Abstract
We hypothesized that the ventromedial prefrontal cortex (vmPFC) is critical for making transitive inferences (e.g., the logical operation that if A > B and B > C, then A > C). To test this, participants with focal vmPFC damage, brain-damaged comparison participants, and neurologically normal participants completed a transitive inference task consisting of an ordered set of arbitrary patterns. Participants first learned through trial-and-error the relationships of the patterns (e.g., Pattern A > Pattern B, Pattern B > Pattern C). After initial learning, participants were presented with novel pairings, some of which required transitive inference (e.g., Pattern A > Pattern C from the relationship above). We observed that vmPFC damage led to a specific deficit in transitive inference, suggesting that an intact vmPFC is necessary for making normal transitive inferences. Given the usefulness of transitivity in inferring social relationships, this deficit may be one of the basic features of social conduct problems associated with vmPFC damage.
Introduction
The ability of the brain to extract as much meaningful information from incoming and internal stimuli as is necessary for an organism’s survival in their adaptive niche is one of its most important functions. The brain embodies the capacity to not only sense information from the external world and internal milieu, but also to process this information to extract relationships and infer values that are not explicitly present in the incoming data. For example, at a simple level, relationships between things can be determined: e.g., A > B. In a slightly more complicated fashion, relationships can be inferred beyond given information. For example, if A > B and B > C, then it can be inferred that the transitive relationship between A and C is such that A > C. This logic is known as transitive inference.
The ability to use transitive inference cuts across many levels of the animal kingdom. It has been demonstrated in non-human primates (Boysen, Berntson, Shreyer, & Quigley, 1993; Gillan, 1981; MacLean, Merritt, & Brannon, 2008; McGonigle & Chalmers, 1992; Treichler & Van Tilburg, 1996), rodents (Davis, 1992; DeVito, Kanter, & Eichenbaum, 2010; Roberts & Phelps, 1994), birds (Bond, Kamil, & Balda, 2003; Lazareva et al., 2004; Paz-y-Miño, 2004; Steirn, Weaver, & Zentall, 1995; von Fersen, Wynne, Delius, & Staddon, 1991; Weiss, Kehmeier, & Schloegl, 2010), and fish (Grosenick, Clement, & Fernald, 2007). In humans, children as young as age 4 have been shown to possess the ability to make transitive inferences (Adams, 1978; Bryant & Trabasso, 1971; Markovits, Dumas, & Malfait, 1995), as have individuals with mental handicaps (Lutkus & Trabasso, 1974). Moreover, in older adults transitive inference has been shown to remain intact as long as the ability to learn the premise relationships is preserved (Ryan, Moses, & Villate, 2009).
In sum, the ability to infer transitivity is observed across diverse vertebrate species and throughout almost the entire human lifespan, even when general cognitive aptitude is relatively modest. Thus, disruption of this basic ability could be predicted to lead to profound disturbances in everyday functioning. Indeed, deficits in transitive inference have been observed in persons with schizophrenia (Titone, Ditman, Holzman, Eichenbaum, & Levy, 2004), Alzheimer’s disease (Waltz et al., 2004), Parkinson’s disease (M. J. Frank, Seeberger, & O’Reilly, 2004), and frontal-variant frontotemporal dementia (Waltz et al., 1999). In everyday life, information is seldom presented in isolation or in totality. Thus, deficits in integrating information and extrapolating useful information via inference could result in maladaptive decision-making and poor adjustment to environmental demands. Ultimately a deficit in transitive inference could explain a good deal of the impaired everyday functioning in individuals with the types of neurological and psychiatric conditions cited above.
