Abstract
Copper is an essential micronutrient. Following entry via the human copper transporter 1 (hCTR1), copper is delivered to several copper chaperones, which subsequently transfer the metal to specific targets via protein:protein interactions. It is has been assumed, but not demonstrated, that chaperones acquire copper directly from hCTR1. However, some reports have pointed to an intermediary role for glutathione (GSH), an abundant copper-binding tri-peptide. To address the issue of how transported copper is acquired by the copper chaperones in vivo, we measured the initial rate of 64Cu uptake in cells in which the cellular levels of copper chaperones or GSH were substantially depleted or elevated. Knockdown or overexpression of copper chaperones ATOX1, CCS, or both had no effect on the initial rate of 64Cu entry into HEK293 cells having endogenous or overexpressed hCTR1. In contrast, depleting cellular GSH using l-buthionine-sulfoximine (BSO) caused a 50% decrease in the initial rate of 64Cu entry in HEK293 cells and other cell types. This decrease was reversed by washout of BSO or GSH replenishment with a permeable ester. BSO treatment under our experimental conditions had no significant effects on the viability, ATP levels, or metal content of the cells. Attenuated 64Cu uptake in BSO was not due to oxidation of the cysteine in the putative metal-binding motif (HCH) at the intracellular hCTR1 COOH terminus, because a mutant lacking this motif was fully active, and 64Cu uptake was still reduced by BSO treatment. Our data suggest that GSH plays an important role in copper handling at the entry step.
Keywords: copper transport, hCTR1, glutathione, copper chaperones
mammalian cells require copper ions as a cofactor for a number of enzymes including cytochrome c oxidase, superoxide dismutase (SOD), lysyl oxidase, and the enzymes necessary for neurotransmitter and neuropeptide synthesis (32). Cellular copper homeostasis in mammals is tightly regulated by various copper binding proteins (19, 24), and free copper is not detectable in cells (42). Free copper is presumed to cause a variety of toxic events through attaching to cellular proteins or via the generation of reactive oxygen species (36).
Copper entry into human cells is mediated mainly via the selective high-affinity human copper transporter 1 (hCTR1; Ref. 49). hCTR1 is a small, 190 amino acid residue membrane protein containing 3 highly conserved membrane-spanning domains. The less conserved amino terminus of each monomer is extracellular, and the highly conserved short COOH-terminal tail is cytoplasmic (15, 26). The protein forms a permeation pore for copper ions through the formation of a homotrimer in the plasma membrane, lined by the second transmembrane domain (1, 13). Studies utilizing mutagenic analysis and heterologous expression have shown that methionine residues in the amino terminus and near the extracellular portion of the pore are essential for copper transport by hCTR1 (14, 30, 41).
The mechanism and energetics of copper transport by hCTR1 are not yet understood. Transport does not appear to require ATP or to be coupled to an ion gradient (27), leaving open the question of what drives the permeation of the metal across the plasma membrane. It is generally thought that cuprous (Cu+), rather than cupric (Cu2+) copper ions are transported by hCTR1 (40). Of numerous metals tested, only monovalent silver (Ag+) inhibits or competes with 64Cu uptake (27), and it was subsequently shown that hCTR1 transports Ag+(8). Furthermore, using spectroscopic techniques, De Feo et al. (13) showed that purified recombinant hCTR1 bound two Cu+ ions per trimer (13).
Following transport via hCTR1, copper is delivered to various cellular destinations. A number of other well-studied proteins and small molecules in addition to hCTR1 participate in copper trafficking in mammalian cells. ATP7A and ATP7B are copper-activated ATPases that deliver copper to enzymes in the secretory pathway and also function to remove excess copper (31). The copper chaperones CCS, ATOX1, and Cox 17 specifically deliver copper to ATP7A and ATP7B, SOD, and Sco1/Cox 11, respectively (21, 23, 45, 47). Metallothionine proteins (4 human isoforms) are believed to store and/or buffer excess copper in vivo (39). In addition to proteins, the thiol-containing tripeptide GSH is highly abundant in the cytoplasm and is known to bind transported copper in vivo (9, 18).
It is unknown exactly how copper is transferred from the hCTR1 transporter to the copper chaperones that subsequently deliver the metal to specific target proteins (21, 38). Specifically, it is not known whether copper is exchanged directly from the transporter to the chaperones via specific transporter:chaperone interactions (22, 43) or via an intermediate ligand. Perhaps because free copper is not detectable in the cytoplasm of cells (42), it has been assumed in the literature that the copper chaperones acquire the metal from the transporter. However, this direct transporter:chaperone exchange has not been tested.
In early studies of copper entry utilizing 67Cu uptake, carried out before hCTR1 or copper chaperones had been identified, it was shown with fractionation procedures that the first identifiable intracellular acceptor of 67Cu was GSH, after which the copper was believed to be transferred to metallothionein (18). More recently, a series of in vitro measurements of the copper binding affinities and cellular concentrations of GSH, copper chaperones, and metallothionein (5) suggested that, in vivo, transported copper could be exchanged between millimolar levels of relatively low-affinity GSH and micromolar concentrations of the chaperones (which have much greater affinity for copper). The chaperones would then exchange the bound copper to target proteins having still higher affinity for the metal (5). There is some dispute in the literature regarding the relative affinities of GSH and the chaperones (48), but we were motivated by these studies to design in vivo experiments (i.e., in cultured cells) that address whether copper transported by hCTR1 is acquired directly by copper chaperones or is first bound by GSH, as suggested by the fractionation experiments mentioned above.
In this work, we measured the initial rates of 64Cu uptake in HEK293 cells in which the levels of copper chaperones or GSH have been manipulated and in which hCTR1 can be overexpressed with tetracycline induction. We reasoned that if chaperones first bind transported copper, then wide variations in chaperone abundance should influence the initial rate of 64Cu uptake mediated by hCTR1. To test whether the copper chaperones ATOX1 or CCS interact directly with hCTR1 to receive copper, we compared the initial rate of 64Cu uptake in untreated cells and in cells having decreased or increased chaperone protein levels, using small interfering (si)RNA knockdown and transient overexpression of the chaperones. We found that altering chaperone protein levels had no significant effect on the initial rates of copper entry. We also tested whether or not GSH levels affect the initial rate of 64Cu uptake by depleting GSH in HEK293 cells with l-buthionine-sulfoximine (BSO), an irreversible inhibitor of γ-glutamylcysteine synthetase (2, 7). We found that depletion of GSH by inhibition of its synthesis caused significant inhibition of copper uptake, which was restored when GSH levels were supplemented with a permeable GSH ester.
EXPERIMENTAL PROCEDURES
Cell culture.
HEK293 FLp-In T-Rex cells (Life Technologies, Grand Island, NY), Huh-7 cells, and A2780 cells were cultured in DMEM (Life Technologies), 25 mM HEPES buffer, and 10% fetal calf serum (Atlanta Biologicals, Atlanta, GA; hereafter, “DEM-10%”). Rat smooth muscle cells were cultured in DMEM with 10% bovine sera (Life Technologies). FLp-In T-Rex cells were maintained with selective antibiotics: 350 μg/ml hygromycin and 10 μg/ml blasticidin. All cells were grown at 37° C in 5% CO2. Cells were subcultured every 3–5 days or thawed from frozen stocks after 12–15 passages.
hCTR1-expressing cells and constructs.
Wild-type (NH2-terminal FLAG-tagged) hCTR1 or a mutant hCTR1 in which the carboxyl-terminal three amino acids HisCysHis were replaced with alanine residues (HCH-AAA) were recombined into the tetracycline-regulated expression site of HEK293 Flp-In T-Rex cells using Invitrogen Flp-In T-Rex vectors as described previously (34). The HCH-AAA mutant was made using PCR as previously described (34) with the wild-type (FLAG-tagged) gene as a template. PCR oligonucleotides were 5′-CTCTAGAACTAGTGGATCCACCATGGACTACAA (forward) and 5′-GCTCTCGAGTTGTCATGCAGCTGCCTCTGTGATATCCACTACCACTGCCTTC (reverse).
Cu uptake assays.
64Cu uptake assays with various cells, treatments, or transfections were performed in 12-well tissue culture plates. For HEK293 Flp-In T-Rex cells in which hCTR1 was overexpressed, cells were induced with media containing 1 μg/ml tetracycline 48 h before the assay. One day before the assays, 12-well tissue culture plates were seeded with 1.0–1.5 × 106 cells/well in DMEM-10%, unless otherwise noted. The following day the cells were washed once with DMEM-10% (without antibiotics or drugs such as BSO) and then incubated in 1 ml DMEM-10% containing 2 or 5 μM added CuCl2 and trace amounts of 64Cu (2–4 × 106 cpm/well) for 5 min at room temperature or 30 min at 37°C (64Cu uptakes from 5-min incubations were subtracted from 30-min incubations to correct for nonspecific binding). Cells from each well were processed for scintillation counting, picomoles copper transported, and protein determination exactly as previously described (34). The Cu uptake was usually calculated as picomoles Cu per milligrams total cellular protein per minute (14). In some instances, copper uptakes are expressed as a percentage of the uptake in untreated cells. Uptake data were also normalized to the relative amount of hCTR1 in the membrane when hCTR1 was elevated by knockdown of ATOX1. Uptake in control siRNA-treated cells was set to 100%, and uptake in other conditions was reported as percentage of control after dividing the quantity of hCTR1 expressed in each condition by the quantity calculated for control cells. Relative Western blot band intensity, and thus relative expression, were determined using Quantity One Software (Bio-Rad, Hercules, CA).
RNAi knockdown of ATOX11I and CCS.
