Abstract
Corticotropin-releasing factor (CRF), a neuropeptide, regulates endocrine and autonomic responses to stress through G-protein coupled receptors, CRF1 or CRF2. A PET ligand able to monitor changes in CRF1 receptor occupancy in vivo would aid in understanding the pathophysiology of stress related diseases as well as in the clinical development of non-peptide antagonists with therapeutic value. We have radiolabeled the CRF1 receptor ligand, BMK-152 ([8-(4-bromo-2,6-dimethoxyphenyl)-2,7-dimethylpyrazolo[1,5-α][1,3,5]triazin-4-yl]-N,N-bis-(2-methoxyethyl)amine; ClogP= 2.6), at both the 3 and 4 position with [76Br]. Using in vitro autoradiography saturation studies the 4-[76Br]BMK-152 exhibited high affinity binding to both rat (Kd = 0.23 ± 0.07 nM; n=3) and monkey frontal cortex (Kd = 0.31 ± 0.08 nM; n=3) consistent with CRF1 receptor regional distribution whereas with the 3-[76Br]BMK-152, the Kd's could not be determined due to high non-specific binding. In vitro autoradiography competition studies using [125I]Tyr0-o-CRF confirmed that 3-Br-BMK-152 (Ki = 24.4 ± 4.9 nM; n=3) had lower affinity (70 fold) than 4-Br-BMK-152 (Ki = 0.35 ± 0.07 nM; n=3) in monkey frontal cortex and similiar studies using [125I]Sauvagine confirmed CRF1 receptor selectivity. In vivo studies with P-glycoprotein (PGP) knockout mice (KO) and their wildtype littermates (WT) showed that the brain uptake of 3-[76Br]BMK/4-[76Br]BMK was increased < 2 fold in KO vs WT indicating that 3-[76Br]BMK-152/4-[76Br]BMK was not a Pgp substrate. Rat brain uptakes of 4-[76Br] BMK-152 from ex vivo autoradiography studies showed regional localization consistent with known published CRF1 receptor distribution and potential as a PET ligand for in vivo imaging of CRF1 receptors.
Keywords: Corticotropin-Releasing Factor Type 1 receptors, CRF1 receptor, Br-76 BMK, PET
Introduction
Corticotropin-releasing factor (CRF), a 41 amino acid peptide first isolated from ovine hypothalamus, activates pituitary corticotrophins which in turn cause the release of proopiomelanocortin-derived peptides, ACTH, and beta-endorphin like peptides (Vale et al., 1981). CRF acts both as a neurohormone and transmitter playing a major role in regulating endocrine, behavioral, and autonomic responses to stress (De Souza and Kuhar, 1986a; Grigoriadis et al., 1996b; Owens and Nemeroff, 1991). The actions of CRF are mediated through specific receptors belonging to the secretin-family of G-protein-coupled receptors (GPCR) which are linked to adenylate cyclase; thus far two distinct CRF receptor subtypes have been identified, CRF1 and CRF2 receptors (CRF1R; CRF2R) (Grigoriadis et al., 1996b). The different regional brain distribution and pharmacological profiles suggest that the subtypes have distinct functional roles with CRF1R implicated as the subtype primarily responsible for initiating the hypothalamic-pituitary-adrenal (HPA) responses to stress (Hauger et al., 2009; Lovenberg et al., 1995). From clinical studies examining stress and feeding disorders, depression, addictive disorders, alcoholism, and insomnia, CRF activation of CRF1 receptor signaling has been identified as playing a key role in the progression of the pathophysiology of these disease states (Hauger et al., 2009; Kolber et al., 2010; Ruggiero et al., 1999; Silberman et al., 2009; Valdez, 2009). Post mortem studies of suicide victims with depression and affective anxiety disorders revealed reduced densities of CRF1R in the prefrontal cortex while CRF1R densities were increased in the cerebral cortex of Alzheimer’s patients (Arborelius et al., 1999; Grigoriadis et al., 1989). These changes in CRF1R appear to reflect compensatory responses to CRF secretion as CRF levels (determined from cerebral spinal fluid) have been found to be increased in suicides, depression and affective anxiety disorders whereas CRF levels were decreased in Alzheimer’s patients. CRF1R antagonists by preventing this activation of CRF1 receptor signaling by CRF may prove promising as therapies for depression, addiction, and other stress related disorders (Hauger et al., 2006; Refojo and Holsboer, 2009; Zorrilla and Koob, 2010). Development of these antagonists has been hampered not only by the lack of suitable animal models but the complexities of the CRF signaling pathways and differential responses depending on regional location. A positron emission tomography (PET) agent would make possible in vivo imaging of CRF1R in human studies and assist in the drug development process by monitoring changes in CRF1 receptor occupancy with treatment.
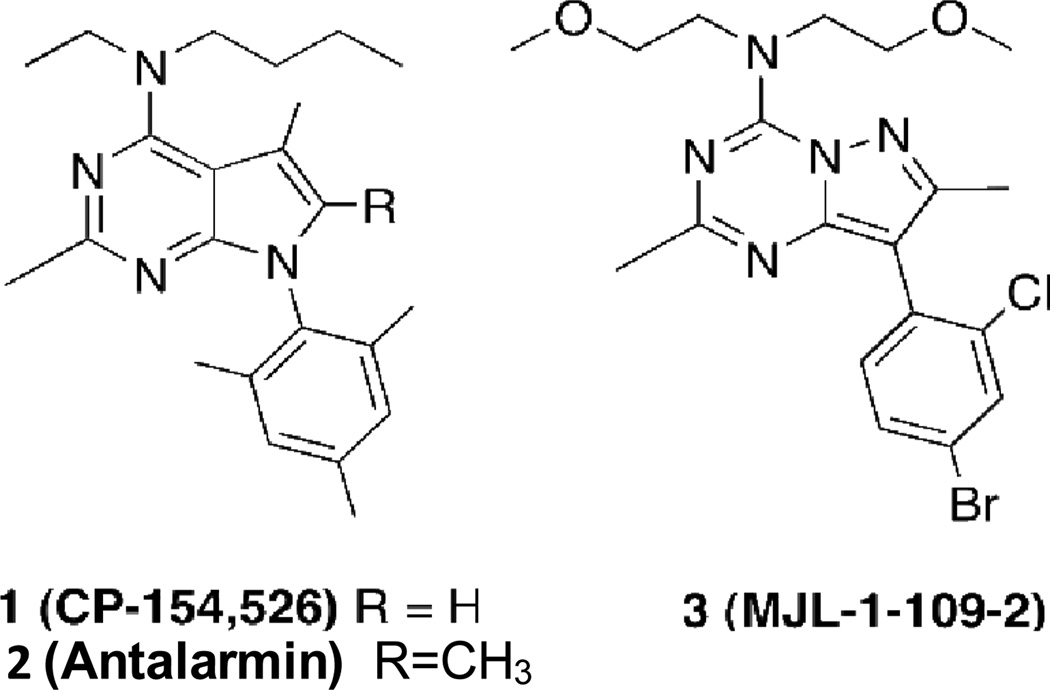
We previously reported studies in which the pyrazololtriazine antagonist, MJL-1-109-2 (Ki= 1.9 nM; ClogP = 3.05; Fig. 1., compound 3), a high affinity lipophilic non-peptide ligand for CRF1 receptors was labeled with 76Br (Jagoda et al., 2003). This lead structure was derived from the same platform as the pyrrolopyrimidine derivatives CP-154,526 (1) and antalarmin (2), specific CRF1 receptor antagonists with high affinity for CRF1 receptors but with high lipophilicity. MJL-1-109-2 (3) represented an improvement in affinity and a lower calculated log P. Although MJL-1-109-2 (3) exhibited specific binding in vitro in rat brain, specific binding in vivo was difficult to discern from non-specific binding.