Patients with frontal-variant frontotemporal dementia (FTD), where hypometabolism in PFC is a defining feature, have been shown to have a specific deficit in transitive inference as opposed to a memory impairment; by contrast, patients with the temporal variant of FTD display the opposite pattern, i.e., impaired memory but normal transitive inference (Waltz et al., 1999). Functional imaging studies point to specific regions within the PFC playing a prominent role in transitive inference, including dorsolateral regions (Acuna, Eliassen, Donoghue, & Sanes, 2002) and rostrolateral regions (Wendelken & Bunge, 2010). Another key region in PFC, not highlighted so far by functional imaging but potentially of considerable importance, is the ventromedial prefrontal cortex (vmPFC), and that is the region targeted by the current investigation.
We have proposed that an important driving force in the evolution of the human brain was a shift from a reliance on perceptual processing, particularly chemosensation, to cognitive computation for conspecific evaluation, which we have termed the Inferential Brain Hypothesis (Koscik & Tranel, submitted). Whereas in most mammals the chemical senses are the important carriers of social information, humans rely very little on the interpersonal transfer of chemicals. Instead, social value must be inferred from indirect sources that can be difficult to detect or interpret and are potentially prone to deception and dissimulation. Mammals evolved the use of chemical communication in support of reproduction. In non-human primates, however, these cues are shifted from the chemical sense to other sensory modalities, particularly vision. This is particularly obvious in the visual signals of reproductive susceptibility (Gilad, Wiebe, Przeworski, Lancet, & Pääbo, 2004) and visual cues of social status (Kuze, Malim, & Kohshima, 2005). In humans, we predict that these cues have been further removed from obvious chemical or visual cues and must be inferred instead. We propose that the brain regions that support social evaluation through chemical communication in mammals have been exapted in primates and humans to provide essentially the same type of information necessary for evolutionary success albeit using different types of sensory information and incorporating different cognitive algorithms.
A particularly important domain of social evaluation for all animals including humans involves understanding the relationships between individuals. Many species exhibit some sort of dominance hierarchy when in social groups and these hierarchies often confer benefits to the individuals on top, e.g., increased reproductive opportunity (e.g., Dewsbury, 1982; Ellis, 1995) and better health (e.g., Adler, Epel, Castellazzo, & Ickovics, 2000; Wilkinson, 1999). Moreover, understanding one’s position in a social hierarchy can benefit many individuals—even though not all individuals reap certain benefits from being on top of the heap, individuals can avoid unnecessary risk and maximize potential by asserting themselves when appropriate and adaptive. Transitivity is particularly relevant when inferring social positions within a group, by allowing an individual to infer relationships without having to explicitly sample all possible permutations between other individuals. The ability to infer relationships provides benefits by saving time and resources by not having to observe all possibilities directly, which is particularly relevant under circumstances when determining relationships incurs personal risk (e.g., male-male aggression to allocate reproductive opportunities).
We predict that cortical regions important for conspecific chemical evaluation in mammals retain their role in social evaluation in humans. However, instead of relying on perceptual processes, these brain regions will implement cognitive processes necessary to infer social value, such as transitive inference. Where neuroimaging work has pointed to dorsolateral and rostrolateral prefrontal involvement in transitive inference, which makes sense given the roles of these structures in relational processing, we predict that the vmPFC is an additional neural structure necessary for drawing transitive inferences. Regions within the vmPFC are necessary for evaluating odors, and orbitofrontal portions of this region comprise secondary olfactory cortex (A. Anderson et al., 2003; Gottfried & Zald, 2005). In addition, the vmPFC is critical for normal social conduct, as indicated by studies showing that damage to the vmPFC often leads to profound social and emotional dysfunction (S. Anderson, Barrash, Bechara, & Tranel, 2006; A. Damasio, 1994; Noonan, Sallet, Rudebeck, Buckley, & Rushworth, 2010; Shamay-Tsoory, Aharon-Peretz, & Perry, 2008; Shamay-Tsoory, Tomer, Goldsher, Berger, & Aharon-Peretz, 2004). Given these dual roles of the vmPFC for social cognition and for olfactory evaluation, we predict that the vmPFC will be a neural substrate that has been exapted from its role in perceptual processing (chemosensation in particular) to implement inferential processes, and will thus be necessary for drawing transitive inferences. Thus, the main objective of the present study was to examine the role of the vmPFC as a critical neural structure for transitive inference.
