Abstract
The 14-membered macrolide 6-deoxyerythronolide B is prepared in 14 steps (longest linear sequence) and 20 total steps. Two different methods for alcohol CH-crotylation via transfer hydrogenation are deployed for the first time in target-oriented synthesis. Enyne metathesis is used to form the 14-membered ring. The present approach represents the most concise construction of any erythronolide reported, to date.
In 1952, the pharmaceutical company Eli Lily commercialized the first macrolide antibiotic, erythromycin A.1 Beyond its impact on human medicine, the challenges in chemical synthesis posed by erythromycin A and related polyketides propelled advances in acyclic stereocontrol via carbonyl addition, especially aldol bond constructions2d and crotylation methods.3 Perhaps fueled further by Woodward’s dim assessment of the prospect of accessing erythromycin A through chemical synthesis,4 the erythromycins have become inextricably tied to the evolution of synthetic organic chemistry and their total syntheses are widely regarded as benchmarks for the state-of-the-art.9f As illustrated in total syntheses of erythromycin A5 and B6, erythronolide A7 and B8, (9S)-dihydroerythronolide A9 and their biogenic precursor 6-deoxyerythronolide B,10 tremendous strides have been made over the past 30 years. However, all reported syntheses remain well over 20 steps in length, suggesting the influence of the erythromycins on chemical synthesis will persist into the future (Figure 1).
Figure 1.
Erythromycin A and B, erythronolide A and B and 6-deoxyerythronolide A and B and prior total syntheses.a
aFor graphical summaries of prior total syntheses, see Supporting Information. For total syntheses of other erythromycin family members and their seco-acids, see the review literature.2 LLS = Longest Linear Sequence; TS = Total Steps.
In the course of exploring C-C bond forming hydrogenations and transfer hydrogenations beyond hydroformylation,11 our laboratory developed a suite of methods for stereoselective polyketide construction, including methods for carbonyl crotylation via redox triggered C-C coupling of primary alcohols and α-methyl allyl acetate 5 or butadiene using iridium12 and ruthenium13 catalysts, respectively. These studies evoked an exceptionally powerful transformation that has no counterpart in conventional allylmetal chemistry:3 the anti-diastereo- and enantioselective iridium catalyzed double crotylation of 2-methyl-1,3-propanediol 6 to form polypropionate stereoquintets.12c To benchmark the utility of this method vis-à-vis polyketide construction, it was applied to the preparation of 6-deoxyerythronolide B. This undertaking has resulted in the most concise route to any erythronolide reported, to date.2,5–10
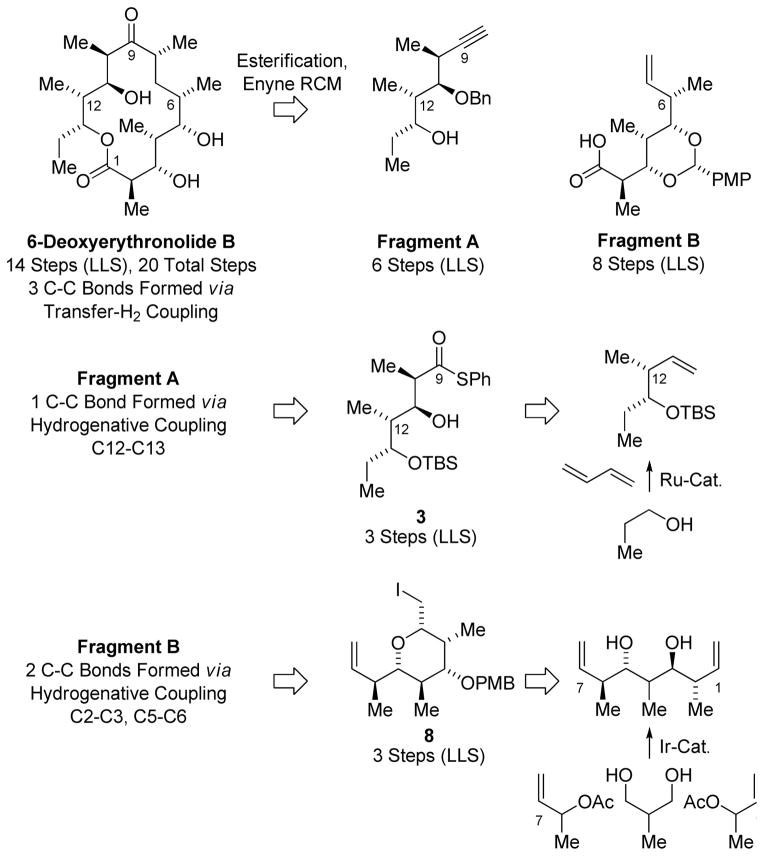
Retrosynthetically, a convergent assembly of 6-deoxyerythronolide B from Fragments A and B was envisioned through esterification followed by ring-closing enyne metathesis to form the 14-membered macrolide.14 Fragment A is prepared in 6 steps from n-propanol 1 through successive introduction of propionate subunits via ruthenium catalyzed butadiene mediated syn-crotylation13d followed by substrate directed syn-aldol addition15 to form thiol ester 3, which incorporates the 4 contiguous stereogenic centers spanning C10-C13. Fragment B, which incorporates the 5-contiguous stereogenic centers spanning C2-C6, is prepared in 8 steps from 2-methyl-1,3-propane diol 6 via iridium catalyzed double crotylation followed by iodoetherification and alkene oxidative cleavage to form the carboxylic acid (Scheme 1).12c
Scheme 1.
