Abstract
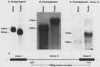
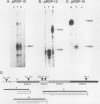
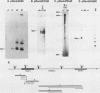
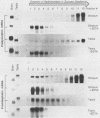
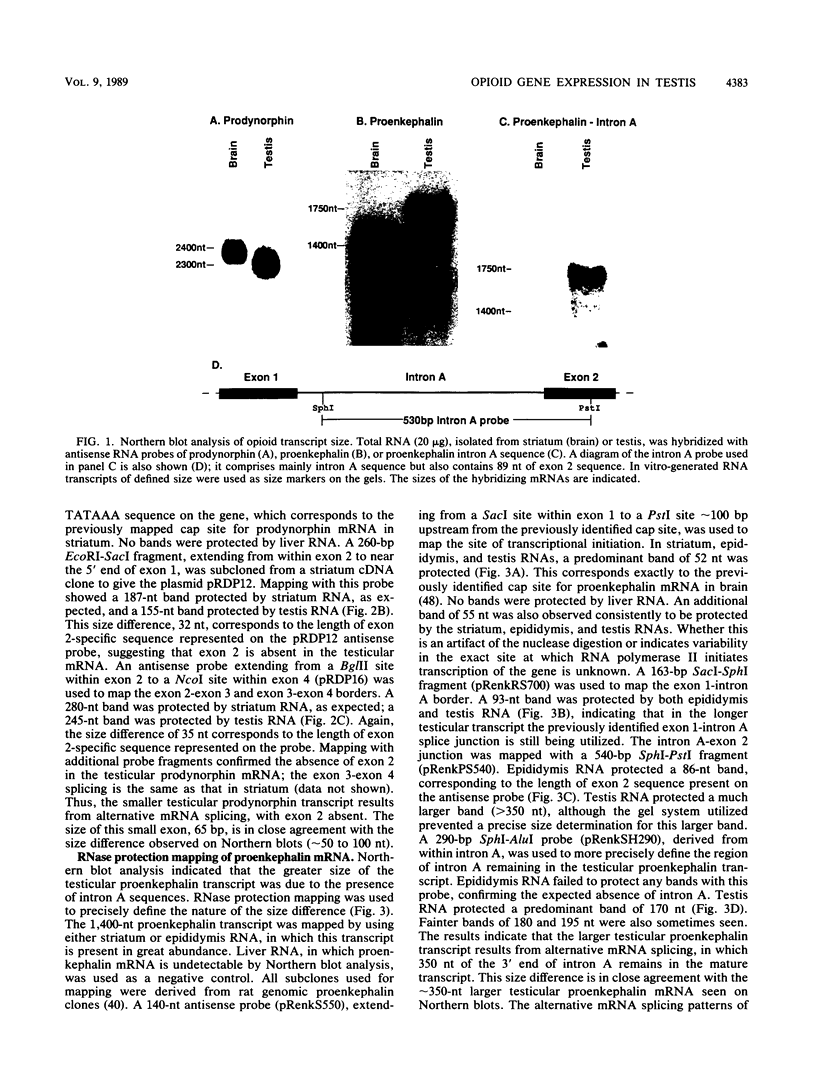
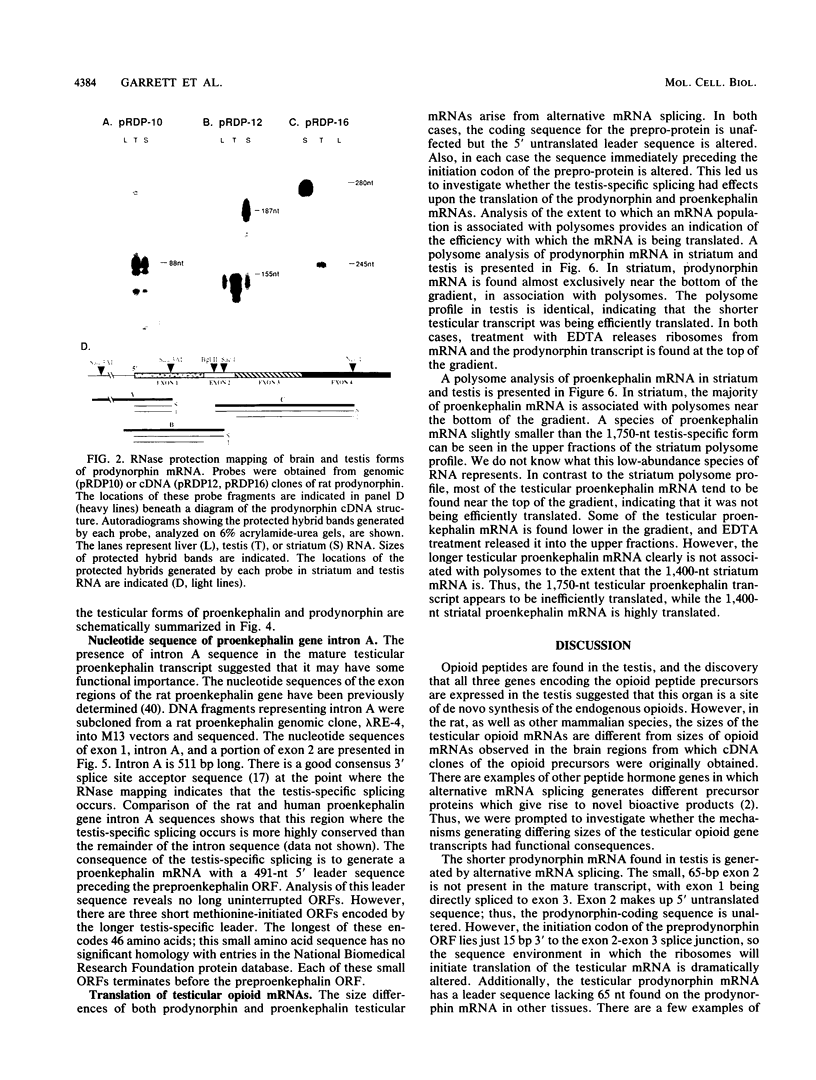
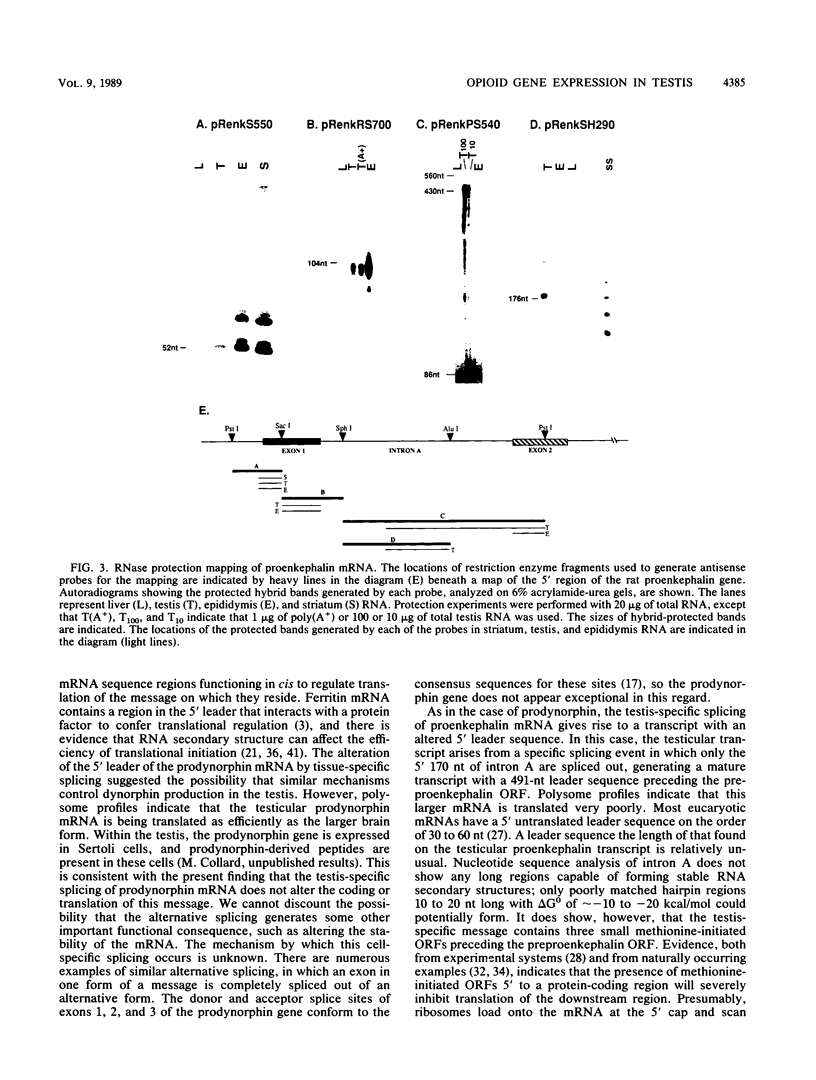
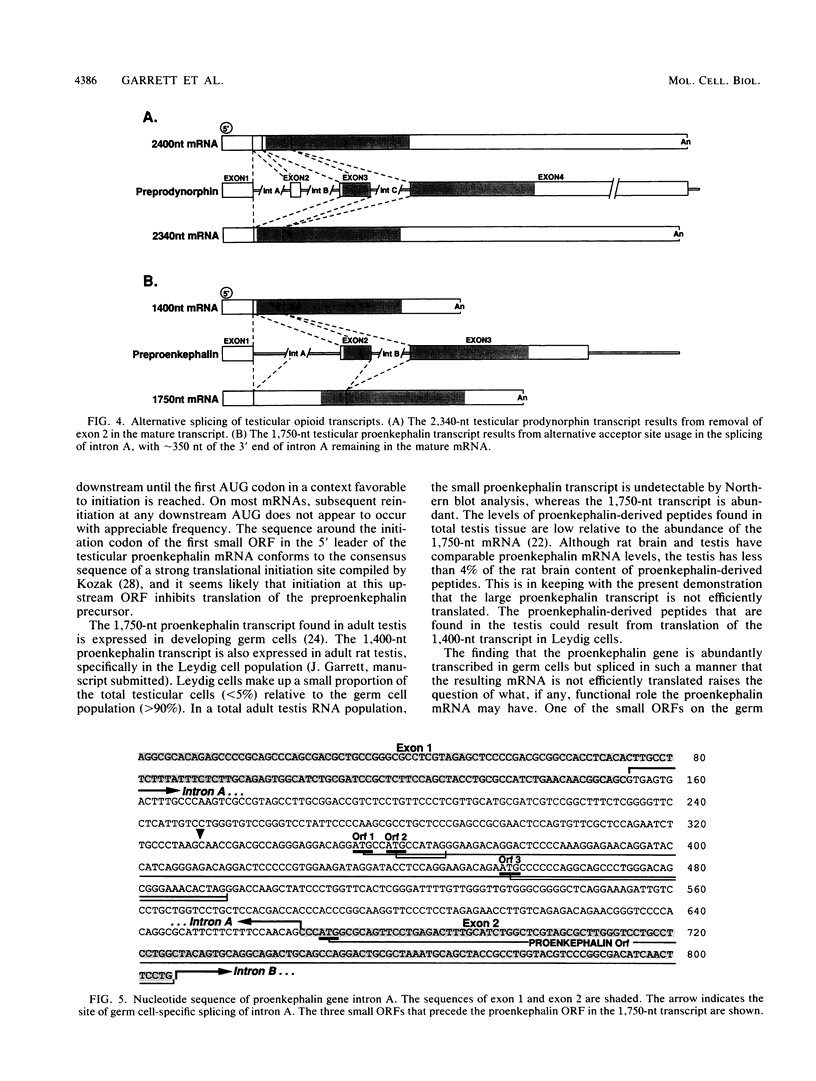
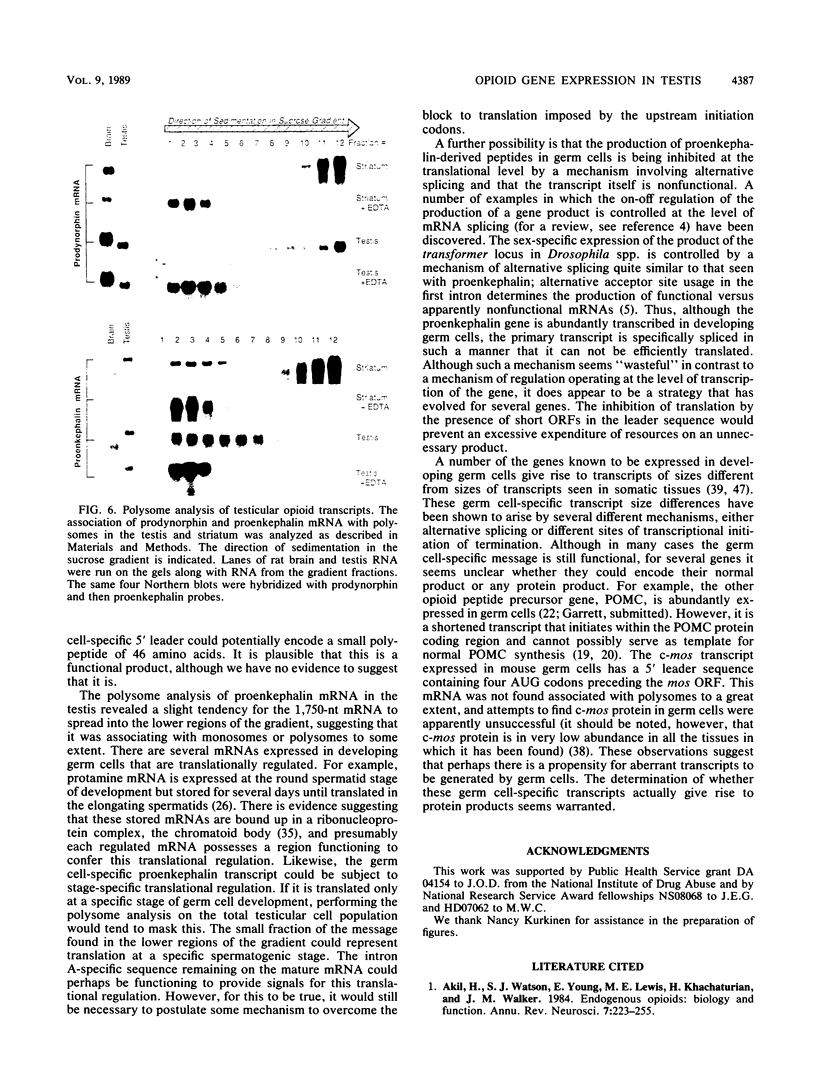
The three genes encoding the opioid peptide precursors (prodynorphin, proenkephalin, and proopiomelanocortin) are expressed in the rat testis. The sizes of the three opioid mRNAs in the testis differ from the sizes of the corresponding mRNAs in other rat tissues in which these genes are expressed. The smaller testicular proopiomelanocortin mRNA has previously been demonstrated to arise from alternative transcriptional initiation. In the present study, we found that the smaller testicular prodynorphin mRNA, expressed in Sertoli cells, results from alternative mRNA processing. Exon 2, which makes up 5' untranslated sequence, is removed from the mature transcript. Polysome analysis of brain and testis RNA indicates that the alteration of the prodynorphin leader sequence in the testis-specific transcript does not affect the efficiency of translation of this mRNA. The larger testicular proenkephalin transcript, expressed in developing germ cells, also results from alternative mRNA processing. Alternative acceptor site usage in the splicing of intron A results in a germ cell-specific proenkephalin transcript with a 491-nucleotide 5' untranslated leader sequence preceding the preproenkephalin-coding