In addition to the body of empirical work examining the PFC, the hippocampus (HPC) has been shown to be involved in transitive inference in studies using fMRI (Greene, Gross, Elsinger, & Rao, 2006; Heckers, Zalesak, Weiss, Ditman, & Titone, 2004; Zalesak & Heckers, 2009), PET (Nagode & Pardo, 2002), and neurological patients with hippocampal amnesia (Smith & Squire, 2005). However, contradictory findings have been reported. Specifically, pharmacological inactivation of the HPC has been shown to cause profound deficits in explicit memory but to improve performance in making inferences (Frank, O’Reilly & Curran 2006); moreover, conscious awareness of the relationships between items may be a critical factor in HPC involvement in transitive inference (e.g., Smith & Squire, 2005). Given these discrepant findings regarding the role of the HPC in transitive inference, and given our ready access to patients with unilateral, focal damage to the medial temporal lobe (including the HPC), we opted to include in the current study a group of HPC patients (in addition to the vmPFC patients), on the premise that we could shed some additional light on the role of the hippocampus in transitive inference. Under the framework of relational memory theory (Cohen & Eichenbaum, 1995; Eichenbaum & Cohen, 2004), the HPC supports memory for all types of relationships (Konkel, Warren, Duff, Tranel, & Cohen, 2008), which is necessary for and would include transitive relationships. Given that some functional imaging studies have indicated differential contributions of left and right HPC to transitive inference (e.g., Zalesak & Heckers, 2009), the question arises as to whether or not unilateral HPC damage is sufficient to disrupt normal transitive inference. Thus, a secondary objective of the current study was to leverage our sample characteristics to examine whether or not unilateral HPC damage is sufficient to impair transitive inference. Since our study was not explicitly designed to tackle the issues of HPC involvement in transitive inference per se, our sample of convenience does not allow us to test whether or not the HPC is necessary for transitive inference or whether bilateral damage would impair performance on this task, only whether or not unilateral HPC damage is sufficient to cause a deficit.
Methods
Participants
Participants consisted of three groups of men and women: (1) a ventromedial prefrontal cortex (vmPFC) group: 15 participants with focal damage to the vmPFC either bilateral (N = 8; 4 women, 4 men) or confined to the left (N = 3; 1 woman, 2 men) or right (N = 4; 1 woman, 3 men) hemisphere; (2) a brain-damaged comparison (BDC) group: 36 participants (19 women, 17 men) with focal brain damage that did not involve the vmPFC (2 bilateral cases, 19 left hemisphere cases, 15 right hemisphere cases); and (3) a group of neurologically normal adults (NC group; N = 44, 23 women, 21 men) (see Table 1). The vmPFC and BDC groups did not differ in lesion chronicity (i.e., time since lesion onset) (equal variances not assumed, t(17.978) = 1.138, p = 0.270). Neuroanatomical, neuropsychological, and experimental data were collected from brain-damaged participants approximately contemporaneously—specifically, during the chronic epoch of recovery (more than 3 months post onset), where the neuroanatomical and neuropsychological profiles were stable.
Table 1.
