Abstract
Busulfan/cyclophosphamide (Bu/Cy) conditioning regimen has been widely used to treat cancer patients, while their effects on major internal organs in females are not fully understood. We treated female mice with Bu/Cy, and examined the histopathology of major internal organs on Day 30 after the treatment. The results show that Bu/Cy treatment affected the ovaries most extensively, while it had less effect on the spleen, lungs, and kidneys, and no effect on the heart, liver, stomach, and pancreas. To better understand the effect of Bu/Cy on the ovaries, we counted follicles, and determined the levels of ovarian steroids. The Bu/Cy-treated mice showed a reduction of primordial and primary follicles (P<0.01) on Day 30 and a marked loss of follicles at all developmental stages (P<0.01) on Day 60. Plasma levels of estradiol and progesterone in Bu/Cy-treated mice decreased by 43.9% and 61.4%, respectively. Thus, there was a gradual process of follicle loss and low estradiol in Bu/Cy-treated mice; this is a profile similar to what is found in women with premature ovarian failure (POF). The Bu/Cy-treated mice may serve as a useful animal model to study the dynamics of follicle loss in women undergoing POF.
Keywords: Premature ovarian failure, Busulfan, Cyclophosphamide, Chemotherapy, Mouse model
1. Introduction
Chemotherapy can improve the long-term survival of cancer patients, but has side effects such as ovarian failure, infertility, and liver toxicity. Chemotherapeutic agents can be classified into five classes depending on their mode of action: alkylating agents, antimetabolites, aneuploidy inducers, radiomimetics, and topoisomerase II inhibitors. These drugs are often used in combination for increased anti-tumour effects (Meirow and Nugent, 2001; Maltaris et al., 2007; Jemal et al., 2010).
Both busulfan and cyclophosphamide (Bu/Cy) are alkylating agents; they have been frequently used in combination as a conditioning regimen before hematopoietic stem cell transplantation for patients with leukemia, inherited metabolic diseases, or chronic granulomatous disease. In comparison to other regimens, the toxicities of Bu and Cy are relatively low (Copelan et al., 1993; de Magalhaes-Silverman et al., 1997). However, like other chemotherapeutic agents, Bu/Cy can cause problems in some organs like the ovaries and lungs in patients. The impairment to the ovaries is the most severe compared with the impact upon other organs (Meirow and Nugent, 2001; Ulrickson et al., 2009).
There are some relevant reports about the effects of Bu and Cy on major internal organs using mice as models. Al-Hashmi et al. (2011) tried to develop a graft-versus-host disease mouse model by treating the mice with Bu/Cy and transplanting with hematopoietic stem cells. They checked the histology of major internal organs of these transplanted mice, and found there were histopathological changes in the liver, pancreas, spleen, lungs, and heart, but not in the kidneys. About the effects of Bu or Cy on ovaries in animals, it was reported that treatment with Bu or Cy alone can cause loss of follicles (Hemsworth and Jackson, 1963; Burkl and Schiechl, 1978; Pelloux et al., 1988; Meirow et al., 1999; Shirota et al., 2003). Until now, there was no systematic study of the effects of the combination of Bu and Cy on major internal organs in mice, especially upon the ovaries, which seem to be the most sensitive to these two drugs. In this study, we examined the effects of a combination of Bu/Cy on major internal organs using female mice as a model and focused on the ovaries. We not only examined the histological changes of the ovaries, but also checked the hormone levels in Bu/Cy-treated mice in comparison with un-treated mice.
2. Materials and methods
2.1. Animals and treatments
All mice used in these studies were of CD-1 background. Forty female mice at two months of age were treated with a single injection of Bu in dimethyl sulfoxide (DMSO; 12 mg/kg subcutaneously) and Cy in 0.9% (9 g/L) sterile sodium chloride solution (120 mg/kg intraperitoneally) (Johnson et al., 2005). Bu powder is difficult to dissolve in water and has been reported to cause occasional deaths by intraperitoneal injection (Jopling and Rosendaal, 2001). We dissolved Bu in DMSO at a concentration of 3.6 mg/ml. We weighed the mice (28–32 g) and injected subcutaneously at the dosage mentioned above (about 0.1 ml for each mouse). Twenty untreated age-matched female mice were used as the control group. On Day 30 after Bu/Cy treatment, twenty mice were weighed, anesthetized with pentobarbital sodium (5 μg/g intraperitoneally) and sacrificed. The major internal organs (the ovary, heart, liver, spleen, lungs, kidneys, stomach, and pancreas) were harvested for histological analysis. We also collected the ovaries of the other twenty Bu/Cy-treated mice on Day 60 to examine their histological changes. All studies were conducted in accordance with the standards of the Shandong University Ethics Committee.