Silencer Select siRNA oligonucleotides targeting ATOX1, CCS, and control siRNA (Life Technologies, Grand Island, NY; product numbers for siRNA available on request) were used to transfect HEK293. To optimize knockdown, cells were transfected with 10 nM siRNA/well in Lipofectamine RNAiMax (Life Technologies). The cells were harvested 24, 48, or 72 h after transfection. The maximun effect of siRNA was seen at 72 h (70–90% knockdown). Thus subsequent siRNA experiments (including surface biotinylation and/or preparation of lysates) were performed with a 72-h siRNA treatment. The cells were plated at 0.8 × 106 cells/well into 12-well culture plates 1 day before the Cu uptake assay. For optimizing siRNA knockdown and for biotinylation of surface proteins to monitor hCTR1 levels in cells in which siRNA was performed, HEK293 cells were plated between 2.5–3.75 × 105 cells/well in six-well culture plates 24 h before transfection. Following a 72-h treatment with siRNA, cells were biotinylated as described previously (37) and were lysed in PBS containing 1% Triton X-100, 0.1% SDS, 1 mM EDTA, and a cocktail of protease inhibitors (Roche, Indianapolis, IN) for 30 min on ice, after which strepavidin beads were added to the lysates to recover biotinylated proteins. Soluble cytoplasmic proteins from the lysate supernatants (e.g., ATOX1 and CCS) were recovered after centrifuging the lysates for 20 min at 16,000 g (4°C). Protein concentration was determined using a protein assay dye binding reagent (Bio-Rad).
Overexpression of ATOX1 and CCS.
For transient transfections of copper chaperone proteins, CMV promoter-driven cDNA clones of ATOX1 (Genbank accession no. NM_004045.3) and CCS (accession no. NM_005125) were obtained from Origene (Rockville, MD) and Genecopia (Rockville, MD), respectively. Each construct was sequenced to verify the reading frames. Vector-only controls were constructed by digestion with restriction enzymes that flanked each coding sequence, blunting of the ends, and religation to produce “empty” expression vector clones. The expression clones and vector controls were transfected 48 h before copper uptake assays using turbofectin (Origene). After 24 h, transfected cells were trypsinized and counted and then plated in 12-well culture plates for 64Cu uptake assays performed on the following day. The fold overexpression relative to endogenous ATOX1 and CCS was estimated by comparing Western blot signals among cell lysate proteins from cells transfected with expression constructs or empty vectors, normalized to actin. Transfected cells from duplicate 12-well plates used in copper uptake assays were lysed as described for siRNA experiments above, and 15–50 μg protein lysate/lane were analyzed in 12% (CCS) or 4–20% (ATOX1) SDS -PAGE. Duplicate wells were also biotinylated.
Biotinylation of surface proteins.
Cells were biotinylated with a membrane-impermeable form of biotin as described previously (37) to assess the effects of various treatments on the level of hCTR1 transporter in the plasma membrane.
SDS-PAGE and Western blot analysis.
Twelve or fifteen percent SDS-PAGE was performed with precast gels (Life Technologies, Grand Island, NY). Sixteen percent Tricine gels (Invitrogen) were used to resolve lower molecular mass proteins <10 kDa in Tricine SDS buffer (Life Technologies). Gels were transferred to Immobilon-P membranes (Millipore, Bedford MA), and Western blots were done as described previously (37). The following primary antibodies were used: rabbit anti-hCTR1 antibody against hCTR1 carboxyl-terminal tail (15), mouse anti-FLAG (Genscript, Piscataway, NJ), mouse anti-CCS (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-ATOX1 (Abcam, Cambridge, MA), mouse anti-Actin (Abcam), and mouse anti-α1-subunit of Na-K-ATPase (Affinity Bioreagents, Golden, CO). Following incubation with primary antibody, membranes were washed in PBS-0.1% Tween and incubated with either donkey anti-rabbit horseradish peroxidase (GE Healthcare) or goat anti-mouse horseradish peroxidase (Thermo-Fisher-Pierce, Rockford, IL). Western blot signals were generated using luminol-based reagents (Thermo-Fisher-Pierce, Millipore) and collected with a Chemi-Doc XRS system (Bio-Rad Laboratories, www.bio-rad.com). Relative band intensity (relative expression and/or copy number of proteins) was determined using Quantity One Software (Bio-Rad) for all Western blots shown.
Manipulation and measurement of cellular GSH levels.
GSH levels in HEK293 and other cell lines cells were reduced by treatment with l-BSO (Santa Cruz Biotechnology, Santa Cruz, CA) To determine the effect of BSO on cytoplasmic GSH concentration, BSO was added to culture media at concentrations ranging from 5 to 1,000 μM, a range of concentrations found not to be toxic to the cells, as discussed below. Cells were incubated in BSO 24–72 h before use in experiments. Most experiments were done after a 48-h BSO treatment. To restore GSH in cells treated with BSO, 1.5 or 4 mM (reduced) GSH-diethyl ester (Bachem, Bubendorf, Switzerland) were added to the medium of BSO-treated cells 12–16 h before Cu-uptake assays (29, 33). Higher concentrations of GSH-diethyl ester (≥5 mM) were toxic to cells. Primary rat smooth muscle cells were very sensitive to GSH-diethyl ester, which caused cell detachment at 1.5 mM in 64Cu uptake assays. Cellular GSH levels were determined with the GSH-Glo and/or GSH-GSSG-Glo luminescence-based glutathione assay kits from Promega (Madison, WI), as directed by the manufacturer. Luminescence signals from 5,000 or 10,000 cells were quantified using a FLUOstar Omega plate reader (BMG Labtech, Cary, NJ) and converted to cellular GSH concentration based on an average cell volume of 2 pl. Average cell volume was calculated with a Scepter Cell Counter (Millipore, Billerica MA) and was close to values previously reported (25). The copy number of GSH was calculated using the GSH concentration determined above and the volume of the number of cells used in the GSH assays.
We attempted to use two other compounds to reduce GSH concentration with or without BSO treatment. 2-AAPA {2-acetylamino-3-[4-(2-acetylamino-2-carboxy-ethylsulfanylthiocarbonylamino)phenylthiocarbamoylsulfanyl] propionic acid, an inhibitor of glutathione reductase, from Sigma/Aldrich} and diethylmaleate (forms GSH adducts, from Santa Cruz Biotechnology) are both reported to decrease cellular GSH levels (33); however, both were toxic to, and caused detachment of HEK293 cells in, our 64Cu uptake assays.
Other cell assays for viability and proliferation.
Viability of cells was assayed using PrestoBlue (Life Technologies, Grand Island, NY), a nontoxic fluorescence-based indicator that is reduced by metabolic activity of viable, growing cells (44). The reagent was added directly to cells grown in 24-well plates as directed by the manufacturer, the cells were incubated for 3–4 h, and the supernatant fluorescence was subsequently quantified in the FLUOstar Omega plate reader described above. Total cell ATP was measured using a fluorescence-based kit from Biovision (Milpitas, CA), according to the manufacturer's directions; 106 cells were used for each sample in triplicate. Fluorescence was quantified in the FLUOstar Omega plate reader, as above. Trypan blue (0.4%, Invitrogen) was added to an equal volume of cells 10 min before counting in a hemocytometer to determine the percentage of cells that excluded the dye.
Quantification of CCS, ATOX1, and hCTR1.
ATOX1 and CCS were quantified using Western blot analysis as follows. Purified ATOX1 (Genway Biotech, San Diego, CA) or CCS (Fitzgerald Industries, Acton, MA) proteins were run in lanes alongside samples containing HEK293 cells (cells were induced with tetracycline to overproduce hCTR1 or were untreated). For each lane, pellets of 5,000 or 10,000 cells were dissolved in sample buffer, heated, and run directly. The amount of ATOX1 or CCS in the sample lanes was estimated based on the Western blot signals from the known amounts of ATOX1 and CCS (e.g., 0.5, 1, or 2 ng) run in adjacent lanes (Western quantification described above). From the estimated mass of ATOX1 or CCS from 5,000 cells, an estimated copy number of each protein per cell was determined. Our estimates from HEK293 cells were in good agreement with published estimates based on a proteomics analysis (6). For determination of hCTR1 copy number in cells overexpressing hCTR1, wells from 12-well culture plates containing 1–2 × 106 HEK293 cells were biotinylated as described above, dissolved in sample buffer, and run alongside purified recombinant hCTR1 protein (kindly provided by V. Unger, Northwestern University) for Western blot quantification. Based on copper uptake rates in HEK293 cells (e.g., see Fig. 2, D vs. B), endogenous hCTR1 is expressed at least 10-fold lower than overexpressed hCTR1 after tetracycline induction. We were not able to use the same Western blot quantification of endogenous hCTR1 due to protein overloading with amounts required to visualize a signal.