Retrosynthetic analysis of deoxyerythronolide B highlighting C-C bonds formed via hydrogenative coupling.
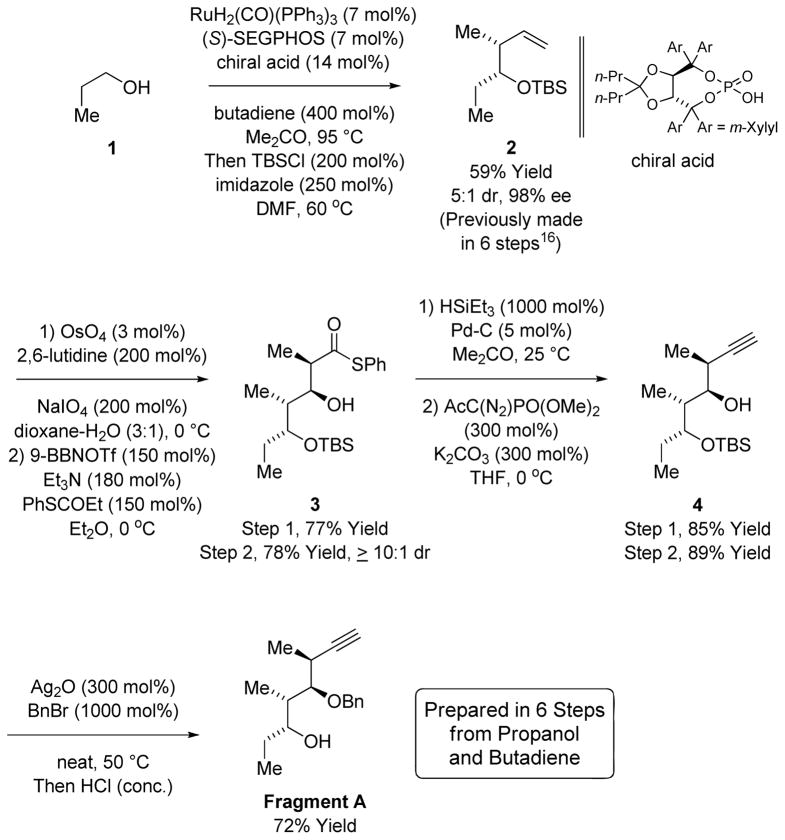
The synthesis of Fragment A begins with the butadiene mediated hydrohydroxyalkylation of n-propanol 1 to form the product of syn-crotylation (Scheme 2).13d As the resulting secondary alcohol is quite volatile, reagents promoting formation of the TBS ether 2 were added to the reaction mixture after the C-C coupling was complete, enabling direct acquisition of TBS ether 2 from n-propanol in 59% isolated yield with 5:1 syn-diastereoselectivity and 98% enantiomeric excess.13d,16 Oxidative cleavage of the terminal olefin followed by treatment of the resulting aldehyde with the (E)-boron enolate derived from S-phenyl propanethioate delivered the product of syn-aldol addition 3 with only trace quantities of the anti-diastereomer detected by 1H NMR analysis.15 The thiol ester 3 was converted to the β-hydroxy aldehyde,17 which was exposed to the Ohira-Bestmann reagent to form the homopropargyl alcohol 4 without protection of the hydroxyl moiety.18b Finally, benzylation of homopropargyl alcohol 4 accompanied by acidic hydrolysis of the TBS ether in the course of isolation provides Fragment A. An even more concise route to Fragment A potentially involves 1,3-enyne hydrohydroxyalkylation to form the C10-C11 bond with concomitant installation of the alkyne, however, this chemistry has not yet been adapted to the use of chiral β-stereogenic alcohols (Scheme 2).19
Scheme 2.