Fig. 1.
Structures of compounds 1 (CP-154,526), 2 (antalarmin) and 3 (MJL-1-109-2).
A suitable CRF1R imaging agent requires not only high affinity to distinguish specific binding from non-specific interactions but lipophilicity (ClogP) between 2 and 4 to efficiently cross the BBB (Hsin et al., 2002). In earlier studies we had shown that binding affinity increased with the addition of a 2,4-dichlorophenyl group to the pyrazolo[1,5-α][1,3,5]triazine so our goal was to further improve the affinity as well as improve hydrophilicity to improve BBB penetration by further modification of this substructure (He et al., 2000).
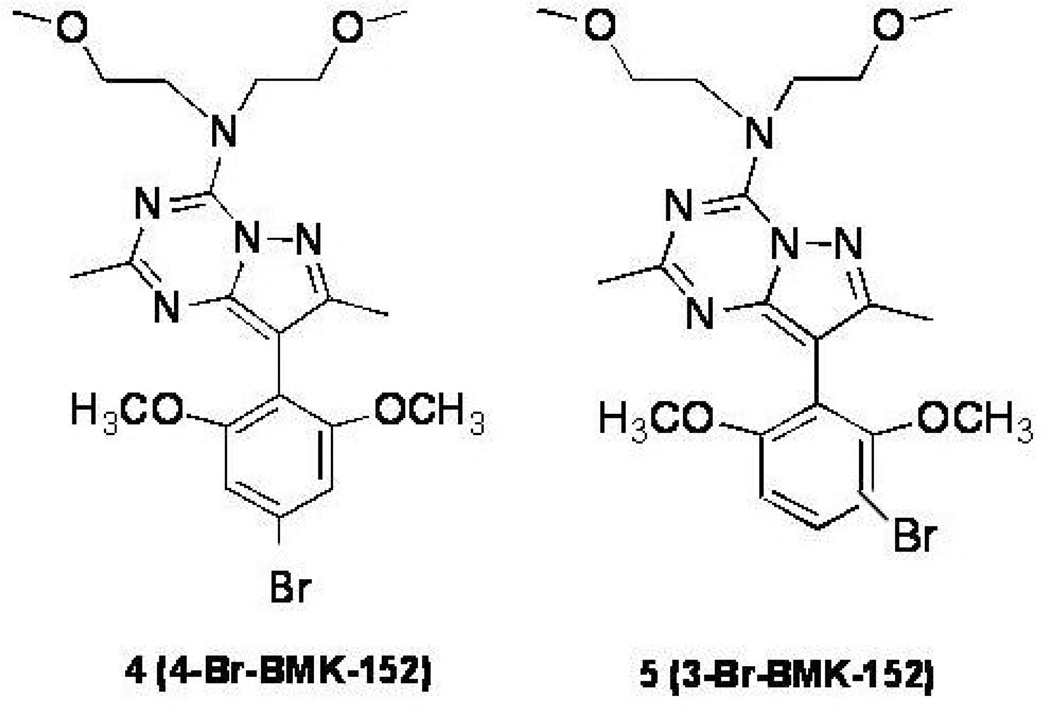
We synthesized 4-Br-BMK-152 (4, 4-BMK) in which the phenyl substructure was modified with the addition of methoxy groups at the 2 and 6 positions (Fig. 2., compound 4). The ClogP of (4) was improved to 2.6, relative to compound (3), and therefore indicative of sufficient hydrophilicity to penetrate the BBB. The Ki was found to range between 0.35 and 0.6 nM representing a 3 to 6 fold improvement in affinity for CRF1 receptors compared to MJL-1-109-2 (3). Since 4-BMK (4) had increased hydrophilicity and affinity for CRF1 receptors, this ligand was selected for further development as a PET imaging agent. Radiobromination of a 4-tributyltin precursor of 4-BMK (4), resulted in 2 brominated species, 4-[76Br]BMK-152 [(4-[76Br]BMK); Fig. 2., compound 4] and 3-[76Br]BMK-152 [(3-[76Br]BMK); Figure 2, compound 5]. Using the same 4-tributyltin precursor and manipulating the reaction conditions, we have previously shown that either 4-[76Br]BMK or 3-[76Br]BMK could be produced in high radiochemical yield (Lang et al., 2009). This manuscript will describe the characterization of 4-[76Br]BMK (4) and 3-[76Br]BMK (5) with respect to CRF1R binding.
Fig. 2.
Structures of compounds 4 (4-Br-BMK-152) and 5 (3-Br-BMK-152).
Materials and Methods
Chemistry and Radiochemistry
Synthesis of the tributyltin precursor used for the bromination procedure, 4-trimethyltin-BMK-152, the authentic standards (3-Br BMK-152 and 4-BrBMK 152) and the [76Br] labeled products,(4-[76Br]BMK-152 and 3-[76Br]BMK-152 have been described previously (Lang et al., 2009). Briefly, [76Br] was produced from the 75As (3He, 2n) 76Br reaction and isolated from the solid target by oxidation and distillation (Szajek et al., 2004). The radionuclide was obtained as aqueous ammonium [76Br]bromide (>5,000 Ci/mmol at EOB). The radiobrominations were carried out with a tin precursor of BMK-152 in which tin was replaced with [76Br]. The same 4-tributyl tin precursor was used for synthesis of both the 4-[76Br]BMK-152 and 3-[76Br]BMK-152 but under different conditions. The 4-[76Br]BMK-152 and 3-[76Br]BMK-152 were isolated by HPLC (Waters Q-TOF MS coupled with a Waters UPLC Acquity system) yielding a single radioactive peak.
In Vitro Autoradiography Studies
The brains from rats (Sprague-Dawley males) or rhesus monkeys (adult male) were rapidly frozen in dry ice and stored until use. The brains were sagitally (rat) or coronally (monkey) sectioned into 20 µm slices, and allowed to air dry before use. The slide-mounted sections (from 3 different rats/rhesus) were pre-incubated in buffer for 15 min, and then incubated for 2 hrs with 3-[76Br]BMK or 4-[76Br]BMK at increasing concentrations for saturation studies; non-specific binding was determined with 4-Br-BMK (10−5M) at each concentration. For competition studies, concentrations ranging from 10−6M to 10−11M of 3-Br-BMK, 4-Br-BMK or ovine corticotropin releasing hormone (o-CRH) were incubated with 0.125 nM [125I]o-CRF ([125I]Tyr0-o-CRF;Perkin Elmer) or 0.1 nM [125I]Sauvagine (Perkin Elmer). The slides were rinsed, air dried, and placed on phosphor-imaging plates with a pixel size of 25 µm (Fuji BAS-SR2025). Following exposure the plates were scanned with a Fuji BAS5000. The CRF1 receptor density was quantified by identifying ROIs expressed as photostimulated luminescence units per mm2 (PSL/mm2). Kds or Kis were determined from ROIs from at least 6 to 10 concentrations of radioligands or cold competitors and analyzed using non-linear regression curve fits including, one-site binding hyperbola for saturation studies and one-site binding competition for competition studies (Graphpad PRISM version 3.02 for Windows, Graphpad software, San Diego, CA, USA; www.graphpad.com).
