagulation. J Biol Chem. 1937;122:177–180. [Google Scholar]
- 15.Hagedorn HCJB, Krarup NB, Wodstrup I. Protamine Insulinate. JAMA. 1936;106(3):177–180. doi: 10.1001/jama.251.3.389. [DOI] [PubMed] [Google Scholar]
- 16.Schnitzler S, Renner H, Pfüller U. Histamine release from rat mast cells induced by protamine sulfate and polyethylene imine. Agents Actions. 1981;11(1-2):73–74. doi: 10.1007/BF01991461. [DOI] [PubMed] [Google Scholar]
- 17.Frater RW, Oka Y, Hong Y, Tsubo T, Loubser PG, Masone R. Protamine-induced circulatory changes. J Thorac Cardiovasc Surg. 1984;87(5):687–692. [PubMed] [Google Scholar]
- 18.Takada A, Takada Y. Interaction of C1s and C1 inactivator in the presence of heparin, dextran sulfate and protamine sulfate. Thromb Res. 1980;18(6):847–859. doi: 10.1016/0049-3848(80)90207-8. [DOI] [PubMed] [Google Scholar]
- 19.Shastri KA, Logue GL, Stern MP, Rehman S, Raza S. Complement activation by heparin-protamine complexes during cardiopulmonary bypass: effect of C4A null allele. J Thorac Cardiovasc Surg. 1997;114(3):482–488. doi: 10.1016/S0022-5223(97)70197-1. [DOI] [PubMed] [Google Scholar]
- 20.Al-Mondhiry H, Pierce WS, Basarab RM. Protamine-induced thrombocytopenia and leukopenia. Thromb Haemost. 1985;53(1):60–64. [PubMed] [Google Scholar]
- 21.Ellerhorst JA, Comstock JP, Nell LJ. Protamine antibody production in diabetic subjects treated with NPH insulin. Am J Med Sci. 1990;299(5):298–301. doi: 10.1097/00000441-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Nell LJ, Thomas JW. Frequency and specificity of protamine antibodies in diabetic and control subjects. Diabetes. 1988;37(2):172–176. doi: 10.2337/diab.37.2.172. [DOI] [PubMed] [Google Scholar]
- 23.Gottschlich GM, Gravlee GP, Georgitis JW. Adverse reactions to protamine sulfate during cardiac surgery in diabetic and non-diabetic patients. Ann Allergy. 1988;61(4):277–281. [PubMed] [Google Scholar]
- 24.Stewart WJ, McSweeney SM, Kellett MA, Faxon DP, Ryan TJ. Increased risk of severe protamine reactions in NPH insulin-dependent diabetics undergoing cardiac catheterization. Circulation. 1984;70(5):788–792. doi: 10.1161/01.cir.70.5.788. [DOI] [PubMed] [Google Scholar]
- 25.Weiss ME, Adkinson NF., Jr Allergy to protamine. Clin Rev Allergy. 1991;9(3-4):339–355. doi: 10.1007/BF02802312. [DOI] [PubMed] [Google Scholar]
- 26.Porsche R, Brenner ZR. Allergy to protamine sulfate. Heart Lung. 1999;28(6):418–428. doi: 10.1016/s0147-9563(99)70031-2. [DOI] [PubMed] [Google Scholar]
- 27.Weiler JM, Freiman P, Sharath MD, et al. Serious adverse reactions to protamine sulfate: are alternatives needed? J Allergy Clin Immunol. 1985;75(2):297–303. doi: 10.1016/0091-6749(85)90061-2. [DOI] [PubMed] [Google Scholar]
- 28.Adourian U, Shampaine EL, Hirshman CA, Fuchs E, Adkinson NF., Jr High-titer protamine-specific IgG antibody associated with anaphylaxis: report of a case and quantitative analysis of antibody in vasectomized men. Anesthesiology. 1993;78(2):368–372. doi: 10.1097/00000542-199302000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Horrow JC. Protamine: a review of its toxicity. Anesth Analg. 1985;64(3):348–361. [PubMed] [Google Scholar]
