Abstract
VEGF receptor (VEGFR) cell surface localization plays a critical role in transducing VEGF signaling toward angiogenic outcomes, and quantitative characterization of these parameters is critical to advancing computational models for predictive medicine. However, studies to this point have largely examined intact muscle; thus, essential data on the cellular localization of the receptors within the tissue are currently unknown. Therefore, our aims were to quantitatively analyze VEGFR localization on endothelial cells (ECs) from mouse hindlimb skeletal muscles after the induction of hindlimb ischemia, an established model for human peripheral artery disease. Flow cytometry was used to measure and compare the ex vivo surface localization of VEGFR1 and VEGFR2 on CD31+/CD34+ ECs 3 and 10 days after unilateral ligation of the femoral artery. We determined that 3 days after hindlimb ischemia, VEGFR2 surface levels were decreased by 80% compared with ECs from the nonischemic limb; 10 days after ischemia, we observed a twofold increase in surface levels of the modulatory receptor, VEGFR1, along with increased proliferating cell nuclear antigen, urokinase plasminogen activator, and urokinase plasminogen activator receptor mRNA expression compared with the nonischemic limb. The significant upregulation of VEGFR1 surface levels indicates that VEGFR1 indeed plays a critical role in the ischemia-induced perfusion recovery process, a process that includes both angiogenesis and arteriogenesis. The quantification of these dissimilarities, for the first time ex vivo, provides insights into the balance of modulatory (VEGFR1) and proangiogenic (VEGFR2) receptors in ischemia and lays the foundation for systems biology approaches toward therapeutic angiogenesis.
Keywords: angiogenesis, peripheral artery disease, endothelial cells, skeletal muscle, quantitative flow cytometry, receptor localization, Quantibrite, hindlimb ischemia, vascular endothelial growth factor receptor
the vegf receptor (VEGFR)-ligand family is a key regulator of angiogenesis, the growth of new blood vessels from preexisting microvasculature. VEGF binding to its receptors activates intracellular signaling pathways inducing the endothelial proliferation and migration necessary for angiogenesis. Therapeutic angiogenesis aims to treat ischemic disease through the supplementation of VEGF and/or other angiogenic growth factors (31, 48). The use of VEGF has successfully stimulated neovascularization in animal models of ischemia (30, 68). However, the use of VEGFs, fibroblast growth factor, hepatocyte growth factor, and other proangiogenic molecules has not yielded successful clinical outcomes (8, 31, 34). These clinical setbacks have been attributed to inadequate dosing, duration, and delivery as well as a need to identify accurate biomarkers of angiogenic progression (31, 48). Systems biology offers unique approaches to surmount these challenges through the coupling of sensitive measurements of the vascular microenvironment with computational modeling. These methods, experimental and computational, provide platforms for testing therapeutic conditions on multiple scales and predicting optimal treatment strategies, thus aiding to advance preclinical understanding of ischemic disease.
Computational models, based on mass-action kinetics of the VEGF-VEGFR signaling axis, have predicted the distribution of soluble VEGFR1 in peripheral vascular disease (75) and the distribution of VEGF within diseased tissue, healthy tissue, and blood (67), and a recent computational model has included in vitro- and ex vivo-derived VEGFR surface levels on the endothelium (23). This experimentally associated model provided insights into the pharmacokinetics of an anti-VEGF agent in the treatment of metastatic breast cancer and suggested novel neoadjuvant treatment approaches (23, 36, 37). The incorporation of experimentally determined VEGFR surface levels into the model significantly affected the distributions of VEGF, with up to sixfold changes in predicted VEGF levels. These data support the need to sensitively determine VEGFR levels in modeled tissues. As such, this study aimed to quantify VEGFR levels on endothelial cells (ECs) isolated from ischemic skeletal muscle compared with nonischemic ECs.
Several studies (12, 26, 57, 73) have aimed to profile the VEGFR response to hypoxia and ischemia. In vitro analysis of the hypoxic VEGFR balance has shown a cell type dependence on the hypoxic response. The exposure of human dermal microvascular ECs (MECs) to hypoxic culture conditions (0.5% O2) results in significant upregulation of VEGFR1 and VEGFR2 mRNA 6–24 h later (57). Whereas the effect of hypoxia on human umbilical vein ECs (HUVECs) remains unclear, Gerber et al. (26) showed increased VEGFR1 mRNA after 24 and 60 h of hypoxia and decreased VEGFR2 mRNA levels after 11–60 h of hypoxia (0% O2). Waltenberger et al. (73) reported upregulated VEGFR2 protein after 24 h of hypoxia (2% O2), and Nilsson et al. (57) and Kremer et al. (42) showed no hypoxia-induced changes in VEGFR mRNA levels. Brogi et al. (12) reported increased surface VEGFR2 after 24 or 48 h of treatment with hypoxic conditioned growth media. Two ex/in vivo rat hypoxia models have shown significant increases in VEGFR1 and VEGFR2 gene expression after myocardial infarct (45) and after both acute and chronic hypoxia induction in the lung (69), and microarray analysis of human skeletal muscle showed significant VEGFR2 upregulation in ischemia (70). Despite the depth of ischemia studies, no studies have quantified the levels of VEGF surface receptors and no studies have examined the changes in VEGFR presentation on ex vivo ECs. Therefore, these results of our study, for the first time, give quantitative insights into the balance of cell surface angiogenic receptors at two different stages in the response to hindlimb ischemia.
MATERIALS AND METHODS
EC isolation.
Gastrocnemius and tibialis anterior muscles were extracted from male and female 12-14-wk-old C57BL/6 mice (Jackson Laboratory). Tissue was collected from both ischemic and nonischemic limbs, thus controlling for any tissue dissociation effects. These muscles were placed in a 50-ml conical tube containing HBSS without calcium and magnesium (Mediatech, Manassas, VA). Muscle tissue was digested as previously described (36). Briefly, tissue was minced into 1-mm sections and added to freshly prepared and filtered 0.2% collagenase type IV (Worthington Biochemical, Lakewood, NJ), which was reconstituted in HBSS. Muscle tissue was digested for 30 min at 37°C with intermittent vortexting. Tissue was passed through a 70-μm strainer (BD). Cells were centrifuged at 300 g for 5 min and resuspended in 30 ml of 0.2 μm filtered isolation buffer containing PBS without calcium and magnesium (Invitrogen), 2 mM EDTA (Mediatech), and 0.1% BSA (Sigma). ECs were isolated from the cell suspension using DSB-X (Invitrogen) biotinylated mouse CD31 antibody (eBioscience and BD Bioscience, San Diego, CA) and FlowComp Dynabeads (Invitrogen) according to the manufacturers' instructions.
Cell staining and flow cytometry.