The harvested organs were fixed in 10% formaldehyde. All the samples were embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin (H&E). Additionally, we counted follicles on ovarian sections. Each ovary produced about 200 sections, and follicles on every 20th section were counted. Follicles were classified as previously assigned (Mayer et al., 2004; Myers et al., 2004). An oocyte, which was surrounded by a single layer of flattened granulosa cells, was defined as primordial follicles. Primary follicles were defined as an oocyte surrounded by a single layer of cuboidal granulosa cells. Secondary follicles possessed an oocyte surrounded by more than one layer of granulosa cells without antral space. Antral follicles were identified as containing an antral space.
2.2. Hormone assay
On Day 30 after Bu/Cy treatment, whole blood samples (1.0–1.2 ml) were harvested after mice were anesthetized. We collected whole blood samples by retro-orbital puncture. The samples were incubated at 37 °C for 1 h. Thereafter, the samples were centrifuged at 3 000 r/min for 30 min at room temperature and the supernatant was collected. The plasma levels of estradiol, progesterone, and testosterone were measured as indicators to assess ovarian senescence in animal models (Danilovich and Ram Sairam, 2006).
2.3. Data analyses
Data analyses included calculations of group means and standard error of means (SEM). Data for follicle numbers and plasma hormone concentrations were analyzed by t-test with significance set at P value <0.05.
3. Results
3.1. Histology of the heart, liver, spleen, lungs, kidneys, stomach, and pancreas in Bu/Cy-treated mice
The mice injected with Bu and Cy had slower locomotor activity and recovered about 3 d later. There is no difference in the body weight of the Bu/Cy-treated mice and the control group.
On Day 30, we harvested the ovaries, hearts, livers, spleens, lungs, kidneys, stomachs, and pancreases of the Bu/Cy-treated mice and the control group. There was no difference in the size and the weight of the heart, liver, spleen, lungs, kidneys, stomach, and pancreas, and only smaller ovaries were observed in the Bu/Cy-treated mice compared with the controls.
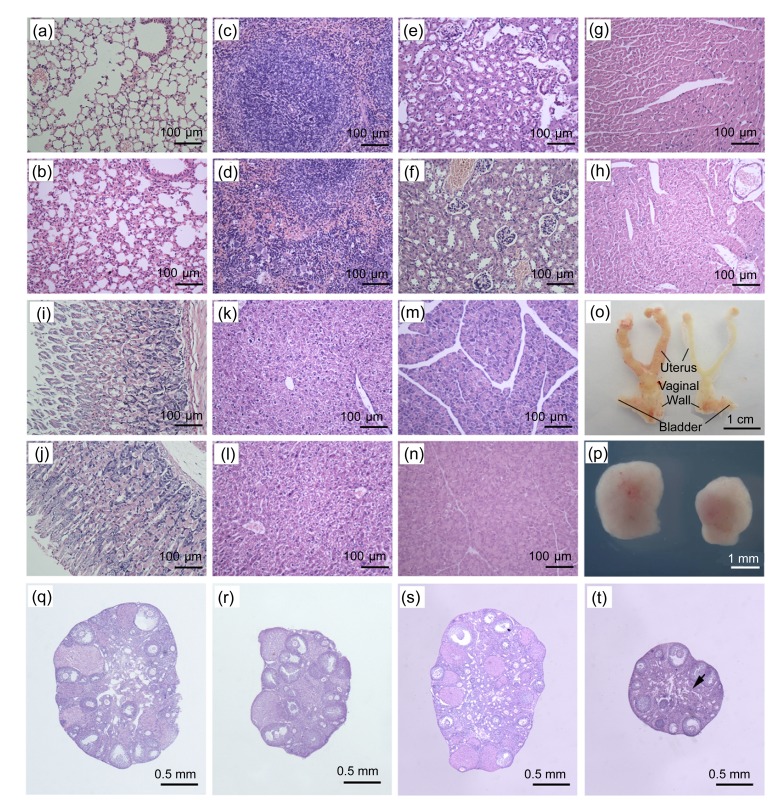
Inflammation in the lungs was observed in the Bu/Cy-treated mice, and there was an accumulation of granulocytes adjacent to alveolar walls, and thicker alveolar wall (Figs. 1a and 1b). In the spleen of the Bu/Cy-treated mice, moderate extramedullar hematopoiesis was detected in the red pulp, and slight hypocellularity was observed in the splenic corpuscle. Additionally, there was reactive hyperplasia of the white pulp in the spleen of the Bu/Cy-treated mice (Figs. 1c and 1d). There were no changes in the renal structure and in the number of renal corpuscles, and only edema of the renal tubules was detected in the kidney of Bu/Cy-treated mice (Figs. 1e and 1f). No histological changes were observed in the heart, stomach, liver, and pancreas (Figs. 1g–1n).
