Abstract
Triclosan is a potent inhibitor of Toxoplasma gondii enoyl reductase (TgENR), which is an essential enzyme for parasite survival. In view of triclosan’s poor druggability, which limits its therapeutic use, a new set of B-ring modified analogs were designed to optimize its physico-chemical properties. These derivatives were synthesized and evaluated by in vitro assay and TgENR enzyme assay. Some analogs display improved solubility, permeability and a comparable MIC50 value to that of triclosan. Modeling of these inhibitors revealed the same overall binding mode with the enzyme as triclosan, but the Bring modifications have additional interactions with the strongly conserved Asn130.
Keywords: TgENR, Triclosan, ADMET, Toxoplasma
Toxoplasma gondii (T. gondii) is an obligate intracellular, protozoan parasite belonging to the phylum Apicomplexa. It infects over one third of the world’s population. It can cause substantial morbidity and mortality.1–6 People are mainly infected by unrecognized and inadvertent ingestion of materials contaminated with oocysts from the feces of cats4, 5 or eating undercooked meat containing bradyzoite-stage parasites6, 7. Generally most infections in healthy individuals are asymptomatic and self-limiting. However, in immunocompromised persons, T. gondii infection can cause eye and brain disease such as toxoplasmic encephalitis, chorioretinitis, and death.8, 9 Infection acquired during pregnancy can be especially severe; it can transmit from mother to the fetus, leading to congenital toxoplasmosis which may result in abortion, neonatal death, or fetal abnormalities.2, 10–16 Currently, there is no vaccine available to prevent human infection from this pathogen. Antifolate agents, sulfadiazine and pyrimethamine, are two primary medicines for treatment of T. gondii infection in humans.2, 15 Although these medicines are effective against tachyzoites in the acute stage of the disease, they do not eradicate encysted, latent bradyzoites. Furthermore, these therapies can be associated with side effects such as bone marrow depression, hypersensitivity and skin rashes.15, 16 There is an urgent need to develop new anti-T. gondii medicines that are both efficacious and nontoxic to humans.
One attractive target for chemotherapeutic intervention against apicomplexan parasites is the prokaryotic-like type II fatty acid biosynthesis (FAS-II) pathway.17–21 In T. gondii, this apicoplast-localized FAS-II pathway has been proved to be essential for parasite survival by both in vitro and in vivo studies.21 Type II FAS is fundamentally divergent from the analogous FAS I pathway in mammals. In eukaryotes, fatty acid biosynthetic enzymes integrate on a single multifunctional polypeptide (FASI), whereas fatty acid synthesis in prokaryotes utilizes a set of distinct enzymes composing the FAS-II pathway.22 Fatty acid biosynthesis is an iterative process beginning with condensation of acetyl-Coenzyme A (acetyl-CoA) with a growing fatty acid chain. In T. gondii, enoyl-acyl carrier protein (ACP) reductase (ENR) is responsible for the final reductive step in each round of the fatty acid chain elongation, the NADH-dependent reduction of trans-2-enoyl-ACP to acyl-ACP.23 There are a number of known bacterial and parasitic ENR inhibitors such as diazaborines, isoniazid and as well as triclosan which is a slow, tight-binding inhibitor of ENR.24–26 Moreover, triclosan inhibits TgENR enzymatic activity with an IC50 value of less than 20 nM 28 and inhibits the growth of T. gondii parasites with an IC50 value of ~200nM.18
Although triclosan is a potent inhibitor of TgENR, the diphenyl ether has low water solubility and a high ClogP value. Another major challenge for the development of medicines against targets which reside within the apicoplast of apicomplexan parasites is the need for the inhibitors to cross the four membranes of the parasite-specific organelle, in addition to the barriers set by both host cell and the parasite.27 In order to overcome these structural drawbacks and address the uptake problem, structure-based modification of triclosan was directed by improving the ADMET (absorption, distribution, metabolism, excretion and toxicity) profiles with special focus on the increase of aqueous solubility and permeability. We have previously shown that the A-ring of triclosan can be modified to exploit an additional space at the base of the inhibitor binding pocket.28 Furthermore, we have shown that substitution on the B-ring may also be tolerated to produce effective triclosan analogues {Stec et al., in preparation}. This study has now been extended to show that modifications of the B-ring can exploit the channel which leads from the substrate binding pocket to the outside solvent. We report herein on our efforts in developing novel 4’- substituted and B-ring substituted triclosan analogs with better physico-chemical properties.
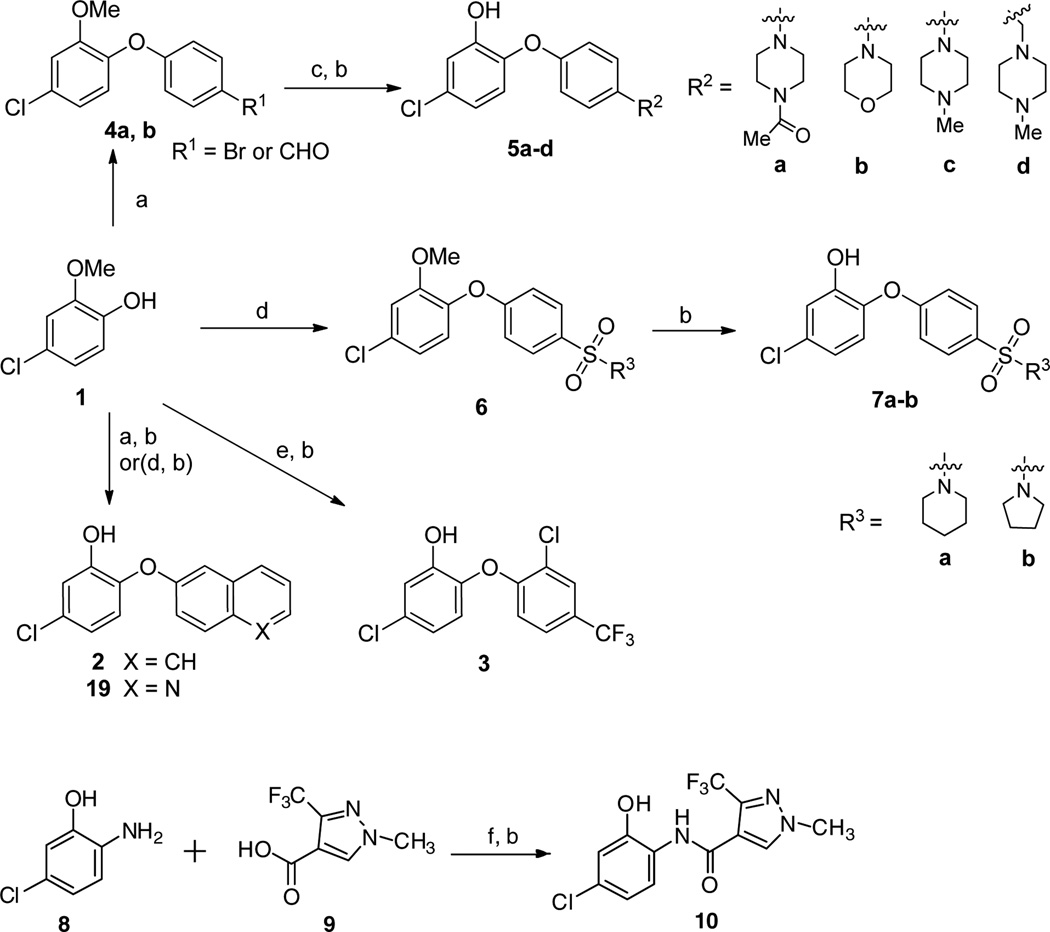
Triclosan analogs substituted at the 4’ position were generated from the commercially available phenol 1 using the methods outlined in Scheme 1.