Ex vivo Autoradiography Studies
Awake rats (Sprague-Dawley male adult) were injected IV with 4-[76Br]BMK (~0.2 mCi) via an indwelling carotid catheter; rats were free roaming during injection and the uptake period to minimize stress responses. Rats were euthanized after 1h uptake; the brains were removed, immediately frozen in dry ice and cut sagittally (to the midline) into 20 µm slices using a cytomicrotome (Bright Instrument Co. and Hacker Instrument Inc, B2589). The slices were placed on slides, air dried, and placed on a phosphorimaging plate with a pixel size of 25 µm (Fuji BAS-SR2025). After exposure the plates were scanned using a Fuji Bio-imaging Analysis System 5000. ROIs were determined and expressed as PSL/mm2.
Mouse Biodistribution Studies
Mice [PGP (P-glycoprotein) knockouts (FVB/TacfBR-[KO]mdr1a-[KO]mdr1b; Taconic Farms, Hudson, NY) and their wildtype littermates (FVB/NTac; Taconic Farms, Hudson, NY) were injected intravenously with ~ 20 uCi of a mixture of 4-[76Br]BMK (30%) and 3-[76Br]BMK (70%) and euthanized (CO2 inhalation) at the appropriate time. The brains were removed, placed in 0.3 M sucrose and dissected; the dissected regions were weighed and counted to determine the total activity. The total activity in each region was expressed as the Differential Uptake Ratio [DUR= (% Injected Dose/g × Body Weight)/100]. The remaining brain tissue was used to determine metabolites. In addition 2 blood samples were taken for: 1) DUR determination; 2) metabolite determination. For metabolite determination: 1) brain tissue was placed in equal volumes of acetonitrile; and homogenized; 2) serum was obtained from the blood samples and mixed with an equal volume of acetonitrile. All samples were centrifuged and the radioactive content of the supernatants and pellets were determined. The supernatants were applied to TLC plates which were developed, and placed on a phosphorimaging plate overnight. The plates were scanned for [76Br] using a Fuji BAS 5000.
Results
Radiochemistry
The 4-[76Br]BMK and 3-[76Br]BMK batches used in the in vitro and in vivo biological studies were obtained with high radiochemical purities of >85% and ~100%, respectively. The major component of radiochemical impurity for 4-[76Br]BMK is the corresponding 3-[76Br]BMK. The specific activities at end of synthesis were 1156 ± 492 (n=5) and 1389 ± 653 Ci/mmol (n=5) for 4-[76Br]BMK and 3- [76Br]BMK, respectively. For the studies with the Pgp knockout (KO) mice and their wildtype littermates (WT) the mass associated with the radioactive injected dose (~ 20 uCi) per mouse was 19.4 pmoles. For the rat studies, the mass associated with the radioactive injected dose (~ 160 uCi) was 184 pmoles.
In Vitro Autoradiography of 4-[76Br]BMK compared to 3-[76Br]BMK
In vitro competition studies were performed to compare the Ki of 4-Br-BMK to the Kis of 3-Br-BMK and o-CRH in the frontal cortex of monkey sections using the radioligand, [125I]o-CRH, a specific CRF1R ligand (De Souza et al., 1985; Rominger et al., 1998) (Table I). The 4-Br-BMK (Ki= 0.35 ± 0.05 nM) exhibited the highest affinity for the CRF1 receptor which was 70 fold and 4 fold greater in affinity compared to 3-Br-BMK and o-CRH, respectively. The Ki of oCRH (1.5 ± 0.7 nM), agrees with reported values of ~ 2 (De Souza and Kuhar, 1986a; Millan et al., 1986). Previous studies have shown o-CRH is comparable to human and rhesus CRF in that it binds with similar affinity to human (cloned) and rhesus CRF1R therefore these results indicate that 4-Br-BMK would compete with endogenous human CRF and bind with similar affinity to human CRF1R in vivo (De Souza and Kuhar, 1986b; Grigoriadis et al., 1995; Grigoriadis et al., 1996a; Primus et al., 1997).
Table I.
Inhibition constants (Ki) for CRF1 receptors determined from in vitro autoradiography competition studies with [125I] o-CRH (CRF1 selective ligand) using rhesus monkey frontal cortex or [125I] Sauvagine (CRF1 and CRF2 ligand) using rat brain. Each value represents the mean ± SD; n=3 (Rhesus or rat brains).
|
[125I]-oCRH |
[125I] Sauvagine |
||
|---|---|---|---|
| unlabeled competitor |
Rhesus Frontal Cortex Ki (nM) |
Rat Frontal Cortex Ki (nM) |
Rat choroid plexus Ki (nM) |
| 3-Br-BMK-152 | 24.40 ± 4.9 | 16.33 ± 3.30 | > 1000 |
| 4-Br-BMK-152 | 0.35 ± 0.05 | 0.15 ± 0.05 | > 1000 |
| o-CRH | 1.46 ± 0.67 | 3.13 ± 1.16 | > 1000 |
In addition, the Kis of the same compounds were determined in similar studies with rat brain sections using [125I] Sauvagine which recognizes both CRF1R and CRF2 R (Table I). The Kis were determined from ROIs in the rat frontal cortex and were comparable to the Kis using monkey frontal cortex. Again the 4-Br-BMK (Ki= 0.15 nM) exhibited the highest affinity which was 110 fold and 5 fold greater in affinity for CRF1 receptors than 3-Br-BMK and o-CRH, respectively. The Ki of o-CRH, 3 nM, using [125I]Sauvagine is lower than the Ki determined with [125I]o-CRH but comparable to previously reported values of ~ 6 in cloned human CRF1R (Grigoriadis et al., 1996a). The 4-Br-BMK, 3-Br-BMK, and o-CRH were found to be selective for CRF1R as all three failed to inhibit [125I] Sauvagine binding in the choroid plexus, a CRF2 R rich region, at 10−6M [Table I: (Lovenberg et al., 1995; Rominger et al., 1998)].