Fig. 1.
Effects of Bu/Cy treatment on histology of the major internal organs
(a, b) The lungs; (c, d) The spleen; (e, f) The kidney; (g, h) The heart; (i, j) The stomach; (k, l) The liver; (m, n) The pancreas; (q, r) The ovary on Day 30; (s, t) The ovary on Day 60. H&E stainings of samples from Bu/Cy-treated mice were shown in (b), (d), (f), (h), (j), (l), (n), (r), and (t), and those from age-matched untreated mice were in (a), (c), (e), (g), (i), (k), (m), (q), and (s). There were thinner uterine walls and vaginal walls in Bu/Cy-treated mice (o, right) compared with the age-matched controls (o, left). The ovary of Bu/Cy-treated mice (p, right) was smaller than that of the controls (p, left). There was a significant reduction of primordial and primary follicles in Bu/Cy-treated mice (r) relative to age-matched untreated controls (q); There was a marked loss of follicles and mild fibrosis in part of interstitial area (arrow) in Bu/Cy-treated mice (t) compared with the controls (s)
3.2. Gross pathology of urogenital systems and histology of ovary in Bu/Cy-treated mice
We checked gross pathology of urogenital systems and histology of ovaries on Day 30 after Bu/Cy treatment. We observed uterine atrophy, thinner uterine walls and vaginal walls in Bu/Cy-treated mice in comparison with the controls (Fig. 1o). There were no gross pathological changes in the bladder of Bu/Cy-treated mice. Additionally, when we dissected the uteri in Bu/Cy-treated mice, we found that they were more fragile than the uteri from the control mice. Ovaries in Bu/Cy-treated mice were smaller in size than those in the control mice. The ovarian size in Bu/Cy-treated mice was about 2/3 the size of the ovaries in the control mice (Fig. 1p).
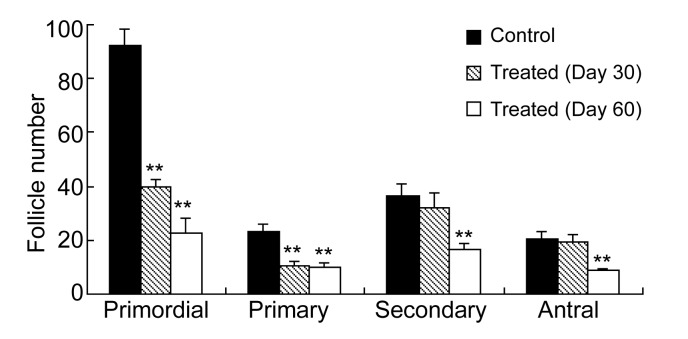
On Day 30 after the Bu/Cy treatment, there was a marked reduction of small follicles (primordial and primary) in the Bu/Cy-treated mice (Fig. 1r) relative to age-matched controls (Fig. 1q), but no significant changes were observed in the number of secondary and antral follicles. By counting the number of follicles on the ovarian sections, we found that primordial and primary follicles in Bu/Cy-treated mice reduced by 56.7% and 54.8%, respectively (P<0.01, Fig. 2). On Day 60 after the onset of Bu/Cy treatment, many follicles, at different developmental stages, were observed on the sections of the controls (Fig. 1s). However, there was a significant loss of follicles at all developmental stages in the Bu/Cy-treated mice (Fig. 1t). Additionally, mild fibrosis was observed in part of interstitial area in the Bu/Cy-treated mice (Fig. 1t, arrow). Our data show that Bu/Cy treatment reduces primordial, primary, secondary, and antral follicles by 75.7%, 57.4%, 53.8%, and 57.4%, respectively (P<0.01, Fig. 2).