sequence. Polysome analysis indicates that this germ cell-specific proenkephalin mRNA is not efficiently translated. Mechanisms by which alternative mRNA splicing may serve to confer translational regulation upon the testicular proenkephalin transcript are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akil H., Watson S. J., Young E., Lewis M. E., Khachaturian H., Walker J. M. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Chou T. B., Mims I., Zachar Z. On/off regulation of gene expression at the level of splicing. Trends Genet. 1988 May;4(5):134–138. doi: 10.1016/0168-9525(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Boggs R. T., Gregor P., Idriss S., Belote J. M., McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987 Aug 28;50(5):739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Chang C. C., Krieger D. T., Bardin C. W. Expression and regulation of proopiomelanocortin-like gene in the ovary and placenta: comparison with the testis. Endocrinology. 1986 Jun;118(6):2382–2389. doi: 10.1210/endo-118-6-2382. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cicero T. J. Effects of exogenous and endogenous opiates on the hypothalamic--pituitary--gonadal axis in the male. Fed Proc. 1980 Jun;39(8):2551–2554. [PubMed] [Google Scholar]
- Civelli O., Douglass J., Goldstein A., Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave J. R., Rubinstein N., Eskay R. L. Evidence that beta-endorphin binds to specific receptors in rat peripheral tissues and stimulates the adenylate cyclase-adenosine 3',5'-monophosphate system. Endocrinology. 