Abstract
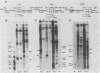
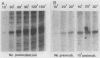
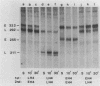
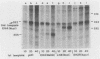
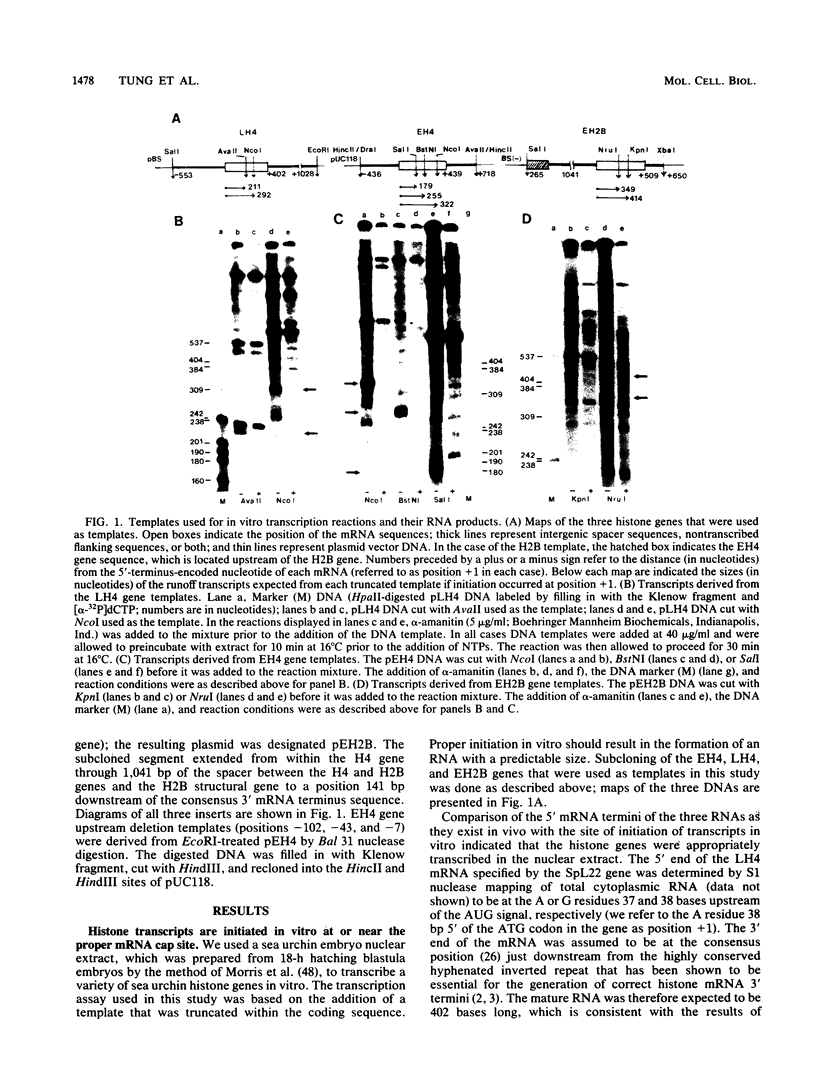
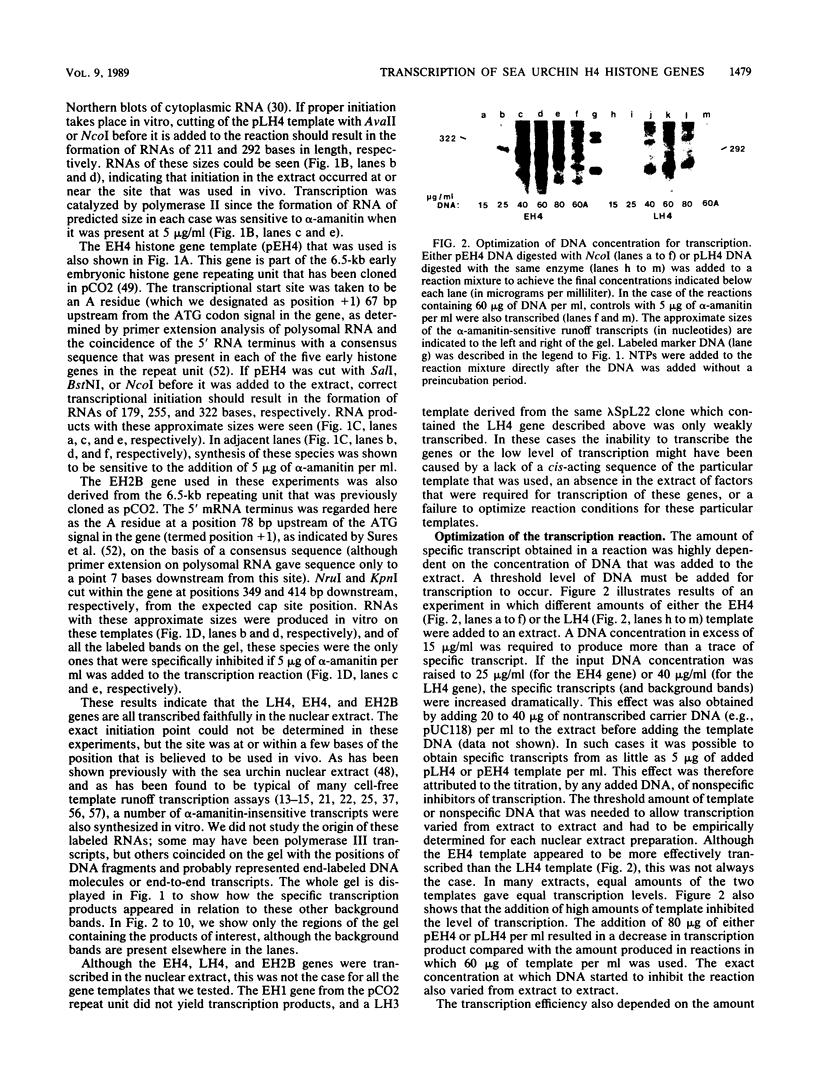
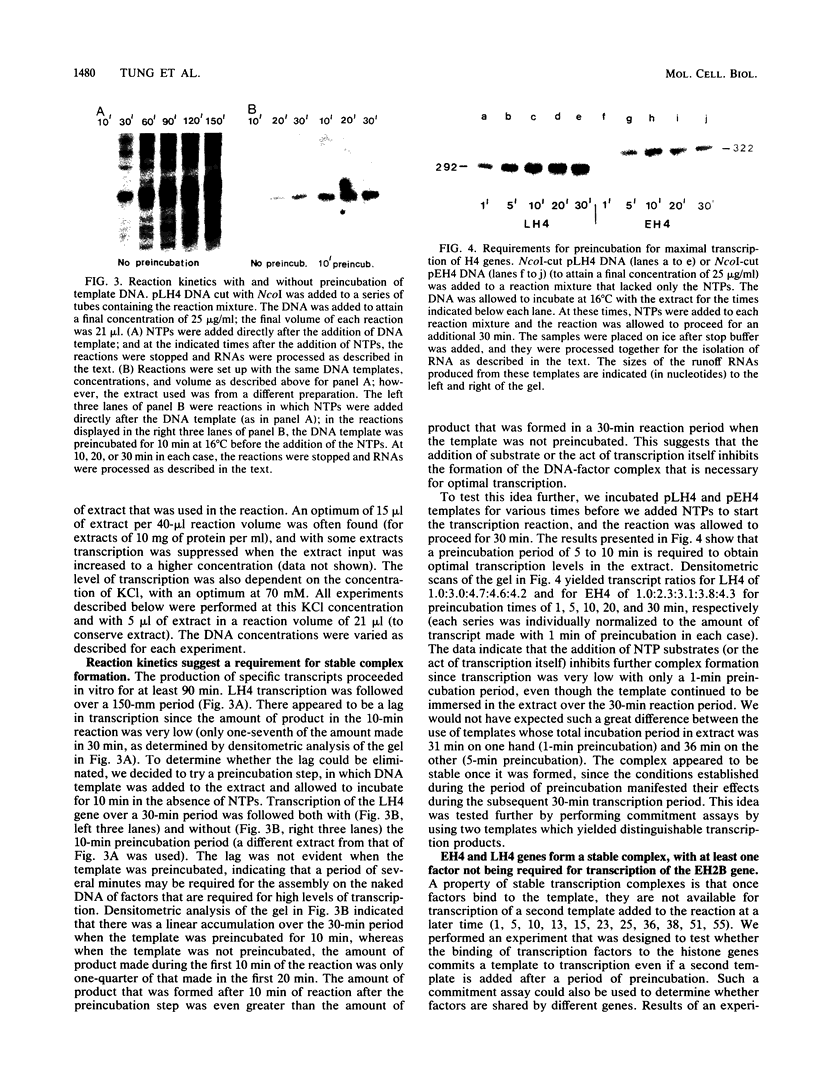
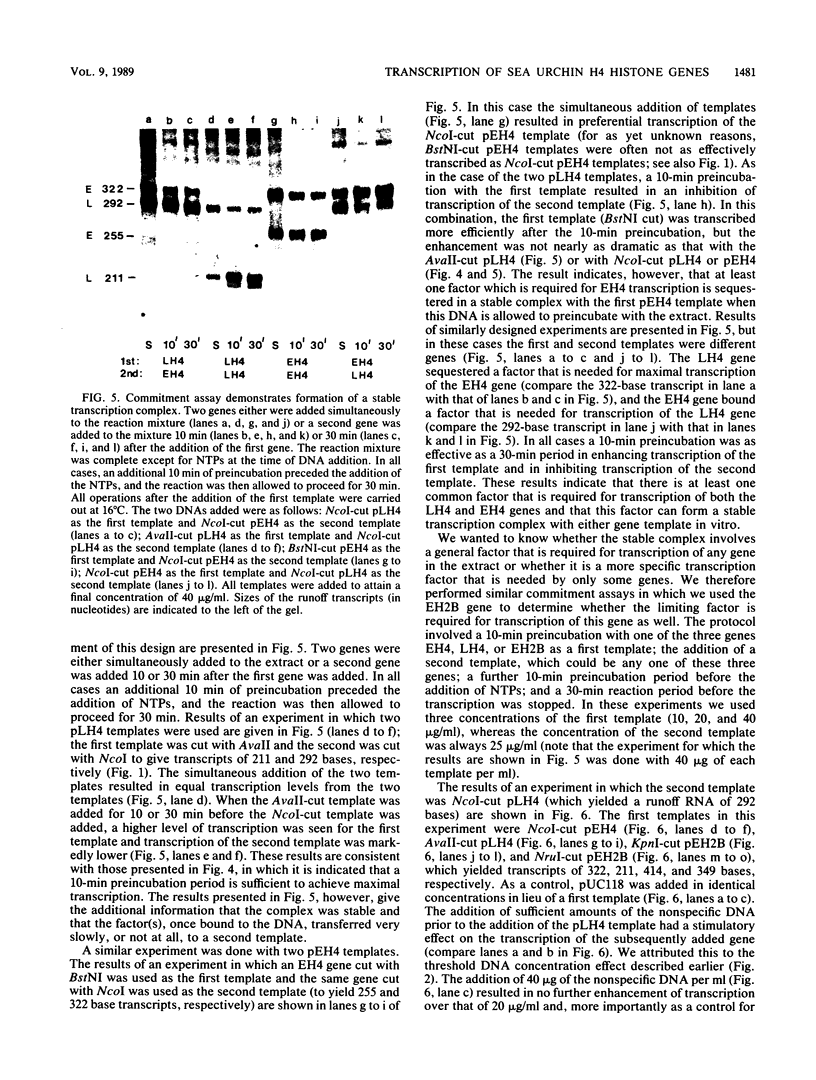
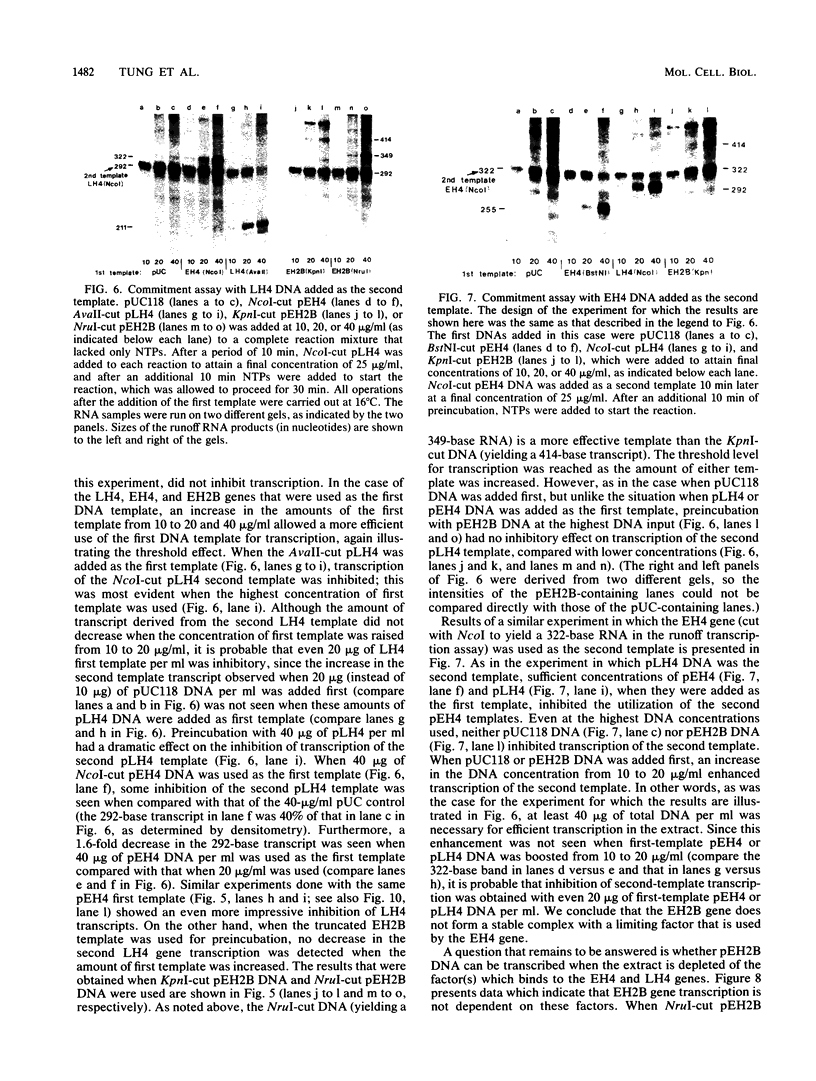
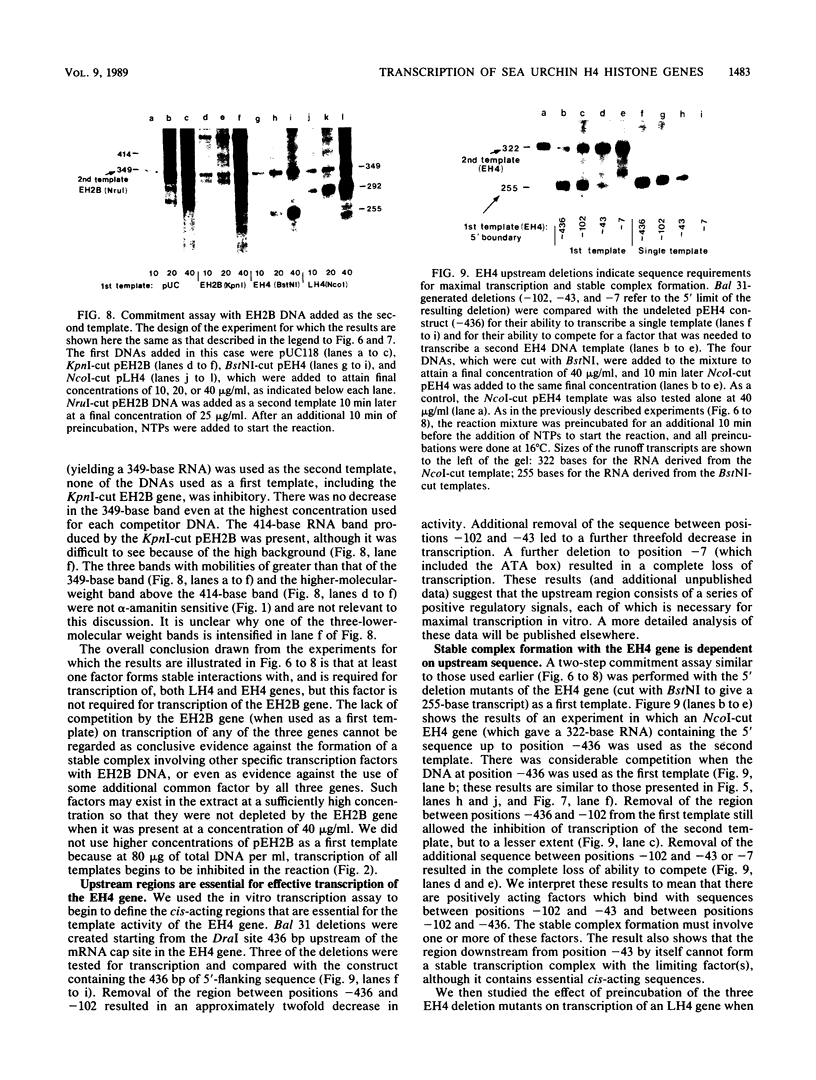
Early embryonic H4 (EH4) and H2B (EH2B) and late embryonic H4 (LH4) histone genes were transcribed in vitro in a nuclear extract from hatching blastula embryos of the sea urchin Strongylocentrotus purpuratus. The extract was prepared by slight modifications of the methods of Morris et al. (G. F. Morris, D. H. Price, and W. F. Marzluff, Proc. Natl. Acad. Sci. USA 83:3674-3678, 1986) that have been used to obtain a cell-free transcription system from embryos of the sea urchin Lytechinus variegatus. Achievement of maximum levels of transcription of the EH4 and LH4 genes required a 5- to 10-min preincubation of template with extract in the absence of ribonucleoside triphosphates. This preincubation allowed the formation of a stable complex which was preferentially transcribed compared with a second EH4 or LH4 template that was added 10 min later. Although the EH4 gene inhibited both EH4 and LH4 gene transcription in this assay and although the LH4 gene inhibited both EH4 and LH4 genes, neither of these genes inhibited transcription of the EH2B gene. Preincubation with the EH2B gene had no effect on the transcription of subsequently added EH4 or LH4 genes. Using this template commitment assay, we showed that the site of binding of at least one essential factor required for transcription of both EH4 and LH4 genes was located between positions -102 and -436 relative to the 5' terminus of the EH4 mRNA. Moreover, deletion of this region resulted in a reduction in EH4 gene transcription in vitro. The sea urchin gene-specific trans-acting factors, in the analysis of the cis-acting sequences with which they