Abstract
Polyamine analogs have demonstrated considerable activity against many important solid tumor models including breast cancer. However, the precise mechanisms of antitumor activities of polyamine analogs are not entirely understood. The cytotoxicity of a newly developed polyamine analog compound, SL11144, against human breast cancer was assessed. Treatment of human breast cancer cell lines in culture with SL11144 decreased cell proliferation and induced programmed cell death in a time- and dose-dependent manner. SL11144 also profoundly inhibited the growth of MDA-MB-231 xenografts in host nude mice without overt toxic effects. Treatment of MDA-MB-435 cells with SL11144 led to the release of cytochrome c from mitochondria into cytosol, activation of caspase-3, and poly(ADP-ribose) polymerase cleavage. SL11144 decreased Bcl-2 and increased Bax protein levels in MDA-MB-231 cells. Furthermore, activator protein 1 transcriptional factor family member c-Jun was up-regulated by SL11144 in MDA-MB-435 and MDA-MB-231 cells, but not in MCF7 cells. In addition, significant inhibition of ornithine decarboxylase activity and a decrease in polyamine pools were demonstrated. These results demonstrate that the novel polyamine analog SL11144 has effective antineoplastic action against human breast cancer cells in vitro and in vivo and that multiple apoptotic mechanisms are associated with its cytotoxic effect in specific human breast cancer cell lines.
INTRODUCTION
The natural polyamines (Put,3 Spd, and Spm) have been shown to be essential for cell growth. The critical role of polyamines in regulation of cell growth has led to the development of a number of inhibitors of key enzymes in the polyamine biosynthetic pathway as a therapeutic strategy (1–3). It has also been demonstrated that synthetic polyamine analogs can down-regulate polyamine biosynthesis by feedback mechanisms but are unable to act as substitutes for natural polyamines to promote cell growth. This approach has become an important means for the study of the physiological roles of natural polyamines and a potent application for creation of new antineoplastic agents (4–6). Indeed, several synthetic polyamine analogs have been reported to inhibit cell proliferation and induce PCD in a variety of tumor cell lines (7–11).
Apoptotic cell death is characterized by chromatin condensation, cytoplasmic blebbing, and internucleosomal DNA fragmentation and occurs in a variety of cellular systems in response to many different stimuli (12). We have demonstrated previously (9) that some polyamine analogs can induce PCD in hormone-responsive or -unresponsive human breast cancer cells. A highly regulated metabolic pathway finely controls intracellular polyamine concentrations. The rate-limiting enzymes ODC and S-Adenosylmethionine decarboxylase regulate biosynthesis, whereas catabolism is regulated by SSAT and human polyamine oxidase h1/spermine oxidase (13). Cell type-specific superinduction of SSAT and the subsequent depletion of natural polyamine pools have been reported in polyamine analog-induced growth inhibition and apoptosis in some tumor cell lines (9, 14). However, in other cell lines, polyamine analogs that do not highly induce SSAT can still inhibit tumor cell growth and produce apoptosis (15, 16). These divergent results suggest that polyamine analog-induced cell death may result from several agent-dependent mechanisms.
SL11144, a leading agent of a new generation of polyamine analogs designated as oligoamines, has shown significant activity against proliferating cells (17). In this study, we have evaluated the antineoplastic efficacy of SL11144 in human breast cancer cells in vitro and in vivo. The data presented in this study suggest that SL11144 significantly inhibits growth and induces PCD in human breast cancer cells.
MATERIALS AND METHODS
Compound, Cell Lines, and Culture Condition
The polyamine analog SL11144 (Fig. 1) was provided by SLIL Biomedical Corp. (Madison, WI). Polyamine analog compounds BENSpm, CPENSpm, and CHENSpm were synthesized as described previously (18). Concentrated stock solutions (10 mM in double-distilled H2O) of polyamine analogs were diluted with medium to the indicated concentrations. Human breast cancer MDA-MB-231 and MCF7 cells were maintained in DMEM supplemented with 5% fetal bovine serum, 2 mM glutamine, and 100 units/ml penicillin/streptomycin. MDA-MB-435 cells were maintained in improved modification of eagle’s medium supplemented with 5% fetal bovine serum, 2 mM glutamine, and 100 units/ml penicillin/streptomycin. Cells were incubated at 37°C in a 5% CO2 atmosphere.
Fig. 1.
Structures of Spm and polyamine analog SL11144.
MTT Survival Assays
MTT assays were performed using a method described previously (19). Briefly, 2000–5000 cells were plated in 96-well dishes and treated with the various concentrations of SL11144 for different lengths of time. At the end of each time point, 100 μl of a 1 mg/ml MTT solution (Sigma Chemical Co., St. Louis, MO), diluted in serum-free culture media, were added to each well. The plates were incubated at 37°C in 5% CO2 atmosphere for 4 h, allowing viable cells to reduce the yellow tetrazolium salt into dark blue formazan crystals. At the end of the 4-h incubation, the MTT solution was removed, and 200 μl of 1:1 (v/v) solution of DMSO:ethanol were added to each well to dissolve the formazan crystals. The absorbance in individual wells was determined at A540 nm. All of the experiments were plated in quadruplicate, and the results of assays were presented as means ± SD.
Analysis of Intracellular Polyamine Pools, SSAT Activity, and ODC Activity
The intracellular polyamine content of treated and untreated cells was determined by precolumn dansylation and reversed phase high-performance liquid chromatography (20). SSAT and ODC activities were measured using cellular extracts as described previously (21, 22). Protein concentrations were determined according to the method of Bradford (23).
Hoechst Staining of Nuclear Chromatin
SL11144-treated cells were fixed with 4% formaldehyde in PBS at 37°C for 10 min and permeabilized with a 19:1 mixture of ethanol/acetic acid at −20°C for 15 min. Fixed cells were stained with 1 μg/ml Hoechst 33258 (Sigma Chemical Co.) in PBS at room temperature for 20 min. Hoechst staining of the cells was analyzed by fluorescence microscopy.