Scheme 1.
Synthesis of 4’- substituted derivatives and compound 10. Reagents and conditions: (a) arylboronic acid, Et3N, Cu(OAc)2, O2, CH2Cl2, room temp, 24 h. For 4a, R = Br, 70%; for 4b, R = CHO, 58%; (b) BBr3, CH2Cl2, −78 °C to room temp, 6 h 30%–80%; (c) For R = Br: amine, 3 mol% Pd2(dba)3, 9 mol% xantphos, t-BuOK, toluene, 110 °C, 5 h; for R = CHO: amine, NaBH(OAc)3, AcOH, CH2Cl2, 0 °C to room temp, 1 h; (d) 4-iodobenzenesulfonamide, 30 mol% picolinic acid, 10 mol% CuI, DMSO, K3PO4, 85 °C, 24 h, 60%–70%; (e) 2-chloro-1-fluoro-4-(trifluoromethyl)benzene, K2CO3, DMF, 120 °C, 10 h. (f) HOBt, EDCI, DIEA, CH2Cl2, 0 °C to rt, overnight.
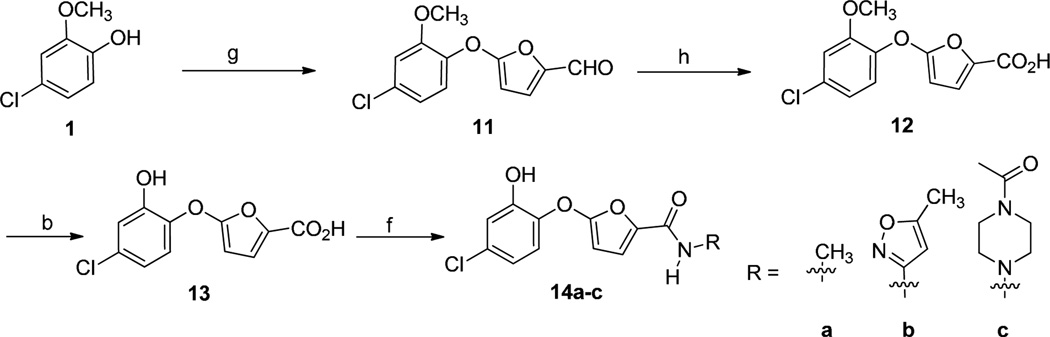
The naphthyl phenyl ether derivative 2 was synthesized by Cu-promoted Chan-Lam coupling29 of the phenol 1 with the 2-naphthyl boronic acid and Subsequent O-demethylation. Compound 3 with a trifluoromethyl substituent at the 4’ position was obtained from phenol 1 and 2-chloro-1-fluoro-4-(trifluoromethyl) benzene via nucleophilic substitution and demethylation. Cu-promoted Chan-Lam coupling29 of the phenol 1 with the 4-bromophenylboronic acid and 4-formylphenylboronic acid gave the corresponding intermediate bromide 4a and aldehyde 4b, respectively. Palladium-catalyzed amination30 of the bromoarene 4a and reductive amination of the benzaldehyde 4b afforded, after deprotection, the piperazine or morpholine inhibitors 5a-d. The 4’-sulfonamide derivatives 7a, b were prepared by Cu-catalyzed arylation of phenol 1 with 4-iodobenzenesulfonamides31 and subsequent demethylation. Compound 10 was prepared by coupling of 2-amino-5-chlorophenol 8 with 1-methyl-3-(trifluoromethyl)-1H-pyrazole-4- carboxylic acid followed by demethylation. Similar to the procedure for 7a, Compound 19 was obtained by treatment of phenol 1 with 6-bromoquinoline and subsequent demethylation. The furyl phenyl ether analogs (13, 14a-c) were obtained in 2−4 steps utilizing the synthetic routes shown in Scheme 2. Reaction of phenol 1 with 5-nitrofuran-2-carbaldehyde provided the furyl phenyl ether aldehyde 11.32 This intermediate was subjected to NaClO2 oxidation and BBr3 mediated demethylation to afford the furan-2-carboxylic acid 13. Next, using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDCI), 1-hydroxybenzotriazole (HOBt) and N, N-diisopropylethylamine (DIEA) carboxylic acid 13 was coupled with three different amines to generate the desired ligands 14a-c.
Scheme 2.
Synthesis of furanyl phenyl ethers. Reagents and conditions: (b) BBr3, CH2Cl2, −78 °C to room temp, 6 h. 30–80%; (f) amine, HOBt, EDCI, DIEA, CH2Cl2, 0 °C to rt, overnight, 40–60%. (g) 5-nitrofuran-2-carbaldehyde, NaH, DMSO, 35%; (h) NaClO2, DMSO, NaH2PO4, THF/tert-BuOH/H2O.