Synthesis of Fragment A via ruthenium catalyzed syn-crotylation of n-propanol 1.a
aIndicated yields are of material isolated by silica gel chromatography. See supporting information for experimental details.
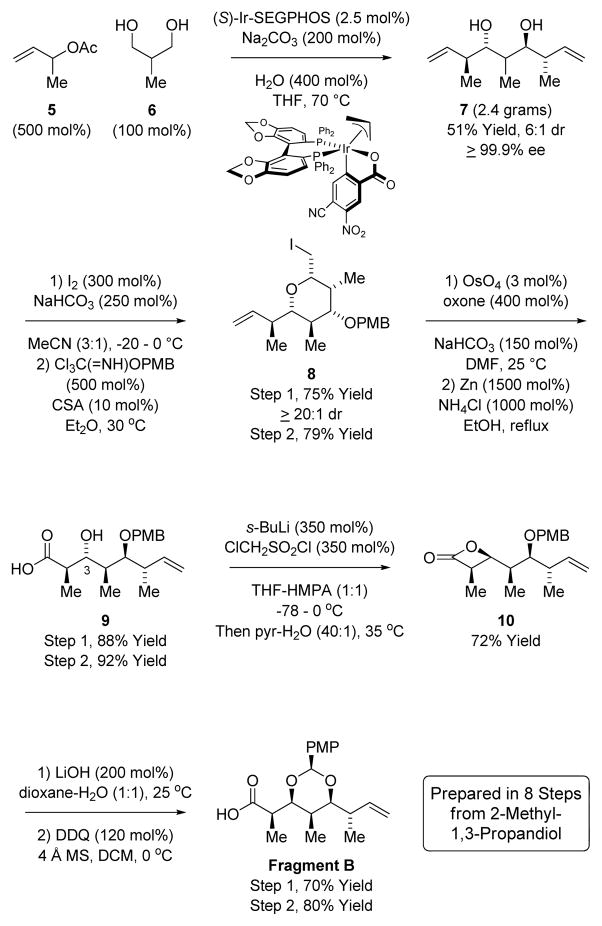
The synthesis of Fragment B begins with the anti-diastereo- and enantioselective iridium catalyzed double crotylation of 2-methyl-1,3-propanediol 6 to furnish the pseudo-C2-symmetric diol 7 (Scheme 3).12c The diol 7 is produced as a single enantiomer as determined by HPLC, as the minor enantiomer of the mono-adduct is converted to the pseudo-meso-diastereomer.20 Iodoetherification of 7, which differentiates the alkene termini and diol moieties and defines the nonstereogenic chirotopic center at C4, followed by benzylation delivers pyran 8. Osmium catalyzed oxidative cleavage of the olefin to form the carboxylic acid21 followed by zinc mediated reductive cleavage of the iodoether provides the β-hydroxy acid 9, which is prone to epimerization. To convert the β-hydroxy acid 9 to Fragment B, an inversion in stereochemistry at C3 is required. To this end, conversion of 9 to the β-lactone 10 was attempted under Mitsunobu conditions, however, decarboxylative Grob type elimination to the cis-alkene was formed in over 70% yield.22 Treatment of the dianion of 9 with methanesulfonyl chloride23 delivered the β-lactone 10 in 15–20% yield along with recovered β-hydroxy acid 9, suggesting a more electrophilic sulfonylchloride was required. Indeed, use of chloromethanesulfonyl chloride led to the formation of β-lactone 10 in 72% yield. It was our hope to directly exploit β-lactone 10 in the acylation of Fragment A. However, although related β-lactone ring openings are known,24a as we learned, cis-disubstituted β-lactones are recalcitrant acylating agents,24b and so β-lactone 10 was converted to the carboxylic acid Fragment B (Scheme 3).
Scheme 3.
Synthesis of Fragment B via iridium catalyzed double anti-crotylation of 2-methyl-1,3-propanediol 6.a
aIndicated yields are of material isolated by silica gel chromatography. See supporting information for experimental details.