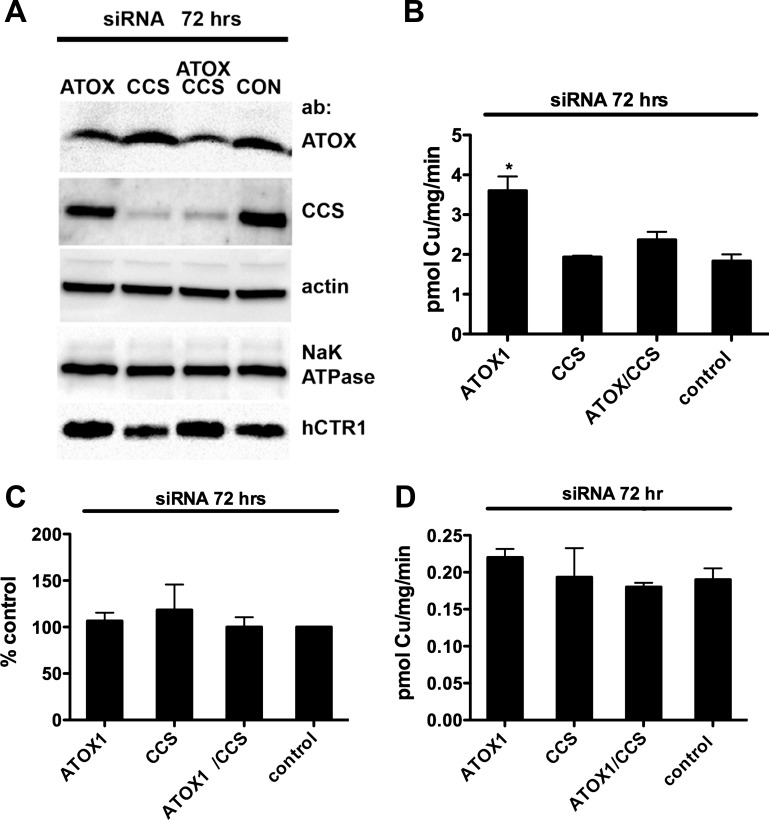
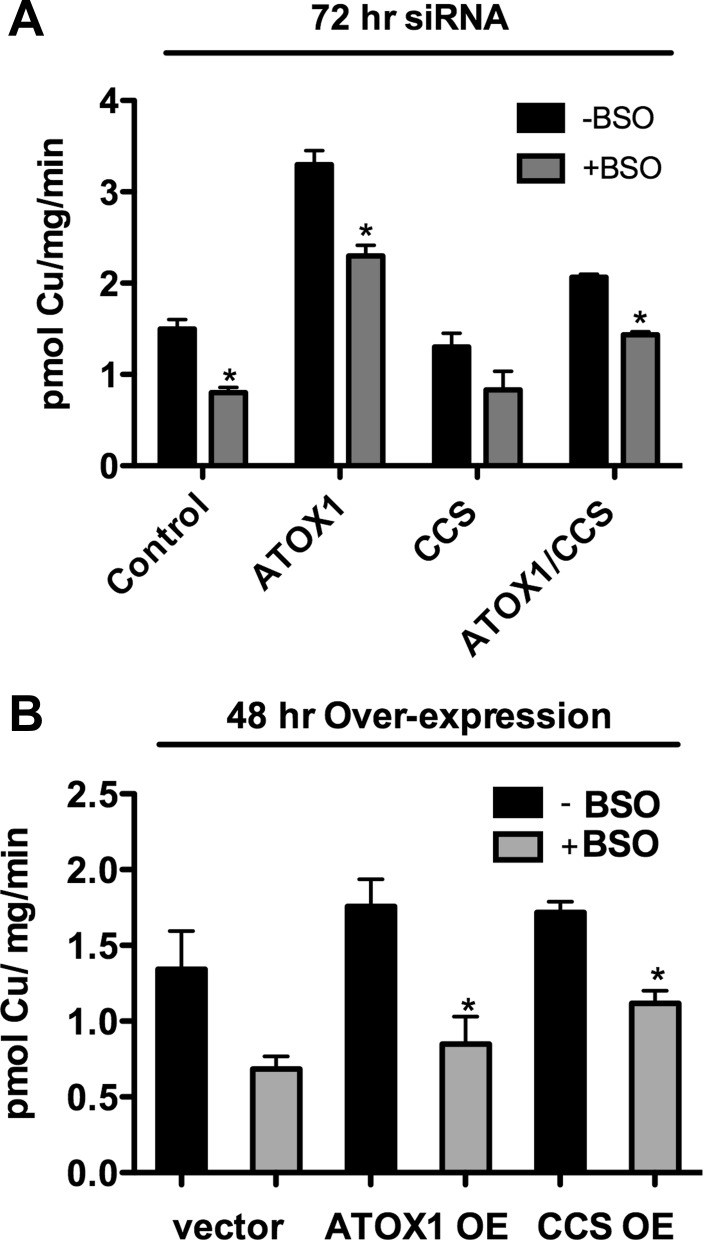
Fig. 2.
Effects of ATOX1, CCS, and ATOX1/CCS knockdown on copper uptake. A: Western blot analysis of ATOX1, CCS, and actin loading controls from lysates of replicate wells used in 64Cu uptakes (50 μg/lane) and surface-biotinylated hCTR1 and Na-K-ATPase (α-subunit) controls from the replicate wells. Primary antibodies (ab) are shown at right. B: 64Cu uptake in siRNA-treated cells. Tet-induced HEK293 cells overexpressing (FLAG-tagged) hCTR1 were transfected with siRNA oligos targeting: ATOX1, CCS, ATOX1, and CCS or control siRNA for 72 h before 64Cu uptake assays (see experimental procedures). In this and each subsequent figure, the means ± SD of a single experiment from triplicate wells are shown and are representative of those from 3–5 separate experiments. C: 64Cu uptake normalized to the amount of hCTR1 in the membrane relative to the control siRNA cells (control set at 100%). Average of 3 experiments shown in B. D: copper chaperone knockdown experiments done exactly as in B, without tet-induced overexpression of hCTR1. *P < 0.05, significantly different value vs. control by Student's t-test, unpaired.
Metal content of cells.
The metal content of cells incubated for 48 h with media having varying 0–1,000 μM BSO was determined using ICP-mass spectroscopy at the Center for Applied Isotope Studies, University of Georgia (Athens, GA). Samples consisted of cells from replicate 12-well plates used in 64Cu uptake experiments (seeded with equal cell numbers 24 h previous to collection) were acidified with 70% nitric acid incubated 3 h, diluted, and used for analysis.
Data analysis.
Data from various experiments were analyzed using Prism 5 (Graphpad Software, La Jolla, CA). The Michaelis-Menten equation was used to determine Km and Vmax values for copper transport by wild-type hCTR1 transporters: pmol copper transported = Vmax * [Cu]/(Km + [Cu]).
Chemicals.
Standard reagent chemicals were from Fisher (Pittsburgh, PA) or Sigma/Aldrich. 64Cu was produced at Washington University (St. Louis, MO).
RESULTS
Measurement of initial rate of copper uptake.
We quantified the initial rates of copper transport by hCTR1 using 64Cu uptake assays in HEK293 cells, as previously reported by ourselves and others in cultured mammalian cells (27, 34, 50). Assays were done in DMEM media with 10% FBS, which has been previously shown to substantially reduce non-hCTR1 64Cu uptake in these assays (27). In addition, 2 μM CuCl2 were added to this media, which itself, on average, contains <0.5 μM copper (Dr. M. Ralle, Oregon Health Sciences University, personal communication). The added Cu2+ is presumably converted to Cu+ before transport by oxidoreductase activity (40).
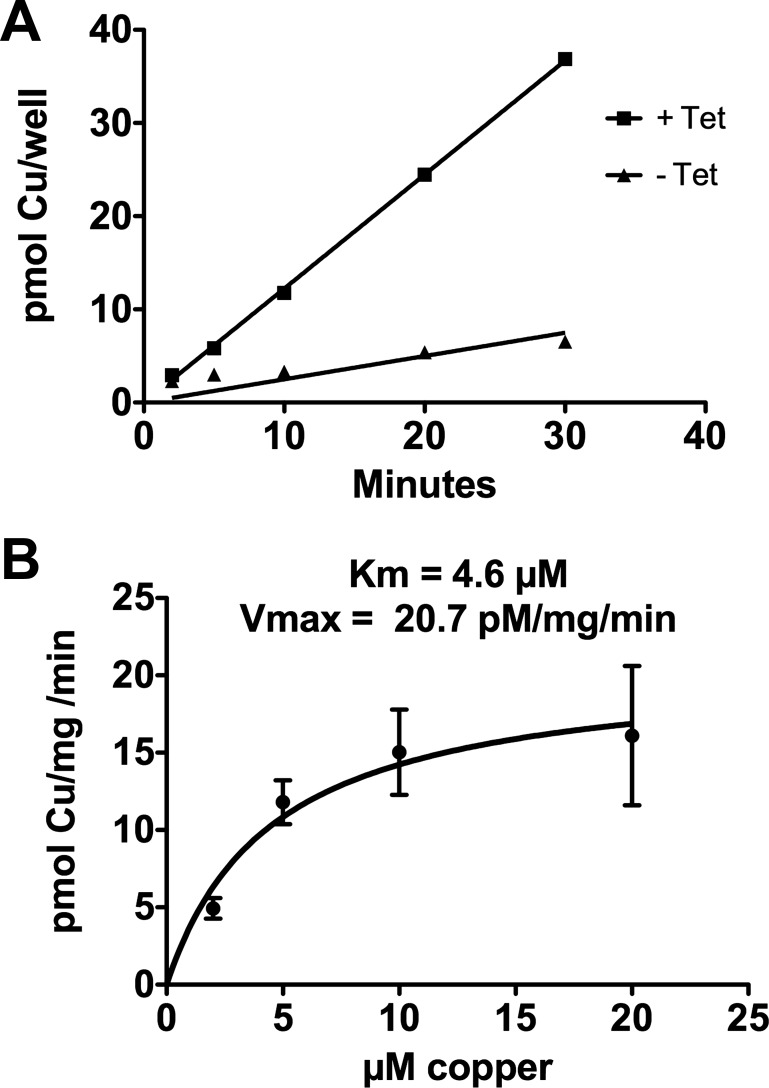
64Cu uptake was linear over the time used in our assay (Fig. 1A) and was saturable (Fig. 1B), with a Km of ∼4.5 μM copper, similar to values reported previously (27, 30, 50) Since free copper is not detectable in cells (42), we hypothesized that a rate-limiting step in 64Cu accumulation in cells would be the exchange of copper from the transporter to the cytoplasmic ligand(s) that distribute transported copper to its targets. It would follow from this hypothesis that significant depletion or elevation of the cytoplasmic concentration of the critical ligands would reduce or increase the initial rate of 64Cu uptake mediated by hCTR1.
Fig. 1.
A: kinetics of 64Cu uptake in HEK293 cells with or without tetracycline (tet)-induced overexpression of human copper transporter 1 (hCTR1); 106 cells were plated in each well 24 h before the assay. B: concentration dependence of copper uptake analyzed with Michaelis-Menten equation in HEK293 cells. In this and subsequent figures, pmol Cu·mg−1·min−1 refers to picomoles copper taken up by the cells per milligram total cell protein per minute.
Effects of varying chaperone levels on initial rate of 64Cu uptake.
To test whether depleting or overexpressing the copper chaperones ATOX1 and/or CCS affected the initial rate of 64Cu uptake by hCTR1, we depleted or elevated their abundance using siRNA knockdown or transient overexpression in HEK293 FLp-In T-Rex cells (hereafter, HEK293 cells). These cells overexpress FLAG-tagged wild-type hCTR1 after tetracycline induction. We also knocked down and overexpressed ATOX1 and CCS in HEK 293 cells not induced with tetracycline that express much lower levels of endogenous hCTR1 (overexpression of hCTR1 by tetracycline induction resulted in an approximately sixfold increase in copper uptake in the experiment shown in Fig. 1A).