interact, and in biochemical studies on the formation of stable transcription complexes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieker J. J., Martin P. L., Roeder R. G. Formation of a rate-limiting intermediate in 5S RNA gene transcription. Cell. 1985 Jan;40(1):119–127. doi: 10.1016/0092-8674(85)90315-0. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Folk W., Birnstiel M. L. The terminal RNA stem-loop structure and 80 bp of spacer DNA are required for the formation of 3' termini of sea urchin H2A mRNA. Cell. 1983 Dec;35(2 Pt 1):433–440. doi: 10.1016/0092-8674(83)90176-9. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Grosschedl R., Birnstiel M. L. Generation of authentic 3' termini of an H2A mRNA in vivo is dependent on a short inverted DNA repeat and on spacer sequences. Cell. 1982 Apr;28(4):739–745. doi: 10.1016/0092-8674(82)90053-8. [DOI] [PubMed] [Google Scholar]
- Bodner M., Karin M. A pituitary-specific trans-acting factor can stimulate transcription from the growth hormone promoter in extracts of nonexpressing cells. Cell. 1987 Jul 17;50(2):267–275. doi: 10.1016/0092-8674(87)90222-4. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Wormington W. M., Brown D. D. Stable transcription complexes of Xenopus 5S RNA genes: a means to maintain the differentiated state. Cell. 1982 Feb;28(2):413–421. doi: 10.1016/0092-8674(82)90359-2. [DOI] [PubMed] [Google Scholar]
- Brown D. D. The role of stable complexes that repress and activate eucaryotic genes. Cell. 1984 Jun;37(2):359–365. doi: 10.1016/0092-8674(84)90366-0. [DOI] [PubMed] [Google Scholar]
- Busslinger M., Barberis A. Synthesis of sperm and late histone cDNAs of the sea urchin with a primer complementary to the conserved 3' terminal palindrome: evidence for tissue-specific and more general histone gene variants. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5676–5680. doi: 10.1073/pnas.82.17.5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs G., Maxson R., Kedes L. H. Histone gene expression during sea urchin embryogenesis: isolation and characterization of early and late messenger RNAs of Strongylocentrotus purpuratus by gene-specific hybridization and template activity. Dev Biol. 1979 Nov;73(1):153–173. doi: 10.1016/0012-1606(79)90144-1. [DOI] [PubMed] [Google Scholar]