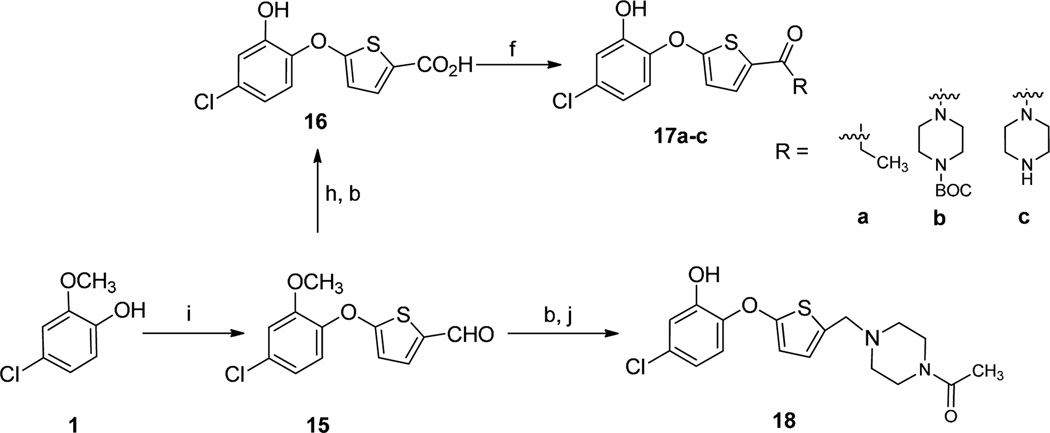
Compounds 17a-c were prepared in an analogous manner as described for 14a, starting from 1 via a sequence of steps involving nucleophilic aromatic substitution, oxidation, demethylation and amide bond formation (Scheme 3). Compound 18 was obtained from 15 through reductive amination and subsequent demethylation.
Scheme 3.
Synthesis of thienyl phenyl ethers. Reagents and conditions: (b) BBr3, CH2Cl2, −78 °C to room temp, 6 h; (f) amine, HOBt, EDCI, DIEA, CH2Cl2, 0 °C to rt, overnight, 40–60%; (h) NaClO2, DMSO, NaH2PO4, THF/t-BuOH/H2O; (i) 5- bromothiophene-2-carbaldehyde, K2CO3, DMSO, 80 °C, overnight, 60%; (j) amine, NaBH(OAc)3, AcOH, CH2Cl2, 0 °C to room temperature, 1 h.
Compounds were tested against TgENR, T. gondi in vitro and in vivo, and P. falciparum in vitro using methods that have been described in the recent literature28, 33 For comparison of parasite burden between treatment groups, analysis of variance (ANOVA) was performed with group and run as factors. Due to evidence of non-normality, natural log-transformed parasite burden was used in the analysis.(Also, see Supplementary Material).
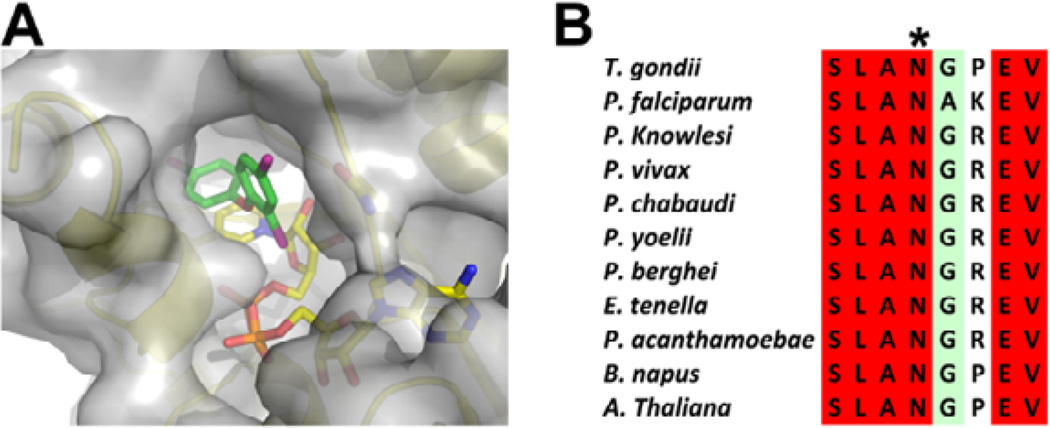
The crystal structure of TgENR in complex with NAD+ and triclosan,34 showed that the 4-chloro phenoxy ring (A-ring) of triclosan participates in a π-π stacking interaction with NAD+, and a hydrogen bond forms between the hydroxyl group and Tyr189. However, the 2,4-dichlorophenoxy ring (B-ring) engages only in van der Waals interactions within a pocket encompassed by the peptide backbone of residues Leu128 to Ala131, the pyrophosphate and nicotinamide moieties of NAD+, and the side chains of Val134, Met193, Ala231 and Ile235. Moreover, there remains some additional space around the B-ring that could be exploited. In particular the B-ring is exposed to the outside solvent via a channel which would allow the fatty acyl substrate attached to the acyl carrier protein to enter the active site (Fig. 1A).
Figure 1.
(A) The TgENR/NAD+/triclosan crystal structure shown in a cartoon representation covered by a transparent surface showing the channel which leads from the triclosan inhibitor to the outside solvent. TgENR Asn130 which has been targeted for the design of new inhibitors is shown in stick format close to the B-ring of triclosan. (B) A multiple sequence alignment in the vicinity of TgENR Asn130 (shown by an asterisk) of a number of parasitic and plant ENR enzymes showing full sequence conservation across parasite and plant families.
In view of the space around the B-ring which could be exploited, we devised a modification strategy to change the B- ring, incorporating additional polar groups to optimize the physicochemical properties (such as permeability and solubility) while keeping the A-ring intact. These modifications were guided by computer-aided ADMET prediction (ADMET suite from ACD/labs). In total, 19 triclosan analogs have been designed with either substitutions at the 4'-position of the B-ring or with the complete replacement of its benzene ring by heteroaromatic groups. The biological test data are shown in Table 1 along with some calculated ADMET properties.