We found that reduction by 70–80% in the cellular CCS protein by siRNA (Fig. 2A, lane 2) had no significant effect on the rate of 64Cu uptake, with or without overexpression of hCTR1 (Fig. 2, B–D). A similar reduction of ATOX1 protein by siRNA resulted in an apparent increase in the rate of 64Cu uptake in cells overexpressing hCTR1 (Fig. 2B). However, siRNA knockdown of ATOX1 resulted in an increase in the abundance of hCTR1 in the plasma membrane compared with control siRNA and CCS siRNA treated cells (Fig. 2A, lane 5). The relative levels of plasma membrane hCTR1 in ATOX1 siRNA knockdown samples were similar to the relative rates of 64Cu uptake in these cells: the twofold increase in 64Cu uptake was mirrored by a twofold increase in plasma membrane hCTR1 following ATOX1 siRNA treatment, as shown in samples normalized for expression of hCTR1 (Fig. 2D). The basis for this phenomenon, which was only seen in cells overexpressing hCTR1 from the CMV promoter in the HEK293 FLp-In T-Rex expression site, is not known.
Thus reduction of ATOX1 protein in HEK293 cells overexpressing hCTR1 did not significantly change the initial rate of 64Cu uptake via (plasma membrane) hCTR1. In the absence of tetracycline-induced overexpression of hCTR1, knockdown of ATOX1 had little or no effect (Fig. 2C). These experiments showed that large reductions in cellular ATOX1 and CCS protein levels in the cells did not cause a reduction in the initial rate of 64Cu uptake mediated by hCTR1.
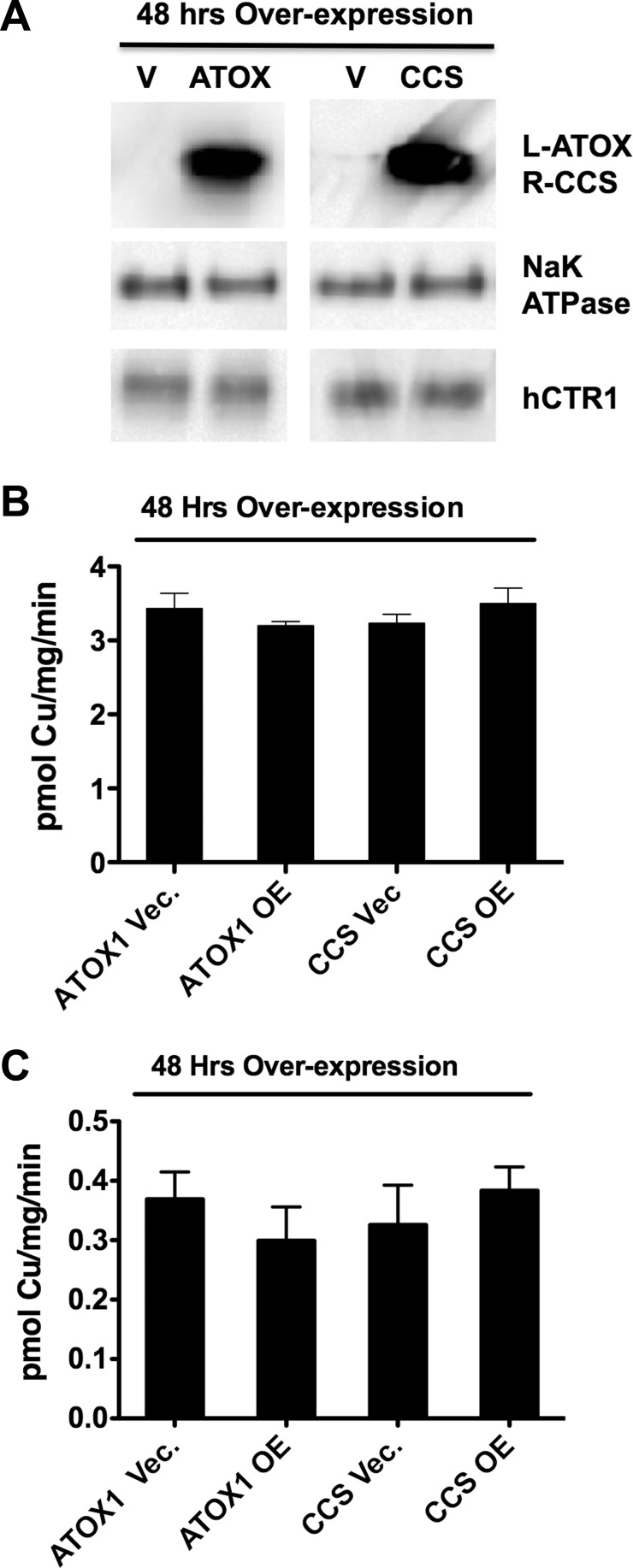
We also overexpressed ATOX1 and CCS in HEK293 cells and examined the effects of increased cellular concentrations of the chaperones on the initial rate of 64Cu uptake. If the chaperones directly interact with hCTR1 to acquire copper, then elevating their concentration might be expected to increase the initial rate of 64Cu uptake. In cells in which both a chaperone and hCTR1 were overexpressed, elevating ATOX1 protein by 14-fold or CCS protein by 17-fold (Fig. 3A) had no significant effect on the initial rate of 64Cu transport (Fig. 3B). In cells grown in the absence of tetracycline, 64Cu transport mediated by the less abundant endogenous hCTR1 transporters showed a similar lack of effect following overexpression of the copper chaperones (Fig. 3C). The levels of hCTR1 in the membrane were unchanged by overexpression of the chaperones (Fig. 3A) Thus neither decreasing nor increasing the cellular concentrations of the copper chaperones ATOX1 and CCS had significant effects on copper transported by hCTR1.
Fig. 3.
Effects of ATOX1 and CCS overexpression on copper uptake. A, top row: Western blots of total cell lysate protein (20 μg/lane) from tet-induced cells transfected with empty vector (V), ATOX1, or CCS. Primary antibody used is shown at right (L- and R-, left and right). A faint band from endogenous CCS is visible in the vector-transfected lane for CCS; no endogenous ATOX1 protein was visible. Bottom row: hCTR1 levels in surface biotinylated proteins from replicate wells from the experiment in B (Na-K-ATPase α-subunit serves as a loading control). The levels of hCTR1 in the plasma membrane in any condition in B were not significantly different. B: 64Cu uptake in HEK293 cells with chaperone overexpression (OE). Tet-induced HEK293 cells overexpressing hCTR1 were transfected with constructs containing ATOX1, CCS, or empty vector controls (Vec.) 48 h before 64Cu uptake assays. C: chaperone overexpression experiments done exactly as in B, without tet-induced overexpression of hCTR1. No values were significantly different than vector alone control (by Student's t-test, unpaired).
Effect of varying the level of GSH on the initial rate of 64Cu uptake.
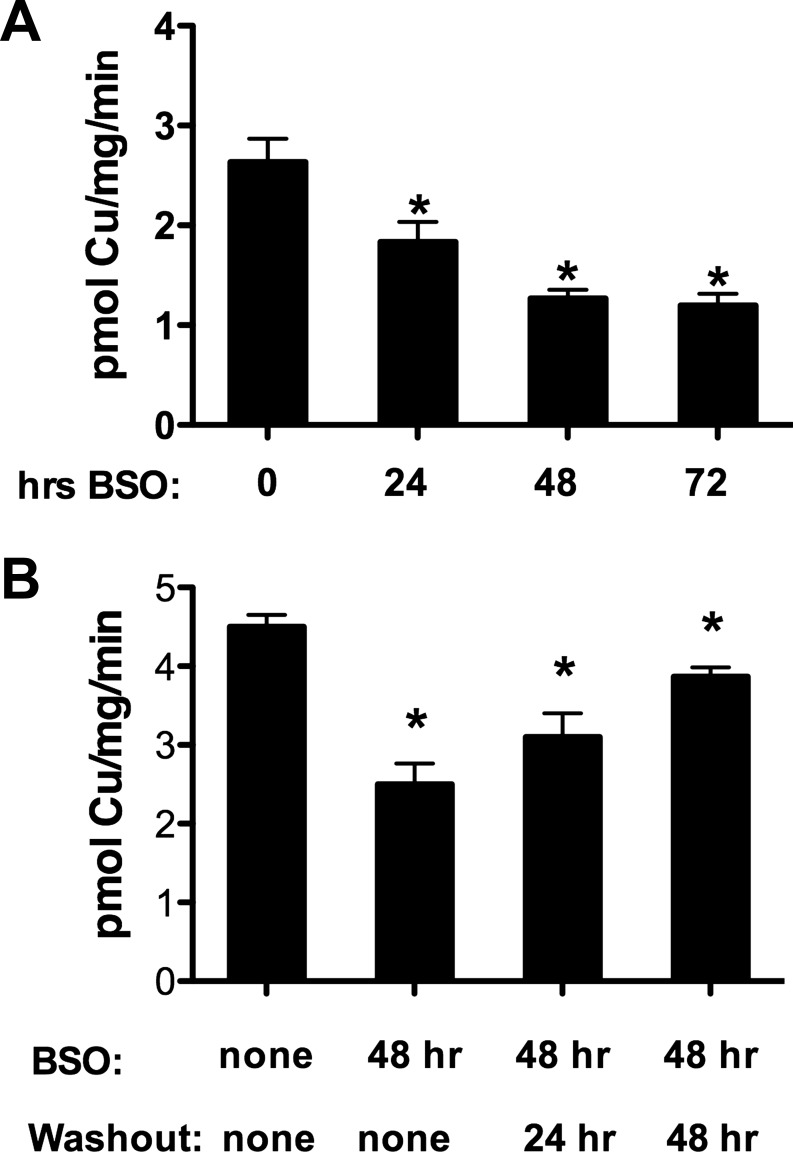
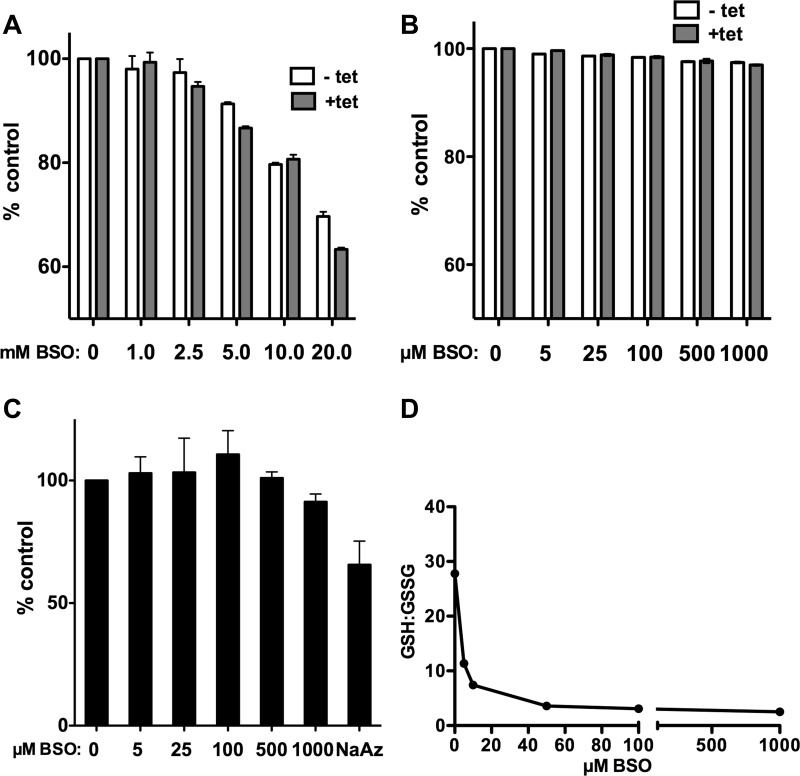
Another well-characterized copper-binding ligand that is abundant in mammalian cytoplasm is GSH. We used BSO, a specific, well-characterized inhibitor of γ-glutamyl cysteine synthetase (2, 7) to deplete cellular GSH and subsequently determined the effects of GSH depletion on the initial rate of copper uptake. If GSH receives copper transported by hCTR1, then reducing the cytoplasmic concentration of GSH could affect the rate of copper uptake. Incubation of HEK293 cells in medium containing 5–1,000 μM BSO for 48 h resulted in a concentration-dependent reduction in cellular GSH concentration, from ∼4.5 mM to ∼250 μM (Fig. 4A). BSO treatments (5–1,000 μM) of HEK293 cells overexpressing hCTR1 resulted in a maximum reduction in the initial rate of 64Cu uptake of ∼50% (Fig. 4B). Most of the reduction in the rate of copper uptake was observed with 25 μM BSO, which reduced GSH to ∼350 μM. Contrary to a previous report (12), BSO treatment did not change the level of hCTR1 in the plasma membrane. In five independent experiments, plasma membrane levels of hCTR1 were unaltered by BSO treatment (Fig. 4, C and D), as determined by surface biotinylation of replicate cells used in 64Cu uptake assays.