1985 Oct;117(4):1389–1396. doi: 10.1210/endo-117-4-1389. [DOI] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Douglass J., Cox B., Quinn B., Civelli O., Herbert E. Expression of the prodynorphin gene in male and female mammalian reproductive tissues. Endocrinology. 1987 Feb;120(2):707–713. doi: 10.1210/endo-120-2-707. [DOI] [PubMed] [Google Scholar]
- Fabbri A., Knox G., Buczko E., Dufau M. L. Beta-endorphin production by the fetal Leydig cell: regulation and implications for paracrine control of Sertoli cell function. Endocrinology. 1988 Feb;122(2):749–755. doi: 10.1210/endo-122-2-749. [DOI] [PubMed] [Google Scholar]
- Fabbri A., Tsai-Morris C. H., Luna S., Fraioli F., Dufau M. L. Opiate receptors are present in the rat testis. Identification and localization in Sertoli cells. Endocrinology. 1985 Dec;117(6):2544–2546. doi: 10.1210/endo-117-6-2544. [DOI] [PubMed] [Google Scholar]
- Gerendai I., Shaha C., Gunsalus G. L., Bardin C. W. The effects of opioid receptor antagonists suggest that testicular opiates regulate Sertoli and Leydig cell function in the neonatal rat. Endocrinology. 1986 May;118(5):2039–2044. doi: 10.1210/endo-118-5-2039. [DOI] [PubMed] [Google Scholar]
- Green M. R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- Howlett T. A., Rees L. H. Endogenous opioid peptides and hypothalamo-pituitary function. Annu Rev Physiol. 1986;48:527–536. doi: 10.1146/annurev.ph.48.030186.002523. [DOI] [PubMed] [Google Scholar]