Aliquots (25 μl) of isolated cells (∼1 × 104–1 × 105 cells) were added to tubes and dually labeled with 10 μl of FITC-conjugated monoclonal antibody to mouse CD34 (BD Pharmingen) and phycoerythrin (PE)-conjugated monoclonal antibody at a final concentration of 14 μg/ml for VEGFR1 and VEGFR2 (R&D). Since CD31 is also expressed on T cells, B cells, natural killer cells, macrophages/monocytes, granulocytes, and platelets, we used CD34-FITC as a secondary marker, which is expressed on ECs, stem cells/precursors, mast cells, and neurons, the latter of which was excluded by the prior CD31 magnetic bead separation (55). The concentrations used have been reported to be saturating by the manufacturer, and we (36) have previously tested anti-human VEGFR (hVEGFR)1-PE, anti-hVEGFR2-PE, anti-hVEGFR3-PE, and anti-human neuropilin 1 (hNRP1)-PE (R&D Systems) at concentrations recommended by the manufacturer and independently confirmed those concentrations to be saturating. Tubes were protected from light and incubated for 40 min on ice. Cells were washed, centrifuged twice with 4 ml FBS stain buffer, and resuspended in 400 μl stain buffer.
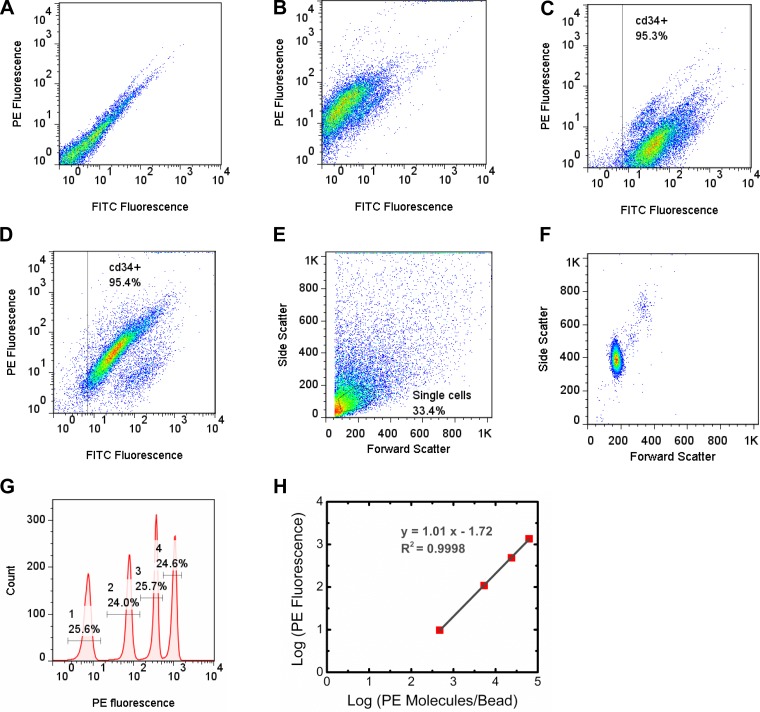
As previously described, flow cytometry was performed on a FACSCalibur; CellQuest (BD) software was used for data acquisition and Flow Jo (TreeStar) was used for data analysis (36, 37). Tubes were vortexted before placement in the flow cytometer, and 5,000–10,000 positive events were collected. Nonlabeled cells were analyzed to establish cell background conditions (Fig. 1A). PE-labeled cells were analyzed to establish the PE fluorescence signal (Fig. 1B). Single cells were selected (gated) for CD34-FITC-positive cells (FL1 channel/FITC fluorescence; Fig. 1, C and D). Cells were further gated in the forward scatter and side scatter channels to select the single cell population (Fig. 1E). Quantibrite PE beads (BD Biosciences) were gated in the forward scatter and side scatter channels to select the bead population (Fig. 1F). Quantibrite bead histograms were used to determine PE (FL2 channel) geometric means for the Quantibrite PE beads using the same compensation and voltage settings for acquiring cell fluorescence data (Fig. 1, G and H). Using PE geometric means and the numbers of PE molecules per bead for fluorescence values of low (474 PE molecules/bead), medium low (5,359 PE molecules/bead), medium high (23,843 PE molecules/bead), and high (62,336 PE molecules/bead) fluorescing beads provided by BD, a calibration curve was formed, which was fitted by linear regression using the following equation: y = mx + b, where x is log10(PE molecules/cell), y is log10(FL2 geometric mean for anti-VEGFR-PE), m is the slope of the PE bead calibration curve, and b is the y-intercept of the PE bead calibration curve (Fig. 1H). PE geometric means from antibody-labeled cells were used to determine the number of receptors bound per cell. The background fluorescence intensity in the PE channel was obtained from cells labeled with CD34-FITC only. This background fluorescence intensity was used to calculate the corresponding number of PE molecules per cell. This value was subtracted from the number of receptors bound per cell.
Fig. 1.
Representative flow cytometry plots for endothelial cells (ECs) and phycoerythrin (PE)-conjugated beads. A: fluorescence of ECs not labeled with either PE or FITC. B: ECs labeled with PE conjugated to a VEGF receptor (VEGFR) antibody. C: ECs labeled with FITC conjugated to a CD34 antibody. The box represents gating for this CD34+ population. D: ECs labeled with both CD34-FITC and VEGFR-PE. E: forward scatter versus side scatter plot of ECs that express CD34. The gate represents the singe cell population. F: forward scatter versus side scatter plot of PE beads. G: fluorescence histogram of PE beads, showing the beads that contained low levels (474 PE molecules/bead), medium low levels (5,359 PE molecules/bead), medium high levels (23,843 PE molecules/bead), and high levels (62,336 PE molecules/bead) of PE molecules. H: calibration curve relating PE fluorescence to the number of PE molecules per bead.
Cell-by-cell analysis.
For a given experiment, single cell fluorescence intensity data from the gated population were extracted using FlowJo (Tree Star, Ashland, OR). EC FL2 fluorescence intensity was converted to receptor localization using the PE bead calibration obtained during the imaging session. Receptor levels were pooled, and data 3 SDs above the mean were excluded. Histograms were created with bins of 500 receptors. Medians and coefficient of variations are shown in Table 1. A two-sample Kolmogorov-Smirnov test was performed in Matlab to determine whether the histograms were from a common distribution. In each case, the Kolmogorov-Smirnov test found the distributions to be significantly different.
Table 1.
Surface receptor statistics
| Ensemble Averaging |
Cell-by-Cell Analysis |
||||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SE | Number of cells | Median | Coefficient of variation | Skewness | Kurtosis | |
| VEGF receptor 1 | |||||||
| Day 3 | |||||||
| Nonischemic limb | 8 | 1,690 ± 130 | 29,189 | 1,780 | 6.05 | 10 | 133 |
| Ischemic limb | 8 | 1,700 ± 220 | 56,269 | 1,730 | 6.22 | 8 | 82 |
| Day 10 | |||||||
| Nonischemic limb | 7 | 1,350 ± 160 | 179,084 | 1,370 | 7.27 | 11 | 215 |
| Ischemic limb | 8 | 2,360 ± 220 | 250,281 | 2,320 | 3.72 | 4 | 26 |
| VEGF receptor 2 | |||||||
| Day 3 | |||||||
| Nonischemic limb | 8 | 1,420 ± 170 | 30,630 | 1,440 | 7.02 | 16 | 337 |
| Ischemic limb | 8 | 320 ± 30 | 57,361 | 520 | 12.9 | 21 | 564 |
| Day 10 | |||||||
| Nonischemic limb | 8 | 900 ± 140 | 222,795 | 830 | 9.49 | 13 | 353 |
| Ischemic limb | 8 | 740 ± 100 | 254,308 | 720 | 8.90 | 11 | 161 |
CD31+/CD34+ cells were isolated from the nonischemic and ischemic hindlimbs of C57BL/6 mice.