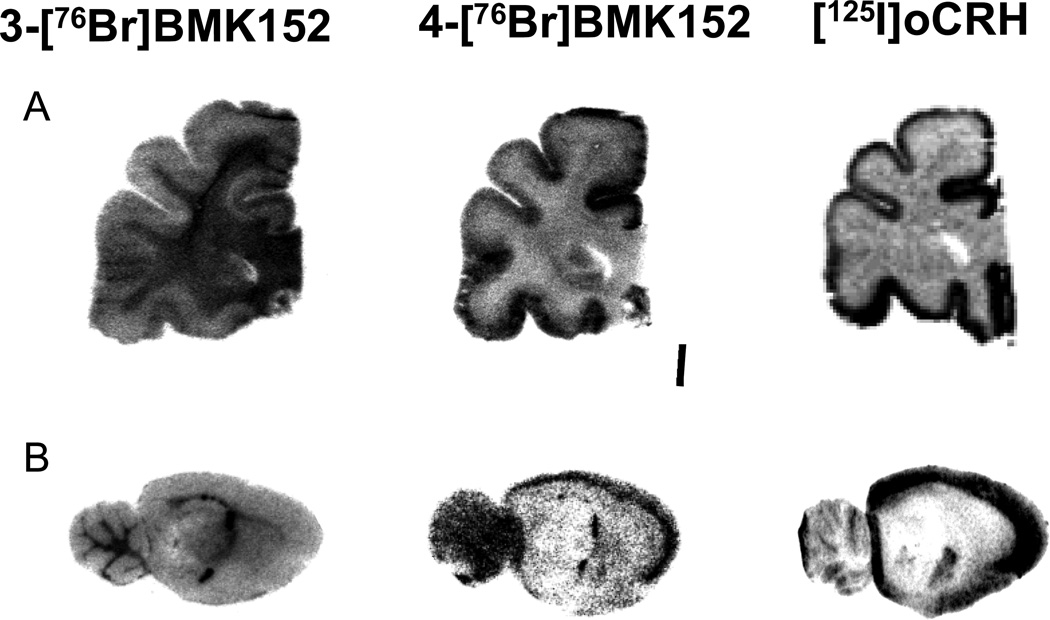
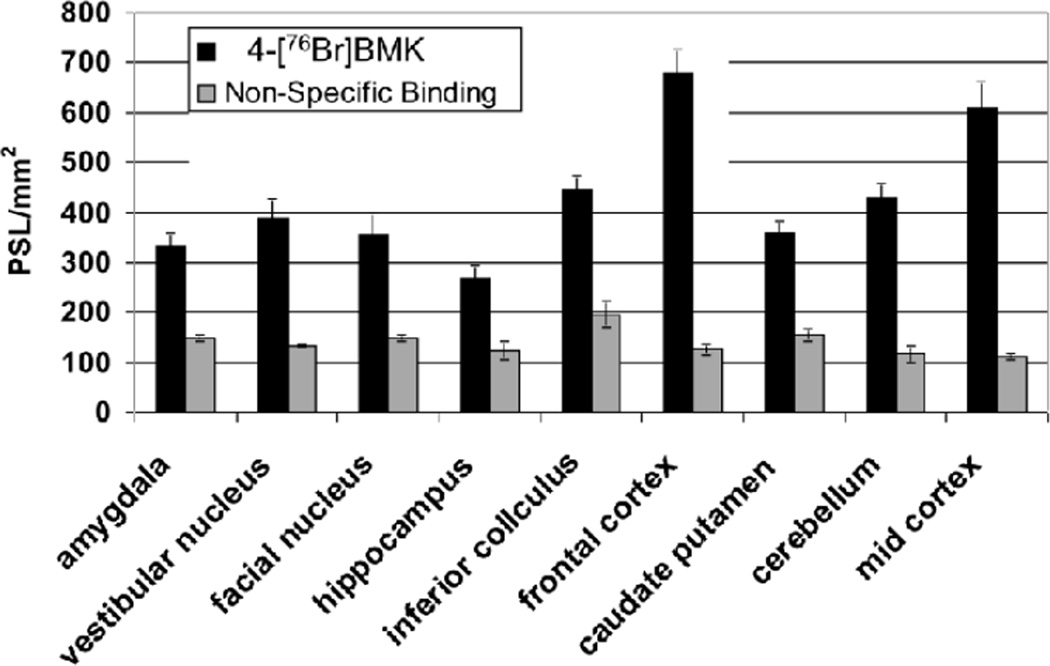
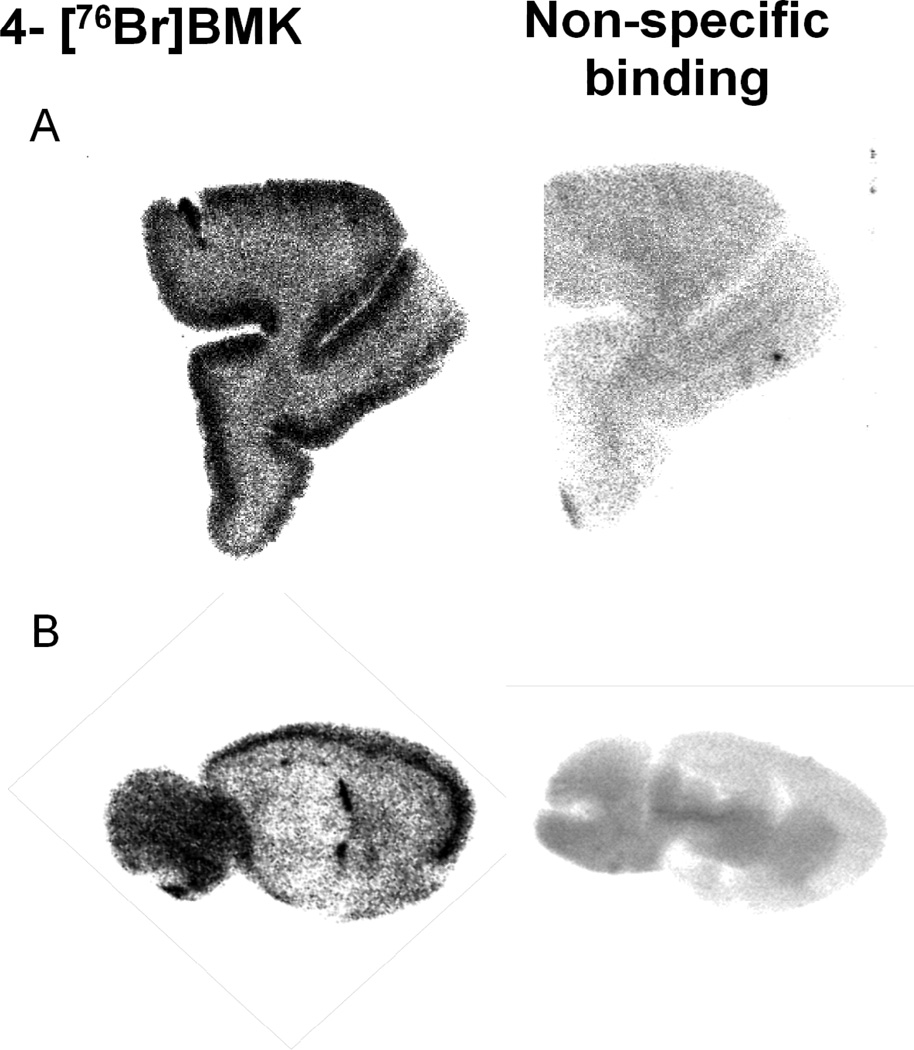
From in vitro autoradiography studies, comparison of the regional distribution of 4-[76Br]BMK to [125I] o-CRH from both rat and monkey brain sections appeared equivalent whereas for the 3-[76Br]BMK, a similar distribution was discernible in some of the cortical regions of the monkey brain sections but could not be visualized in comparable regions in the rat brain (Fig. 3.). In addition when cortical monkey ROIs were quantitated, the 3-[76Br]BMK specific binding was decreased 2 to 3 fold compared to the 4-[76Br]BMK or [125I]o-CRH in analogous ROIs. With this decrease in 3-[76Br]BMK specific binding the uptake in the white matter appears more prominent but as this region lacks CRF1R, this does not represent specific CRF1R binding (Fig. 3.). For the rat brain sections, further quantitative analysis was performed and the density of the 4-[76Br]BMK binding was determined from ROIs (PSL/mm2) for the pre-frontal cortex, mid cortex, inferior colliculus, amygdala, vestibular nucleus, facial nucleus, hippocampus, and caudate putamen; the non-specific binding was determined in the presence of 4-BMK (10−5M) and quantitated from similar ROIs in adjacent sections (Fig. 4.). This regional distribution was consistent with established densities for CRF1R with the highest densities of 4-[76Br]BMK in the rat frontal and mid cortex and also the highest specific binding of > 85% [when blocked with 4-BMK (10−5M: Fig. 4. and Fig. 5. B) or o-CRH (10−5M); data not shown] (De Souza and Kuhar, 1986a; Primus et al., 1997). With rhesus monkey tissue the highest densities of 4-[76Br]BMK binding were observed in the neocortical laminae of the prefrontal cortex with specific binding ~80% (Fig. 3. and Fig. 5. A) which compared favorably with published results in which high densities of CRF receptors were found in cortical layers I–V using [125I] Sauvagine and [125I]o-CRH. (Grigoriadis et al., 1995; Millan et al., 1986; Sanchez et al., 1999). Additionally the regional distribution of 4-[76Br]BMK in monkey cerebellum, hippocampus, and entorhinal cortex sections was characteristic of the known CRF1R regional distribution (data not shown).