Determination of Internucleosomal DNA Cleavage
After tumor cells were treated with increasing concentrations of SL11144 for increasing times, cells were harvested, counted, and washed with PBS at 4°C. Cells were then suspended in lysis buffer (5 mM Tris-HCl, 20 mM EDTA, and 0.5% Triton X-100) and incubated for 20 min on ice. After incubation, samples were centrifuged at 14,000 × g for 20 min, and the supernatant was transferred to a reaction tube followed by phenol/chloroform/isoamyl (25:24:1) extraction. Two volumes of 100% ethanol were added to supernatant, followed by 5-min centrifugation at 14,000 × g. The pellet was resuspended in 0.1× SSC buffer and incubated with RNase for at least 30 min at 37°C. Then 50 μl of 5 M NaCl was added, followed by phenol/chloroform/isoamyl (25:24:1) extraction. After ethanol precipitation and centrifugation, the pellet was washed with 70% ethanol and dried. DNA samples were analyzed by electrophoresis in a 1.2% agarose slab gel containing 0.2 μg/ml ethidium bromide and visualized under UV illumination. This method isolates only DNA ladder fragments without genomic DNA.
Animal Studies
Female 4–6-week-old BALB c nu/nu athymic nude mice (Harlan Bioproducts for Science Inc., Indianapolis, IN) weighing between 16 and 18 g received injection in the right flank with 3.75 × 106 MDA-MB-231 cells. Cells were allowed to grow for 10 days to an average volume of 50–100 mm3. Animals were then randomly assigned (eight mice for control group and seven mice for treatment groups) to receive vehicle control or SL11144 (2.5, 5, or 10 mg/kg) via i.p. injections twice weekly for 5 weeks. Tumor volumes were regularly assessed twice weekly by measuring 0.5 × length (mm) × width (mm) × width (mm). Mice were also weighed twice weekly.
Nuclear and Cytoplasmic Protein Extraction
The extractions of nuclear and cytoplasmic protein were performed using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Pierce, Rockford, IL). MDA-MB-435 cells treated with 10 μM SL11144 for different times were harvested by trypsinization and washed with PBS. Two hundred μl of ice-cold Cytoplasmic Extraction Reagent I with protease inhibitors (0.5 mg/ml benzamidine, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 0.2 M phenylmethylsulfonyl fluoride) were added to the cell pellets. After a 10-min incubation on ice, 11 μl of ice-cold Cytoplasmic Extraction Reagent II without protease inhibitors were added, followed by a 5-min centrifugation at 14,000 × g. The supernatant containing the cytoplasmic extract was retained, and the insoluble pellet was resuspended in 100 μl of Nuclear Extraction Reagent and incubated on ice for 40 min. After a 10-min centrifugation, the supernatant that contained the nuclear extract was saved. Both cytoplasmic and nuclear extracts were analyzed by Western blot using anti-c-Jun and anti-c-Fos antibodies as described below.
Detection of Cytochrome c Release
To avoid artifacts due to mechanical breakage of the outer mitochondrial membrane, selective plasma membrane permeabilization with digitonin was used to examine the release of cytochrome c from mitochondria into cytosol (24). Briefly, cells treated with different concentrations of SL11144 for the desired exposure time were harvested by trypsinization, washed with PBS, and subsequently incubated in 100 μl of permeabilization buffer [210 mM D-mannitol, 70 mM sucrose, 10 mM HEPES, 5 mM succinate, 0.2 mM EGTA, and 100 μg/ml digitonin (pH 7.2)] for 5 min. After centrifugation for 10 min at 14,000 × g, the supernatant with protein content was saved, and protein concentrations were determined using the Pierce Micro Protein Assay Kit. Equal amounts of protein were fractionated using 12% SDS-PAGE and analyzed by Western blot as described below.
Western Blotting
Cells treated with different concentrations of SL11144 for the desired exposure times were harvested by trypsinization and washed with PBS. Cellular protein was isolated using the protein extraction buffer containing 150 mM NaCl, 10 mM Tris (pH 7.2), 5 mM EDTA, 0.1% Triton X-100, 5% glycerol, and 2% SDS. Protein concentrations were determined using the Pierce Micro Protein Assay Kit. Equal amounts of proteins (50 μg/lane) were fractionated using 12% SDS-PAGE and transferred to PVDF membranes. The membranes were incubated with primary antibodies against caspase-3, PARP, Bcl-2, Bax, caspase-8, caspase-9, cytochrome c, c-jun, c-fos, or FasL (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA). After washing with PBS, the membranes were incubated with peroxidase-conjugated goat antimouse or antirabbit secondary antibody (1:3000; DAKO Corp., Carpinteria, CA) followed by enhanced chemiluminescence staining using the enhanced chemiluminescence system (Amersham Biosciences). Actin was used to normalize for protein loading.