Hindlimb ischemia and laser-Doppler imaging.
Hindlimb ischemia was performed by unilateral femoral artery ligation and excision on days 3 and 10 postischemia, as previously described (20, 32). Day 3 is a time point that is well studied (32, 47, 52), providing insights into the early response to ischemic injury at a point when much of the variability that occurs in the postoperative period has resolved and flow and, in most cases, VEGF levels are significantly increased. Day 10 provides an intermediate time point after ischemic injury, where there is an acceleration in perfusion recovery (20, 32). Perfusion flow in ischemic and contralateral nonischemic limbs was measured with the use of laser-Doppler perfusion imaging (Perimed, Stockholm, Sweden) at baseline and at preselected postoperative time points, as previously described (20, 32).
RNA isolation and quantitative RT-PCR.
Total RNA was isolated and analyzed by real-time quantitative RT-PCR, as previously described (19). Briefly, after extraction, RNA was treated with DNase and purified with use of an RNeasy Mini kit (Qiagen), according to the manufacturer's protocol. Total RNA (1 μg) was used for first-strand cDNA synthesis by reverse transcription with Multi-Scribe reverse transcriptase and random hexamer primers following the manufacturer's instructions (GeneAmp Gold RNA PCR reagent kit, AB Applied Biosystems, Foster City, CA). Taqman probes and primer sets for VEGFR2 and CD31 were from Applied Biosystems. Real-time quantitative RT-PCR is performed on an I cycler (Bio-Rad, Hercules, CA). For each sample, an amplification plot was generated. From each amplification plot, a threshold cycle value was calculated, representing the PCR cycle number at which fluorescence was detectable above threshold. Negative controls lacking template cDNA were included in each experiment.
Statistical analysis.
Values are expressed as means ± SE. Unless otherwise noted, P values of <0.05 were considered statistically significant using Student-Newman-Keuls ANOVA. P values are shown in the figures. Comparison of significance between two groups was analyzed with Student's t-test.
RESULTS
Optimization of quantification ex vivo.
Our previous quantification of VEGFR levels, in vitro, determined conditions for performing receptor quantification. Antibody specificity was confirmed by testing porcine aortic ECs (kindly provided by Dr. Shay Soker, Wake Forest University) expressing hVEGFR2 and hNRP1. Saturating antibody conditions were identified by measuring fluorescence intensity over a range of antibody concentrations. Cell dissociation conditions were optimized to ensure the preservation of target receptors, and monomeric antibody binding was established through flow cytometry-fluorescence resonance energy transfer experiments (37). Compared with quantification in vitro, additional steps are required for quantification ex vivo; these steps include tissue harvest and tissue dissociation. To identify whether receptor levels are compromised during enzymatic digestion, we (36) previously tested HUVECs under the same enzymatic dissociation conditions that we used on the dissected skeletal muscle tissue and determined that VEGFR1 and VEGFR2 surface levels were unaffected by collagenase digestion. This was a significant finding, since serine proteases (e.g., trypsin), which are intrinsic to collagenase, may significantly affect cell isolation yields (62). This finding mirrored our previous work (37) in vitro, which showed that VEGFR1 and VEGFR2 surface levels were unaffected by trypsin. However, NRP1 surface levels on HUVECs were significantly affected by each of the enzymes tested. The collagenase-mediated decrease in NRP1 levels was consistent with our previous finding in vitro of trypsin significantly decreasing NRP1 levels (37). As such, we will not report levels of the NRP1 coreceptor ex vivo.
Determination of the effectiveness of antibody-bead cell isolation.
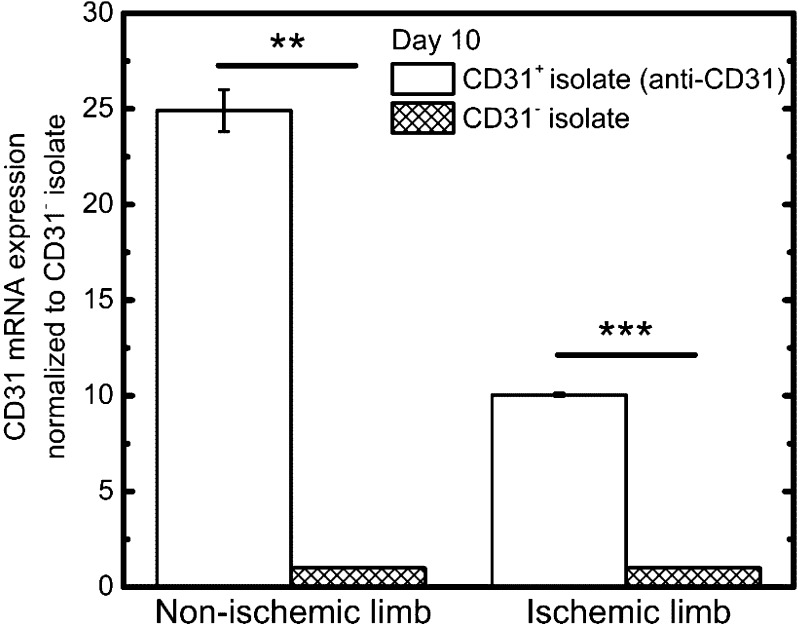
To determine the effectiveness of the antibody-bead separation, we assess CD31 mRNA expression levels in two cell fractions: cells positively isolated with CD31 antibody (CD31+ isolate) and cells that were not captured by the CD31 cell surface antibody (CD31− isolate). mRNA analysis confirmed significant CD31+ cell enrichment, with the nonischemic limb showing 25-fold higher CD31 mRNA levels in the CD31+ isolate compared with the CD31− isolate (Fig. 2). Similarly, significant CD31+ cell enrichment was seen in the ischemic limb, with ∼10-fold higher CD31 mRNA levels in the CD31+ isolate compared with the CD31− isolate (Fig. 2).
Fig. 2.
Validation of EC isolation through RT-PCR. CD31 mRNA expression was 25-fold higher in nonischemic limb cells isolated using the anti-CD31 antibody magnetic bead separation (CD31+ isolate) compared with the corresponding nonischemic limb cell fraction that did not bind the anti-CD31 antibody (CD31− isolate). Similarly, CD31 mRNA expression was 10-fold higher in ischemic limb cells isolated using the anti-CD31 antibody magnetic bead separation (CD31+ isolate) compared with the corresponding ischemic limb CD31− isolate. **P < 0.01; ***P < 0.001.
Quantification of VEGFR surface levels.