- Childs G., Nocente-McGrath C., Lieber T., Holt C., Knowles J. A. Sea urchin (lytechinus pictus) late-stage histone H3 and H4 genes: characterization and mapping of a clustered but nontandemly linked multigene family. Cell. 1982 Dec;31(2 Pt 1):383–393. doi: 10.1016/0092-8674(82)90132-5. [DOI] [PubMed] [Google Scholar]
- Cizewski V., Sollner-Webb B. A stable transcription complex directs mouse ribosomal RNA synthesis by RNA polymerase I. Nucleic Acids Res. 1983 Oct 25;11(20):7043–7056. doi: 10.1093/nar/11.20.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin A. M., Catlin T. L., Kidson S. H., Maxson R. Closely linked early and late histone H2B genes are differentially expressed after microinjection into sea urchin zygotes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):507–510. doi: 10.1073/pnas.85.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L., Hanly S. M., Roeder R. G., Heintz N. Distinct transcription factors bind specifically to two regions of the human histone H4 promoter. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7241–7245. doi: 10.1073/pnas.83.19.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison B. L., Egly J. M., Mulvihill E. R., Chambon P. Formation of stable preinitiation complexes between eukaryotic class B transcription factors and promoter sequences. Nature. 1983 Feb 24;301(5902):680–686. doi: 10.1038/301680a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A., Samuels M., Sharp P. A. Interactions between RNA polymerase II, factors, and template leading to accurate transcription. J Biol Chem. 1984 Feb 25;259(4):2509–2516. [PubMed] [Google Scholar]