Table 1.
Activity data and ADMET parameter predictions for new diaryl ethers inhibitors of TgENR
| Compound | Structure | Parasite tissue challenge assay |
TgENR Enzyme assay |
Calculated ADMET properties (ACD/Labs)d |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (µM) |
Toxicitya (µM) |
Inhibition (%) b at 1 µM |
IC50 (nM) |
ClogPc | TPSA [Å2] |
Sw (mg/L) |
Caco-2 (×10−6 cm/s) |
F>30% | PPB[%] | ||
| Triclosan | 5 | >10 | 98 | 15 | 5.53 | 53.25 | 4.6e | 174 | 0.81 | 99 | |
| 2 | 10 | >10 | 91 | 41 | 5.51 | 29.46 | 2.8 | 188 | 0.81 | 99 | |
| 3 | 4 | >10 | 95 | 30 | 5.70 | 29.46 | 3.1 | 192 | 0.81 | 99.6 | |
| 5a | 10-1 | >10 | 25 | - | 3.72 | 35.94 | 360 | 211 | 0.35 | 95 | |
| 5b | >10 | >10 | 74 | - | 4.15 | 41.93 | 78 | 236 | 0.35 | 97 | |
| 5c | 1.5 | >10 | 81 | 225 | 2.32 | 53.01 | 160 | 232 | 0.70 | 97 | |
| 5d | >10 | >10 | 32 | - | 4.64 | 35.94 | 1300 | 188 | 0.35 | 89 | |
| 7a | 10 | >10 | 78 | - | 4.16 | 75.22 | 15 | 227 | 0.94 | 97 | |
| 7b | 10 | >10 | 81 | - | 4.72 | 75.22 | 8.3 | 234 | 0.94 | 98 | |
| 10 | >10 | >10 | 27 | - | 1.54 | 67.15 | 270 | 173 | 0.95 | 93 | |
| 13 | >10 | >10 | 79 | - | 3.25 | 79.90 | 710 | 18 | 0.91 | 98 | |
| 14a | >10 | >10 | 46 | - | 2.23 | 71.70 | 680 | 162 | 0.95 | 95 | |
| 14b | 10 | >10 | 90 | 58 | 3.07 | 97.73 | 210 | 202 | 0.95 | 98 | |
| 14c | >10 | >10 | 45 | - | 1.72 | 83.22 | 1600 | 140 | 0.7 | 95 | |
| 16 | 10 | >10 | 48 | - | 3.84 | 95.00 | 610 | 17 | 0.91 | 98 | |
| 17a | 10 | 10 | 75 | - | 3.42 | 96.80 | 290 | 195 | 0.85 | 97 | |
| 17b | >10 | >10 | 76 | - | 4.71 | 107.55 | 130 | 233 | 0.35 | 96 | |
| 17c | >10 | >10 | 60 | - | 2.73 | 90.04 | 1900 | 31 | 0.51 | 93 | |
| 18 | 5 | >10 | 73 | 965 | 3.31 | 81.25 | 2200 | 222 | 0.7 | 96 | |
| 19 | 7.5 | >10 | 87 | 135 | 4.22 | 42.35 | 21 | 236 | 0.81 | NA | |
Toxicity to human foreskin fibroblasts.
At compound concentration (µM), enzyme inhibition percentage (%).
Calculated by ChemDraw Ultra 7.0.
These data were predicted by ADMET suite 5.0 (ACD/Labs). TPSA = topological polar surface area; Sw = solubility in water; Caco-2 permeability; F = probability of bioavailability more than 30%; Log BB = log of the ratio of the drug’s concentration in the brain to that in the blood; PPB = plasma protein binding.
The actual water solubility for triclosan is 12 mg/L at 20°C, according to US EPA - Registration Eligibility Decision (RED) for Triclosan.
Modeling studies indicated that these inhibitors should have similar binding positions as triclosan with respect to the A-ring. While the changes on the B-ring in these derivatives might protrude out from the triclosan binding pocket using the natural channel which the fatty acid substrate uses when attached to the acyl carrier protein (Fig 1A). An attractive additional interaction which these substituted inhibitors may acquire is with Asn130 (TgENR numbering) which is fully conserved within the apicomplexan family (Fig 1A, 1B). The inhibitory properties and cytotoxicity data of the tested inhibitors are shown in Table 1.
Compounds 2, 3 and 10 and 19
Initially, the B-ring of triclosan was substituted with a bulkier naphthalene ring system (Compound 2) to explore the size of the pocket around the B-ring. This had the adverse effect of making the inhibitor even less soluble than triclosan itself, which is unpractical for a therapeutic drug. Despite the good activity of this compound, it was therefore not consider worthwhile to develop this family of compounds further. This result did however, confirm that the B-ring of triclosan can accommodate significant change and still be an effective ENR inhibitor. To increase the solubility, we prepared compound 19 in which the naphthalene ring was replaced by a quinoline ring. However, it lost some enzyme activity. In order to investigate the role of the oxygen atom in the ether linker and to further understand the role of the B-ring, we prepared compound 10. Despite the A-ring system being intact, compound 10 was relatively inactive compared to triclosan and thus further modifications of this compound were not attempted. The low activity of compound 10 is primarily due to the loss of the bridging oxygen which has been shown to play an important role in triclosan’s efficacy35. Excellent activity was observed for compound 3 in which the chlorine atom attached to the C4 of triclosan was replaced by a trifluoromethyl group. The comparable activity of this derivative to triclosan is not surprising due to the close similarity in size between the trifluoromethyl and chloro substituent. Since compound 3 has similar physico-chemical properties to triclosan, and therefore is thought to share many of the known problems, we did not further optimize this compound. Instead, we exploited the fact that we were able to substitute the 4-chlorine atom of the B-ring without significant loss of efficacy, and we made a series of substitutions at this position of the triclosan B-ring.