RESULTS
Inhibition of Growth by SL11144
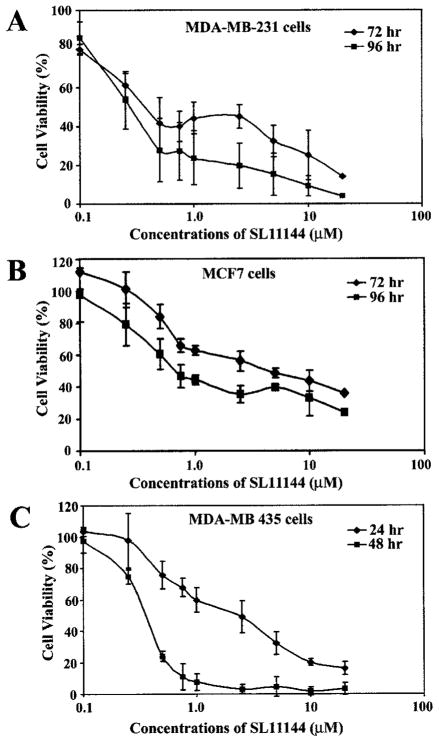
The sensitivity of three human breast cancer cell lines (MDA-MB-231, MDA-MB-435, and MCF7 cells) to the newly synthesized polyamine analog SL11144 (Fig. 1) was assessed by using a MTT cellular survival assay. These cells were chosen because they represent hormone-responsive (MCF7) and -unresponsive (MDA-MB-231 and MDA-MB-435) human breast cancer cell lines. All three cell lines exhibited time- and concentration-dependent growth inhibition by SL11144 (Fig. 2, A–C ). The IC50 values for MDA-MB-231 and MCF7 cells are about 1–5 μM for a 72-h treatment and 0.5–0.75 μM for a 96-h treatment. MDA-MB-435 cells were more sensitive to SL11144 with IC50 values of 2.5 μM for 24-h drug exposure and 0.25–0.5 μM for 48-h drug exposure. In addition, the cytotoxicity of SL11144 against MDA-MB-231 cells was compared with the identified polyamine analogs BENSpm, CPENSpm, and CHENSpm. MTT studies demonstrated that SL11144 had a lower IC50 at 96-h treatment in MDA-MB-231 cells than BENSpm (1–2.5 μM), CPENSpm (1–2.5 μM), or CHENSpm [~2.5 μM (data not shown)]. These data indicate that SL11144 is a more potent inhibitor of human breast tumor cell proliferation than previously synthesized polyamine analogs.
Fig. 2.
SL11144 inhibits growth of human breast cancer cells in a time-and dose-dependent manner. MDA-MB-231 cells (A) and MCF7 cells (B) were treated with increasing concentrations of SL11144 for 72 or 96 h. MDA-MB-435 cells (C) were treated with increasing concentrations of SL11144 for 24 or 48 h. MTT assays were performed as described in “Materials and Methods.” Shown are means ± SD of independent experiments performed in quadruplicate.
Regulation of Intracellular Polyamine Pools and Metabolic Enzymes by SL11144
To address whether the observed growth-inhibitory effects of SL11144 in human breast cancer cells reflect its effects on the polyamine metabolic pathway, intracellular SL11144 accumulation, polyamine pools (Put, Spd, and Spm), and regulatory enzyme (SSAT and ODC) activities were assessed. As shown in Table 1, the accumulation rates of SL11144 in the three cell lines are similar after exposure of cells to 10 μM SL11144 for 24 h. SL11144 reduced all intracellular polyamines in MDA-MB-231 cells. Put and Spm were decreased, and Spd was slightly increased by SL11144 in MCF7 cells. In MDA-MB-435 cells, Spd level was down-regulated, and Spm was slightly up-regulated by SL11144. ODC activity was significantly inhibited, and SSAT activity was modestly increased in all three cell lines.
Table 1.
Effects of SL11144 on polyamine pools and SSAT and ODC activities in human breast cancer cells Intracellular polyamine levels, SSAT activity, and ODC activity were determined as described in “Materials and Methods” after incubation of tumor cells for 24 h in the presence or absence of 10 μM SL11144. Values represent the means of duplicate determinations.
| Cell lines | Treatment | SL11144 (nmol/mg protein) | Polyamines (nmol/mg protein)
|
SSAT activity (pmol/mg protein/min) | ODC activity (pmol CO2/mg protein/h) | ||
|---|---|---|---|---|---|---|---|
| Put | Spd | Spm | |||||
| MDA-MB-231 | Control | NDa | 3.65 | 52.04 | 21.17 | 2.22 | 2290.98 |
| SL11144 | 2.32 | ND | 15.27 | 8.14 | 6.02 | ND | |
| MCF7 | Control | ND | 6.22 | 49.38 | 28.52 | 1.39 | 2359.10 |
| SL11144 | 4.81 | 0.95 | 58.53 | 16.58 | 14.72 | 6.53 | |
| MDA-MB-435 | Control | ND | ND | 22.03 | 3.24 | 2.73 | 558.79 |
| SL11144 | 3.62 | ND | 8.84 | 4.35 | 23.39 | 4.58 | |
ND, not detected.
SL11144 Induces Apoptotic Cell Death
To determine whether observed SL11144-induced decrease in growth rate was a result of apoptosis, DNA fragmentation assays were performed. DNA ladders isolated from untreated and SL11144-treated cells were processed by agarose gel electrophoresis to detect the typical oligonucleosomal DNA fragmentation. The results (Fig. 3) indicate that SL11144 induces DNA fragmentation in all three human breast cancer cell lines, but the time and dose required for the induction of apoptosis varied by cell type. DNA fragmentation was clearly detected only after a 96-h exposure to SL11144 in MDA-MB-231 and MCF7 cells but was detectable in 12 h in MDA-MB-435 cells. A minimum concentration of 5 μM is required for SL11144 to induce DNA fragmentation in MDA-MB-231 and MDA-MB-435 cells, but MCF7 cells are more sensitive in that DNA fragmentation was observed with 0.1–0.25 μM SL11144 for 96 h.
Fig. 3.
SL11144 induces internucleosomal DNA fragmentation. MDA-MB-231 cells (A) and MCF7 cells (B) were treated with increasing concentrations of SL11144 (0.1–10 μM) for 96 h or treated with 10 μM SL11144 for 48, 72, and 96 h. MDA-MB-435 cells (C) were treated with increasing concentrations of SL11144 (0.1–10 μM) for 24 h or treated with 10 μM SL11144 for 3, 6, 12, 24, and 48 h. Cells were harvested, and fragmented DNA was extracted as described in “Materials and Methods.” Fragmented DNA was analyzed by electrophoresis in a 1.2% agarose gel containing 0.1% ethidium bromide. Each experiment was done twice with similar results.