- Flytzanis C. N., Britten R. J., Davidson E. H. Ontogenic activation of a fusion gene introduced into sea urchin eggs. Proc Natl Acad Sci U S A. 1987 Jan;84(1):151–155. doi: 10.1073/pnas.84.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flytzanis C. N., McMahon A. P., Hough-Evans B. R., Katula K. S., Britten R. J., Davidson E. H. Persistence and integration of cloned DNA in postembryonic sea urchins. Dev Biol. 1985 Apr;108(2):431–442. doi: 10.1016/0012-1606(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Franks R. R., Hough-Evans B. R., Britten R. J., Davidson E. H. Spatially deranged though temporally correct expression of Strongylocentrotus purpuratus actin gene fusion in transgenic embryos of a different sea urchin family. Genes Dev. 1988 Jan;2(1):1–12. doi: 10.1101/gad.2.1.1. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Hatching in the sea urchin Lytechinus pictus is accompanied by a shift in histone H4 gene activity. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4135–4139. doi: 10.1073/pnas.75.9.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanly S. M., Bleecker G. C., Heintz N. Identification of promoter elements necessary for transcriptional regulation of a human histone H4 gene in vitro. Mol Cell Biol. 1985 Feb;5(2):380–389. doi: 10.1128/mcb.5.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Functional steps in transcription initiation and reinitiation from the major late promoter in a HeLa nuclear extract. J Biol Chem. 1987 Mar 15;262(8):3452–3461. [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Separation and partial characterization of three functional steps in transcription initiation by human RNA polymerase II. J Biol Chem. 1985 Jul 5;260(13):8163–8172. [PubMed] [Google Scholar]
- Heberlein U., Tjian R. Temporal pattern of alcohol dehydrogenase gene transcription reproduced by Drosophila stage-specific embryonic extracts. Nature. 1988 Feb 4;331(6155):410–415. doi: 10.1038/331410a0. [DOI] [PubMed] [Google Scholar]