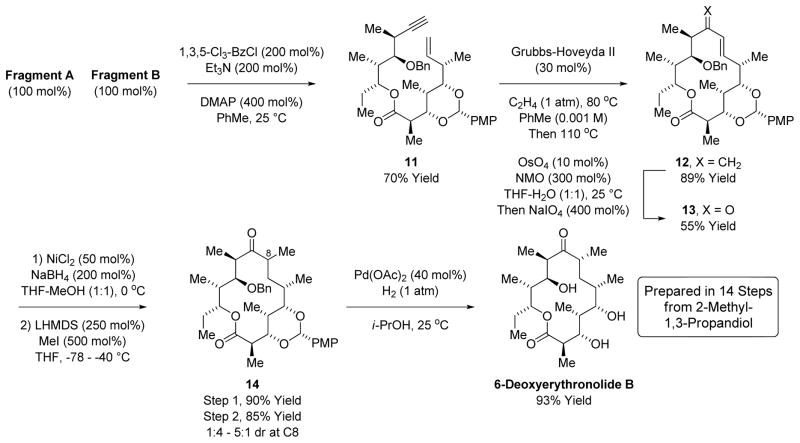
The convergent assembly of Fragments A and B is achieved through esterification under Yamaguchi’s conditions to form the tethered enyne 11 (Scheme 4).25 Initial attempts at ring-closing enyne metathesis14 in the absence of ethylene led to isomerization of the terminal olefin. Under an atmosphere of ethylene at 80 °C the terminal alkyne is converted to the conjugated diene in nearly quantitative yield, but macrocyclization is not observed. Hence, upon complete conversion to the conjugated diene at 80 °C, the reaction vessel was purged with nitrogen and the reaction temperature was increased to 110 °C, which induced formation of the 14-membered macrolide 12 as a single regioisomer in a remarkable 89% yield. Osmium catalyzed oxidative cleavage of the C9 methylidene residue provides the conjugated enone 13.21 Reductive methylation of enone 13 to form ketone 14 under the conditions of dissolving metal reduction26a,b or through the agency of arene anion radicals26c,d was explored. Although efficient reductive alkylation was achieved in a model system (4,4-dimethylcyclohexenone), enone 13 underwent benzyl cleavage or decomposed upon exposure to dissolving metal conditions, and upon treatment with arene anion radicals enone 13 was converted to the product of enone 1,2-reduction. Consequently, the conversion of enone 13 to ketone 14 was accomplished by nickel catalyzed conjugate reduction27 followed by conventional enolate methylation. Interestingly, highly variable levels of diastereoselectivity were associated with the newly formed C8-stereocenter of ketone 14, suggesting facile epimerization at this position. Indeed, irrespective of the diastereomeric ratio at C8, exposure of ketone 14 to the slightly acidic conditions of palladium catalyzed homogenous hydrogen provides 6- deoxyerythronlide B in 93% isolated yield as a single diastereomer. Thus, 6-deoxyerythronlide B is prepared in 14 steps (longest linear sequence) and 20 total steps, representing the most concise route to any erythromycin family member reported, to date.
Scheme 4.
Union of Fragment A and Fragment B and total synthesis of 6-deoxyerythronolide B.a
aIndicated yields are of material isolated by silica gel chromatography. See supporting information for experimental details.
New reactivity is the principal basis for new functional group interconversions and, hence, new strategies that can shift the retrosynthetic paradigm, ultimately simplifying longstanding challenges in chemical synthesis. As illustrated in the present total synthesis of 6-deoxyerythronolide B and recent total syntheses of the macrolides roxaticin28a and bry-ostatin 7,28b alcohol C-H functionalization via transfer hydrogenation augments synthetic efficiency by opening novel routes to polyketide natural products that bypass stoichiometric use of chiral auxiliaries, premetallated C-nucleophiles and discrete alcohol-to-aldehyde redox reactions. As organic molecules are defined as compounds composed of carbon and hydrogen, the reactivity embodied by such processes where C-C bond cleavage is accompanied by hydrogen redistribution evokes numerous possibilities in terms of related transformations, including imine addition from the amine oxidation and the direct C-C coupling of alcohols to α-olefins. These and other topics are currently under investigation in our laboratory.
Supplementary Material
Acknowledgments
The Robert A. Welch Foundation (F-0038) and the NIH-NIGMS (RO1-GM093905) are acknowledged for partial support of this research.