Fig. 3.
Representative autoradiograms comparing 3-[76Br]BMK-152 and 4-[76Br]BMK-152 regional distribution to [125I]oCRH, a known CRF1R ligand in: A) rhesus monkey prefrontal cortex; B) rat brain (sagittal slice ~3.8 mm from midline).
Fig. 4.
Quantitative regional distribution of 4-[76Br]BMK in rat brain determined by in vitro autoradiography which corresponds to CRF1 receptor binding. The quanitative data was obtained by drawing ROI’s expressed as photostimulated luminescence units per mm2 (PSL/mm2). Non-specific binding determined using 4-BMK (10−5M) and quantitated in the same manner (Each bar represents the mean ± SD; n=3).
Fig. 5.
Regional Localization of 4-[76Br]BMK and non-specific binding (10−5M 4-BMK) determined from in vitro autoradiography: A) rhesus monkey prefrontal cortex; B) Rat brain (sagittal section ~3.6 mm from midline).
Saturation studies were performed with 4-[76Br]BMK using both rat brain and rhesus frontal cortex sections with non-specific binding determined using 4-BMK (10−5 M) (Fig. 5. A and B). In Fig. 5. B (representative of one concentration) the increased densities observed in rat prefrontal cortex and cerebellum were blocked ~70% with 4-Br-BMK; the Kd was 0.23 nM ± 0.07 (n=3) determined from ROIs drawn in the prefrontal cortex from at least 6 concentrations of 4-[76Br]BMK. In similar saturation studies with monkey tissue, 4-[76Br]BMK was found to have a comparable Kd [0.31 nM ± 0.08 (n=3)] determined using ROIs from the prefrontal cortex. In contrast in analogous studies using 3-[76Br]BMK, although specific binding was observed (ranging from 70% to 40%) saturation could not be achieved and therefore a Kd could not be determined (Fig. 3.). Further this is indicative of low affinity binding by 3-[76Br]BMK and in agreement with the 3-Br-BMK Ki which was 70 to 100 fold lower in affinity than the 4-Br-BMK. In similar saturation studies using [125I]o-CRH, the Kd (0.68 ± 0.22 nM; n=3) using monkey frontal cortex sections was 2 to 3 fold lower than the Kd for 4-[76Br]BMK and comparable to published findings (Grigoriadis et al., 1996b).
Rodent in vivo studies with 4-[76Br]BMK and 3-[76Br]BMK
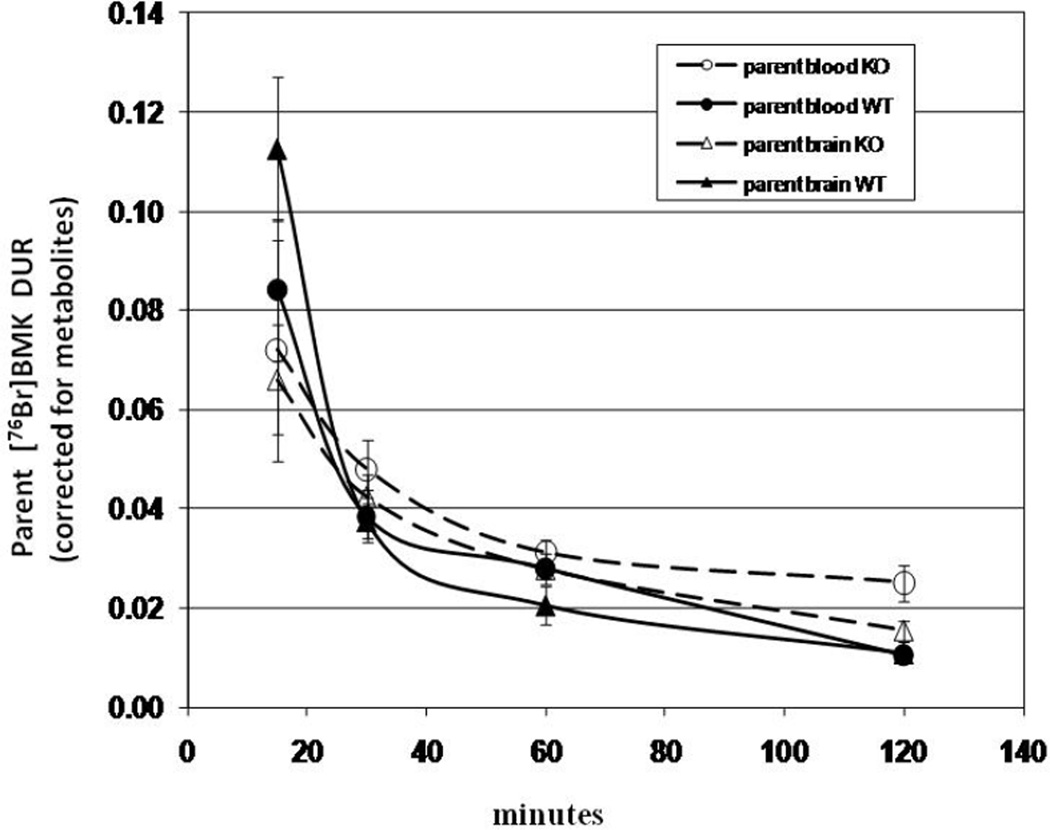
Although 4-[76Br]BMK and 3-[76Br]BMK were designed with an appropriate log P for BBB penetration, P-glycoprotein (Pgp) may also play a role in excluding these ligands from the brain (Elsinga et al., 2004). Studies with Pgp knockout (KO) mice and their wildtype littermates (WT) were undertaken to determine if [76Br]BMK [mixture of 4-[76Br]BMK(30%) and 3-[76Br]BMK (70%)] acts as a Pgp substrate. If [76Br]BMK is a substrate for Pgp, substantial increases in the brain uptake of [76Br]BMK would be expected in the KO mice compared to the WT. Brain uptakes of [76Br]BMK in WT mice decreased from 0.25 DUR to 0.04 DUR over the 15 to 120 min time course representing an 83% decrease in radioactivity; KO mice brain uptakes decreased from 0.15 to 0.07 DUR representing 53% decrease over the same time course. Brain uptakes in KO vs WT mice increased 43%, 65%, and 75% at 30, 60, and 120 min, respectively. To insure that the differences in [76Br]BMK brain uptakes observed between the KO and WT mice were not influenced by metabolism, additional blood and brain samples were taken from KO or WT mice at each time point to determine by TLC the fraction of the radioactivity which is parent [76Br]BMK (unmetabolized). The highest measured fraction of parent [76Br]BMK in the brain samples occurred at 15 min ranging from 44% to 54% in both KO and WT. After 120 min the fraction of radioactivity remaining in the blood and brain which was parent [76Br]BMK ranged from 3% to 9% and 21% to 25%, respectively for both KO and WT mice. In Fig. 6., the [76Br]BMK uptakes (DURs) in blood and brain of the WT and KO mice after correction for metabolites are compared; the brain uptakes of parent[76Br]BMK in the KO vs WT increased 13%, 35%, and 42% at 30, 60, and 120 min., respectively.