Piperazine and morpholine based inhibitors 5a-d
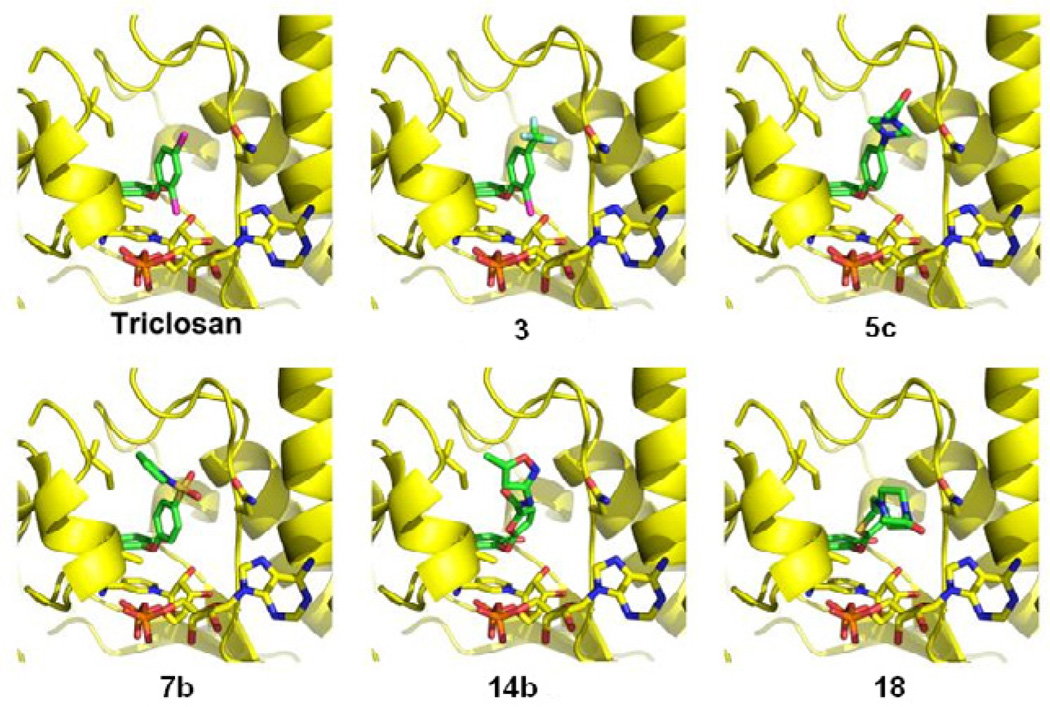
One of the most promising compound families developed involved the replacement of chlorine at the C4-position of the Bring with a piperazine moiety (5a and 5c). Incorporation of this polar group would greatly improve the water solubility according the ADMET prediction (360 mg/L for 5a and 160 mg/L for 5c versus 4.6 mg/L for triclosan). Furthermore, placing an acetyl group onto the N4 position of the piperazine derivative 5c improved its inhibitory properties in the enzyme assay (81% inhibition at 1 µM for 5c vs. 25% for 5a) (Table 1). However, this increased potency is offset by a slight difference in solubility between 5a and 5c. This highlights the fine balance between the inhibitory properties and other important features such as solubility and cellular permeability. Modeling of these two compounds using the TgENR/NAD+/triclosan complex crystal structure34 as a guide indicated that these compounds should be able to form a hydrogen bond between the N4 of the piperazine ring and the oxygen atom of the Asn130 side chain (Figure 2).
Figure 2.
Modeling of the designed inhibitors into the TgENR/NAD+/triclosan crystal structure. TgENR is shown in cartoon format with inhibitors, NAD+ cofactor, Asn130, Ala231 and Ile235 shown in stick format and colored red, blue, orange, pale blue, magenta, gold, yellow and green for oxygen, nitrogen, phosphorus, fluorine, chlorine, sulfur, carbon (TgENR) and carbon (modeled inhibitors), respectively.
The replacement of N4 with oxygen (5b) results in a significant decrease in efficacy (Table 1). This is likely due to the loss of a hydrogen bond to the O4 atom through the subtle differences in a morpholine ring structure when compared to a piperazine structure. Insertion of a flexible carbon linker between the piperazine group and the B-ring (5d) resulted in a significant loss of potency when compared to 5a. Since the only difference between these inhibitors is the methylene linker, the reduction in potency can be explained by the shift of the piperazine group further out of the binding pocket so that it can no longer maintain a hydrogen bond with Asn130. These results show that the N4 position of the rigidly attached piperazine group is important for good inhibitor activity and that replacement of the chlorine atom at the C4’-position on the B-ring of triclosan by a large piperazine group yields a scaffold suited for further development of TgENR inhibitors.
Benzenesulfonamide based inhibitors (7a and 7b)
Two compounds were designed that feature a replacement of the chlorine atom at the C4-position on the B-ring of triclosan by a pyrrolidinylsulfonyl (7a) and piperidinylsulfonyl group (7b). These inhibitors displayed similar activities in both the enzyme-based and cell-based assays (Table 1) but were significantly less active than compounds 5b and 5c. We concluded that the observed reduction in potency is unlikely to be an effect of their piperidine or pyrrole ring motifs but instead an effect of their shared sulfonyl motifs suggesting that this is an unsuitable substitution. The modeling of these inhibitors showed no steric clashes within the binding pocket which would cause a decrease in binding affinity. However, the sulfonyl groups would be able to form a hydrogen bond with the main chain nitrogen of Asn130 and Gly131. This may inadvertently cause a shift of the B-ring moiety which has a secondary effect on the A-ring such that it no longer has optimum stacking interactions with the NAD+ cofactor. Further crystallographic studies would be required to test this hypothesis.