- Ivell R., Walther N., Morley S. Proopiomelanocortin cDNA sequences from the bovine ovary indicate alternative non-functional transcriptional initiation and a new polymorphism. Nucleic Acids Res. 1988 Aug 11;16(15):7747–7747. doi: 10.1093/nar/16.15.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannotte L., Burbach J. P., Drouin J. Unusual proopiomelanocortin ribonucleic acids in extrapituitary tissues: intronless transcripts in testes and long poly(A) tails in hypothalamus. Mol Endocrinol. 1987 Oct;1(10):749–757. doi: 10.1210/mend-1-10-749. [DOI] [PubMed] [Google Scholar]
- Jobling S. A., Gehrke L. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature. 1987 Feb 12;325(6105):622–625. doi: 10.1038/325622a0. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Borland K., Jin D. F. Differential expression of opioid peptide genes by testicular germ cells and somatic cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5695–5699. doi: 10.1073/pnas.84.16.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Howells R. D., Noe M., Bailey L. C., Udenfriend S. Expression of preproenkephalin-like mRNA and its peptide products in mammalian testis and ovary. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7467–7469. doi: 10.1073/pnas.82.21.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Millette C. F. Expression of proenkephalin messenger RNA by mouse spermatogenic cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5015–5018. doi: 10.1073/pnas.83.14.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Rosenthal J. L. The proenkephalin gene is widely expressed within the male and female reproductive systems of the rat and hamster. Endocrinology. 