Fig. 6.
Uptake of parent 3- and 4-[76Br]BMK (corrected for metabolites) in brain and blood of PgP knockout mice and wildtypes from 15 min to 120 min. Mice were injected IV (via tail vein with mixture of 3- and 4-[76Br]BMK) awake under temporary restraint and then allowed to free roam for the appropriate uptake period. Each time point represents DUR ± SD of parent 3- and 4-[76Br]BMK [n= 3 (15 min groups only) or 4 (all other time points)].
Preliminary in vivo rat studies were undertaken to determine the brain uptake and metabolism of 4-[76Br]BMK after 1 hr in awake free roaming rats. Rats were injected via in dwelling catheters to minimize stress responses, which could affect uptake. Ex vivo autoradiograms of the brain revealed increased uptake in the frontal cortex (189% ± 24%, n=3) and cerebellum (176% ± 20%, n=3) compared to the choroid plexus (region with neglible CRF1R) which is consistent with the regional distribution of CRF1 receptors. TLC results of brain homogenates revealed that parent 4-[76Br]BMK comprised >70% of the total radioactivity, while in the blood, metabolism was more rapid with <35% of the remaining radioactivity parent 4-[76Br]BMK.
Discussion
From the in vitro autoradiography studies both 4-[76Br]BMK and 3-[76Br]BMK were found selective for CRF1 receptors, but only 4-[76Br]BMK displayed high sub-nanomolar affinity binding (Kd~ 0. 2 nM and 0.3nM, rat and monkey pre-frontal cortex, respectively). For these in vitro studies the use of autoradiography techniques allowed drawing discrete ROI’s in which the cortical layers of high specific binding of CRF1 receptors could be easily isolated whereas with cerebellum or cortical membrane preparations, these high density layers are diluted and therefore usually associated with high non-specific binding and would require further purifications.
The in vivo studies with rats and mice would indicate 4-[76Br]BMK is able to penetrate the BBB and in the rat brain at1hr post injection remains primarily unmetabolized 4-[76Br]BMK (> 70% at 1 hr). In the studies with the P-gp knockout (KO) mice and their wildtype littermates (WT), [76Br]BMK and parent[76Br]BMK brain uptakes in the KO group were increased, ranging from 40% to 75% and 13% to 42%, respectively, compared to the control group. This increase of less than 1.75 fold would indicate that [76Br]BMK is not a P-gp substrate as the increases would be expected to be greater. For example paclitaxel, a known P-gp substrate was found to be increased 640% in P-gp KO mice compared to WT mice (Kiesewetter et al., 2003). Passchier et al. showed that the brain uptakes of [18F]MPPF (a 5-HT1A antagonist) was increased from 2 to 3 fold in P-gp KO vs WT mice and that by inhibiting the P-gp pump with administration of cyclosporine A, the [18F]MPPF brain uptake could be enhanced by 5 to 10 fold (Passchier et al., 2000). In this case with such small increases in the brain uptakes (< 2 fold) of [76Br]BMK in P-gp KO vs WT mice, the addition of P-gp modulators with the radioligand would not be expected to increase brain uptakes in normal mice.
The ex vivo studies in rats revealed a regional distribution of 4-[76Br]BMK suggestive of CRF1R binding with almost a 2-fold increase observed in prefrontal cortex compared to a non-receptor region. For these studies the 4-[76Br]BMK concentration in the frontal cortex at 60 min was calculated to be 0.23 nM [184 pmoles (mass injected) × 0.127 %ID/g in frontal cortex; see radiochemistry section]. This could represent as much as 4% to 8 % receptor saturation assuming a 6 nM to 3 nM frontal cortex Bmax concentration [derived from the highest and lowest density values from autoradiography data of frontal parietal cortex regions (De Souza et al., 1985)]. Since the concentration of the 4-[76Br]BMK could exceed a 5% saturation level of CRF1 receptors (Bmax =3 nM), this could result in lowering target to non-target ratios (Jagoda et al., 2004). While this modest increase of 4-[76Br]BMK binding may actually represent a low density of receptors sensitive to mass effects, it may also represent only the fraction of unoccupied receptors with the remaining fraction occupied by CRF due to activation of acute stress responses of the rats during the study (Altholtz et al., 2006; Hauger et al., 2009). In reported PET imaging studies in baboons using [11C]R121920 and [11C]DMP696, high nM affinity CRF1 ligands, no regional differences in the brain were observed in the distribution of radioactivity which was attributed to low receptor densities (Sullivan et al., 2007). In these studies the monkeys were anesthetized with isoflurane during the imaging studies which would not necessarily prevent stress responses, potentially lowering unoccupied receptors (Altholtz et al., 2006; Hotchkiss et al., 1998; Preckel and Bolten, 2005; Uetsuki et al., 2005). In addition, visualization of a lower density of receptors may require a high specific activity preparation to avoid mass effects as well as increased imaging time to allow for non-target activity to clear which would not be possible with the short half-life of [11C]. Further studies in rodents would have several limitations including: 1) the inability to detect volumetrically small cortical layers or other brain regions containing high CRF1R densities using small animal imaging. The binding to small cortical layers is key to determining specific binding because they contain high CRF1 receptor densities, 2) there are technical complexities of controlling stressors in rodents. For example, the release of CRF under stress will compete with the radiotracer and decrease binding, 3) predicting human metabolism and blood-brain barrier penetration from rodent data is especially difficult in this case of relatively low CRF1R densities and potential competitive inhibition by endogenous ligands. 4) With the advent of the exploratory IND (xIND), human studies can be carried out early in the development timeline because of the reduction in costly toxicity data needed for approval. Pharmacokinetic and pharmacodynamic studies of 4-[76Br]BMK in humans will determine the clinical utility of this promising targeted radiotracer.