The effect of SL11144 on cell morphology was also investigated. Both control and SL11144-treated cells were stained with the fluorescent dye Hoechst 33258 and visualized by fluorescence microscopy. Typical morphological changes of apoptosis including chromatin condensation and nuclear fragmentation were observed in all three treated cell lines (Fig. 4B), but not in the untreated control cells (Fig. 4A). Taken together, the DNA fragmentation and fluorescence results suggest that SL11144 induces apoptotic cell death in all three human breast cancer cell lines.
Fig. 4.
Fluorescent micrographs of SL11144-treated cells. MDA-MB-231 cells, MDA-MCF7 cells, and MB-435 cells were exposed to 10 μM SL11144 for the indicated times. Then cells were fixed in formaldehyde and stained with Hoechst dye 33258. A, untreated cells; B, SL11144-treated cells.
Therapeutic Effect of SL11144 against Human Breast Cancer MDA-MB-231 Xenografts
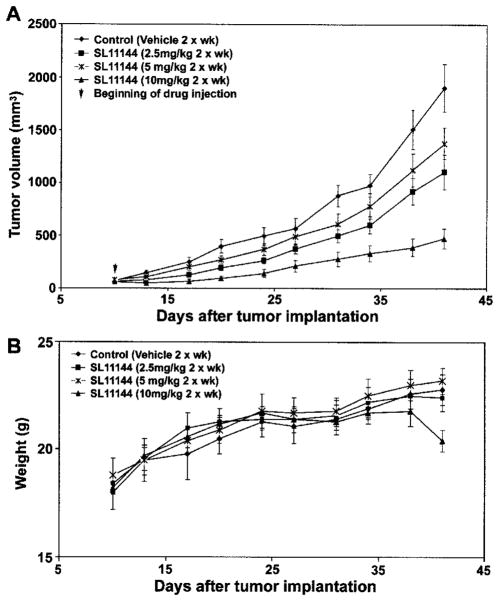
The in vivo therapeutic effect of SL11144 was evaluated using human breast cancer MDA-MB-231 xenografts in athymic nude mice. By 10 days after tumor cell inoculation, the average tumor sizes reached approximately 50–100 mm3. Mice were then randomized into treatment (n = 7) and control (n = 8) groups. Different doses of SL11144 (2.5, 5, and 10 mg/kg) were administered via i.p. injections twice a week. SL11144 displayed antiproliferative effects against MDA-MB-231 xenografts in a dose-dependent manner (Fig. 5A). Whereas partial suppressions of tumor growth were observed at the doses of 2.5 and 5 mg/kg, increasing the dose to 10 mg/kg significantly inhibited the tumor growth. During the course of treatment, there was no obvious weight loss observed, except a slight decrease of weight (~5% of body weight) in the group treated with a dose of 10 mg/kg at 41 days (Fig. 5B). These results indicate that the treatment of SL11144 possesses significant in vivo growth suppression efficacy against MDA-MB-231 cells with no overt toxic effects.
Fig. 5.
Effects of SL11144 in nude mice bearing MDA-MB-231 xenografts. A, MDA-MB-231 cells were transplanted into the flank region of nude mice. Ten days after implantation, different doses of SL11144 (2.5, 5, or 10 mg/kg) or vehicle were given via i.p. injection twice weekly. Tumor volumes of mice were measured twice weekly. The vertical bars indicate mean tumor size (in mm3) ± SE. B, weights of mice were measured twice weekly. The vertical bars indicate mean mouse weight (in g) ± SE.
Effects of SL11144 on Apoptosis-related Proteins
Several apoptosis-associated genes or proteins have been shown to play critical roles in regulating apoptosis. These include caspases, bcl-2 family members, FasL, cytochrome c, and PARP (25–29). To determine whether these proteins are involved in the mediation of SL11144-induced cell death in human breast cancer cells, we examined their expression by Western blotting. In MDA-MB-231 cells, as shown in Fig. 6A, treatment with 10 μM SL11144 decreased the amount of caspase-3 protein by 96 h of treatment, but no cleaved, active caspase-3 or its downstream target, PARP, was detected. We next examined whether two upstream proteases, caspase-9 and caspase-8, were affected by SL11144 in MDA-MB-231 cells. SL11144 treatment increased the cleavage of caspase-9 after 72 h, whereas caspase-8 was essentially undetectable under the conditions examined. Cytochrome c release from mitochondria was enhanced at 48 h and returned to its baseline level thereafter. Furthermore, Bcl-2 protein was down-regulated, and Bax was up-regulated by SL11144 beginning at 24 h. Finally, expression of FasL was increased by SL11144 after 48 h. The changes observed precede DNA fragmentation, which was not observed until 96 h of treatment.
Fig. 6.
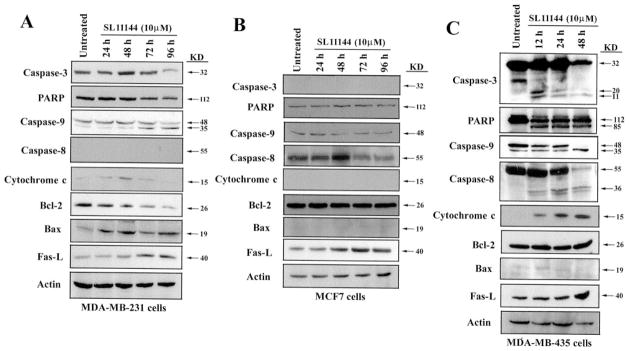
Effects of SL11144 on apoptosis proteins. MDA-MB-231 cells (A) and MCF7 cells (B) were treated with 10 μM SL11144 for 24, 48, 72, and 96 h. MDA-MB-435 cells (C) were treated 10 μM SL11144 for 12, 24, and 48 h. Equal amounts (50 μg/lane) of cellular protein were fractionated on 12% SDS-PAGE gels and transferred to PVDF membranes followed by immunoblotting with anti-caspase-3, PARP, caspase-9, caspase-8, cytochrome c, Bcl-2, Bax, or FasL monoclonal or polyclonal antibodies and analyzed as described in “Materials and Methods.” Actin protein was blotted as a control. Each experiment was repeated twice with similar results.