Demographic and Neuropsychological Data
| VMPC | BDC | NC | ||
|---|---|---|---|---|
| WAIS-R | N (Women, Men) | 15 (6, 9) | 36 (19, 17) | 44 (23, 21) |
| Handedness (R, L, M) | 15, 0, 0 | 30, 2, 4 | 41, 3, 0 | |
| Age (years) | 60.7 (13.8) | 54.0 (13.6) | 60.4 (13.4) | |
| Education (years) | 14.1 (2.3)* | 15.0 (3.0) | 16.0 (2.4) | |
| Chronicity (years) | 12.3 (9.4) | 10.3 (7.8) | - | |
| FSIQ | 109.5 (17.3) | 106.0 (13.4) | - | |
| PIQ | 110.0 (14.5) | 106.9 (13.4) | - | |
| VIQ | 108.6 (18.4) | 104.9 (15.8) | - | |
| BDI | 6.5 (6.5) | 6.8 (5.5) | - | |
| BAI | 4.4 (3.4) | 6.0 (4.4) | - | |
| AVLT | 13.5 (2.5) | 14.5 (2.9) | - | |
| WCST | 4.6 (2.3) | 5.4 (1.6) | - | |
| Face Disc. | 45.6 (3.7) | 44.8 (5.2) | - | |
| COWA | 42.8 (14.1) | 39.9 (11.2) | - | |
| BNT | 55.7 (3.7) | 52.3 (9.2) | - |
Mean (Std. Dev.)
p < 0.05
Handedness: R = Right handed, L = Left Handed, M = Mixed Handed
The vmPFC group had fewer years of education than the NC group. All other group differences were not significant. WAIS = Wechsler Adult Intelligence Scale – III; FSIQ = Full Scale Intelligence Quotient; PIQ = Performance Intelligence Quotient; VIQ = Verbal Intelligence Quotient; BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; AVLT = Auditory Verbal Learning Test, 30 minute recognition, # correct responses; WCST = Wisconsin Card Sorting Test, # categories completed; Face Disc. = Benton Facial Discrimination Test; COWA = Controlled Oral Word Association Test; BNT = Boston Naming Test. For all neuropsychological measures, references can be found in Tranel (2009).
All participant groups were predominantly right-handed and comparable in age, though there was a weak trend for the BDC group to be younger (F(2,94) = 2.361, p = 0.100). Education was also broadly comparable across groups—the vmPFC group had statistically fewer years of education (F(2,94) = 3.597, p = 0.031), but post hoc tests revealed that this difference is only significant between the vmPFC and NC groups (p = 0.036), and the mean difference was less than two years (1.98 years).
Brain-damaged participants have, for the most part, intact psychometric intelligence, memory, executive functions, and verbal abilities (see Table 1). Five participants (4 BDC and 1 vmPFC, not included in the participant counts reported above) were excluded from all analyses as they reported elevated scores on measures of depression and/or anxiety. All lesions were stable and were clearly identifiable on either magnetic resonance (MR) images or computerized tomographic (CT) images. All brain-damaged participants had the onset of their brain lesion in adulthood, after age 18. Damage to the vmPFC was mainly caused by benign tumor resection (N = 9) or cerebrovascular accident (typically aneurysm-related) (N = 6).
The regions of brain damage in the BDC group allowed us to identify two distinct subgroups: one with medial temporal lobe (MTL) damage, which includes the hippocampus (HPC), and another group whose damage excluded both vmPFC and MTL. These BDC subgroups allowed us to test our secondary objective regarding whether unilateral HPC damage would impair transitive inference: an MTL group (N = 17; 12 women, 5 men; 8 right and 9 left hemisphere cases) and group whose damage excluded the MTL (BDC*) (N = 19; 7 women, 12 men; 2 bilateral, 7 right, and 10 left hemisphere cases). Damage in the MTL was primarily caused by surgical resection for pharmacoresistant epilepsy (N = 15); the remaining two cases in the MTL group were due to an ischemic stroke and aneurysm rupture. Brain damage in the BDC* group tended to be due to cerebral vascular accident (typically stroke or hemorrhage, N = 14) or benign tumor resection (N = 5). We did not expect that lesion etiology would affect performance on our tasks over and above lesion locus. Indeed, there was no significant effect of lesion etiology on any of the neuropsychological variables or on the dependent measures (all ps > 0.1).