As 4-[76Br]BMK, is a structural analogue of antalarmin, we would expect it to behave as a small molecule non-peptide CRF1R antagonist and compete for binding with endogenous CRF. Antalarmin has been shown to reduce anxiety-like behaviors and hypthalamic-pituitary-adrenal (HPA) stress responses by binding to CRF1R and preventing subsequent activation of the associated signaling pathways (Deak et al., 1999; Ducottet et al., 2003; Jutkiewicz et al., 2005; Valdez, 2009; Webster et al., 1996; Wong et al., 1999; Zorrilla et al., 2002). Further studies have found that the antagonistic or allosteric actions of non-peptide antagonists like antalarmin are dependent on the G-protein which is coupled to the CRF1R. In particular, antalarmin inhibits the Gs pathway in a competitive manner while inhibition of the Gi pathway is non-competitive (Berger et al., 2006; Hoare et al., 2004; Zorrilla and Koob, 2010). Non-peptide antagonist ligands like antalarmin bind almost exclusively at a single transmembrane site, the juxtamembrane domain (J-domain), while peptide ligands bind first at an extracellular N-domain of the CRF1R facilitating binding to the J-domain which is essential for G-protein coupling and receptor activation (Beyermann et al., 2007; Hoare, 2005). In this case antalarmin inhibits competitively or allosterically peptide binding at the J-domain, either strongly when the receptor is G-protein coupled or weakly in the uncoupled state (Hoare et al., 2003a; Hoare et al., 2003b).
With this in mind we would expect 4-[76Br]BMK to measure in vivo unoccupied receptors in either G-protein coupled or uncoupled states while competing for binding with endogenous CRF would be dependent on the conformational state of the CRF1R. We would expect 4-[76Br]BMK to bind at the J-domain and interact competitively with both peptide and non-peptide ligands at this domain, while the interaction at the N-domain with peptide ligands would be allosteric and dependent on the conformational state of the CRF1R (Grigoriadis et al., 2009; Hoare et al., 2004).
Since CRF1R non-peptide antagonists have therapeutic potential and are in clinical development, 4-[76Br]BMK would be useful in the drug development process and, with stable Br, possibly as a pharmaceutical as well. PET studies with 4-[76Br]BMK would be expected to detect dose dependent changes in CRF1R occupancy following treatment with non-peptide antagonists and thereby assist in establishing efficacious doses in humans although correlation to behavioral responses maybe more complicated since the regional location of the CRF1R and its associated G-proteins also play a role in regulating these responses. In conclusion, the high affinity, selectivity for CRF1 receptors, in vivo brain uptake, and longer half-life of 4-[76Br]BMK would warrant its further development for imaging CRF1 receptors, although for monkey and rat studies additional measures should be considered to control stressors during the studies.
References
- Altholtz LY, Fowler KA, Badura LL, Kovacs MS. Comparison of the stress response in rats to repeated isoflurane or CO2:O2 anesthesia used for restraint during serial blood collection via the jugular vein. J Am Assoc Lab Anim Sci. 2006;45(3):17–22. [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Berger H, Heinrich N, Wietfeld D, Bienert M, Beyermann M. Evidence that corticotropin-releasing factor receptor type 1 couples to Gs- and Gi-proteins through different conformations of its J-domain. Br J Pharmacol. 2006;149(7):942–947. doi: 10.1038/sj.bjp.0706926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyermann M, Heinrich N, Fechner K, Furkert J, Zhang W, Kraetke O, Bienert M, Berger H. Achieving signalling selectivity of ligands for the corticotropin-releasing factor type 1 receptor by modifying the agonist's signalling domain. Br J Pharmacol. 2007;151(6):851–859. doi: 10.1038/sj.bjp.0707293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci. 1985;5(12):3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB, Kuhar MJ. Corticotropin-releasing factor receptors in the pituitary gland and central nervous system: methods and overview. Methods Enzymol. 1986a;124:560–590. doi: 10.1016/0076-6879(86)24040-9. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Kuhar MJ. Corticotropin-releasing factor receptors: autoradiographic identification. Res Publ Assoc Res Nerv Ment Dis. 1986b;64:179–198. [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, Licinio J, Wong ML, Chrousos GP, Webster E, Gold PW. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140(1):79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):625–631. doi: 10.1016/S0278-5846(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Elsinga PH, Hendrikse NH, Bart J, Vaalburg W, van Waarde A. PET Studies on P-glycoprotein function in the blood-brain barrier: how it affects uptake and binding of drugs within the CNS. Curr Pharm Des. 2004;10(13):1493–1503. doi: 10.2174/1381612043384736. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, Dent GW, Turner JG, Uno H, Shelton SE, De Souza EB, Kalin NH. Corticotropin-releasing factor (CRF) receptors in infant rhesus monkey brain and pituitary gland: biochemical characterization and autoradiographic localization. Dev Neurosci. 1995;17(5–6):357–367. doi: 10.1159/000111306. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, Hoare SR, Lechner SM, Slee DH, Williams JA. Drugability of extracellular targets: discovery of small molecule drugs targeting allosteric, functional, and subunit-selective sites on GPCRs and ion channels. Neuropsychopharmacology. 2009;34(1):106–125. doi: 10.1038/npp.2008.149. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, Liu XJ, Vaughn J, Palmer SF, True CD, Vale WW, Ling N, De Souza EB. 125I-Tyro-sauvagine: a novel high affinity radioligand for the pharmacological and biochemical study of human corticotropin-releasing factor 2 alpha receptors. Mol Pharmacol. 1996a;50(3):679–686. [PubMed] [Google Scholar]
- Grigoriadis DE, Lovenberg TW, Chalmers DT, Liaw C, De Souze EB. Characterization of corticotropin-releasing factor receptor subtypes. Ann N Y Acad Sci. 1996b;780:60–80. doi: 10.1111/j.1749-6632.1996.tb15112.x. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, Struble RG, Price DL, De Souza EB. Normal pattern of labeling of cerebral cortical corticotropin-releasing factor (CRF) receptors in Alzheimer's disease: evidence from chemical cross-linking studies. Neuropharmacology. 1989;28(7):761–764. doi: 10.1016/0028-3908(89)90164-0. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS & neurological disorders drug targets. 2006;5(4):453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Gilligan PJ, Zaczek R, Fitzgerald LW, McElroy J, Shen HS, Saye JA, Kalin NH, Shelton S, Christ D, Trainor G, Hartig P. 4-(1,3-Dimethoxyprop-2-ylamino)-2,7-dimethyl-8-(2, 4-dichlorophenyl)pyrazolo[1,5-a]-1,3,5-triazine: a potent, orally bioavailable CRF(1) receptor antagonist. J Med Chem. 2000;43(3):449–456. doi: 10.1021/jm9904351. [DOI] [PubMed] [Google Scholar]