Effects of SL11144 on apoptotic protein expression were further assessed in MCF7 cells. Our results (Fig. 6B) confirmed the previous finding (30) that caspase-3 is not expressed in MCF7 cells. The activities of caspase-8, caspase-9, and PARP were not affected by SL11144. SL11144 did not change Bcl-2 protein levels, whereas Bax expression was minimal. No release of cytochrome c was observed in SL11144-treated MCF7 cells. However, FasL level was increased by SL11144 after 48 h of treatment. These data suggest that SL11144 may induce apoptosis in MCF7 cells through caspase- and cytochrome c release-independent pathways. Another possibility is that the DNA fragmentation and apoptotic morphological changes noted in MCF7 cells are induced directly by SL11144. Many polyamine analogs bind strongly to DNA and are capable of inducing structural changes in chromatin (6).
In contrast to the above-mentioned results in MCF7 and MDA-MB-231 cells, SL11144 treatment of MDA-MB-435 cells led to caspase-3 activation and cleavage of PARP within 12 h of drug exposure (Fig. 6C). Also, caspase-8 was activated, and the proform of caspase-9 was completely cleaved by 48 h of exposure. Although Bcl-2 expression did not change, and Bax expression was essentially undetectable, cytochrome c was released into cytoplasm from mitochondria by 12 h. FasL expression was induced at 48 h. These results suggest that both caspase and mitochondrial pathways are activated by SL11144 in MDA-MB-435 cells. The time course of caspase activation and cytochrome c release parallels the course of DNA fragmentation, which was detected at 12 h of SL11144 treatment.
SL11144 Up-Regulates c-Jun and c-Fos in MDA-MB-231 and MDA-MB-435 Cells
Because the effects of SL11144 on apoptotic pathways varied greatly between different human breast cancer cell lines, we examined the impact of SL11144 on other important apoptosis-related factors, particularly c-Jun and c-Fos. Both c-Jun and c-Fos are important members of the AP-1 transcription factor family, which plays a critical role in regulating transcription of a variety of genes involved in growth, differentiation, apoptosis, and so forth. In MDA-MB-231 cells, SL11144 treatment induced c-Jun phosphorylation after 48 h but did not alter the protein expression of either c-Jun or c-Fos (Fig. 7A). In MCF7 cells, no obvious changes in c-Jun and c-Fos were observed (Fig. 7B). In contrast, SL11144 significantly induced the phosphorylation of c-Jun and enhanced the protein level of c-Jun and c-Fos in MDA-MB-435 cells within 6–12 h (Fig. 7C). To study whether SL11144-enhanced c-Jun and c-Fos protein expression led to the increased nuclear localization of these proteins in MDA-MB-435 cells, the subcellular localization of c-Jun and c-Fos was examined. c-Jun was induced and expressed largely in the nucleus, whereas c-Fos was induced in both the cytoplasm and the nucleus (Fig. 7D). These results imply that up-regulations of AP-1 family proteins by SL11144 are cell type specific and may play an active role in the mediation of growth-inhibitory activities of SL11144.
Fig. 7.

Effects of SL11144 on c-Jun and c-Fos. Tumor cells were treated with 10 μM SL11144 for the times indicated. Equal amounts (50 μg/lane) of whole cell (A–C), cytoplasmic or nuclear protein (D) were fractionated on 12% SDS-PAGE gels and transferred to PVDF membranes followed by immunoblotting with anti-c-Jun and c-Fos polyclonal antibodies and analyzed as described in “Materials and Methods.” Actin protein was blotted as a control.
DISCUSSION
Results of previous studies from our laboratory demonstrated that first and second generations of N-acetyl substituted polyamine analogs could inhibit growth and induce apoptosis in MCF7 and other human breast cancer cell lines (3, 9, 31). However a Phase II clinical trial of one early polyamine analog, N1,N11-diethylnorspermine (DENSPM; also known as BENSpm), showed that it was not effective as a single agent in women with advanced breast cancer (32). Recently, a group of new polyamine analogs designated as oligoamines has been developed (17). Oligoamines were synthesized with longer chains than natural cellular polyamine molecules that occur in mammalian cells and are effective against a variety of proliferating cells (17). In this study, we demonstrate that one of the leading oligoamine compounds, SL11144, significantly inhibits the growth of and induces PCD in human breast cancer cells. It displays more potent antiproliferative activity against breast tumor line proliferation than the previously reported polyamine analogs, BENSpm, CPENSpm, and CHENSpm. SL11144 induced DNA fragmentation and typical apoptotic morphological changes in both hormone-responsive (MCF7) and hormone-unresponsive (MDA-MB-231 and MDA-MB-435) breast cancer cell lines. It appears that there is no relationship between hormone receptor status and cytotoxic effects of SL11144. SL11144 also inhibits the growth of MDA-MB-231 xenografts in nude mice in a dose-dependent fashion without apparent toxicity.
SL11144-induced apoptosis, based on morphological and DNA fragmentation criteria, was not detected until 96 h of treatment in MDA-MB-231 and in MCF7 cells, but 12 h of treatment with SL11144 resulted in apoptosis in MDA-MB-435 cells. Although the mechanisms of differential susceptibility among tumor cells to polyamine analog-induced cell death are unclear, this could reflect varied effects on apoptotic pathway members including caspases, bcl-2 family members, cytochrome c, and FasL, which have been demonstrated to play critical roles in regulating PCD (25–28). Caspases have been characterized as the effectors and executioners of apoptosis, and caspase-3 is a critical downstream apoptotic effector that cleaves specific substrates such as PARP. The observation that caspase-3 activation was followed by PARP cleavage in MDA-MB-435 cells indicates that caspase-3 may play a key role as an important executioner in SL11144-induced apoptosis in this cell line. However, the failure of SL11144 to activate caspase-3 in MDA-MB-231 cells and the absence of caspase-3 expression in MCF7 suggest that other factors or pathways can also function as apoptotic effectors in these two cell lines.