Furan ring based inhibitors (13 and 14a-c)
The next set of compounds was designed with a 2-furyl group to replace the B-ring of triclosan. The furan ring was then further substituted at C5 with several chemical motifs. Attachment of an N-acetylpiperazine ring through a carboxyl group (14c) would greatly increase the compound’s solubility to 1600mg/L according to the calculated ADMET predication. However, a significant loss of potency was observed when 14c was tested in the TgENR assay. A second approach was to attach a carboxyl group (13) or related amide group (14a) to the furan ring at its C5-position. These inhibitors showed similar results in the parasite tissue culture assay and shared similar calculated solubility values. However, they varied in their ability to inhibit TgENR (compound 13 displayed 79% inhibition at 1 uM whereas compound 14a displayed only 46% inhibition). The improved inhibitory activity of compound 13 is likely to be due to the carboxyl group. However, the best compound of this family, 14b, contains the amide motif at this position with an additional isoxazole group. We conclude that either a carboxyl or amide group is tolerated at this position, with the substituent on the amide nitrogen playing an important role in inhibitor efficacy, as an amide group not bearing additional functionality is not sufficient to improve inhibitor binding. In order to further understand the possible role that the additional isoxazolyl group at the amine nitrogen might play, this inhibitor was modeled into the TgENR/NAD+/triclosan complex crystal structure. The modeling result showed that as with the piperazine based inhibitors (5a, 5c), Asn130 may play an important role in inhibitor binding by forming a hydrogen bond to the inhibitor. Inhibitor 14b also showed a greatly improved solubility (210 mg/L) when compared to triclosan (4.6 mg/L) without a significant effect on its predicted cellular permeability properties. This compound family has shown that not just substituents added to the B-ring of triclosan are tolerated but that the B-ring itself can be replaced without significant loss of potency. This is an important insight when trying to avoid some of the major downsides of triclosan such as low solubility, stability.
Thiophene ring based inhibitors (16, 17a-c, 18)
Since triclosan can accommodate the replacement of the B-ring with a 2-furyl group, our next step in designing novel ENR inhibitors was to replace the B-ring with a 2-thienyl group, to which a variety of chemically distinct groups were attached at its C5-position. The smallest group that we attached to the thiophene ring was carboxyl (16), which did not produce an effective inhibitor like the similar compound 13. This is likely the case because no additional inhibitor–enzyme interactions can be formed to the small carboxyl group. The most promising of the thiophene-based inhibitors is compound 18 which showed a good inhibitory effect in the parasite tissue cell culture assay and moderate activity in the enzyme assay, coupled with a significant improvement in solubility compared to triclosan. Modeling of compound 18, which contains a 4-acetylpiperazin-1-yl group attached to the thiophene ring through a methylene linker, showed again the importance of Asn130 on the efficacy of these inhibitors as it may hydrogen bond to the piperazine moiety (Table 1, Fig. 2). Interestingly, 14c is similar to 18 in that it also contains a N-acetylated piperazine ring at its terminal. However, this group is attached through a furan ring and carbonyl group in compound 14c rather than a thiophene ring and methylene group in compound 18. Since the furan and thiophene ring systems are similar, it is conceivable that the flexible methylene linker permits the piperazine ring in 18 to adopt a favorable position with respect to hydrogen bonding with Asn130 while the rigid carbonyl linker in 14c does not. The carbonyl linker in the effective furan-based compound 14b does not have this negative effect due to the different orientation of the hydrogen bond acceptors in its isoxazolyl group compared to the N4-atom of the piperazinyl group in compound 18. Moreover, compounds 17a, 17b, and 17c, which contain a carbonyl rather than methylene linker between their B rings and terminal amine moieties, also exhibit significant decreases in activity.
In vivo experiments
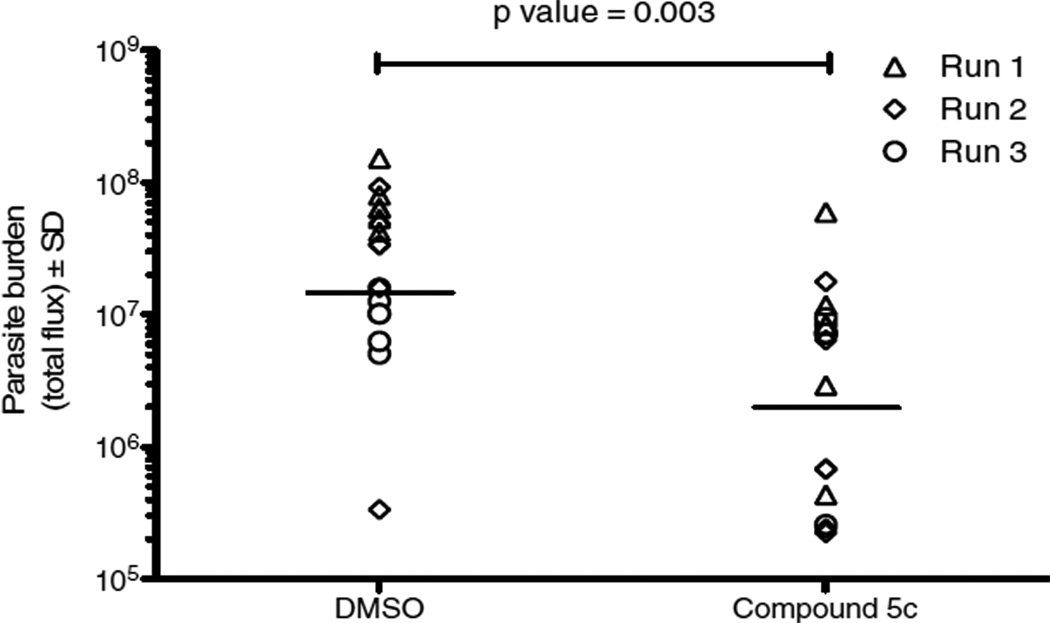
Compound 5c was selected for further biological evaluation in a T. gondii murine infection model (Figure 3). In 3 separate, replicate experiments mice treated with compound 5c had fewer parasites on average when compared with the DMSO control on the 6th day after infection. Although parasite number varied between individual mice, these modest differences reached significance (p<0.05), in two of the three replicate experiments. These data are shown pooled in Figure 3 with a separate symbol for each experiment. When the data were pooled the difference between the amount of parasite burden in DMSO control and compound 5c treated mice was significant. The p-value for the treatment group comparison was 0.003. A lower dose of 50mg/kg was not effective and a higher dose of 100mg/kg was not completely soluble. The compound was toxic at 75mg/kg by the seventh day after infection. Thus, although the predicted ADMET properties were superior to those of triclosan, this compound did not prove to be superior in these in vivo tests where triclosan is active at 25mg/kg.The relatively lower efficacy may be due to metabolic instability of compound 5c.
Figure 3.