Fig. 4.
Effect of glutathione depletion on copper uptake in HEK293 cells overexpressing hCTR1. A: glutathione concentrations (mM) averaged from 3 experiments in HEK293 cells overexpressing hCTR1 and treated with l-buthionine-sulfoximine (BSO). Cells were incubated for 48 h in media containing increasing concentrations of BSO before being assayed for GSH content. B: 64Cu uptake after 48 h incubation in media containing increasing concentrations of BSO, expressed as % uptake of control cells without BSO. C: Western blot showing biotinylated hCTR1 protein (and Na-K-ATPase α-subunit loading control from the same Western blot gel) from cells treated for 48 h with indicated concentrations of BSO in the medium. D: quantification of hCTR1 levels (%untreated cells) in cells incubated in medium containing indicated amounts of BSO for 48 h. The average of 5 experiments is shown.
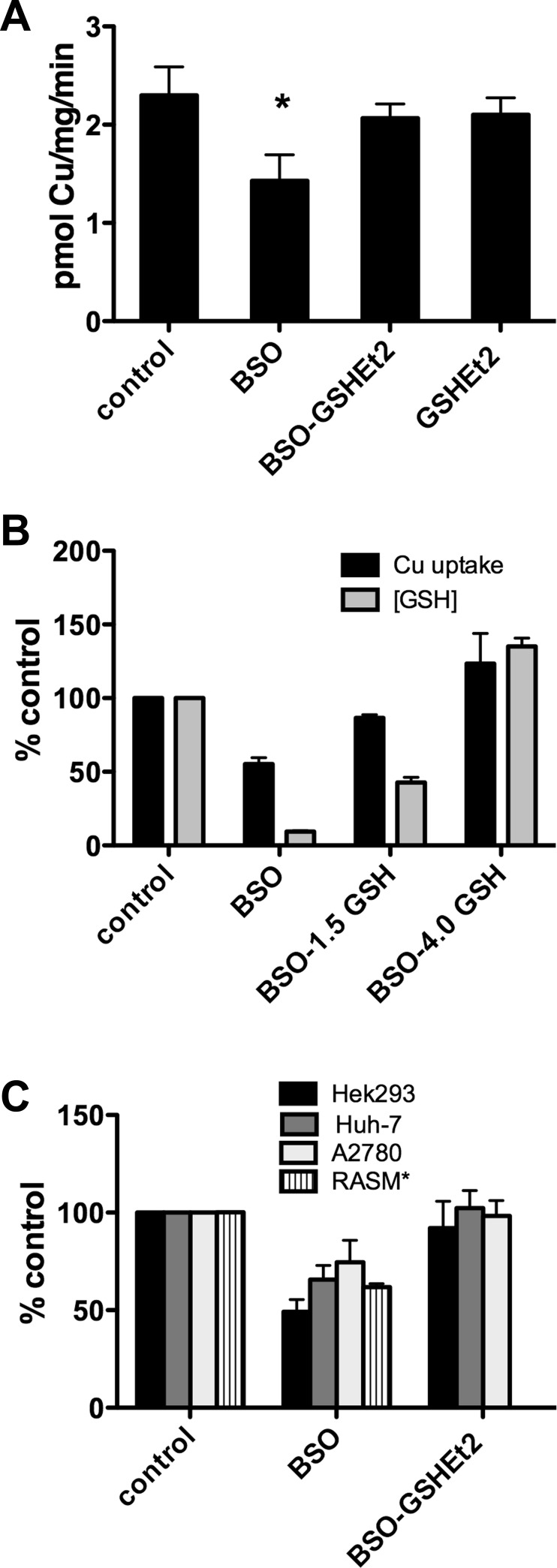
The effect of BSO on 64Cu uptake was reversible by washout or GSH supplementation. The time-dependent effect of BSO on 64Cu uptake (Fig. 5A) was reversed over time after BSO was washed out of the cells (Fig. 5B). Furthermore, supplementation of BSO-treated cells with 1.5 mM membrane-permeable glutathione diethyl ester (GSHEt2; Ref. 29), which is hydrolyzed to GSH following entry, also restored the initial rate of 64Cu transport in the presence of BSO (Fig. 6A). Treatment with GSHEt2 alone had no effect.
Fig. 5.
Reversibility of BSO effect on copper uptake by BSO washout. A: time-dependent BSO effect. 64Cu uptake in cells incubated in medium containing 250 μM BSO for 24–72 h, as indicated below each column. B: copper uptake following BSO treatment with or without drug washout. Cells were incubated in medium without or with 500 mM BSO for 48 h (1st 2 columns). For washout samples, BSO-treated cells were transferred to normal media 24 or 48 h before assay (3rd and 4th columns). *P < 0.05, significantly different value vs. control by Student's t-test, unpaired.
Fig. 6.
Restoration of 64Cu uptake copper uptake in BSO- inhibited cells with GSH supplementation. A: cells were incubated for 48 h in media without or with 500 μM BSO (1st 2 bars) followed by overnight treatment with 1.5 mM GSH diethyl ester, a membrane permeable form of GSH which is hydrolyzed to release GSH in the cytoplasm (3rd and 4th bars) before 64Cu uptake assay. B: 64Cu uptake copper uptake and cellular GSH levels in cells treated with BSO or BSO and 1.5 or 4.0 mM GSHEt2 (GSH). Controls (100%) are cells with no treatments. C: BSO effect on copper uptake in other cell lines expressing only endogenous CTR1. Cells were incubated for 48 h in medium with or without 500 μM BSO or with BSO treatment and overnight incubation in 1.5 mM GSHEt2. Because GSHEt2 was toxic to rat smooth muscle cells, no results are shown for those cells. * P < 0.05, significantly different value vs. control by Student's t-test, unpaired.
We also measured both 64Cu uptake and cellular GSH concentration (in replicate wells) of the same cells overexpressing hCTR1 after 48-h incubation in BSO-containing media and overnight supplementation with 1.5 or 4.0 mM GSHEt2 (Fig. 6B) We found that cellular GSH levels were partially restored after supplementation with 1.5 mM GSH. Supplementation with 4.0 mM GSHEt2 increased cellular GSH above that of the untreated cells and also resulted in an increase in 64Cu uptake over that seen in untreated cells (Fig. 6B).
To confirm the generality of the role of GSH in copper transport, we investigated the effects of GSH depletion on 64Cu uptake by endogenous hCTR1 in several cell lines. We found that, in addition to HEK293 kidney-derived cells, BSO treatment of liver, ovarian, and rat smooth muscle cells also reduced the initial rate of 64Cu uptake and that supplementation of BSO-treated cells with GSHEt2 reversed the effect (Fig. 6C). Cell lines varied in the extent of BSO-mediated 64Cu uptake reduction, but the BSO inhibition, and restoration by GSHEt2, were qualitatively similar in the cell lines (GSHEt2 caused detachment of rat smooth muscle cells in 64Cu uptake assays and was therefore not reported in Fig. 6C. BSO alone had no similar toxic effect).