- Heintz N., Roeder R. G. Transcription of human histone genes in extracts from synchronized HeLa cells. Proc Natl Acad Sci U S A. 1984 May;81(9):2713–2717. doi: 10.1073/pnas.81.9.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Hendricks M. B., Hemminki K., Weinberg E. S. Histone gene switch in the sea urchin embryo. Identification of late embryonic histone messenger ribonucleic acids and the control of their synthesis. Biochemistry. 1979 Jun 26;18(13):2707–2716. doi: 10.1021/bi00580a004. [DOI] [PubMed] [Google Scholar]
- Hough-Evans B. R., Franks R. R., Cameron R. A., Britten R. J., Davidson E. H. Correct cell-type-specific expression of a fusion gene injected into sea urchin eggs. Dev Biol. 1987 Jun;121(2):576–579. doi: 10.1016/0012-1606(87)90193-x. [DOI] [PubMed] [Google Scholar]
- Ito M., Bell J., Lyons G., Maxson R. Synthesis and turnover of late H2B histone mRNA in developing embryos of the sea urchin, Strongylocentrotus purpuratus. Dev Biol. 1988 Sep;129(1):147–158. doi: 10.1016/0012-1606(88)90169-8. [DOI] [PubMed] [Google Scholar]
- Katula K. S., Hough-Evans B. R., Britten R. J., Davidson E. H. Ontogenic expression of a CyI actin fusion gene injected into sea urchin eggs. Development. 1987 Nov;101(3):437–447. doi: 10.1242/dev.101.3.437. [DOI] [PubMed] [Google Scholar]
- Kaumeyer J. F., Weinberg E. S. Sequence, organization and expression of late embryonic H3 and H4 histone genes from the sea urchin, Strongylocentrotus purpuratus. Nucleic Acids Res. 1986 Jun 11;14(11):4557–4576. doi: 10.1093/nar/14.11.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler I., Busslinger M. Characterization of two nonallelic pairs of late histone H2A and H2B genes of the sea urchin: differential regulation in the embryo and tissue-specific expression in the adult. Mol Cell Biol. 1986 Nov;6(11):3746–3754. doi: 10.1128/mcb.6.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. A., Childs G. J. Comparison of the late H1 histone genes of the sea urchins Lytechinus pictus and Strongelocentrotus purpuratus. Nucleic Acids Res. 1986 Oct 24;14(20):8121–8133. doi: 10.1093/nar/14.20.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. A., Childs G. J. Temporal expression of late histone messenger RNA in the sea urchin Lytechinus pictus. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2411–2415. doi: 10.1073/pnas.81.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. A., Lai Z. C., Childs G. J. Isolation, characterization, and expression of the gene encoding the late histone subtype H1-gamma of the sea urchin Strongylocentrotus purpuratus. Mol Cell Biol. 1987 Jan;7(1):478–485. doi: 10.1128/mcb.7.1.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z. C., Maxson R., Childs G. Both basal and ontogenic promoter elements affect the timing and level of expression of a sea urchin H1 gene during early embryogenesis. Genes Dev. 1988 Feb;2(2):173–183. doi: 10.1101/gad.2.2.173. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Martin P. L., Roeder R. G. Transcription of class III genes: formation of preinitiation complexes. Science. 1983 Nov 18;222(4625):740–748. doi: 10.1126/science.6356356. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W., Lienhard S., Jiricny J., De Robertis E. M. An enhancer-like sequence within the Xenopus U2 gene promoter facilitates the formation of stable transcription complexes. Nature. 1985 Jul 11;316(6024):163–167. doi: 10.1038/316163a0. [DOI] [PubMed] [Google Scholar]
- Mauron A., Kedes L., Hough-Evans B. R., Davidson E. H. Accumulation of individual histone mRNAs during embryogenesis of the sea urchin Strongylocentrotus purpuratus. Dev Biol. 1982 Dec;94(2):425–434. doi: 10.1016/0012-1606(82)90359-1. [DOI] [PubMed] [Google Scholar]
- Maxson R. E., Jr, Wilt F. H. Accumulation of the early histone messenger RNAs during the development of Strongylocentrotus purpuratus. Dev Biol. 1982 Dec;94(2):435–440. doi: 10.1016/0012-1606(82)90360-8. [DOI] [PubMed] [Google Scholar]
- Maxson R. E., Jr, Wilt F. H. The rate of synthesis of histone mRNA during the development of sea urchin embryos (Strongylocentrotus purpuratus). Dev Biol. 1981 Apr 30;83(2):380–386. doi: 10.1016/0012-1606(81)90485-1. [DOI] [PubMed] [Google Scholar]