Fig. 2.
Effect of Bu/Cy treatment on different follicle types
On Day 30, there was a significant reduction (P<0.01) of primordial and primary follicles in Bu/Cy-treated mice (hatched bar) compared with age-matched untreated mice (black bar). On Day 60, there was a significant reduction (P<0.01) of primordial, primary, secondary, and antral follicles in Bu/Cy-treated mice (white bar) compared with the controls (black bar). Each bar represents the mean±standard error of the mean (SEM) (n=5). ** P<0.01 compared with untreated female mice at the age of three months
3.3. Plasma levels of estradiol, progesterone, and testosterone
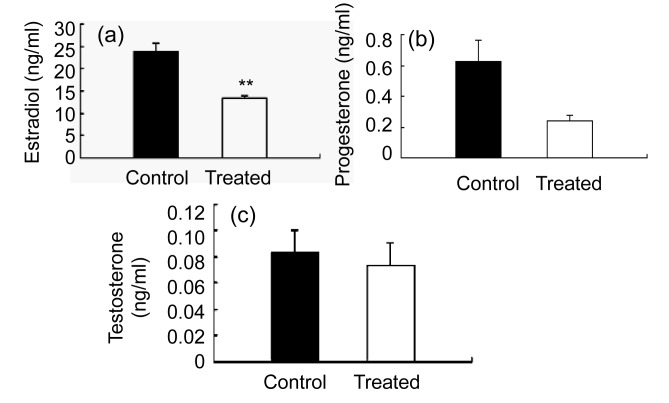
On Day 30 after the onset of Bu/Cy treatment, the plasma samples were collected and tested. Plasma estradiol and progesterone levels were lower in Bu/Cy-treated mice than in the controls. Plasma levels of estradiol and progesterone in Bu/Cy-treated mice decreased by 43.9% and 61.4%, respectively (Figs. 3a and 3b), but there was no significant change in testosterone levels (Fig. 3c).
Fig. 3.
Plasma levels of estradiol (a), progesterone (b), and testosterone (c) in mice
Levels of estradiol and progesterone were tested on Day 30. (a) The plasma level of estradiol in Bu/Cy-treated mice (white bar) decreased by 43.9% compared with the control (black bar). (b) The plasma level of progesterone in Bu/Cy-treated mice (white bar) decreased by 61.4% compared with the control (black bar). (c) There were no significant changes of testosterone levels between Bu/Cy-treated mice and the control. Values are the mean±SEM (n=6). ** P<0.01 compared with untreated age-matched female mice. Control represents the age-matched untreated mice. Treated represents the Bu/Cy-treated mice
4. Discussion
Chemotherapy is an important method to treat cancers. The combination of Bu and Cy (Bu/Cy) is a common regimen that is used before hematopoietic stem cell transplantation for patients with leukemia, inherited metabolic diseases, or chronic granulomatous disease. The clinical observations indicated that cancer patients treated with Bu/Cy can potentially suffer from ovarian failure and infertility (Grigg et al., 2000; Meirow and Nugent, 2001; Sklar, 2005; Maltaris et al., 2007). However, there are no stuides on the effects of Bu/Cy combination on major internal organs (especially ovaries) using mice as the model.
In this research, the Bu/Cy treatment was provided as a single injection; it was found to cause a gradual process of follicle loss and low estradiol and progesterone in the treated mice. The Bu/Cy-treated mice showed a significant loss of primordial and primary follicles on Day 30, while they showed a significant reduction of follicles at all stages by Day 60.
The development and maturation of ovarian follicles involved several stages, including primordial, primary, secondary, and antral. With the loss of primordial and primary follicles, all growing follicles (secondary and antral) were eventually depleted due to the lack of the precursor follicle populations for recruitment. Estrogen and progesterone were mainly secreted by granulosa cells in the ovaries. With impairment to granulosa cells by the chemotherapeutic agents Bu/Cy, the levels of progesterone and estradiol were reduced. Our results were in accordance with the previous report that Cy could induce granulosa cell apoptosis and cause ovarian damage (Lopez and Luderer, 2004). The thinner uterine walls most likely resulted from the withdrawal of estradiol, since one of estradiol’s functions was to act as the growth hormone for the uterine wall (Kaaks et al., 2002). The smaller size of ovaries may be the result of Bu/Cy-induced follicle loss.