HEK293 cells in which ATOX1 and CCS were knocked down (Fig. 7A) or overexpressed (Fig. 7B) also showed reductions in the rate of 64Cu transport following treatment with BSO. Thus lowering the concentration of cellular GSH significantly reduces the initial rate of 64Cu uptake by hCTR1, regardless of the abundance of the copper chaperones ATOX1 and CCS.
Fig. 7.
Effects of BSO treatment on copper uptake cells in which ATOX1 and CCS levels were varied. In A and B, cells were incubated in medium with or without 500 μM BSO for 48 h before the assay. A: cells were transfected with siRNA (targeted or control) indicated below each pair of columns 72 h before uptake assays. B: cells were transfected with expression or empty vector control plasmids 48 h before uptake assays, as indicated below each pair of columns. Note: experiments with ATOX1 knockdown have higher copper uptake than do control and CCS knockdown experiments, due to a higher level of hCTR1 expressed in the plasma membrane after knockdown (see experimental procedures and Fig. 2). *P < 0.05, significantly different value vs. no BSO treatment, Student's t-test, unpaired. In A, CCS: P = 0.133. In B, vector: P = 0.057.
Minimal toxic effects of BSO treatment.
BSO has been reported to be toxic in some cells (2, 7, 11). To test whether our BSO treatment of the HEK293 cells used here had indirect (toxic) effects that might have affected copper transport, we performed a series of experiments to determine whether BSO treatment affects general cell viability or metabolism. First, we measured the fraction of cells excluding trypan blue after treatment with 0–1,000 μM BSO. Under the conditions of our experiments, ∼98% of BSO-treated and untreated cells used in uptake assays excluded trypan blue (data not shown). Second, as a more sensitive measure of cell viability and proliferation, we incubated BSO-treated cells with PrestoBlue a fluorometric redox indicator that changes emission following chemical reduction by growing, metabolically active cells (44). At high concentrations of BSO (5–20 mM), HEK293 had reduced viability/proliferation, with or without hCTR1 tetracycline-induced overexpression of hCTR1 (Fig. 8A). As shown in Fig. 8B, BSO at the concentrations (5–1,000 μM) used in our experiments has little effect on cell viability as judged by fluorescence of the indicator. Cells treated for 48 h with 1,000 μM BSO exhibited a 3% decrease in fluorescence. Third, as a measure of the overall metabolic status of the cells we measured total ATP in BSO-treated and untreated cells. As with the viability assay, cellular ATP levels were only slightly decreased (Fig. 8C). Fourth, the BSO treatments did not obviously affect general protein synthesis (another measure of cell proliferation and growth), as judged by equivalent protein contents measured for control and BSO-treated cells used in numerous 64Cu uptake assays (see experimental procedures).
Fig. 8.
Effects of BSO treatment on HEK293 FLp-In T-Rex cells. Unless noted (A and B), cells were induced with tet resulting in overexpression of hCTR1. A and B: cell viability as measured using Presto Blue fluorometric indicator. After a 48-h incubation in media containing indicated concentrations of BSO, with or without tet, the reagent was added to cells for 4 h before fluorescence measurement. Cells were treated with mM (A) or μM (B) concentrations of BSO as indicated for 48 h. C: total ATP measured using a fluorometric assay and expressed as %untreated cells. D: GSH-to-GSSG ratio was determined by measuring total GSH and GSSG in cells treated with indicated concentrations of BSO, then subtracting GSSG from total to obtain GSH in each sample, from which the GSH-to-GSSG ratio was calculated.
We also measured the GSH-to-GSSG ratio in cells treated with BSO. We found that the ratio of GSH to GSSG declined with increasing concentration of BSO from ∼28:1 to ∼3:1 (Fig. 8D). In spite of the change in the GSH-to-GSSG ratio, the redox-based PrestoBlue indicator (44) was not similarly affected, suggesting that, under the conditions of the experiments, the redox potential of the cell was not changed by BSO to the same extent as was the GSH-to-GSSG ratio.
As another indicator of cell health and copper status, we measured the metal content of cells following a 48-h treatment with media containing 5–1,000 μM BSO under the conditions used in 64Cu uptake assays (see experimental procedures). We found no significant changes (i.e., more than ±3.5%) in Cu, Fe, or Zn, or in metals such as Mg or K, or other physiologically relevant elements such as Ca, P, Mn, and Na in samples of equal cell numbers from various conditions, (data not shown).
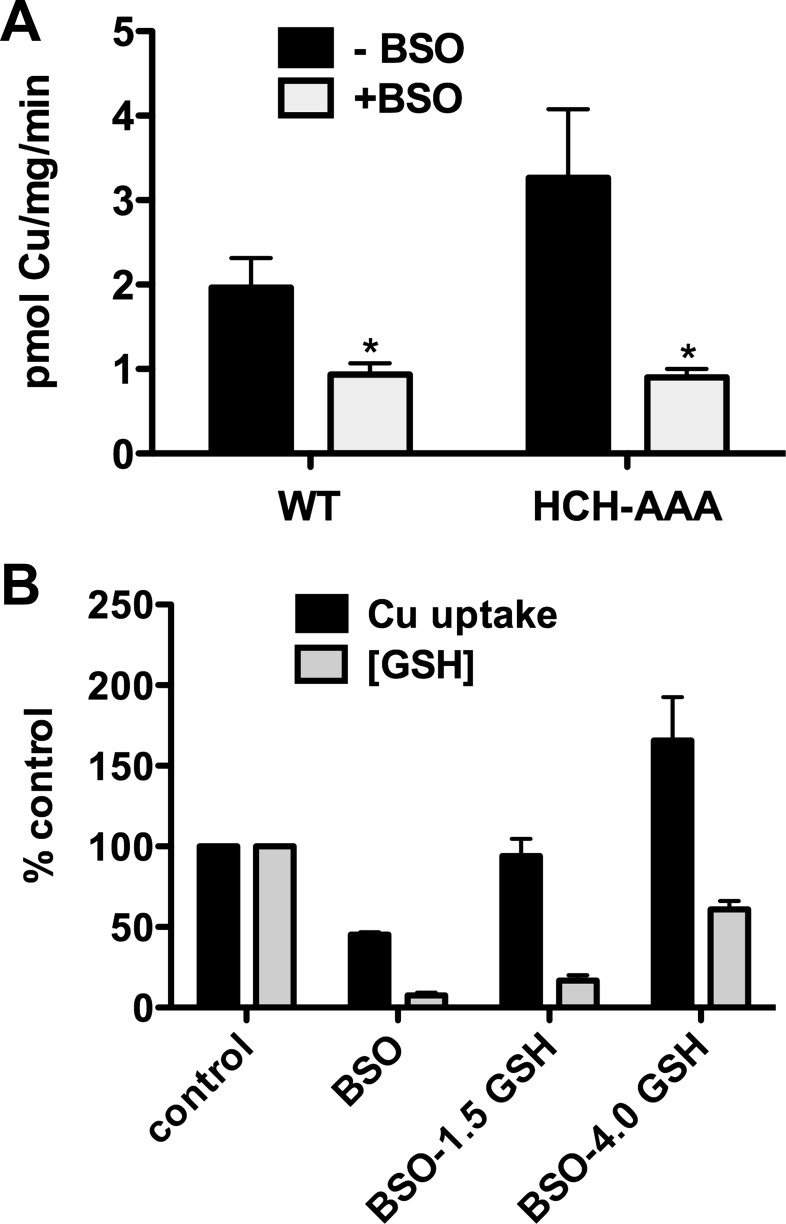
Role of COOH-terminal HCH motif.
The intracellular COOH-terminal tails of hCTR1 homotrimers terminate with a putative metal-binding sequence HCH (13, 49). It was possible that BSO-induced depletion of GSH resulted in oxidation of the COOH-terminal cysteine residues in the HCH tripeptide. If these cysteines were essential for copper transport, then the reduction in 64Cu uptake we observed in BSO could be related to oxidation of the cysteine thiols, rather than a direct role for GSH in 64Cu uptake. To test this possibility, we expressed an hCTR1 mutant in HEK cells that had the COOH-terminal HCH sequence replaced by alanine residues (AAA). Interestingly, the AAA mutant hCTR1 had a higher initial rate of 64Cu transport than wild-type hCTR1, which was inhibited (by ∼70%) when cellular GSH concentration was reduced by BSO (Fig. 9A). Supplementation of GSH with 1.5 or 4.0 mM GSHEt2 increased cellular GSH and also increased 64Cu uptake (Fig. 9B). Addition of 4.0 mM GSHEt2 resulted in a rate of 64Cu uptake that was higher than that of untreated cells, as was observed for cells overexpressing wild-type hCTR1 (Fig. 6B). Thus the inhibitory effects of GSH depletion that we observed are not caused by oxidation of the COOH-terminal cysteine residues. Furthermore, the COOH-terminal HCH motif is not essential for hCTR1-mediated copper transport.
Fig. 9.
Effects of BSO treatment on copper uptake by HCH-AAA mutant. A: 64Cu uptake in HEK293 cells overexpressing wild type hCTR1 or mutant hCTR1 in which the carboxyl-terminal HCH (putative) metal binding motif is substituted with alanine residues (HCH-AAA). Cells were treated in media with or without added BSO (500 μM) for 48 h. B: 64Cu uptake in HEK293 cells overexpressing the HCH-AAA mutant cells treated with BSO or BSO and 1.5 or 4.0 mM GSH-diethyl ester (GSH). Control (100%) cells had no BSO or GSHEt2 treatment. *P < 0.05, significantly different value vs. no BSO treatment by Student's t-test, unpaired.
Quantification of molecules involved in copper homeostasis.