Compound 5c protected mice from T. gondii in vivo. Mice were infected intraperitoneally with 2x104 tachyzoites (Pru strain expressing firefly luciferase) and imaged daily following intraperitonal injection of luciferin (3mg) in an IVIS spectrum (Caliper Biosciences).
Activity against Plasmodium falciparum
Compound 5c was also tested against P. falciparum strain D6 (CDC/Sierra Leone) and TM91C235 (WRAIR, Thailand). Results are in Table 2.
Table 2.
Effects on Plasmodium falciparum strain D6 (Sierra Leone) and C235 (Thailand; chloroquine resistant)
| Compound | SYBR Green D6 IC50 (ng/ml) |
SYBR Green C235 IC50 (ng/ml) |
|---|---|---|
| 5c | >10000 | >10000 |
Compound 5c was inactive against the chloroquine sensitive D6 and the chloroquine resistant C235 strains. Modeling of 5c within the PfENR active site shows that there is one subtle difference which may affect 5c binding whereby ASN which H-bonds to the inhibitor is followed by Gly-Pro in TgENR and Ala-Lys in PfENR. This change alters the main chain position about the exit of 5c such that it makes less favourable packing interactions. However, it is unlikely that this small change would abolish activity and it is more likely that this can be explained by the non-essential nature of ENR within P. falciparum during its blood stage development 37. A crystal structure would be needed to determine if that prediction is correct and explains the lack of activity of 5c for P. falciparum. Since the fatty acid biosynthesis enzyme ENR plays a key role in the development of liver stage malarial parasites, it also will be of interest in the future to learn whether the new inhibitors that utilize the space in the enzyme that accepts B ring modifications would improve the activity of such inhibitors against the liver stage organisms.
The use of triclosan-based compounds as possible inhibitors of apicomplexan parasites has become an intense area of study since the parasitic ENR was validated as a novel target.17, 18 However, despite its potency, triclosan displays a range of poor ADMET properties. In particular, it is very poorly soluble in water. In order to address some of these problems we synthesized a range of inhibitors bearing modifications to the B-ring of the triclosan scaffold. Compounds 2 and 3 showed that, as previously reported,36 the B-ring of triclosan can be modified without a significant reduction in inhibitor efficacy. The structural changes that were explored were based on chemical units comprised of piperazine, furan and thiophene ring systems. These added chemical groups were designed to occupy the channel which leads from the triclosan binding site to the area exposed to outside solvent; this area is thought to be the portal through which insertion of fatty acid substrates by the ACP takes place.
Because of the important role that this portal appears to play, as it is lined by relatively well conserved amino acid residues, the use of this region in the design of new therapeutics appears justified. In particular, the strongly conserved amino acid residue Asn130 (Fig. 1A) may play an important role in binding a number of these ligands. For those compounds which are incapable of forming an H-bond interaction with Asn130, only poor inhibitory activity is observed. Of the compounds disclosed herein, three compounds, namely 14b, 5c, and 18 may serve as leads candidates for further rounds of SAR optimization, as these compounds appear to show drug-like properties along with the lack of toxicity to human fibroblasts in comparison to their toxicity to the parasite.
Supplementary Material
Acknowledgments
The authors are grateful for the financial support provided by NIAID grant U01 AI082180-0101 and the Wellcome Trust and the support of the Mann and Cornwell, Rooney-Alden, Taub, Engel and Mussilami families. SPM is funded by an MRC Career Development Fellowship (G1000567).The authors also thank Alan Kozikowski for his contributions. They also thank K. Wroblewski for her assistance with statistical analyses of the in vivo data. The opinions of the authors from Walter Reed Army Institute of Research are their own and do not reflect the official views of the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data (experimental procedures and spectral data) associated with this article can be found, in the online version.
References
- 1.Hill D, Dubey JP. Clin. Microbiol. Infect. 2002;8:634. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 2.McLeod R, Boyer K, Karrison T, Kasza K, Swisher C, Roizen N, Jalbrzikowski J, Remington J, Heydemann P, Noble AG, Mets M, Holfels E, Withers S, Latkany P, Meier P. Clin. Infect. Dis. 2006;42:1383. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 3.Boyer K, Marcinak J, McLeod R. In: Principles and Practice of Pediatric Infectious Diseases. 4th Ed. Long S, Pickering LK, Prober CG, editors. Churchill Livingstone, New York: 2009. section 274. [Google Scholar]
- 4.Hill D, Coss C, Dubey JP, Wroblewski K, Sautter M, Hosten T, Munoz-Zanzi C, Mui E, Withers S, Boyer K, Hermes G, Coyne J, Jagdis F, Burnett A, McLeod P, Morton H, Robinson D, McLeod R. J. Parasitol. 2011;97:328. doi: 10.1645/GE-2782.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer K, Hill D, Mui E, Wroblewski K, Karrison T, Dubey JP, Sautter M, Noble AG, Withers S, Swisher C, Heydemann P, Hosten T, Babiarz J, Lee D, Meier P, McLeod R. Clin. Infect. Dis. 2011;53:1081. doi: 10.1093/cid/cir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmonts G, Couvreur J. N. Engl. J. Med. 1974;290:1110. doi: 10.1056/NEJM197405162902003. [DOI] [PubMed] [Google Scholar]