Footnotes
Supporting Information Available: Experimental procedures and spectral data. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Isolation: McGuire JM, Bunch RL, Anderson RC, Boaz HE, Flynn EH, Powell HM, Smith JW. Antibiot Chemother. 1952;2:281.
- 2.For selected reviews on the synthesis of erythromycin family natural products, see: Masamune S, Bates GS, Corcoran JW. Angew Chem Int Ed. 1977;16:585. doi: 10.1002/anie.197705851.Paterson I, Mansuri MM. Tetrahedron. 1985;41:3569.Mulzer J. Angew Chem Int Ed. 1991;30:1452.Schetter B, Mahrwald R. Angew Chem Int Ed. 2006;45:7506. doi: 10.1002/anie.200602780.Pal S. Tetrahedron. 2006;62:3171.Ward D. Chem Comm. 2011;47:11375. doi: 10.1039/c1cc13323c.
- 3.For selected reviews on enantioselective carbonyl allylation and crotylation, see: Yamamoto Y, Asao N. Chem Rev. 1993;93:2207.Ramachandran PV. Aldrichim Acta. 2002;35:23.Kennedy JWJ, Hall DG. Angew Chem Int Ed. 2003;42:4732. doi: 10.1002/anie.200301632.Denmark SE, Fu J. Chem Rev. 2003;103:2763. doi: 10.1021/cr020050h.Yu CM, Youn J, Jung HK. Bull Korean Chem Soc. 2006;27:463.Marek I, Sklute G. Chem Commun. 2007:1683. doi: 10.1039/b615042j.Hall DG. Synlett. 2007:1644.Li J, Menche D. Synthesis. 2009:2293.Leighton JL. Aldrichim Acta. 2010;43:3.Yus M, Gonzá-lez-Gómez JC, Foubelo F. Chem Rev. 2011;111:7774. doi: 10.1021/cr1004474.
- 4.Woodward RB. In: Perspectives in Organic Chemistry. Todd A, editor. Wiley-Interscience; New York: 1956. p. 160. “Erythromycin, with all of our advantages, looks at present time quite hopelessly complex, particularly in view of its plethora of asymmetric centers”. [Google Scholar]
- 5.Erythromycin A: Woodward RB, Logusch E, Nambiar KP, Sakan K, Ward DE, Au-Yeung B-W, Balaram P, Browne LJ, Card PJ, Chen CH, Chênevert RB, Fliri A, Froble K, Gais HJ, Garratt DG, Hayakawa K, Heggie W, Hesson DP, Hoppe D, Hoppe I, Hyatt JA, Ikeda D, Jacobi PA, Kim KS, Kobuke Y, Kojima K, Krowicki K, Lee VJ, Leutert T, Malchenko S, Martens J, Matthews RS, Ong BS, Press JB, Rajan Babu TV, Rousseau G, Sauter HM, Suzuki M, Tatsuta K, Tolbert LM, Truesdales EA, Uchida I, Ueda Y, Uyehara T, Vasella AT, Vladuchick WC, Wade PA, Williams RM, Wong HN-C. J Am Chem Soc. 1981;103:3215.and references cited therein Formal Syntheses: Bernet B, Bishop PM, Caron M, Kawamata T, Roy BL, Ruest L, Sauvé G, Soucy P, Deslongchamps P. Can J Chem. 1985;63:2818.and references cited therein Nakata T, Fukui M, Oishi T. Tetrahedron Lett. 1988;29:2223. and references cited therein.
- 6.Erythromycin B: Martin SF, Hida T, Kym PR, Loft M, Hodgson A. J Am Chem Soc. 1997;119:3193.Hergenrother PJ, Hodgson A, Judd AS, Lee WC, Martin SF. Angew Chem Int Ed. 2003;42:3278. doi: 10.1002/anie.200351136.Breton P, Hergenrother PJ, Hida T, Hodgson A, Judd AS, Kraynack E, Kym PR, Lee WC, Loft MS, Yamashita M, Martin SF. Tetrahedron. 2007;63:5709.