A quantitative issue arose from our observations regarding the extent of inhibition of 64Cu uptake after BSO treatment. We observed a maximum reduction of ∼50% in the initial rate of 64Cu uptake in BSO-treated HEK293 cells overexpressing wild-type hCTR1 (e.g., Fig. 4B), while GSH levels were reduced by up to 95% (Fig. 4A). To better understand this difference, we determined the copy numbers of GSH, ATOX1, CCS, and (overexpressed) hCTR1 in the HEK293 cells, using a GSH assay and quantitative Western blot analysis with purified recombinant ATOX1, CCS, and hCTR1 as protein standards (see experimental procedures). The GSH concentration in HEK293 cells averaged 4.5 mM or ∼5.5 × 109 GSH molecules/cell (assuming a cell volume of 2 pl; see experimental procedures and Ref. 25). Maximal BSO-mediated depletion reduced GSH by 95%, to ∼250 μM, or 3 × 108 molecules per cell (Fig. 3A). As shown in Table 1, we estimated that there are 3.2 × 105 ATOX1 copies/cell and 1.5 × 104 CCS polypeptides/cell, which agreed reasonably well with values obtained for ATOX1 (9.2 × 105/cell) and CCS (3.4 × 103/cell) from a human cell quantitative proteome analysis in U2OS osteosarcoma cells (6). We also estimated from Western blot quantification that in cells overexpressing hCTR1, there are 1.6 × 104 hCTR1 trimers/cell and at least 10-fold fewer trimers/cell in cells expressing endogenous levels of hCTR1, based on 64Cu uptake rates (e.g., Fig. 2, D vs. B). Thus GSH molecules are normally present at 4–5 orders of magnitude greater than both hCTR1 and the chaperones. Presumably, the limited decrease (∼50%) in the initial rate of 64Cu transport following GSH depletion reflects the fact that even after depletion by 95%, GSH is still in excess of hCTR1 and the chaperones by 2–4 orders of magnitude.
Table 1.
Copy number of GSH, ATOX1, CCS, and hCTR1 in HEK293 cells
| Molecule |
||||
|---|---|---|---|---|
| GSH (n = 3) | ATOX1 (n = 6) | CCS (n = 5) | hCTR1 (n = 5) | |
| Copy #/cell | 5.5 ± 1.9 × 109 | 3.2 ± 0.9 × 105 | 1.5 ± 0.3 × 104 | 1.6 ± 0.5 × 104 |
Values are means ± SE. hCTR1, human copper transporter 1. For GSH, copy number was calculated from cellular GSH concentrations determined using a luminescence-based glutathione assay kit (see experimental procedures). Cellular concentration of GSH was calculated based on a cell volume of 2 pl (see experimental procedures and Ref. 25). Levels of ATOX1, CCS, and hCTR1 were quantified using Western blots as described in experimental procedures.
DISCUSSION
In the present work we set out to test the hypothesis that copper ions transported by hCTR1 are first accepted by copper-specific chaperone proteins that deliver copper ions to specific target proteins. This mechanism of copper distribution has been widely assumed (e.g., 22, 43) but not specifically tested. Surprisingly, in cells having either endogenous or overexpressed levels of hCTR1, wide variations in the cellular concentrations of the copper-specific chaperones ATOX1 and CCS had no effect on the initial rate of copper entry (Figs. 2 and 3). These results are not consistent with a direct transfer of copper from hCTR1 to the chaperones following transport, which would be expected to be a rate-limiting step in copper acquisition by the cell. In contrast, treatment of these cells with BSO caused a concentration-dependent decrease in both the cellular level of GSH and the initial rate of copper entry, which was reversed by supplementation of GSH levels (Figs. 4–6), suggesting that GSH plays a role in initial copper acquisition from hCTR1. The well-established copper-trafficking role of chaperones comes from a series of studies in a variety of eukaryotic systems, including yeast and mammalian cells. CCS, the copper chaperone for SOD, and ATOX1, which delivers copper to the copper-dependent ATPases ATP7A and ATP7B, are well-studied examples (31, 45, 47). However, just how the chaperones acquire their copper has been somewhat mysterious. Either the chaperones interact directly with hCTR1 to accept transported copper in a similar way that they interact with their cognate acceptors to deliver copper, or some other intermediary agent accepts the metal ion from the transporter and subsequently delivers it to the chaperones. Alternatively, copper dissociates from the transporter and the cytosolic copper-binding ligands compete for the metal. This last possibility seems unlikely, as cell-permeable chelators do not appear to have access to free copper (42). Our observations that the cellular levels of CCS and ATOX1, separately or together, could be elevated by more than an order of magnitude or reduced by 70–80% (Figs. 2 and 3) and had no effect on the rate of cellular copper entry make it less likely that these chaperones interact directly with the transporter. In contrast, reduction in GSH levels with BSO resulted in a reduction in the rate of copper entry, providing evidence for an initial, important role for GSH following copper entry, possibly by direct interaction with the transporter.
It is well understood that GSH plays a number of important roles in cell metabolism, including affecting the redox potential of the cell (2, 4). Previous reports are equivocal regarding the effects of BSO on cells. In some cells, toxic effects were reported; in other cells, BSO treatment had few effects (2, 7, 11, 17, 33). Treatment of cells with BSO and the subsequent reduction in cellular GSH (and a decrease in GSH-to-GSSG ratio) could cause nonspecific toxicity, whereby cell metabolism and the cellular redox potential are compromised and a decrease in copper uptake results. We therefore performed a number of experiments in the HEK293 cells used in our experiments to determine whether BSO, under the conditions used, affected cell metabolism and or viability. First, we ascertained what concentrations of BSO could be used with minimal effects on the viability/proliferation of HEK293 cells and found little or no effect on the cells with the concentrations (≤ 1 mM) we used in our experiments (Fig. 8, A and B). The viability/proliferation assay is based on the reductive capacity of metabolically active cells (44), which suggests that the BSO treatments did not have severe effects on the redox potential of the cells, in spite of lowering the total cellular GSH-to-GSSG ratio (Fig. 8D). The lack of significant depletion of ATP (Fig. 8C) or significant changes in cellular metal content, suggested that cell viability and metabolic status were not compromised. It is also important to note that that most of the reduction in the rate of Cu entry (∼50%) takes place using lower concentrations of BSO (5–25 μM; Fig. 4B) and hence with the initial decreases of GSH from its physiological levels (Fig. 4A). Furthermore, following BSO treatment, when GSH is returned to physiological levels with GSHEt2 (Figs. 6 and 10), the recovery of the copper uptake rate suggests that no irreversible damage has occurred to cellular metabolic machinery by BSO treatment.
Fig. 10.
Model. Copper is transported from the extracellular side of the cell by hCTR1 and is bound by GSH on the cytoplasmic side of the membrane. GSH then delivers the metal to copper chaperones, such as ATOX1 and CCS, which subsequently transfer it to target proteins. Examples of target proteins shown are ATP7A, ATP7B, and SOD.
We also tested the possibility that the reduction in the copper uptake rate following BSO treatment resulted from a change in the oxidation state of hCTR1 cysteines in the intracellular COOH-terminal HCH tripeptide, a putative metal-binding site that has been proposed to be directly involved in the copper transport mechanism (13). BSO treatment of cells overexpressing an hCTR1 mutant in which the HCH sequence was substituted with AAA (Fig. 9) resulted in a 60% reduction in the rate of copper uptake. This result showed that oxidation of the HCH cysteines in wild-type hCTR1 (by cross-linking 2 cysteines in the hCTR1 trimer or oxidation of single residues) could not have explained the decrease in the copper uptake after BSO treatment. Furthermore, the copper uptake rate was higher in cells expressing the AAA mutant than in cells expressing wild type, demonstrating that the HCH sequence is not essential for hCTR1-mediated copper transport. It is probable that this highly conserved motif has some other role in copper homeostasis or in hCTR1 regulation. To summarize these experiments, we found no evidence for indirect effects of BSO on cell metabolism or on the transporter itself that might have explained the reduction in the initial rate of copper uptake when cellular GSH levels are reduced by BSO.
The effects of lowering GSH levels with BSO include an effect on the redox status of ATOX1 thiols (22). If it were assumed that ATOX1 directly receives copper from hCTR1, the BSO inhibition of copper transport may seem to point to a mechanism in which copper binding by ATOX1 protein is impaired by oxidation, leading to the reduction in copper uptake we observed in the presence of BSO. However, complete ablation of ATOX1 protein in Atox−/− mouse embryonic fibroblasts (MEFs) does not prevent relatively normal copper homeostasis, such as a normal distribution of ATP7A, the copper ATPase that receives copper from Atox1 (20). This result led to the suggestion that ATP7A can obtain the metal from some other donor (20). Interestingly, moderate levels of BSO are toxic to Atox1−/− MEF cells but not to the parental Atox1+/+ MEF line (22). This toxicity might be explained by a large reduction in (copper-bound) GSH by BSO, which could be the unknown ATP7A copper donor in Atox1−/− cells. GSH has also been implicated in delivery of copper to the CCS target protein SOD1 in CCS null mouse cells (10), and to metallothionien (16), results consistent with the idea that GSH is capable of delivering copper to cellular copper-binding proteins.
Previous work supports the hypothesis that copper transported by hCTR1 is first acquired by GSH. In early studies of copper entry, carried out before copper chaperones (or hCTR1) were identified, it was proposed that the first intracellular acceptor of transported 67Cu was GSH (18). More recently, in vitro measurements of the relative copper-binding affinities of GSH, copper chaperones, and their target proteins are consistent with a model in which transported copper would be first bound by cytoplasmic GSH and then transferred to the higher affinity sites on the less abundant chaperones (5). Our experiments provide kinetic support to these studies.
It is instructive to compare the differences in abundance of proteins involved in copper distribution in the cell in vivo. In the present work we estimated the copies per cell of ATOX1 (3.2 × 105), CCS (1.5 × 104), and hCTR1 (1.6 × 104). Our estimates of ATOX1 and CCS compare well with recent measurements obtained using a proteomics approach (6). However, these numbers, which correspond to less than or equal to the micromolar concentration, are dwarfed by the millimolar concentrations of GSH (5.5 × 109 copies/cell) that we determined and that have been reported elsewhere (2, 7). Thus the most abundant copper ligand in the mammalian cell cytosol is GSH. It is perhaps not surprising that when GSH is reduced by 95%, only a 50% reduction in the initial rate of copper entry is seen. Under these conditions, GSH is still 2–4 orders of magnitude in excess (∼3 × 108 copies/cell) of the other copper ligands. In addition, some level of non-hCTR1-mediated copper transport occurs in cultured cells. For example, 64Cu uptake in mCTR1−/− mouse embryo fibroblast cells was ∼20% the level of uptake in mCTR1+/+ cells (28). Thus the remaining copper uptake in the presence of maximal GSH reduction by BSO is likely the combination of a reduced rate of hCTR1-mediated copper transport and (BSO-insensitive) copper uptake that is independent of GSH. In our studies we have focused on measuring the initial rates of copper uptake to study kinetic effects on the transport pathway. The subsequent fate of copper that enters the cells is complex, as exchange reactions take place among the molecules involved in copper homeostasis, including copper efflux transporters ATP7A and ATP7B (19, 31).