- Maxson R., Cohn R., Kedes L., Mohun T. Expression and organization of histone genes. Annu Rev Genet. 1983;17:239–277. doi: 10.1146/annurev.ge.17.120183.001323. [DOI] [PubMed] [Google Scholar]
- Maxson R., Mohun T., Gormezano G., Childs G., Kedes L. Distinct organizations and patterns of expression of early and late histone gene sets in the sea urchin. Nature. 1983 Jan 13;301(5896):120–125. doi: 10.1038/301120a0. [DOI] [PubMed] [Google Scholar]
- McConkey G. A., Bogenhagen D. F. TFIIIA binds with equal affinity to somatic and major oocyte 5S RNA genes. Genes Dev. 1988 Feb;2(2):205–214. doi: 10.1101/gad.2.2.205. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Flytzanis C. N., Hough-Evans B. R., Katula K. S., Britten R. J., Davidson E. H. Introduction of cloned DNA into sea urchin egg cytoplasm: replication and persistence during embryogenesis. Dev Biol. 1985 Apr;108(2):420–430. doi: 10.1016/0012-1606(85)90045-4. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Marzluff W. F. A factor in sea urchin eggs inhibits transcription in isolated nuclei by sea urchin RNA polymerase III. Biochemistry. 1983 Feb 1;22(3):645–653. doi: 10.1021/bi00272a019. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Price D. H., Marzluff W. F. Synthesis of U1 RNA in a DNA-dependent system from sea urchin embryos. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3674–3678. doi: 10.1073/pnas.83.11.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton G. C., Weinberg E. S. Length and sequence heterogeneity of the histone gene repeat unit of the sea urchin, S. purpuratus. Cell. 1978 Jun;14(2):247–257. doi: 10.1016/0092-8674(78)90111-3. [DOI] [PubMed] [Google Scholar]
- Perry M., Thomsen G. H., Roeder R. G. Genomic organization and nucleotide sequence of two distinct histone gene clusters from Xenopus laevis. Identification of novel conserved upstream sequence elements. J Mol Biol. 1985 Oct 5;185(3):479–499. doi: 10.1016/0022-2836(85)90065-8. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Tower J. Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem. 1986;55:801–830. doi: 10.1146/annurev.bi.55.070186.004101. [DOI] [PubMed] [Google Scholar]