- 7.Erythronolide A: Corey EJ, Hopkins PB, Kim S, Yoo SE, Nambiar KP, Falck JR. J Am Chem Soc. 1979;101:7131.and references cited therein Nakata M, Arai M, Tomooka K, Ohsawa N, Kinoshita M. Bull Chem Soc Jpn. 1989;62:2618.Muri D, Lohse-Fraefel N, Carreira EM. Angew Chem Int Ed. 2005;117:4036. doi: 10.1002/anie.200500172.Muri D, Carreira EM. J Org Chem. 2009;74:8695. doi: 10.1021/jo901817b.Formal Synthesis: Hikota M, Tone H, Horita K, Yonemitsu O. Tetrahedron. 1990;46:4613.Hikota M, Tone H, Horita K, Yonemitsu O. J Org Chem. 1990;55:7.Sviridov AF, Borodkin VS, Ermolenko MS, Yashunsky DV, Kochetkov NK. Tetrahedron. 1991;47:2317. and references cited therein.
- 8.Erythronolide B: Corey EJ, Kim S, Yoo S, Nicolaou KC, Melvin LS, Brunelle DJ, Falck JR, Trybulski EJ, Lett R, Sheldrake PW. J Am Chem Soc. 1978;100:4620.and references cited therein Sviridov AF, Ermolenko MS, Yashunsky DV, Borodkin VS, Kochetkov NK. Tetrahedron Lett. 1987;28:3839.and references cited therein Mulzer J, Kirstein HM, Buschmann J, Lehmann C, Luger P. J Am Chem Soc. 1991;113:910.Formal Synthesis: Chandra B, Fu D, Nelson SG. Angew Chem Int Ed. 2010;49:2591. doi: 10.1002/anie.200906245.
- 9.(9S)-Dihydroerythronolide A: Stork G, Rychnovsky SD. J Am Chem Soc. 1987;109:1565.Stork G, Rychnovsky SD. Pure & Appl Chem. 1987;59:345.Tone H, Nishi T, Oikawa Y, Hikota M, Yonemitsu O. Tetrahedron Lett. 1987;28:4569.Tone H, Nishi T, Oikawa Y, Hikota M, Yonemitsu O. Chem Pharm Bull. 1989;37:1167.Paterson I, Rawson DJ. Tetrahedron Lett. 1989;30:7463.Stürmer R, Ritter K, Hoffmann RW. Angew Chem Int Ed. 1993;32:101.Peng ZH, Woerpel KA. J Am Chem Soc. 2003;125:6018. doi: 10.1021/ja034865z.
- 10.6-Deoxyerythronolide B: Masamune S, Hirama M, Mori S, Ali SA, Garvey DS. J Am Chem Soc. 1981;103:1568.Myles DC, Danishefsky SJ. J Org Chem. 1990;55:1636.Evans DA, Kim AS. Tetrahedron Lett. 1997;38:53.Evans DA, Kim AS, Metternich R, Novack VJ. J Am Chem Soc. 1998;120:5921.Stang EM, White MC. Nat Chem. 2009;1:547. doi: 10.1038/nchem.351.Formal Synthesis: Crimmins MT, Slade DJ. Org Lett. 2006;8:2191. doi: 10.1021/ol0607241.
- 11.For recent reviews of C-C bond forming hydrogenation and transfer hydrogenation, see: Bower JF, Krische MJ. Top Organomet Chem. 2011;43:107. doi: 10.1007/978-3-642-15334-1_5.Hassan A, Krische MJ. Org Proc Res Devel. 2011;15:1236. doi: 10.1021/op200195m.Moran J, Krische MJ. Pure Appl Chem. 2012;84:1729. doi: 10.1351/PAC-CON-11-10-18.
- 12.For iridium catalyzed carbonyl crotylation from the alcohol oxidation level employing α-methyl allyl acetate as the crotyl donor, see: Kim IS, Han SB, Krische MJ. J Am Chem Soc. 2009;131:2514. doi: 10.1021/ja808857w.Gao X, Townsend IA, Krische MJ. J Org Chem. 2011;76:2350. doi: 10.1021/jo200068q.Gao X, Han H, Krische MJ. J Am Chem Soc. 2011;133:12795. doi: 10.1021/ja204570w.
- 13.For ruthenium catalyzed hydrohydroxyalkylations of butadiene to form products of crotylation, see: Shibahara F, Bower JF, Krische MJ. J Am Chem Soc. 2008;130:6338. doi: 10.1021/ja801213x.Zbieg JR, Moran J, Krische MJ. J Am Chem Soc. 2011;133:10582. doi: 10.1021/ja2046028.Zbieg JR, Yamaguchi E, McInturff EL, Krische MJ. Science. 2012;336:324. doi: 10.1126/science.1219274.McInturff EL, Yamaguchi E, Krische MJ. J Am Chem Soc. 2012;134:20628. doi: 10.1021/ja311208a.