We also studied the effects of Bu/Cy treatment on other major organs. We found that the treatment of Bu/Cy affected the ovary the most, while it had lesser effects on the spleen, lungs, and kidneys, and no effects on the heart, liver, stomach, and pancreas. Al-Hashmi et al. (2011) treated mice with Bu/Cy, and found there were histopathological changes in the liver, pancreas, spleen, lungs, and heart, but not in the kidneys. These different results may attribute to the difference in dosage and the time of examination used in the two laboratories. Also various organs have different repairing capacity after the treatment. Al-Hashmi et al. (2011) examined the tissues shortly after treatment, while we examined 1–2 months later. Bu is well known for lung injury shortly after the clinical treatment (Soble and Perry, 1977; Hankins et al., 1978; Schallier et al., 1983; Vergnon et al., 1988). However, the literature is lacking a clear understanding of the mechanisms by which Bu induces lung impairment, because there are no animal models of Bu-induced pulmonary toxicity. The Bu/Cy-treated mice in this study showed lung alveolar thickening (similar to those symptoms caused by Bu in clinical observation), and they may be used as animal models to study the mechanisms involved in Bu-induced pulmonary disorders.
The Bu/Cy-treated mice showed a gradual process of follicle loss and low estradiol; these conditions were similar to premature ovarian failure (POF) in women. Thus, the Bu/Cy-treated mice can potentially be used to examine the dynamics of follicle loss that is not easy to perform (if not impossible) in women with POF and help us to understand this disease. It is widely accepted that mammalian ovaries contain a fixed number of primordial follicles at birth. The majority of these follicles undergo a continual process of degeneration termed atresia. Once the oocyte reserve is used up, ovarian senescence occurs; this is referred to as menopause. Natural menopause usually occurs in women at the age of 45–55 years. However, some women under the age of 40 years suffer from ovarian senescence; this is called POF. POF is also called premature menopause, which refers to a group of conditions that include amenorrhoea, hypo-oestrogenism and elevated gonadotrophin levels in women younger than 40 years of age (Shelling, 2010). It was reported that 1%–2% of women younger than 40 years and 0.1% of women younger than 30 years have suffered from POF (Coulam et al., 1986).
The etiology of POF is largely unknown, with different factors interacting to produce the clinical symptoms. The known causes of POF included some gene defects, permanent damage to the ovary by toxic chemicals, X-chromosome abnormalities and autoimmune conditions (Woad et al., 2006; Dixit et al., 2010). However, we still lack a clear understanding of POF, such as the causes and the dynamics of follicle depletion. It is important to develop animal models for studying POF, because there is an urgent need to examine the dynamics of follicle loss and the endocrine profiles that can help to evaluate the risks versus benefits of some therapies (like long-term steroid hormone replacement) for this disease (Barrett-Connor and Stuenkel, 1999; Nair and Herrington, 2000; Rossouw et al., 2002). Additionally, animal models are especially useful in some research that is impossible or unethical to perform in humans.
There are some reports about the construction of animal models for accelerated reproductive aging. However, there are shortcomings in every case. The ovariectomized animal was the most commonly used model, but no ovary remained to be analyzed in this model, and it can only be used to study effects of ovarian loss on other tissues or systems. Some genetically modified mouse models have been reported, such as follitropin receptor knockout (FORKO) mouse and dioxin/aryl hydrocarbon receptor (AhR) knockout mouse (Dierich et al., 1998; Benedict et al., 2000). The reproductive systems in these models have been affected at early developmental stage, and normal reproductive systems have never formed. While in POF, the reproductive function is normal in young females, and ovarian failure happens at the age 30–40 years. Additionally, 4-vinylcyclohexene diepoxide (VCD) treated mice were reported to be an animal model of accelerated reproductive aging (Mayer et al., 2004). Yet depletion of follicles in VCD-treated mice happened so quickly, no ovarian primordial and primary follicles remained on Day 37 after the initiation of treatment and almost all were lost 46 d after the treatment, a condition which is different from that in POF. Additionally there was a practical shortcoming because these mice had to be treated each day for 15 d.