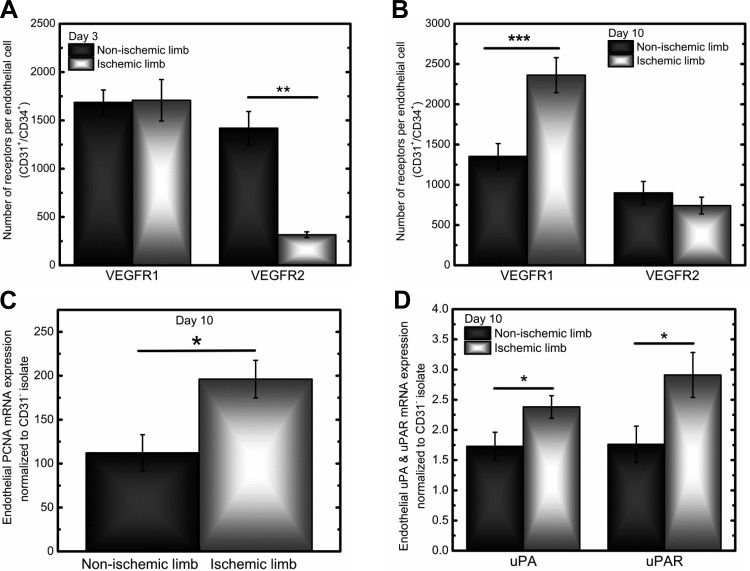
On day 3 of hindlimb ischemia, we observed 80% lower VEGFR2 surface levels on ECs from the ischemic hindlimb relative to the nonischemic hindlimb (P < 0.01; Fig. 3A): ischemic hindlimb ECs contained ∼300 VEGFR2/EC versus 1,400 VEGFR2/EC from nonischemic hindlimb ECs (Table 1). Cell-by-cell analysis revealed that this decrease in VEGFR2 surface levels occurred across the entire ischemic endothelial population (Fig. 4B). Furthermore, these ischemic ECs displayed greater homogeneity in VEGFR2 levels relative to nonischemic ECs. Surface levels of VEGFR1 on ECs were comparable in the nonischemic and ischemic hindlimb, with ∼1,700 VEGFR1/EC after 3 days of ischemia (Table 1). Comparable VEGFR1 surface levels were also observed in the cell-by-cell analysis with overlapping EC distributions (Fig. 4A).
Fig. 3.
Cell surface levels of VEGFR1 and VEGFR2 on ECs in nonischemic and ischemic hindlimbs. A: at 3 days postischemia, ischemic and nonischemic CD31+/CD34+ cells (ECs) had similar VEGFR1 surface levels (1,700 ± 200 and 1,690 ± 130 VEGFR1/cell, respectively). VEGFR2 surface levels were significantly downregulated on ECs in the ischemic hindlimb, with 320 ± 30 VEGFR2/cell compared with 1,420 ± 170 VEGFR2/cell in the nonischemic hindlimb. B: at 10 days postischemia, VEGFR1 surface levels were significantly upregulated in the ischemic limb, with ischemic ECs displaying 2,360 ± 220 VEGFR1/cell versus 1,350 ± 160 VEGFR1/cell in the nonischemic hindlimb. VEGFR2 surface levels on ECs were comparable, with 740 ± 100 VEGFR2/cell in the ischemic limb and 900 ± 140 VEGFR2/cell on the nonischemic hindlimb. C: at 10 days postischemia, ECs from the ischemic limb expressed 40% higher mRNA levels of the proliferation marker proliferating cell nuclear antigen (PCNA) compared with ECs from the nonischemic limb. D: at 10 days postischemia, ECs from the ischemic limb expressed ∼30% and ∼40% higher mRNA levels of the migration markers urokinase plasminogen activator (uPA) and uPA receptor (uPAR), respectively, compared with ECs from the nonischemic limb. *P < 0.05; **P < 0.01; ***P < 0.001.
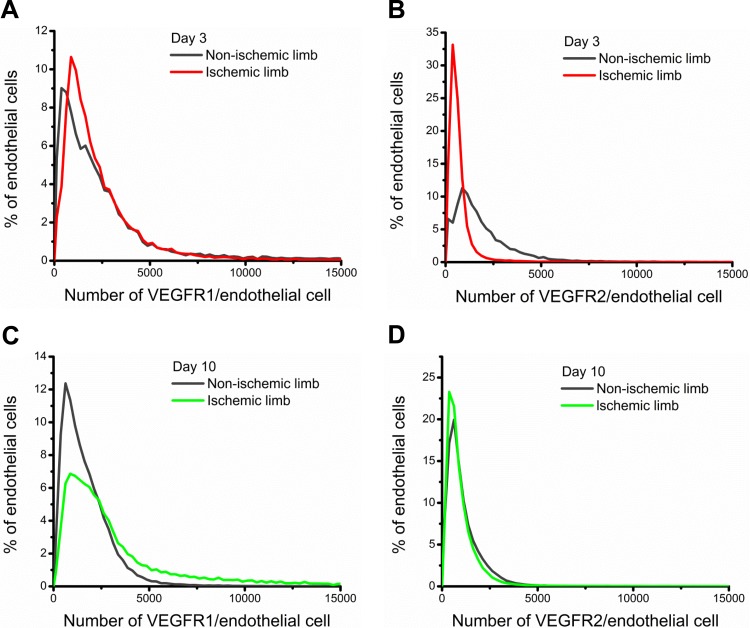
Fig. 4.
Cell-by-cell analysis of VEGFR1 and VEGFR2 distributions on mouse ECs after ischemia. The distributions showed that there were significant differences in the surface expression of VEGFRs in the ischemic and nonischemic limbs. A and B: surface levels of VEGFR1 (A) and VEGFR2 (B) 3 days postischemia. C and D: surface levels of VEGFR1 (C) and VEGFR2 (D) 10 days postischemia.
By day 10 of hindlimb ischemia, ECs from the ischemic hindlimb presented 40% higher VEGFR1 surface levels (P < 0.001; Fig. 3B). These upregulated levels of surface VEGFR1 on ischemic ECs also coincided with ∼40% higher mRNA expression of the proliferation marker proliferating cell nuclear antigen/cyclin (PCNA) (14) and ∼30% higher urokinase plasminogen activator (uPA) and ∼40% higher uPA receptor (u-PAR) mRNA levels in ischemic ECs compared with nonischemic ECs, suggesting that 10 days of ischemia induces increases in endothelial proliferation and migration (Fig. 3, C and D) (60).
Cell-by-cell analysis showed that the 40% increase in endothelial surface VEGFR1 levels could be attributed to the increased frequency of ECs displaying high surface levels of VEGFR1 in the ischemic limb compared with the nonischemic limb following the density crossing point (the intersection of the distributions; Fig. 4C). Surface levels of VEGFR2 on ECs on day 10 of hindlimb ischemia were comparable in the nonischemic and ischemic limbs, presenting ∼700–800 VEGFR2/EC (Table 1). On a cell-by-cell level, we observed overlapping endothelial distributions in the nonischemic and ischemic limbs (Fig. 4D). Between days 3 and 10, average levels of VEGFR2 appeared to decrease in the nonischemic hindlimb (∼450 VEGFR2 decrease); however, ANOVA showed that the decrease seen across these days was not statistically significant (P = 0.056).