Mitochondria can be induced to release cytochrome c in response to many anticancer drugs and to other stresses by the opening of channels on the outer mitochondrial membrane (33). Release of cytochrome c activates the caspase adaptor, caspase-9, which then activates downstream caspases such as caspase-3 and caspase-8 (34). Our studies found that cytochrome c release was transiently enhanced by SL11144 with a 48-h drug exposure in MDA-MB-231 cells, whereas it was rapidly and consistently induced in MDA-MB-435 cells. In both cell lines, time-dependent activation of caspase-9 was observed, but caspase-8 activation was only seen in MDA-MB-435 cells. However, in MCF7 cells, SL11144 has no effect on cytochrome c release or on caspase-8 or -9 activation. The simultaneous activation of both caspase cascades and of the mitochondrial pathway in MDA-MB-435 cells by SL11144 might explain why cell death was more rapidly induced in these cells than in MDA-MB-231 or MCF7 cells. A recent study by Ellison et al. (35) reported that MDA-MB-435 cells might be of melanoma origin based on differential expressions of genes characteristic of breast cancer cells. If more compelling evidence ultimately confirmed this conclusion, the different sensitivity of MDA-MB-435 cells to SL11144 may possibly reflect its non-breast cancer identity.
Members of the Bcl-2 family play a central role in regulating the mitochondrial pathway of apoptosis. More than 20 Bcl-2 family members have been identified to date, including antiapoptosis members (Bcl-2, Bcl-XL, Bcl-W, Bcl-G, Mcl-1, and so forth) and proapoptosis members [Bax, Bak, Bok, Bad, Bid, Bik, Bim, Bcl-Xs, and so forth (33, 34, 36–39)]. In response to various stimuli and stresses, Bcl-2 family proteins usually translocate to the outer mitochondrial membrane and modulate membrane permeabilization, leading to the release of cytochrome c. SL11144 decreased Bcl-2 and increased Bax expression in MDA-MB-231 cells but did not affect Bcl-2 and Bax in MDA-MB-435 and MCF7 cells, suggesting that the regulation of Bcl-2 family members by polyamine analog is cell type specific. Our data also demonstrate that SL11144 enhances FasL (the only protein to be uniformly affected) expression in all three cell lines. The Fas/FasL (CD95-CD95 ligand) system is another critical pathway that leads to the activation of apoptotic machinery. Binding of FasL to Fas and to other death receptors results in receptor trimerization, recruitment of adaptor protein to the cytoplasmic death domain, and activation of a series of downstream apoptotic events (40, 41). Recent studies have shown that overexpression of FasL can lead to suicidal or fratricidal destruction in melanoma and leukemia cells via autocrine or fratricidal interactions between FasL and Fas (42, 43). Up-regulation of FasL level by SL11144 in all three human breast cancer cell lines implies that activation of Fas/FasL system might be a common mechanism for the cell death induced by SL11144.
We further investigated whether other important upstream regulatory or signaling events were involved in the mediation of SL11144-induced growth inhibition and apoptosis. SL11144 induces expression and phosphorylation of c-Jun, an important member of the AP-1 family, in both MDA-MB-231 and MDA-MB-435 cells. It also significantly increased the protein expression of another important AP-1 family member, c-Fos, after 12 h in MDA-MB-435 cells. Nuclear extraction analysis showed that c-Jun protein was located largely in the nucleus, where it can potentially play an active role in mediation of a wide range of gene expressions. However, neither c-Jun or c-Fos levels nor phosphorylation status was significantly affected by SL11144 treatment in MCF7 cells. c-Jun-NH2-terminal kinase signaling and AP-1 transcription factors have been implicated in the regulation of cell proliferation, differentiation, and apoptosis (44). The proapoptotic targets of c-jun include FasL, tumor necrosis factor α, c-Myc, p53, and members of the bcl-2 family (45–49). The activation of c-Jun in MDA-MB-231 and MDA-MB-435 cells, but not in MCF7 cells, suggests that c-Jun-NH2-terminal kinase/AP-1 and the upstream regulator mitogen-activated protein kinase family might be a major polyamine analog response pathway in some but not all breast cancer cell lines.
The intracellular polyamines are highly regulated by several polyamine metabolic enzymes. ODC, the first and rate-limiting step of polyamine biosynthesis, increases levels of polyamines in cells during rapid proliferation or differentiation (6). High expression of ODC characterizes some cancers including breast cancer. As a result, there has been extensive effort to design compounds that can inhibit ODC activity in tumor cells. α-Difluoromethylornithine, an irreversible inhibitor of ODC, has proven to be effective in inhibiting growth in several in vitro and in vivo tumor models (6, 50). In this study, the effect of SL11144 on natural polyamine levels was variable in different cell lines. SL11144 treatment led to a decrease in all natural polyamines in MDA-MB-231 cells and had inconsistent effects in MCF7 and MDA-MB-435 cells. The increased level of Spd in MCF7 cells by SL11144 might explain why MCF7 cells are less sensitive to SL11144 than the other two cell lines. Although ODC activities were significantly suppressed by SL11144 in all these cells, it is not clear whether the attenuation of ODC activities contributes to SL11144 cytotoxicity. In addition, the activity of another critical polyamine metabolic enzyme, SSAT, was only modestly up-regulated by SL11144 exposure, indicating that SSAT activity is not responsible for the observed cytotoxic response. All these results imply that the effects of SL11144 may not be solely a function of its effect on polyamine pools.