In summary, the follicle-reduced, ovary-intact mouse model in this study displayed the endocrine profile and some functions of ovaries that resemble those in women with POF. It could be used to study the dynamics of follicle loss in women undergoing POF, and to assess the risks versus benefits of some therapies (like long-term steroid hormone replacement) that are hard or expensive to perform (if not impossible) in women. The Bu/Cy-treated mice, in this study, may prove to be an alternative, inexpensive, and sensitive animal model of accelerated ovarian aging.
Acknowledgments
We thank Drs. Bo HAN (School of Medicine, Shandong University) and Zhong-ke CHEN (School of Life Science, Shandong University) for some help with histological analysis and Prof. Donald HUISINGH (Institute for a Secure and Sustainable Environment, University of Tennessee, USA) for critically editing.
Footnotes
Project (No. 2010CB945002) supported by the National Basic Research Program (973) of China
Compliance with ethics guidelines: Yan JIANG, Jing ZHAO, Hui-jing QI, Xiao-lin LI, Shi-rong ZHANG, Daniel W. SONG, Chi-yang YU, and Jian-gang GAO declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Al-Hashmi S, Hassan Z, Sadeghi B, Rozell B, Hassan M. Dynamics of early histopathological changes in GVHD after busulphan/cyclophosphamide conditioning regimen. Int J Clin Exp Pathol. 2011;4(6):596–605. [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett-Connor E, Stuenkel C. Hormones and heart disease in women: heart and estrogen/progestin replacement study in perspective. J Clin Endocrinol Metab. 1999;84(6):1848–1853. doi: 10.1210/jc.84.6.1848. [DOI] [PubMed] [Google Scholar]
- 3.Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56(2):382–388. doi: 10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- 4.Burkl W, Schiechl H. The growth of follicles in the rat ovary under the influence of busulphan and endoxan. Cell Tissue Res. 1978;186(2):351–359. doi: 10.1007/BF00225543. [DOI] [PubMed] [Google Scholar]
- 5.Copelan EA, Biggs JC, Szer J, Thompson JM, Crilley P, Brodsky I, Klein JL, Kapoor N, Harman GS, Avalos BR. Allogeneic bone marrow transplantation for acute myelogenous leukemia, acute lymphocytic leukemia, and multiple myeloma following preparation with busulfan and cyclophosphamide (BuCy2) Semin Oncol. 1993;20(Suppl. 4):33–38. [PubMed] [Google Scholar]
- 6.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604–606. [PubMed] [Google Scholar]
- 7.Danilovich N, Ram Sairam M. Recent female mouse models displaying advanced reproductive aging. Exp Gerontol. 2006;41(2):117–122. doi: 10.1016/j.exger.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 8.de Magalhaes-Silverman M, Lister J, Rybka W, Wilson J, Ball E. Busulfan and cyclophosphamide (Bu/Cy2) as preparative regimen for patients with lymphoma. Bone Marrow Transplant. 1997;19(8):777–781. doi: 10.1038/sj.bmt.1700733. [DOI] [PubMed] [Google Scholar]
- 9.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. PNAS. 1998;95(23):13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixit H, Rao L, Padmalatha V, Raseswari T, Kapu AK, Panda B, Murthy K, Tosh D, Nallari P, Deenadayal M, et al. Genes governing premature ovarian failure. Reprod Biomed Online. 2010;20(6):724–740. doi: 10.1016/j.rbmo.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Grigg AP, McLachlan R, Zaja J, Szer J. Reproductive status in long-term bone marrow transplant survivors receiving busulfan-cyclophosphamide (120 mg/kg) Bone Marrow Transplant. 2000;26(10):1089–1095. doi: 10.1038/sj.bmt.1702695. [DOI] [PubMed] [Google Scholar]
- 12.Hankins DG, Sanders S, MacDonald FM, Drage CW. Pulmonary toxicity recurring after a six week course of busulfan therapy and after subsequent therapy with uracil mustard. Chest. 1978;73(3):415–416. doi: 10.1378/chest.73.3.415. [DOI] [PubMed] [Google Scholar]