We also analyze the skewness and kurtosis to quantitatively describe the population distributions relative to a Gaussian distribution. The positive skewness for each of the distributions (Fig. 4) illustrated the decreasing frequency of ECs with high surface VEGFRs (18), whereas the positive kurtosis across all EC populations demonstrated the heavier tails and higher peak of the distributions relative to a normal distribution (Table 1) (5).
DISCUSSION
The VEGFR-ligand family serves as a key mediator of angiogenesis. Under ischemic or hypoxic conditions, VEGFs are secreted and bind to VEGFR1, VEGFR2, and VEGFR3 as well as coreceptors NRP1 and NRP2. The activation of these receptors leads to EC proliferation and migration and the sprouting of new capillaries, which constitutes the process of neovascularization within the tissue, relieving the ischemic burden. As such, the VEGF-VEGFR signaling axis has been extensively explored toward the treatment of ischemic disease (71), with the goal of increasing collateral vessel density, thus providing increased blood flow to ischemic tissues (2). A number of factors are critical in rendering the extracellular VEGF signal to an intracellular response, but none is more important than the absolute amounts of the two main receptors, VEGFR1 and VEGFR2, as well as their ratio. As such, their surface levels significantly affect the signaling within the skeletal muscle microvasculature that drives angiogenesis.
We identified an 80% downregulation of VEGFR2 surface levels across all ECs at 3 days postischemia induction, and at 10 days we observed a 40% upregulation of VEGFR1 surface levels, which was associated with the presence of an endothelial population with high VEGFR1 surface levels and increased expression of proliferation (PCNA) and migration markers (uPA and uPAR). These results provide complimentary insights with what has been previously reported from intact muscles, where VEGFR2 expression was shown to increase by ∼13-fold at both days 3 and 10 after hindlimb ischemia with no changes in VEGFR1 expression at either time point (32). The sensitivity intrinsic to resolving receptor changes in isolated ECs combined with the evaluation of cellular heterogeneity on a cell-by-cell level provides improved insights into the ischemia-induced shifts in VEGFR levels.
Cell-to-cell variability may play an important biological role, and it has been the subject of systems biology research (56, 59). Stochasticity in gene expression leads to cell-to-cell variability in mRNA and protein levels, which can translate to heterogeneous receptor levels across similar ECs (21, 61). Furthermore, within tissue, ECs can take on differing roles: ECs involved in sprouting angiogenesis can take on either a primarily proliferative phenotype of stalk cells or a primarily migrating phenotype of tip cells. These differing EC phenotypes may contribute to innate heterogeneity in surface receptor levels.
The vascular structure can also contribute significant functional heterogeneities, with large vessels driving arteriogenesis and microvessels driving angiogenesis, distinct processes that may result in differential changes in vascular network resistance (4). In addition to such functional heterogeneities, the endothelium derived from macrovascular versus microvascular circulation may also present variability at the protein level (54). Despite this potential for microvascular versus macrovascular ischemic responses, we do not expect such heterogeneities to underlie our VEGFR surface expression ischemic responses for two reasons. First, our previous profiling of VEGFR surface expression in vitro examined both primary macrovascular cells (HUVECs) and primary MECs (human dermal MECs). The results showed similar levels of VEGFR1 on both cell types. Although we also observed significantly higher levels of VEGFR2 in MECs compared with HUVECs, the order of magnitude of these receptors was similar across these cells (37). Second, we analyzed macrovascular and microvascular endothelial responses to VEGF and observed a significant upregulation of VEGFR1 after 24 h of exposure to 1 nM VEGF, with a significant downregulation of VEGFR2 under the same conditions. These VEGF-mediated changes in VEGFR levels occurred in both macrovascular ECs and MECs (37), indicating that both cell types have a comparable potential for surface receptor regulatory responses.
While classically defined differences in endothelial structure may not dictate VEGFR heterogeneity, cell-by-cell analysis tools can inform on differential expression patterns have revealed increased heterogeneity in endothelial VEGFR1 surface localization aftr VEGF-A-mediated upregulation of this receptor in cultured primary ECs (37). Additionally, when cell-by-cell analysis was coupled with the isolation of ECs from skeletal muscle, a subpopulation of ECs with high surface levels of VEGFR1 was uncovered (36). These previous studies convey the necessity of tracking endothelial changes in receptor levels and profiling endothelial populations, both of which we have addressed in this study.
Upregulation of VEGFR1 at day 10 of hindlimb ischemia.
Given the role of VEGFRs as the initial conduit of the extracellular VEGF signals, several studies have sought to monitor changes in VEGFR gene and protein levels in ischemia. Therefore, the increased levels of surface VEGFR1 on day 10 of ischemia suggests a significant inductive role for VEGFR1 in ischemic signaling outcomes. Previous studies (32, 46) have reported no changes in VEGFR1 protein levels in ischemic skeletal muscle compared with nonischemic skeletal muscle on days 3 and 10 after femoral artery ligation, despite an upregulation of VEGF-A protein levels at 3 days postischemia. Although total protein levels may not directly correlate with surface receptor levels, the recruitment of intracellularly localized VEGFR1 may explain the increased VEGFR1 surface levels that we observed.
VEGFR1 trafficking may play a significant role in angiogenic signaling. Intracellular VEGFR1 stores represent a significant fraction of total VEGFR1, with ∼80% of total VEGFR1 being localized intracellularly in the Golgi apparatus (53) and in the nucleus (76). VEGFR endocytosis occurs through a clathrin-mediated pathway, and VEGFR1 shows little to no constitutive recycling in vitro (39). However, VEGFR1 levels can be regulated by VEGF, which stimulates trans-Golgi network to plasma membrane translocation of VEGFR1. Trafficking components, such as syntaxin and synaptosomal-associated protein 25, are regulated by neuronal ischemia (1, 13). However, the components of VEGFR1 endocytosis and exocytosis have not been fully characterized; as such, any ischemia-induced changes in VEGFR1 trafficking are yet to be established. In light of the upregulation of VEGFR1 that we report here, additional work is necessary to determine any ischemia-induced regulation of VEGFR1 trafficking.
The upregulation of endothelial VEGFR1 on day 10 of ischemia also coincides with increased angiogenic readouts: increased mRNA expression of migration and proliferation markers in the ECs. Critical to the process of angiogenesis is the degradation of the extracellular matrix, allowing for endothelial migration. This process is mediated by several enzymes, including matrix metaloproteinases and serine proteases, through the action of the uPA-uPAR axis (28, 43). Activated uPA converts plasminogen to the protease plasmin, allowing for both degradation of the extracellular matrix and activation of matrix metaloproteinases. Furthermore, uPAR has been reported to localize on the leading edge of migrating ECs, possibly serving a role in VEGF-mediated migration (11). Therefore, the upregulated uPAR and uPA mRNA expression that we observed are indicative of increased proteolytic activity and a migratory phenotype in these ECs on day 10 of ischemia. Similarly, the upregulated PCNA indicates significant endothelial proliferative activity (14).