- Hoare SR. Mechanisms of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug discovery today. 2005;10(6):417–427. doi: 10.1016/S1359-6446(05)03370-2. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Sullivan SK, Ling N, Crowe PD, Grigoriadis DE. Mechanism of corticotropin-releasing factor type I receptor regulation by nonpeptide antagonists. Mol Pharmacol. 2003a;63(3):751–765. doi: 10.1124/mol.63.3.751. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Sullivan SK, Pahuja A, Ling N, Crowe PD, Grigoriadis DE. Conformational states of the corticotropin releasing factor 1 (CRF1) receptor: detection, and pharmacological evaluation by peptide ligands. Peptides. 2003b;24(12):1881–1897. doi: 10.1016/j.peptides.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Sullivan SK, Schwarz DA, Ling N, Vale WW, Crowe PD, Grigoriadis DE. Ligand affinity for amino-terminal and juxtamembrane domains of the corticotropin releasing factor type I receptor: regulation by G-protein and nonpeptide antagonists. Biochemistry. 2004;43(13):3996–4011. doi: 10.1021/bi036110a. [DOI] [PubMed] [Google Scholar]
- Hotchkiss CE, Brommage R, Du M, Jerome CP. The anesthetic isoflurane decreases ionized calcium and increases parathyroid hormone and osteocalcin in cynomolgus monkeys. Bone. 1998;23(5):479–484. doi: 10.1016/s8756-3282(98)00124-0. [DOI] [PubMed] [Google Scholar]
- Hsin LW, Tian X, Webster EL, Coop A, Caldwell TM, Jacobson AE, Chrousos GP, Gold PW, Habib KE, Ayala A, Eckelman WC, Contoreggi C, Rice KC. CRHR1 Receptor binding and lipophilicity of pyrrolopyrimidines, potential nonpeptide corticotropin-releasing hormone type 1 receptor antagonists. Bioorganic & medicinal chemistry. 2002;10(1):175–183. doi: 10.1016/s0968-0896(01)00261-9. [DOI] [PubMed] [Google Scholar]
- Jagoda E, Contoreggi C, Lee MJ, Kao CH, Szajek LP, Listwak S, Gold P, Chrousos G, Greiner E, Kim BM, Jacobson AE, Rice KC, Eckelman W. Autoradiographic visualization of corticotropin releasing hormone type 1 receptors with a nonpeptide ligand: synthesis of [(76)Br]MJL-1-109-2. J Med Chem. 2003;46(17):3559–3562. doi: 10.1021/jm034077k. [DOI] [PubMed] [Google Scholar]
- Jagoda EM, Vaquero JJ, Seidel J, Green MV, Eckelman WC. Experiment assessment of mass effects in the rat: implications for small animal PET imaging. Nuclear medicine and biology. 2004;31(6):771–779. doi: 10.1016/j.nucmedbio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Wood SK, Houshyar H, Hsin LW, Rice KC, Woods JH. The effects of CRF antagonists, antalarmin, CP154,526, LWH234, and R121919, in the forced swim test and on swim-induced increases in adrenocorticotropin in rats. Psychopharmacology (Berl) 2005;180(2):215–223. doi: 10.1007/s00213-005-2164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesewetter DO, Jagoda EM, Kao CH, Ma Y, Ravasi L, Shimoji K, Szajek LP, Eckelman WC. Fluoro-, bromo-, and iodopaclitaxel derivatives: synthesis and biological evaluation. Nuclear medicine and biology. 2003;30(1):11–24. doi: 10.1016/s0969-8051(02)00351-7. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles SA, Vogt SK, Muglia LJ. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci. 2010;30(7):2571–2581. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang L, Ma Y, Kim BM, Jagoda EM, Rice KC, Szajek LP, Contoreggi C, Gold PW, Chrousos GP, Eckelman WC, Kiesewetter DO. [Br-76]BMK-I-152, a non-peptide analogue for PET imaging of corticotropin-releasing hormone type 1 receptor (CRHR1) J Labelled Compd Rad. 2009;52(9–10):394–400. [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92(3):836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MA, Jacobowitz DM, Hauger RL, Catt KJ, Aguilera G. Distribution of corticotropin-releasing factor receptors in primate brain. Proc Natl Acad Sci U S A. 1986;83(6):1921–1925. doi: 10.1073/pnas.83.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43(4):425–473. [PubMed] [Google Scholar]
- Passchier J, van Waarde A, Doze P, Elsinga PH, Vaalburg W. Influence of P-glycoprotein on brain uptake of [18F]MPPF in rats. Eur J Pharmacol. 2000;407(3):273–280. doi: 10.1016/s0014-2999(00)00752-4. [DOI] [PubMed] [Google Scholar]
- Preckel B, Bolten J. Pharmacology of modern volatile anaesthetics. Best Pract Res Clin Anaesthesiol. 2005;19(3):331–348. doi: 10.1016/j.bpa.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology. 1997;17(5):308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Refojo D, Holsboer F. CRH signaling. Molecular specificity for drug targeting in the CNS. Ann N Y Acad Sci. 2009;1179:106–119. doi: 10.1111/j.1749-6632.2009.04983.x. [DOI] [PubMed] [Google Scholar]
- Rominger DH, Rominger CM, Fitzgerald LW, Grzanna R, Largent BL, Zaczek R. Characterization of [125I]sauvagine binding to CRH2 receptors: membrane homogenate and autoradiographic studies. J Pharmacol Exp Ther. 1998;286(1):459–468. [PubMed] [Google Scholar]
- Ruggiero DA, Underwood MD, Rice PM, Mann JJ, Arango V. Corticotropic-releasing hormone and serotonin interact in the human brainstem: behavioral implications. Neuroscience. 1999;91(4):1343–1354. doi: 10.1016/s0306-4522(98)00703-9. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408(3):365–377. [PubMed] [Google Scholar]
- Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, Kash T, Lack AK, Messing RO, Siggins GR, Winder D, Roberto M, McCool BA, Weiner JL. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol (Fayetteville, NY. 2009;43(7):509–519. doi: 10.1016/j.alcohol.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Parsey RV, Kumar JS, Arango V, Kassir SA, Huang YY, Simpson NR, Van Heertum RL, Mann JJ. PET Imaging of CRF1 with [11C]R121920 and [11C]DMP696: is the target of sufficient density? Nuclear medicine and biology. 2007;34(4):353–361. doi: 10.1016/j.nucmedbio.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajek LP, Kao CH, Kiesewetter DO, Sassaman MB, Lang L, Plascjak P, Eckelman WC. Radiochimica Acta. 2004;92:291–295. [Google Scholar]
- Uetsuki N, Segawa H, Mayahara T, Fukuda K. The role of CRF1 receptors for sympathetic nervous response to laparotomy in anesthetized rats. Brain Res. 2005;1044(1):107–115. doi: 10.1016/j.brainres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Valdez GR. CRF receptors as a potential target in the development of novel pharmacotherapies for depression. Curr Pharm Des. 2009;15(14):1587–1594. doi: 10.2174/138161209788168083. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996;137(12):5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- Wong ML, Webster EL, Spokes H, Phu P, Ehrhart-Bornstein M, Bornstein S, Park CS, Rice KC, Chrousos GP, Licinio J, Gold PW. Chronic administration of the non-peptide CRH type 1 receptor antagonist antalarmin does not blunt hypothalamic-pituitary-adrenal axis responses to acute immobilization stress. Life Sci. 1999;65(4):PL53–PL58. doi: 10.1016/s0024-3205(99)00268-4. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug discovery today. 2010 doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952(2):188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]