- 13.Hemsworth BN, Jackson H. Effect of busulphan on the developing ovary in the rat. J Reprod Fertil. 1963;6(2):229–233. doi: 10.1530/jrf.0.0060229. [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, Forkert R, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122(2):303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Jopling C, Rosendaal M. A cautionary tale: how to delete mouse haemopoietic stem cells with busulphan. Br J Haematol. 2001;113(4):970–974. doi: 10.1046/j.1365-2141.2001.02825.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–1543. [PubMed] [Google Scholar]
- 18.Lopez SG, Luderer U. Effects of cyclophosphamide and buthionine sulfoximine on ovarian glutathione and apoptosis. Free Radic Biol Med. 2004;36(11):1366–1377. doi: 10.1016/j.freeradbiomed.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 19.Maltaris T, Seufert R, Fischl F, Schaffrath M, Pollow K, Koelbl H, Dittrich R. The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol. 2007;130(2):148–155. doi: 10.1016/j.ejogrb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71(1):130–138. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 21.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7(6):535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 22.Meirow D, Lewis H, Nugent D, Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999;14(7):1903–1907. doi: 10.1093/humrep/14.7.1903. [DOI] [PubMed] [Google Scholar]
- 23.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- 24.Nair GV, Herrington DM. The ERA trial: findings and implications for the future. Climacteric. 2000;3(4):227–232. doi: 10.1080/13697130008500132. [DOI] [PubMed] [Google Scholar]
- 25.Pelloux MC, Picon R, Gangnerau MN, Darmoul D. Effects of busulfan on ovarian folliculogenesis, steroidogenesis and anti-müllerian activity of rat neonates. Acta Endocrinol (Copenh) 1988;118(2):218–226. doi: 10.1530/acta.0.1180218. [DOI] [PubMed] [Google Scholar]
- 26.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 27.Schallier D, Impens N, Warson F, van Belle S, de Wasch G. Additive pulmonary toxicity with melphalan and busulfan therapy. Chest. 1983;84(4):492–493. doi: 10.1378/chest.84.4.492. [DOI] [PubMed] [Google Scholar]
- 28.Shelling AN. Premature ovarian failure. Reproduction. 2010;140(5):633–641. doi: 10.1530/REP-09-0567. [DOI] [PubMed] [Google Scholar]
- 29.Shirota M, Soda S, Katoh C, Asai S, Sato M, Ohta R, Watanabe G, Taya K, Shirota K. Effects of reduction of the number of primordial follicles on follicular development to achieve puberty in female rats. Reproduction. 2003;125(1):85–94. doi: 10.1530/rep.0.1250085. [DOI] [PubMed] [Google Scholar]
- 30.Sklar C. Maintenance of ovarian function and risk of premature menopause related to cancer treatment. J Natl Cancer Inst Monogr. 2005;2005(34):25–27. doi: 10.1093/jncimonographs/lgi018. [DOI] [PubMed] [Google Scholar]
- 31.Soble AR, Perry H. Fatal radiation pneumonia following subclinical busulfin injury. AJR Am J Roentgenol. 1977;128(1):15–18. doi: 10.2214/ajr.128.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Ulrickson M, Aldridge J, Kim HT, Hochberg EP, Hammerman P, Dube C, Attar E, Ballen KK, Dey BR, McAfee SL, et al. Busulfan and cyclophosphamide (Bu/Cy) as a preparative regimen for autologous stem cell transplantation in patients with non-Hodgkin lymphoma: a single-institution experience. Biol Blood Marrow Transplant. 2009;15(11):1447–1454. doi: 10.1016/j.bbmt.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Vergnon JM, Boucheron S, Riffat J, Guy C, Blanc P, Emonot A. Interstitial pneumopathies caused by busulfan. Histologic, developmental and bronchoalveolar lavage analysis of 3 cases. Rev Med Interne. 1988;9(4):377–383. doi: 10.1016/S0248-8663(88)80137-1. [DOI] [PubMed] [Google Scholar]
- 34.Woad KJ, Watkins WJ, Prendergast D, Shelling AN. The genetic basis of premature ovarian failure. Austr N Z J Obstet Gynaecol. 2006;46(3):242–244. doi: 10.1111/j.1479-828X.2006.00585.x. [DOI] [PubMed] [Google Scholar]