In summary, a newly developed polyamine analog, SL11144, exhibits significant inhibitory actions against human breast cancer cell growth in vitro and in vivo. Apoptotic cell death was induced by SL11144 in a time- and dose-dependent manner. SL11144 modulated expression of apoptotic proteins in a cell type-specific manner, suggesting that multiple apoptotic pathways might be involved in SL1144-induced apoptosis in different human breast cancer cell lines.
Footnotes
Supported by NIH Grants P50CA88843 (to N. E. D.) and CA51085 (to R. A. C.), Army DOD Grant DAMD 17-99-1-9242 (to N. E. D.), and The Breast Cancer Research Foundation (N. E. D.).
The abbreviations used are: Put, putrescine; Spd, spermidine; Spm, spermine; PCD, programmed cell death; ODC, ornithine decarboxylase; SSAT, spermidine/spermine N1-acetyltransferase; BENSpm, N1,N11-bis(ethyl)norspermine; CPENSpm, N1-ethyl-N11-[(cyclopropyl)methyl]-4,8,-diazaundecane; CHENSpm, N1-ethyl-N11-[(cyclohepthyl)methyl]-4,8,-diazaundecane; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PARP, poly(ADP-ribose) polymerase; AP-1, activator protein 1; PVDF, polyvinylidene difluoride; FasL, Fas ligand.
References
- 1.Porter CW, Herrera-Ornelas L, Pera P, Petrelli NF, Mittelman A. Polyamine biosynthetic activity in normal and neoplastic human colorectal tissues. Cancer (Phila) 1987;60:1275–1281. doi: 10.1002/1097-0142(19870915)60:6<1275::aid-cncr2820600619>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- 3.Davidson NE, Mank AR, Prestigiacomo LJ, Bergeron RJ, Casero RA., Jr Growth inhibition of hormone-responsive and -resistant human breast cancer cells in culture by N1,N12-bis(ethyl)spermine. Cancer Res. 1993;53:2071–2075. [PubMed] [Google Scholar]
- 4.Seiler N, Delcros J, Vaultier M, Le Roch N, Havouis R, Douaud F, Moulinoux JP. Bis(7-amino-4-azaheptyl) dimethylsilane and bis(7-ethylamino-4-azaheptyl) dimethylsilane: inhibition of tumor cell growth in vitro and in vivo. Cancer Res. 1996;56:5624–5630. [PubMed] [Google Scholar]
- 5.Casero RA, Jr, Woster PM. Terminally alkylated polyamine analogues as chemotherapeutic agents. J Med Chem. 2001;44:1–26. doi: 10.1021/jm000084m. [DOI] [PubMed] [Google Scholar]
- 6.Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 7.Ha HC, Woster PM, Yager JD, Casero RA., Jr The role of polyamine catabolism in polyamine-induced programmed cell death. Proc Natl Acad Sci USA. 1997;94:1557–1562. doi: 10.1073/pnas.94.21.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai H, Kramer DL, Yang C, Murti KG, Porter CW, Cleveland JL. The polyamine oxidase inhibitor MDL-72,527 selectively induces apoptosis of transformed hematopoietic cells through lysosomotropic effects. Cancer Res. 1999;59:4944–4954. [PubMed] [Google Scholar]
- 9.McCloskey DE, Casero RA, Woster PM, Davidson NE. Induction of programmed cell death in human breast cancer cells by an unsymmetrically alkylated polyamine analogue. Cancer Res. 1995;55:3233–3236. [PubMed] [Google Scholar]
- 10.Bergeron RJ, McManis JS, Liu CZ, Feng Y, Weimar WR, Luchetta GR, Wu Q, Ortiz-Ocasio J, Vinson JRT, Kramer D, Porter CW. Antiproliferative properties of polyamine analogues: a structure-activity study. J Med Chem. 1994;37:3464–3476. doi: 10.1021/jm00047a004. [DOI] [PubMed] [Google Scholar]
- 11.McCloskey DE, Yang J, Woster PM, Davidson NE, Casero RA., Jr Polyamine analogue induction of programmed cell death in human lung tumor cells. Clin Cancer Res. 1996;2:441–446. [PubMed] [Google Scholar]
- 12.McConkey DJ, Orrennius S, Johdal M. Cellular signaling in programmed cell death (apoptosis) Immunol Today. 1990;11:120–121. doi: 10.1016/0167-5699(90)90048-e. [DOI] [PubMed] [Google Scholar]
- 13.Casero RA, Jr, Pegg AE. Spermidine/spermine N1-acetyl-transferase: the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- 14.Gabrielson EW, Pegg AE, Casero RA., Jr The induction of spermidine/spermine N1-acetyltransferase (SSAT) is a common event in the response of human primary non-small cell lung carcinomas to exposure to the new antitumor polyamine analogue N1,N11-bis-(ethyl)norspermine. Clin Cancer Res. 1999;5:1638–1641. [PubMed] [Google Scholar]
- 15.Wallace HM, Duthie J, Evans DM, Lamond S, Nicoll KM, Heys SD. Alterations in polyamine catabolic enzymes in human breast cancer tissue. Clin Cancer Res. 2000;6:3657–3661. [PubMed] [Google Scholar]
- 16.Webb HK, Wu Z, Sirisoma N, Ha HC, Casero RA, Jr, Woster PW. 1-(N-Alkylamino)-11-(N-ethylamine)-4,8-diazaundecanes: simple synthetic polyamine analogues that differentially alter tubulin polymerization. J Med Chem. 1999;42:1415–1421. doi: 10.1021/jm980603+. [DOI] [PubMed] [Google Scholar]
- 17.Bacchi CJ, Weiss LM, Lane S, Frydman B, Valasinas A, Reddy V, Sun JS, Marton LJ, Khan IA, Moretto M, Yarlett N, Wittner M. Novel synthetic polyamines are effective in the treatment of experimental microsporidiosis, an opportunistic AIDS-associated infection. Antimicrob Agents Chemother. 2002;46:55–61. doi: 10.1128/AAC.46.1.55-61.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saab MH, West EE, Bieszk NC, Preuss C, Mank AR, Casero RA, Jr, Woster PM. Synthesis and evaluation of unsymmetrically substituted polyamine analogues as modulators of human/spermine-N1-acetyltransferase (SSAT) and as potential anti-tumor agents. J Med Chem. 1993;36:2998–3004. doi: 10.1021/jm00072a020. [DOI] [PubMed] [Google Scholar]