- 30.Hellema HW, Samuel T, Rumke P. Sperm autoantibodies as a consequence of vasectomy. II. Long-term follow-up studies. Clin Exp Immunol. 1979;38(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- 31.Bakchoul T, Zöllner H, Amiral J, et al. Anti-protamine–heparin antibodies: incidence, clinical relevance and pathogenesis. Blood. 2013 doi: 10.1182/blood-2012-10-460691. January [Epub ahead of pring] [DOI] [PubMed] [Google Scholar]
- 32.Pouplard C, Leroux D, et al. Incidence of antibodies to protamine sulfate/heparin complexes in cardiac surgery patients and impact on platelet activation and clinical outcome. J Thromb Haemost. 2012 doi: 10.1160/TH12-11-0844. (in press) [DOI] [PubMed] [Google Scholar]
- 33.Kelton JG, Sheridan D, Santos A, et al. Heparin-induced thrombocytopenia: laboratory studies. Blood. 1988;72(3):925–930. [PubMed] [Google Scholar]
- 34.Pouplard C. Leroux D, Rollin J, et al. Incidene of antibodies to protamine/heparin complexes in cardiac surgery patients and impact on platelet activation in vitro and clinical outcome. In: 53rd American Society of Hematology Annual Meeting and Exposition; December 10–13, 2011; San Diego, CA. Abstract 2217. [Google Scholar]
- 35.Gluckman TJ, Segal JB, Schulman SP, et al. Effect of anti-platelet factor-4/heparin antibody induction on early saphenous vein graft occlusion after coronary artery bypass surgery. J Thromb Haemost. 2009;7(9):1457–1464. doi: 10.1111/j.1538-7836.2009.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pouplard C, Amiral J, Borg JY, Vissac AM, Delahousse B, Gruel Y. Differences in specificity of heparin-dependent antibodies developed in heparin-induced thrombocytopenia and consequences on cross-reactivity with danaparoid sodium. Br J Haematol. 1997;99(2):273–280. doi: 10.1046/j.1365-2141.1997.4183225.x. [DOI] [PubMed] [Google Scholar]
- 37.Schwab C, Bosshard HR. Caveats for the use of surface-adsorbed protein antigen to test the specificity of antibodies. J Immunol Methods. 1992;147(1):125–134. doi: 10.1016/s0022-1759(12)80037-8. [DOI] [PubMed] [Google Scholar]
- 38.Alberio L, Kimmerle S, Baumann A, Taleghani BM, Biasiutti FD, Lämmle B. Rapid determination of anti-heparin/platelet factor 4 antibody titers in the diagnosis of heparin-induced thrombocytopenia. Am J Med. 2003;114(7):528–536. doi: 10.1016/s0002-9343(03)00080-9. [DOI] [PubMed] [Google Scholar]
- 39.Zwicker JI, Uhl L, Huang WY, Shaz BH, Bauer KA. Thrombosis and ELISA optical density values in hospitalized patients with heparin-induced thrombocytopenia. J Thromb Haemost. 2004;2(12):2133–2137. doi: 10.1111/j.1538-7836.2004.01039.x. [DOI] [PubMed] [Google Scholar]
- 40.Warkentin TE, Sheppard JA, Moore JC, Moore KM, Sigouin CS, Kelton JG. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: how much class do we need? J Lab Clin Med. 2005;146(6):341–346. doi: 10.1016/j.lab.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Keeler JF, Hansbro SD, Shah MV. Protamine–What dose? J Cardiothorac Anesth. 1990;4:41. [Google Scholar]
- 42.Singla A, Sullivan M, Lee GM, et al. Protamine-induced immune thrombocytopenia. Transfusion. 2013 doi: 10.1111/trf.12112. February 4 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.