The upregulated proliferation and migration markers along with the upregulated VEGFR1 on day 10 postischemia further advance the question of the role of VEGFR1 in angiogenic and/or arteriogenic responses. VEGFR1 is widely believed to function as a negative regulator of angiogenesis. VEGFR1-null mice exhibit a disorganized embryonic vasculature marked by endothelial overgrowth and are embryonically lethal (24, 65). This indicates a role for VEGFR1 in the regulation of vascular network organization during development. Second, VEGFR1 tyrosine kinase domain-deficient homozygous mice develop a normal vasculature (35), indicating a role for VEGFR1 in the negative regulation of endothelial proliferation during development. Finally, the >10-fold higher VEGF-binding affinity to VEGFR1 compared with VEGFR2 (VEGFR1 Kd: ∼10 pM vs. VEGFR2 Kd: 75–700 pM ) (72) but 10-fold lower VEGFR1 kinase activity relative to VEGFR2 suggests that VEGFR1 may serve as a decoy receptor, sequestering VEGF to produce an antiangiogenic response (72), or that it may serve to modulate VEGFR2 activation (3).
The expression pattern of VEGFR1 may also provide insights to its functional role. VEGFR1 is highly expressed on the plasma membrane of BALB/c-derived fibroblasts (36), and primary fibroblasts express both VEGFR1 mRNA and protein with a functional role of inducing fibroblast migration (38). In corneal fibroblasts, migration can be abolished with bevacizumab treatment (9). This VEGFR1-mediated migratory function has also been observed in macrophages (64), monocytes, and ECs (41, 66). These previous studies provide significant insights into the principles underlying the ischemia-induced upregulation of VEGFR1: as a potential contributor to endothelial proliferation, migration, and vascular patterning through negative regulation or modulation of aberrant neovascularization.
Downregulation of VEGFR2 on day 3 of hindlimb ischemia.
Previous studies (32, 74) of hindlimb ischemia have shown significant upregulation of VEGFR2 protein levels in the ischemic limb relative to the nonischemic limb on days 3, 8, and 10 after femoral artery ligation. VEGF protein levels are increased in ischemic tissue (17), and a previous study (32) using ELISA has shown significantly increased VEGF-A protein expression 3 days postischemia in the ischemic limb. Our cell-by-cell analysis revealed significant VEGFR2 downregulation within the entire EC population. As such, the downregulation of surface VEGFR2 that we observed, despite reports of whole tissue upregulation of VEGFR2 protein expression, may be linked to changes in trafficking, or we may have uncovered a previously masked population due to our analysis of enriched ECs.
VEGFR surface levels are regulated by trafficking processes unique to each VEGFR. VEGF significantly regulates VEGFR2 trafficking by translocating endosomal VEGFR2 to the plasma membrane (25) and to the nucleus (76). VEGF also routes VEGFR2 to late endosomes and ultimately to degradation, generally via lysosomes and possibly through proteasomal mechanisms (22). Trafficking studies have revealed a significant fraction, 40% of total VEGFR2, residing intracellularly (40, 53) in endosomal storage compartments (10, 22, 25, 44). A portion of those intracellular VEGFR2 stores (∼50%) is then constitutively recycled. Assuming that similar processes govern the ex vivo trafficking of VEGFR2, our results may point to increased internalization of VEGFR2, possibly mediated by the increased levels of VEGF in the tissue, since VEGF can downregulate VEGFR surface levels (25, 37).
Studies of VEGFR2 association with vascular-endothelial cadherin have shown increased VEGFR2 internalization and sustained phosphorylation of VEGFR2 in the absence of vascular-endothelial cadherin, suggesting the presence of VEGFR2 intracellular signaling (44). Intracellular tyrosine kinase receptor signaling has also been established through studies of EGF receptor (EGFR) endosomal accumulation. One study (63) has identified cell surface EGFRs as promoting cell growth and intracellular EGFRs as inducing apoptosis. Another study (66) has identified the presence of ERK-MAPK components on EGFR-containing endosomes and the presence of ligand-bound EGFRs on endosomes, suggesting the occurrence of EGFR endosomal signaling. These previously published data suggest the possibility of VEGFR2 intracellular/endosomal signaling. As such, the downregulation of VEGFR2 that we observed may be indicative of this type of process. These data underlie a need to determine whether intracellular stores of VEGFR2 contribute to angiogenic signaling.
Implications of ischemic ex vivo quantification.
Our research team has developed whole body models of VEGF-VEGFR binding kinetics, which have predicted the distribution of VEGF in the body upon administration of an anti-VEGF antibody or bevacizumab, informing on the mechanism of action of this therapeutic agent (23, 67). Such models have the power to similarly predict the optimal drug and properties for which therapeutic angiogenesis agents may have an advantageous effect. However, the predictive power of these models has previously been limited by insufficient knowledge of cell surface receptor levels. Therefore, the data that we report here provide critical parameters needed to advance systems biology models of ischemic disease (7, 27, 49, 58, 75).
Sensitivity analysis performed on these computational models has shown that blood, healthy tissue, and diseased tissue levels of VEGF depend significantly on VEGFR surface levels on the endothelium and in stromal cells. The incorporation of experimentally determined VEGFR surface levels, levels that were one order of magnitude lower than previous estimates, resulted in up to sixfold changes in predictions of total levels of VEGF-A165 and VEGF-A121 in tissue (23). In the context of our previous models, the loss of >1,000 VEGFR2 on postischemic day 3 and the gain of ∼1,000 VEGFR1 on day 10 lie within the dynamic range with which changes in VEGF levels would be observed; however, these therapeutic angiogenesis models must be compiled and simulated with these new data to precisely predict the angiogenic response and accurately determine treatment approaches. Therefore, uncovering these time-dependent VEGFR changes in ischemia will allow for more sensitive therapeutic angiogenesis models to be developed.
Quantitative profiling of VEGFRs.
Prior approaches toward understanding VEGFR surface levels have largely used in vitro radioligand-binding analyses, which reported surface densities of 500–50,000 VEGFR1/cell and 6,000–150,000 VEGFR2/cell; these variations can be attributed to the profiling of nonhuman, clonal, and transfected cells (29, 72). Scatchard analysis on HUVECs has previously reported 4,200 VEGFR1/HUVEC and 12,400 VEGFR2/HUVEC (16). We have recently optimized a state-of-the-art fluorescence approach, presented here, toward quantitatively profiling VEGFRs on ECs in vitro (37) and ex vivo (36). Our in vitro analysis revealed significant VEGF-mediated shifts in endothelial heterogeneity (37), and our ex vivo analysis identified endothelial heterogeneity in two different mouse strains, whose extensively studied differences in vascular properties and ischemic responses (6, 15, 20, 33, 50, 51) may serve as proxies for human population variability.