- 19.Hahm HA, Dunn VR, Butash KA, Devereux WL, Woster PM, Casero RA, Jr, Davidson NE. Combination of standard cytotoxic agents with polyamine analogues in the treatment of breast cancer cell lines. Clin Cancer Res. 2001;7:391–399. [PubMed] [Google Scholar]
- 20.Bergeron RJ, Neims AH, McManis JS, Hawthorne TR, Vinson JRT, Bortell R, Ingeno MJ. Synthetic polyamine analogues as antineoplastics. J Med Chem. 1988;31:1183–1190. doi: 10.1021/jm00401a019. [DOI] [PubMed] [Google Scholar]
- 21.Casero RA, Jr, Celano P, Ervin SJ, Wiest L, Pegg AE. High specific induction of spermidine/spermine N1-acetyltransferase in a human large cell lung carcinoma. Biochem J. 1990;270:615–620. doi: 10.1042/bj2700615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seely JE, Pegg AE. Ornithine decarboxylase (mouse kidney) Methods Enzymol. 1983;94:158–161. doi: 10.1016/s0076-6879(83)94025-9. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Leist M, Volbracht C, Fava E, Nicotera P. 1-Methyl-4-phenylpyridinium induces autocrine excitotoxicity, protease activation, and neuronal apoptosis. Mol Pharmacol. 1998;54:789–801. doi: 10.1124/mol.54.5.789. [DOI] [PubMed] [Google Scholar]
- 25.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 26.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 27.Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 28.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ, Fritz LC. Cell-specific induction of apoptosis by microinjection of cytochrome c. Bcl-xL has activity independent of cytochrome c release. J Biol Chem. 1997;272:30299–30305. doi: 10.1074/jbc.272.48.30299. [DOI] [PubMed] [Google Scholar]
- 31.Davidson NE, Hahm HA, McCloskey DE, Woster PM, Casero RA., Jr Clinical aspects of cell death in breast cancer: the polyamine pathway as a new target for treatment. Endocr Relat Cancer. 1999;6:69–73. doi: 10.1677/erc.0.0060069. [DOI] [PubMed] [Google Scholar]
- 32.Wolff AC, Bowling MK, DeClue C, Amstrong DK, Fetting JH, Casero RA, Jr, Davidson NE. A Phase II study of diethylnorspermine (DENSPM) in previously treated patients with metastatic breast cancer (MBC) Breast Cancer Res Treat. 2001;69:286. [Google Scholar]
- 33.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 34.Green DR, Reed JC. Mitochondria and apoptosis. Science (Wash DC) 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 35.Ellison G, Klinowska T, Westwood RF, Docter E, French T, Fox JC. Further evidence to support the melanocytic origin of MDA-MB-435. Mol Pathol. 2002;55:294–299. doi: 10.1136/mp.55.5.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strobel T, Swanson L, Korsmeyer S, Cannistra SA. BAX enhances paclitaxel-induced apoptosis through a p53-independent pathway. Proc Natl Acad Sci USA. 1996;90:14094–14099. doi: 10.1073/pnas.93.24.14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256:50–57. doi: 10.1006/excr.2000.4839. [DOI] [PubMed] [Google Scholar]
- 39.Guo B, Godzik A, Reed JC. Bcl-G, a novel pro-apoptotic member of the Bcl-2 family. J Biol Chem. 2000;276:2780–2785. doi: 10.1074/jbc.M005889200. [DOI] [PubMed] [Google Scholar]
- 40.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 41.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 42.Buechner SA, Wernli M, Harr T, Hahn S, Itin P, Erb P. Regulation of basal cell carcinoma by intralesional interferon-α treatment is mediated by CD95 (Apo-1/Fas)-CD95 ligand-induced suicide. J Clin Investig. 1997;100:2691–2696. doi: 10.1172/JCI119814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 44.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 45.Harwood FG, Kasibhatla S, Petak I, Vernes R, Green DR, Houghton JA. Regulation of FasL by NF-κB and AP-1 in Fas-dependent thymineless death of human colon carcinoma cells. J Biol Chem. 2000;275:10023–10029. doi: 10.1074/jbc.275.14.10023. [DOI] [PubMed] [Google Scholar]
- 46.Zagariya A, Mungre S, Lovis R, Birrer M, Ness S, Thimmapaya B, Pope R. Tumor necrosis factor α gene regulation: enhancement of C/EBPβ-induced activation by c-Jun. Mol Cell Biol. 1998;18:2815–2824. doi: 10.1128/mcb.18.5.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H, Arsura M, Wu M, Duyao M, Buckler AJ, Sonenshein GE. Role of Rel-related factors in control of c-myc gene transcription in receptor-mediated apoptosis of the murine B cell WEHI 231 line. J Exp Med. 1995;181:1169–1177. doi: 10.1084/jem.181.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreiber M, Kolbus A, Piu F, Szabowski A, Mohle-Steinlein U, Tian J, Karin M, Angel P, Wagner EF. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999;13:607–619. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park J, Kim I, Oh YJ, Lee K, Han PL, Choi EJ. Activation of c-Jun N-terminal kinase antagonizes an anti-apoptotic action of Bcl-2. J Biol Chem. 1997;272:16725–16728. doi: 10.1074/jbc.272.27.16725. [DOI] [PubMed] [Google Scholar]
- 50.Mamont PS, Duchesne MC, Grove J, Bey P. Anti-proliferative properties of DL-α-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978;81:58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]