- 7.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Emerg. Infect. Dis. 1999;5:607. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luft BJ, Remington JS. Clin. Infect. Dis. 1992;15:211. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 9.Porter SB, Sande MA. N. Engl. J. Med. 1992;327:1643. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 10.Swisher CN, Boyer K, McLeod R. Semin. Pediatr. Neurol. 1994;1:4. [PubMed] [Google Scholar]
- 11.Olariu TR, Remington JS, McLeod R, Alam A, Montoya JG. Pediatr. Infect. Dis. J. 2011;30:1056. doi: 10.1097/INF.0b013e3182343096. [DOI] [PubMed] [Google Scholar]
- 12.McLeod R, Mack DG, Boyer K, Mets M, Roizen N, Swisher C, Patel D, Beckmann E, Vitullo D, Johnson D, et al. J. Lab. Clin. Med. 1990;116:623. [PubMed] [Google Scholar]
- 13.McGee T, Wolters C, Stein L, Kraus N, Johnson D, Boyer K, Mets M, Roizen N, Beckman J, Meier P, et al. Otolaryngol. Head Neck Surg. 1992;106:75. doi: 10.1177/019459989210600131. [DOI] [PubMed] [Google Scholar]
- 14.McAuley J, Boyer KM, Patel D, Mets M, Swisher C, Roizen N, Wolters C, Stein L, Stein M, Schey W, et al. Clin. Infect. Dis. 1994;18:38. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Remington JS, McLeod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington J, Klein J, editors. Infectious Diseases of the Fetus and Newborn Infant. 7th Ed. Philadelphia, WB: Saunders; 2011. [Google Scholar]
- 16.McLeod R, Khan AR, Noble GA, Latkany P, Jalbrzikowski J, Boyer K. Pediatr. Infect. Dis. J. 2006;25:270. doi: 10.1097/01.inf.0000202070.59190.9a. [DOI] [PubMed] [Google Scholar]
- 17.Zuther E, Johnson JJ, Haselkorn R, McLeod R, Gornicki P. Proc. Natl. Acad. Sci. USA. 1999;96:13387. doi: 10.1073/pnas.96.23.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLeod R, Muench SP, Rafferty JB, Kyle DE, Mui EJ, Kirisits MJ, Mack DG, Roberts CW, Samuel BU, Lyons RE, Dorris M, Milhous WK, Rice DW. Int. J. Parasitol. 2001;31:109. doi: 10.1016/s0020-7519(01)00111-4. [DOI] [PubMed] [Google Scholar]
- 19.Ben Mamoun C, Prigge ST, Vial H. Drug Dev. Res. 2010;71:44. doi: 10.1002/ddr.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu JZ, Muench SP, Allary M, Campbell S, Roberts CW, Mui E, McLeod RL, Rice DW, Prigge ST. Parasitology. 2007;134:1949. doi: 10.1017/S0031182007003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazumdar J, E HW, Masek K, C AH, Striepen B. Proc. Natl. Acad. Sci. USA. 2006;103:13192. doi: 10.1073/pnas.0603391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnuson K, Jackowski S, Rock CO, Cronan JJE. Microbiol. Rev. 1993;57:522. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massengo-Tiasse RP, Cronan JE. Cell Mol. Life Sci. 2009;66:1507. doi: 10.1007/s00018-009-8704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward WH, Holdgate GA, Rowsell S, McLean EG, Pauptit RA, Clayton E, Nichols WW, Colls JG, Minshull CA, Jude DA, Mistry A, Timms D, Camble R, Hales NJ, Britton CJ, Taylor IW. Biochemistry. 1999;38:12514. doi: 10.1021/bi9907779. [DOI] [PubMed] [Google Scholar]
- 25.D A, Grant GA, Barton DH, Jacobs WR, Jr, Sacchettini JC. Science. 1998;279:98. doi: 10.1126/science.279.5347.98. [DOI] [PubMed] [Google Scholar]
- 26.Grassberger MA, Turnowsky F, Hildebrandt J. J. Med. Chem. 1984;27:947. doi: 10.1021/jm00374a003. [DOI] [PubMed] [Google Scholar]
- 27.Samuel BU, Hearn B, Mack D, Wender P, Rothbard J, Kirisits MJ, Mui E, Wernimont S, Roberts CW, Muench SP, Rice DW, Prigge ST, Law AB, McLeod R. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14281. doi: 10.1073/pnas.2436169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tipparaju SK, Muench SP, Mui EJ, Ruzheinikov SN, Lu JZ, Hutson SL, Kirisits MJ, Prigge ST, Roberts CW, Henriquez FL, Kozikowski AP, Rice DW, McLeod RL. J. Med. Chem. 2010;53:6287. doi: 10.1021/jm9017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans DA, Katz JL, West TR. Tetrahedron Lett. 1998;39:2937. [Google Scholar]
- 30.Guari Y, van Es DS, Reek JNH, Kamer PCJ, van Leeuwen PWNM. Tetrahedron Lett. 1999;40:3789. [Google Scholar]
- 31.Maiti D, Buchwald SL. J. Org. Chem. 2010;75:1791. doi: 10.1021/jo9026935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo S-C, Wu J-S, Huang L-J, Wu C-H, Huang S-C, Chou T-C. J. Heterocycl. Chem. 1989;26:605. [Google Scholar]
- 33.Fomovska A, Huang Q, El Bissati K, Mui EJ, Witola WH, Cheng G, Zhou Y, Sommerville C, Roberts CW, Bettis S, Prigge ST, Afanador GA, Hickman MR, Lee PJ, Leed SE, Auschwitz JM, Pieroni M, Stec J, Muench SP, Rice DW, Kozikowski AP, McLeod R. Antimicrob. Agents Chemother. 2012;56:2666. doi: 10.1128/AAC.06450-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muench SP, Prigge ST, McLeod R, Rafferty JB, Kirisits MJ, Roberts CW, Mui EJ, Rice DW. Acta. Crystallogr. Sect. D. 2007;63:328. doi: 10.1107/S0907444906053625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heath RJ, Li J, Roland GE, Rock CO. J. Biol. Chem. 2000;275:4645. doi: 10.1074/jbc.275.7.4654. [DOI] [PubMed] [Google Scholar]
- 36.Freundlich JS, Anderson JW, Sarantakis D, Shieh HM, Yu M, Valderramos JC, Lucumi E, Kuo M, Jacobs WR, Jr, Fidock DA, Schiehser GA, Jacobus DP, Sacchettini JC. Bioorg. Med. Chem. Lett. 2005;15:5247. doi: 10.1016/j.bmcl.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 37.Yu M, Santha Kumar TR, Nkrumah LJ, Coppi A, Retzlaff S, Li CD, Kelly BJ, Moura PA, Lakshmanan V, Freundlich JS, Vilcheze J-C, Siedner S, Tsai JH-C, Falkard B, Sidhu BS, Purcell LA, Gratraud P, Kremer L, Waters AP, Schiehser G, Jacobus DP, Janse CJ, Ager A, Jacobs WR, Jr, Sacchettini JC, Heussler V, Sinnis P, Fidock DA. Cell Host Microbe. 2008;4:567–578. doi: 10.1016/j.chom.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.