Heterogeneity in cell populations has gained interest as a regulator of systemic response, and mapping these heterogeneities underlies a grand challenge across cardiovascular and biomedical research. The parameters obtained from our quantitative, proteomic surface mapping of ischemia will be incorporated into our computational models, offering a better understanding of the physiological and pathological adaptations occurring within the vascular microenvironment during angiogenesis and vascular remodeling. As such, the coupling of sensitive cell isolation with quantitative, fluorescent profiling advances a novel cellular-proteomic paradigm for mapping and interpreting heterogeneity while progressing computational frameworks for predictive medicine.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant R01-HL-101200 (to A. S. Popel) and by a United Negro College Fund/Merck Postdoctoral Fellowship, Federation of American Societies for Experimental Biology Postdoctoral Professional Development Award, and NHLBI Grant T32-HL-007581 (to P. I. Imoukhuede). A. O. Dokun was supported by NHLBI Grant RO1-HL-101200S1 and a Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.I.I., A.O.D., B.H.A., and A.S.P. conception and design of research; P.I.I. and A.O.D. performed experiments; P.I.I. and A.O.D. analyzed data; P.I.I., A.O.D., B.H.A., and A.S.P. interpreted results of experiments; P.I.I. and A.O.D. prepared figures; P.I.I. drafted manuscript; P.I.I., A.O.D., B.H.A., and A.S.P. edited and revised manuscript; P.I.I., A.O.D., B.H.A., and A.S.P. approved final version of manuscript.
REFERENCES
- 1. Antonucci F, Cerri C, Vetencourt JFM, Caleo M. Acute neuroprotection by the synaptic blocker botulinum neurotoxin E in a rat model of focal cerebral ischaemia. Neuroscience 169: 395–401, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Attanasio S, Snell J. Therapeutic angiogenesis in the management of critical limb ischemia: current concepts and review. Cardiol Rev 17: 115–120, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Communi D, Shibuya M, Collen D, Conway EM, Carmeliet P. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med 9: 936–943, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bailey AM, O'Neill TJ, Morris CE, Peirce SM. Arteriolar remodeling following ischemic injury extends from capillary to large arteriole in the microcirculation. Microcirculation 15: 389–404, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balanda KP, MacGillivray HL. Kurtosis: a critical review. Am Stat 42: 111–119, 1988 [Google Scholar]
- 6. Barone FC, Knudsen DJ, Nelson AH, Feuerstein GZ, Willette RN. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab 13: 683–692, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Bauer AL, Jackson TL, Jiang Y. A cell-based model exhibiting branching and anastomosis during tumor-induced angiogenesis. Biophys J 92: 3105–3121, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, Van Belle E. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet 377: 1929–1937, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Berthaut A, Mirshahi P, Benabbou N, Azzazene D, Bordu C, Therwath A, Legeais JM, Mirshahi M. Vascular endothelial growth factor receptor-1 (VEGFR-1) expression in human corneal fibroblast decreased with age. Mol Vis 15: 1997–2007, 2009 [PMC free article] [PubMed] [Google Scholar]
- 10. Bhattacharya R, Kang-Decker N, Hughes DA, Mukherjee P, Shah V, McNiven MA, Mukhopadhyay D. Regulatory role of dynamin-2 in VEGFR-2/KDR-mediated endothelial signaling. FASEB J 19: 1692–1694, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Binder B, Mihaly J, Prager G. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist's view. Thromb Haemost 97: 336–342, 2007 [PubMed] [Google Scholar]
- 12. Brogi E, Schatteman G, Wu T, Kim EA, Varticovski L, Keyt B, Isner JM. Hypoxia-induced paracrine regulation of vascular endothelial growth factor receptor expression. J Clin Invest 97: 469–476, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao F, Hata R, Zhu P, Niinobe M, Sakanaka M. Up-regulation of syntaxin1 in ischemic cortex after permanent focal ischemia in rats. Brain Res 1272: 52–61, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Celis JE, Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci USA 82: 3262–3266, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics 30: 179–191, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Chen JH, Wang XC, Kan M, Sato JD. Effect of FGF-1 and FGF-2 on VEGF binding to human umbilical vein endothelial cells. Cell Biol Int 25: 257–260, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 27: 563–584, 2011 [DOI] [PubMed] [Google Scholar]
- 18. D'Agostino RB, Belanger A, D'Agostino RB., Jr A suggestion for using powerful and informative tests of normality. Am Stat 44: 316–321, 1990 [Google Scholar]
- 19. Dai Q, Huang J, Klitzman B, Dong C, Goldschmidt-Clermont PJ, March KL, Rokovich J, Johnstone B, Rebar EJ, Spratt SK, Case CC, Kontos CD, Annex BH. Engineered zinc finger-activating vascular endothelial growth factor transcription factor plasmid DNA induces therapeutic angiogenesis in rabbits with hindlimb ischemia. Circulation 110: 2467–2475, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Dokun AO, Keum S, Hazarika S, Li Y, Lamonte GM, Wheeler F, Marchuk DA, Annex BH. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation 117: 1207–1215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science 297: 1183–1186, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Ewan LC, Jopling HM, Jia H, Mittar S, Bagherzadeh A, Howell GJ, Walker JH, Zachary IC, Ponnambalam S. Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting and degradation in endothelial cells. Traffic 7: 1270–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Finley S, Engel-Stefanini M, Imoukhuede PI, Popel A. Pharmacokinetics and pharmacodynamics of VEGF-neutralizing antibodies. BMC Sys Biol 5: 193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66–70, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Gampel A, Moss L, Jones MC, Brunton V, Norman JC, Mellor H. VEGF regulates the mobilization of VEGFR2/KDR from an intracellular endothelial storage compartment. Blood 108: 2624–2631, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. J Biol Chem 272: 23659–23667, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Gevertz JL, Torquato S. Modeling the effects of vasculature evolution on early brain tumor growth. J Theor Biol 243: 517–531, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Ghajar CM, Kachgal S, Kniazeva E, Mori H, Costes SV, George SC, Putnam AJ. Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp Cell Res 316: 813–825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gitay-Goren H, Soker S, Vlodavsky I, Neufeld G. The binding of vascular endothelial growth factor to its receptors is dependent on cell surface-associated heparin-like molecules. J Biol Chem 267: 6093–6098, 1992 [PubMed] [Google Scholar]