1986 Jul;119(1):370–374. doi: 10.1210/endo-119-1-370. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol. 1984 Sep;105(1):71–79. doi: 10.1016/0012-1606(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M. J., Herz A. The endocrinology of the opioids. Int Rev Neurobiol. 1985;26:1–83. doi: 10.1016/s0074-7742(08)60072-0. [DOI] [PubMed] [Google Scholar]
- Orth J. M. FSH-induced Sertoli cell proliferation in the developing rat is modified by beta-endorphin produced in the testis. Endocrinology. 1986 Oct;119(4):1876–1878. doi: 10.1210/endo-119-4-1876. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Young N. Translational regulation of B19 parvovirus capsid protein production by multiple upstream AUG triplets. J Biol Chem. 1988 Aug 5;263(22):10922–10926. [PubMed] [Google Scholar]
- Parvinen M., Parvinen L. M. Active movements of the chromatoid body. A possible transport mechanism for haploid gene products. J Cell Biol. 1979 Mar;80(3):621–628. doi: 10.1083/jcb.80.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Pintar J. E., Schachter B. S., Herman A. B., Durgerian S., Krieger D. T. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science. 1984 Aug 10;225(4662):632–634. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- Propst F., Rosenberg M. P., Iyer A., Kaul K., Vande Woude G. F. c-mos proto-oncogene RNA transcripts in mouse tissues: structural features, developmental regulation, and localization in specific cell types. Mol Cell Biol. 1987 May;7(5):1629–1637. doi: 10.1128/mcb.7.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst F., Rosenberg M. P., Vande Woude G. F. Proto-oncogene expression in germ cell development. Trends Genet. 1988 Jul;4(7):183–187. doi: 10.1016/0168-9525(88)90073-x. [DOI] [PubMed] [Google Scholar]