Based on the work we have presented here, it appears likely that copper chaperones acquire copper from GSH, bearing the transported copper ion from hCTR1. At equilibrium, the chaperones would have a higher fractional occupancy than would GSH (5, 48). The results of our experiments and previous studies suggest that GSH is the first intracellular acceptor of copper from hCTR1. The delivery of copper from GSH to copper-binding proteins has been previously suggested (5, 9, 16, 18).
The proposed initial delivery of copper from hCTR1 to GSH is an attractive mechanism to explain how several structurally different copper binding chaperones have access to the transported copper. That this is a general mechanism is supported by our observations that depletion of GSH caused a decrease in the rate of 64Cu uptake by several other cell lines of diverse cell types (Fig. 6C). A model for the role of GSH in intracellular copper trafficking is shown in Fig. 10. The proposed transfer of copper from hCTR1 to GSH, rather than to copper chaperones, may be simply a consequence of the relative abundance of GSH, or that the geometry of the transporter precludes larger molecular weight chaperones from directly accessing the metal, or that GSH has specific interactions with the transporter. In any case, the relative affinities for copper of each of the known copper chaperones are much higher than GSH (5, 48), which would result in transfer of the transported copper from GSH to the chaperones.
In recent years it has become apparent that copper plays an important role in a number of diverse physiological processes, including vascular smooth muscle migration and wound-healing (3) and macrophage-pathogen responses (46). The pathological disruptions seen in the human genetic diseases of Wilson Disease and Menkes Disease speak to the profound consequences of disruptions in copper homeostasis (35). In the present work we have provided evidence that the small molecule GSH, known to be important in cellular copper homeostasis, may play an important role in the mechanism of copper entry into human cells.
GRANTS
This work was supported by National Institute of General Medical Sciences Grant P01-GM-067166 (to J. H. Kaplan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.B.M., S.A.M., and J.H.K. conception and design of research; E.B.M. and S.A.M. performed experiments; E.B.M., S.A.M., and J.H.K. analyzed data; E.B.M., S.A.M., and J.H.K. interpreted results of experiments; E.B.M. and S.A.M. prepared figures; E.B.M., S.A.M., and J.H.K. drafted manuscript; E.B.M. and J.H.K. edited and revised manuscript; E.B.M. and J.H.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Vinzenz Unger for purified recombinant hCTR1 protein.
REFERENCES
- 1. Aller SG, Unger VM. Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc Natl Acad Sci USA 103: 3627–3632, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 111–112: 1–14, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Ashino T, Sudhahar V, Urao N, Oshikawa J, Chen GF, Wang H, Huo Y, Finney L, Vogt S, McKinney RD, Maryon EB, Kaplan JH, Ushio-Fukai M, Fukai T. Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ Res 107: 787–799, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 390: 191–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P. Affinity gradients drive copper to cellular destinations. Nature 465: 645–648, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A, Herzog F, Rinner O, Ellenberg J, Aebersold R. The quantitative proteome of a human cell line. Mol Syst Biol 7: 549, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berger SJ, Gosky D, Zborowska E, Willson JK, Berger NA. Sensitive enzymatic cycling assay for glutathione: measurements of glutathione content and its modulation by buthionine sulfoximine in vivo and in vitro in human colon cancer. Cancer Res 54: 4077–4083, 1994 [PubMed] [Google Scholar]
- 8. Bertinato J, Cheung L, Hoque R, Plouffe LJ. Ctr1 transports silver into mammalian cells. J Trace Elem Med Biol 24: 178–184, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Brouwer M, Brouwer-Hoexum T. Glutathione-mediated transfer of copper(I) into American lobster apohemocyanin. Biochemistry 31: 4096–4102, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Carroll MC, Girouard JB, Ulloa JL, Subramaniam JR, Wong PC, Valentine JS, Culotta VC. Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc Natl Acad Sci USA 101: 5964–5969, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Celli A, Que FG, Gores GJ, LaRusso NF. Glutathione depletion is associated with decreased Bcl-2 expression and increased apoptosis in cholangiocytes. Am J Physiol Gastrointest Liver Physiol 275: G749–G757, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Chen HH, Song IS, Hossain A, Choi MK, Yamane Y, Liang ZD, Lu J, Wu LY, Siddik ZH, Klomp LW, Savaraj N, Kuo MT. Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1. Mol Pharmacol 74: 697–704, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA 106: 4237–4242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisses JF, Kaplan JH. The mechanism of copper uptake mediated by human CTR1: a mutational analysis. J Biol Chem 280: 37159–37168, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the humancopper uptake protein. J Biol Chem 277: 29162–29171, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Ferreira AM, Ciriolo MR, Marcocci L, Rotilio G. Copper(I) transfer into metallothionein mediated by glutathione. Biochem J 292: 673–676, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ford JM, Yang JM, Hait WN. Effect of buthionine sulfoximine on toxicity of verapamil and doxorubicin to multidrug resistant cells and to mice. Cancer Res 51: 67–72, 1991 [PubMed] [Google Scholar]
- 18. Freedman JH, Ciriolo MR, Peisach J. The role of glutathione in copper metabolism and toxicity. J Biol Chem 264: 5598–5605, 1989 [PubMed] [Google Scholar]
- 19. Gupta A, Lutsenko S. Human copper transporters: mechanism, role in human diseases and therapeutic potential. Future Med Chem 1: 1125–1142, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamza I, Prohaska J, Gitlin JD. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci USA 100: 1215–1220, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrison MD, Jones CE, Solioz M, Dameron CT. Intracellular copper routing: the role of copper chaperones. Trends Biochem Sci 25: 29–32, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Hatori Y, Clasen S, Hasan NM, Barry AN, Lutsenko S. Functional partnership of the copper export machinery and glutathione balance in human cells. J Biol Chem 287: 26678–26687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J Biol Chem 279: 35334–35340, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kaplan JH, Lutsenko S. Copper transport in mammalian cells: special care for a metal with special needs. J Biol Chem 284: 25461–25465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimelberg HK, O'Connor ER, Sankar P, Keese C. Methods for determination of cell volume in tissue culture. Can J Physiol Pharmacol 70 Suppl: S323–333, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Klomp AE, Juijn JA, van der Gun LT, van den Berg IE, Berger R, Klomp LW. The N-terminus of the human copper transporter 1 (hCTR1) is localized extracellularly, and interacts with itself. Biochem J 370: 881–889, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee J, Pena MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem 277: 4380–4387, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem 277: 40253–40259, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Levy EJ, Anderson ME, Meister A. Transport of glutathione diethyl ester into human cells. Proc Natl Acad Sci USA 90: 9171–9175, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang ZD, Stockton D, Savaraj N, Tien Kuo M. Mechanistic comparison of human high-affinity copper transporter 1-mediated transport between copper ion and cisplatin. Mol Pharmacol 76: 843–853, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Linz R, Lutsenko S. Copper-transporting ATPases ATP7A and ATP7B: cousins, not twins. J Bioenerg Biomembr 39: 403–407, 2007 [DOI] [PubMed] [Google Scholar]
- 32. MacPherson IS, Murphy ME. Type-2 copper-containing enzymes. Cell Mol Life Sci 64: 2887–2899, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Markovic J, Mora NJ, Broseta AM, Gimeno A, de-la-Concepcion N, Vina J, Pallardo FV. The depletion of nuclear glutathione impairs cell proliferation in 3t3 fibroblasts. PLos One 4: e6413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maryon EB, Molloy SA, Kaplan JH. O-linked glycosylation at threonine 27 protects the copper transporter hCTR1 from proteolytic cleavage in mammalian cells. J Biol Chem 282: 20376–20387, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Mercer JF. The molecular basis of copper-transport diseases. Trends Mol Med 7: 64–69, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Merker K, Hapke D, Reckzeh K, Schmidt H, Lochs H, Grune T. Copper related toxic effects on cellular protein metabolism in human astrocytes. Biofactors 24: 255–261, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Molloy SA, Kaplan JH. Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem 284: 29704–29713, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Halloran TV, Culotta VC. Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem 275: 25057–25060, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Ogra Y, Aoyama M, Suzuki KT. Protective role of metallothionein against copper depletion. Arch Biochem Biophys 451: 112–118, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Pope CR, Flores AG, Kaplan JH, Unger VM. Structure and function of copper uptake transporters. Curr Top Membr 69: 97–112, 2012 [DOI] [PubMed] [Google Scholar]
- 41. Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem 277: 26021–26030, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284: 805–808, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Safaei R, Howell SB. Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol Hematol 53: 13–23, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Shiloh MU, Ruan J, Nathan C. Evaluation of bacterial survival and phagocyte function with a fluorescence-based microplate assay. Infect Immun 65: 3193–3198, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wernimont AK, Huffman DL, Lamb AL, O'Halloran TV, Rosenzweig AC. Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nat Struct Biol 7: 766–771, 2000 [DOI] [PubMed] [Google Scholar]
- 46. White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem 284: 33949–33956, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wong PC, Waggoner D, Subramaniam JR, Tessarollo L, Bartnikas TB, Culotta VC, Price DL, Rothstein J, Gitlin JD. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA 97: 2886–2891, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xiao Z, Brose J, Schimo S, Ackland SM, La Fontaine S, Wedd AG. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J Biol Chem 286: 11047–11055, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA 94: 7481–7486, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zimnicka AM, Maryon EB, Kaplan JH. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J Biol Chem 282: 26471–26480, 2007 [DOI] [PubMed] [Google Scholar]