- 30. Gowdak LHW, Poliakova L, Wang X, Kovesdi I, Fishbein KW, Zacheo A, Palumbo R, Straino S, Emanueli C, Marrocco-Trischitta M, Lakatta EG, Anversa P, Spencer RGS, Talan M, Capogrossi MC. Adenovirus-mediated VEGF121 gene transfer stimulates angiogenesis in normoperfused skeletal muscle and preserves tissue perfusion after induction of ischemia. Circulation 102: 565–571, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res 105: 724–736, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res 101: 948–956, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol 26: 520–526, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER; VIVA investigators The VIVA Trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 107: 1359–1365, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA 95: 9349–9354, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Imoukhuede PI, Popel AS. Expression of VEGF receptors on endothelial cells in mouse skeletal muscle. PLoS One 7: e44791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Imoukhuede PI, Popel AS. Quantification and cell-to-cell variation of vascular endothelial growth factor receptors. Exp Cell Res 317: 955–965, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin X, Ge X, Zhu Dl Yan C, Chu YF, Chen Wd Liu J, Gao Pj. Expression and function of vascular endothelial growth factor receptors (Flt-1 and Flk-1) in vascular adventitial fibroblasts. J Mol Cell Cardiol 43: 292–300, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Jones MC, Caswell PT, Moran-Jones K, Roberts M, Barry ST, Gampel A, Mellor H, Norman JC. VEGFR1 (Flt1) regulates Rab4 recycling to control fibronectin polymerization and endothelial vessel branching. Traffic 10: 754–766, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Jopling HM, Howell GJ, Gamper N, Ponnambalam S. The VEGFR2 receptor tyrosine kinase undergoes constitutive endosome-to-plasma membrane recycling. Biochem Biophys Res Commun 410: 170–176, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood 103: 4527–4535, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Kremer C, Breier G, Risau W, Plate KH. Up-regulation of flk-1/vascular endothelial growth factor receptor 2 by its ligand in a cerebral slice culture system. Cancer Res 57: 3852–3859, 1997 [PubMed] [Google Scholar]
- 43. Kroon ME, Koolwijk P, van der Vecht B, van Hinsbergh VWM. Urokinase receptor expression on human microvascular endothelial cells is increased by hypoxia: implications for capillary-like tube formation in a fibrin matrix. Blood 96: 2775–2783, 2000 [PubMed] [Google Scholar]
- 44. Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol 174: 593–604, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li J, Brown LF, Hibberd MG, Grossman JD, Morgan JP, Simons M. VEGF, flk-1, and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am J Physiol Heart Circ Physiol 270: H1803–H1811, 1996 [DOI] [PubMed] [Google Scholar]
- 46. Li Y, Hazarika S, Xie D, Pippen AM, Kontos CD, Annex BH. In mice with type 2 diabetes, a vascular endothelial growth factor (VEGF)-activating transcription factor modulates VEGF signaling and induces therapeutic angiogenesis after hindlimb ischemia. Diabetes 56: 656–665, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Luo D, Luo Y, He Y, Zhang H, Zhang R, Li X, Dobrucki WL, Sinusas AJ, Sessa WC, Min W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol 169: 1886–1898, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mac Gabhann F, Annex B, Popel A. Gene therapy from the perspective of systems biology. Curr Opin Mol Ther 12: 570–577, 2010 [PMC free article] [PubMed] [Google Scholar]
- 49. Mac Gabhann F, Ji J, Popel A. Multi-scale computational models of pro-angiogenic treatments in peripheral arterial disease. Anna Biomed Eng 35: 982–994, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Mac Gabhann F, Peirce SM. Collateral capillary arterialization following arteriolar ligation in murine skeletal muscle. Microcirculation 17: 333–347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Majid A, He YY, Gidday JM, Kaplan SS, Gonzales ER, Park TS, Fenstermacher JD, Wei L, Choi DW, Hsu CY. Differences in vulnerability to permanent focal cerebral ischemia among 3 common mouse strains. Stroke 31: 2707–2714, 2000 [DOI] [PubMed] [Google Scholar]
- 52. McClung J, McCord T, Keum S, Johnson S, Annex B, Marchuk D, Kontos C. Skeletal muscle-specific genetic determinants contribute to the differential strain-dependent effects of hindlimb ischemia in mice. Am J Pathol 180: 2156–2169, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mittar S, Ulyatt C, Howell GJ, Bruns AF, Zachary I, Walker JH, Ponnambalam S. VEGFR1 receptor tyrosine kinase localization to the Golgi apparatus is calcium-dependent. Exp Cell Res 315: 877–889, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Morello JP, Plamondon J, Meyrick B, Hoover R, O'Connor-McCourt MD. Transforming growth factor-β receptor expression on endothelial cells: heterogeneity of type III receptor expression. J Cell Physiol 165: 201–211, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Nielsen JS, McNagny KM. CD34 is a key regulator of hematopoietic stem cell trafficking to bone marrow and mast cell progenitor trafficking in the periphery. Microcirculation 16: 487–496, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Niepel M, Spencer SL, Sorger PK. Non-genetic cell-to-cell variability and the consequences for pharmacology. Curr Opin Chem Biol 13: 556–561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nilsson I, Shibuya M, Wennström S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res 299: 476–485, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Peirce SM. Computational and mathematical modeling of angiogenesis. Microcirculation 15: 739–751, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pelkmans L. Using cell-to-cell variability–a new era in molecular biology. Science 336: 425–426, 2012 [DOI] [PubMed] [Google Scholar]
- 60. Pepper MS, Sappino AP, Stocklin R, Montesano R, Orci L, Vassalli JD. Upregulation of urokinase receptor expression on migrating endothelial cells. J Cell Biol 122: 673–684, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raj A, van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135: 216–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rose NL, Palcic MM, Shapiro AMJ, Lakey JR. An evaluation of the activation of endogenous pancreatic enzymes during human islet isolations. Transplant Proc 35: 2455–2457, 2003 [DOI] [PubMed] [Google Scholar]
- 63. Rush JS, Quinalty LM, Engelman L, Sherry DM, Ceresa BP. Endosomal accumulation of the activated epidermal growth factor receptor (EGFR) induces apoptosis. J Biol Chem 287: 712–722, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, Nakahata T, Shibuya M. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97: 785–791, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66, 1995 [DOI] [PubMed] [Google Scholar]
- 66. Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 10: 609–622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stefanini MO, Wu FT, Mac Gabhann F, Popel AS. Increase of plasma VEGF after intravenous administration of bevacizumab is predicted by a pharmacokinetic model. Cancer Res 70: 9886–9894, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Takeshita S, Pu LQ, Stein LA, Sniderman AD, Bunting S, Ferrara N, Isner JM, Symes JF. Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation 90: 228–234, 1994 [PubMed] [Google Scholar]
- 69. Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest 95: 1798–1807, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tuomisto TT, Rissanen TT, Vajanto I, Korkeela A, Rutanen J, Ylä-Herttuala S. HIF-VEGF-VEGFR-2, TNF-α and IGF pathways are upregulated in critical human skeletal muscle ischemia as studied with DNA array. Atherosclerosis 174: 111–120, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Uchida C, Haas TL. Evolving strategies in manipulating VEGF/VEGFR signaling for the promotion of angiogenesis in ischemic muscle. Curr Pharm Des 15: 411–421, 2009 [DOI] [PubMed] [Google Scholar]
- 72. Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 269: 26988–26995, 1994 [PubMed] [Google Scholar]
- 73. Waltenberger J, Mayr U, Pentz S, Hombach V. Functional upregulation of the vascular endothelial growth factor receptor KDR by hypoxia. Circulation 94: 1647–1654, 1996 [DOI] [PubMed] [Google Scholar]
- 74. Willmann JK, Chen K, Wang H, Paulmurugan R, Rollins M, Cai W, Wang DS, Chen IY, Gheysens O, Rodriguez-Porcel M, Chen X, Gambhir SS. Monitoring of the biological response to murine hindlimb ischemia with 64Cu-labeled vascular endothelial growth factor-121 positron emission tomography. Circulation 117: 915–922, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu FTH, Stefanini MO, Gabhann FM, Kontos CD, Annex BH, Popel AS. VEGF and soluble VEGF receptor-1 (sFlt-1) distributions in peripheral arterial disease: an in silico model. Am J Physiol Heart Circ Physiol 298: H2174–H2191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang Z, Neiva K, Lingen M, Ellis L, Nor J. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ 17: 499–512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]