- Rosen H., Douglass J., Herbert E. Isolation and characterization of the rat proenkephalin gene. J Biol Chem. 1984 Nov 25;259(22):14309–14313. [PubMed] [Google Scholar]
- Saito H., Hayday A. C., Wiman K., Hayward W. S., Tonegawa S. Activation of the c-myc gene by translocation: a model for translational control. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7476–7480. doi: 10.1073/pnas.80.24.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaha C., Margioris A., Liotta A. S., Krieger D. T., Bardin C. W. Demonstration of immunoreactive beta-endorphin- and gamma 3-melanocyte-stimulating hormone-related peptides in the ovaries of neonatal, cyclic, and pregnant mice. Endocrinology. 1984 Jul;115(1):378–384. doi: 10.1210/endo-115-1-378. [DOI] [PubMed] [Google Scholar]
- Simonds W. F. The molecular basis of opioid receptor function. Endocr Rev. 1988 May;9(2):200–212. doi: 10.1210/edrv-9-2-200. [DOI] [PubMed] [Google Scholar]
- Tsong S. D., Phillips D., Halmi N., Liotta A. S., Margioris A., Bardin C. W., Krieger D. T. ACTH and beta-endorphin-related peptides are present in multiple sites in the reproductive tract of the male rat. Endocrinology. 1982 Jun;110(6):2204–2206. doi: 10.1210/endo-110-6-2204. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Williams C., Sabol S. L. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984 Nov 25;259(22):14301–14308. [PubMed] [Google Scholar]