- 14.For an authoritative review of enyne metathesis, see: Diver ST, Giessert AJ. Chem Rev. 2004;104:1317. doi: 10.1021/cr020009e.For a related enone-olefin cross-metathesis, see: Xuan R, Oh H-S, Lee Y, Kang H-Y. J Org Chem. 2008;73:1456. doi: 10.1021/jo702384d.
- 15.Hirama M, Garvey DS, Lu LDL, Masamune S. Tetrahedron Lett. 1979;20:3937.For a review, see: Masamune S, Choy W, Petersen JS, Sita LR. Angew Chem Int Ed. 1985;24:1.
- 16.TBS ether 2 could not be prepared via Brown crotylation, as chromatographic separation from the byproduct isopinocampheol or the TBS ether of isopinocampheol could not be achieved. Consequently, the reported synthesis of TBS ether 2 requires a six step preparation from a chiral thiazole-2-thione auxiliary derived from cysteine: Narasimhulu CP, Das P. Synthesis. 2009:474.
- 17.Fukuyama T, Lin SC, Li L. J Am Chem Soc. 1990;112:7050.For a review, see: Tokuyama H, Yokoshima S, Lin SC, Li L, Fukuyama T. Synthesis. 2002:1121.
- 18.(a) Müller S, Liepold B, Roth GJ, Bestmann HJ. Synlett. 1996:251. [Google Scholar]; (b) Micoine K, Fürstner A. J Am Chem Soc. 2010;132:14064. doi: 10.1021/ja107141p. [DOI] [PubMed] [Google Scholar]
- 19.Geary LM, Woo SK, Leung JC, Krische MJ. Angew Chem Int Ed. 2012;51:2972. doi: 10.1002/anie.201200239. [DOI] [PubMed] [Google Scholar]
- 20.(a) Kogure T, Eliel EL. J Org Chem. 1984;49:576. [Google Scholar]; (b) Midland MM, Gabriel J. J Org Chem. 1985;50:1144. [Google Scholar]; (c) Poss CS, Schreiber SL. Acc Chem Res. 1994;27:9. [Google Scholar]
- 21.Travis BR, Narayan RS, Borhan B. J Am Chem Soc. 2002;124:3824. doi: 10.1021/ja017295g. [DOI] [PubMed] [Google Scholar]
- 22.Mulzer J, Lammer O. Angew Chem Int Ed. 1983;22:628. [Google Scholar]
- 23.Wu Y, Sun YP. Chem Comm. 2005:1906. doi: 10.1039/b416383d. [DOI] [PubMed] [Google Scholar]
- 24.(a) White JD, Johnson AT. J Org Chem. 1994;59:3347. [Google Scholar]; (b) Roush WR, Pfeifer LA, Marron TG. J Org Chem. 1998;63:2064. doi: 10.1021/jo961841k. [DOI] [PubMed] [Google Scholar]
- 25.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull Chem Soc Jpn. 1979;52:1989. [Google Scholar]
- 26.(a) Stork G, Rosen P, Goldman NL. J Am Chem Soc. 1961;83:2965. [Google Scholar]; (b) Stork G, Rosen P, Goldman N, Coombs RV, Tsuji J. J Am Chem Soc. 1965;87:275. [Google Scholar]; (c) Freeman PK, Hutchinson LL. J Org Chem. 2002;67:5015. [Google Scholar]; (d) Donohoe TJ, House D. J Org Chem. 2002;45:1924. doi: 10.1021/jo0257593. [DOI] [PubMed] [Google Scholar]
- 27.Brown CA, Brown HC. J Am Chem Soc. 1963;85:1003.Brown HC, Brown CA. J Am Chem Soc. 1963;85:1005.Brown CA, Ahuja NK. J Org Chem. 1973;2:2226.For review, see: Ganem B, Osby JO. Chem Rev. 1986;86:763.
- 28.(a) Han SB, Hassan A, Kim IS, Krische MJ. J Am Chem Soc. 2010;132:15559. doi: 10.1021/ja1082798. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lu Y, Woo SK, Krische MJ. J Am Chem Soc. 2011;133:13876. doi: 10.1021/ja205673e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.