- Sures I., Levy S., Kedes L. H. Leader sequences of Strongylocentrotus purpuratus histone mRNAs start at a unique heptanucleotide common to all five histone genes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1265–1269. doi: 10.1073/pnas.77.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Vitelli L., Kemler I., Lauber B., Birnstiel M. L., Busslinger M. Developmental regulation of micro-injected histone genes in sea urchin embryos. Dev Biol. 1988 May;127(1):54–63. doi: 10.1016/0012-1606(88)90188-1. [DOI] [PubMed] [Google Scholar]
- Wandelt C., Grummt I. Formation of stable preinitiation complexes is a prerequisite for ribosomal DNA transcription in vitro. Nucleic Acids Res. 1983 Jun 11;11(11):3795–3809. doi: 10.1093/nar/11.11.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Kédinger C., Corden J., Brison O., Chambon P. Specific in vitro initiation of transcription on conalbumin and ovalbumin genes and comparison with adenovirus-2 early and late genes. Nature. 1980 Jun 5;285(5764):367–373. doi: 10.1038/285367a0. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Weinberg E. S., Hendricks M. B., Hemminki K., Kuwabara P. E., Farrelly L. A. Timing and rates of synthesis of early histone mRNA in the embryo of Strongylocentrotus purpuratus. Dev Biol. 1983 Jul;98(1):117–129. doi: 10.1016/0012-1606(83)90340-8. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Brown D. D. Developmental regulation of two 5S ribosomal RNA genes. Science. 1988 Sep 23;241(4873):1626–1632. doi: 10.1126/science.241.4873.1626. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Brown D. D. Differential 5S RNA gene expression in vitro. Cell. 1987 Dec 4;51(5):733–740. doi: 10.1016/0092-8674(87)90096-1. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P. Transcription fraction TFIIIC can regulate differential Xenopus 5S RNA gene transcription in vitro. EMBO J. 1988 Apr;7(4):1071–1079. doi: 10.1002/j.1460-2075.1988.tb02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]