All participants with focal brain damage were recruited from the Patient Registry of the Division of Behavioral Neurology and Cognitive Neuroscience in the Department of Neurology at the University of Iowa. All participants were free of dementia, psychiatric disorder, substance abuse, and significant intellectual impairments. Normal comparison participants were recruited from the Iowa City area through advertisement, and were compensated for their participation. All participants provided informed consent prior to participation in accordance with the Institutional Review Board of the University of Iowa.
Lesion Analysis
Neuroanatomical analysis was based on MR or CT images obtained in the chronic epoch of recovery. Each brain lesion was reconstructed in three dimensions using Brainvox (H. Damasio & Frank, 1992; R. Frank, Damasio, & Grabowski, 1997) and manually warped to a normal template brain using the MAP-3 technique (H. Damasio, Tranel, Grabowski, Adolphs, & Damasio, 2004). Following manual transfer to the normal template space, the template brain was warped to the MNI152 standard 1mm T1-weighted atlas (Collins, Neelin, Peters, & Evans, 1994; Evans, Dai, Collins, Neelin, & Marrett, 1991; Mazziotta et al., 2001) to provide a more direct comparison to a large portion of the literature that also uses this standard space. This warping was accomplished using BRAINSDemonWarp (Johnson & Zhao, 2009), which is a high dimension image registration algorithm that generates displacement vectors for each voxel to define the transform from the moving to fixed image (Thirion, 1998). This transform, from the lesion template to the MNI152 template, was then applied to each of the lesion maps. Lesion maps were then processed with Matlab (r2007b, The Mathworks), in order to create overlap maps of appropriate participants.
Naturally occurring brain lesions do not respect functional or anatomically boundaries, thus an all-or-none approach to classifying lesions is inappropriate. Likewise, anatomical parcellation schemes give a false impression of distinct, abrupt boundaries between regions, thus, lesions that have their focus in an adjacent, non-target region may spill-over in the periphery of the region of interest, but not affect the core of this region to a significant extent. Our solution to this classification problem was twofold. First our recruitment procedures targeted participants with known damage to the vmPFC, our region of interest. Second, to exclude participants with damage limited to the periphery of our region of interest, we set a lower limit on the proportion of damaged voxels for inclusion at 5%. In our sample, of the lesions confined entirely to the vmPFC, the smallest proportion of the vmPFC that is covered is ~15% (a unilateral lesion taken as proportion of bilateral vmPFC volume); four subjects with foci of damage in regions adjacent to the vmPFC have some spill-over (with an average 1.3% coverage of bilateral vmPFC, maximum 2.6%). These data were then visualized using MRIcroN (Rorden, 2007, 2008) (see Figure 1).
Figure 1.
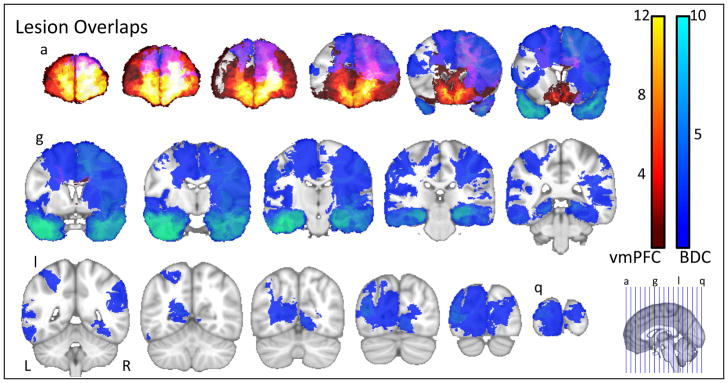
Lesion Overlaps. Lesions of participants in the vmPFC group are shown in the warm colours, where the maximum overlap (12) is within ventromedial prefrontal sector. Lesions in the BDC group cover a much broader swath of cortical territory, though the maximum overlap (10) is located in the MTL. Purple represents regions of overlap between the vmPFC and BDC groups, and covers bilateral dorsolateral PFC.
Transitive Inference Task
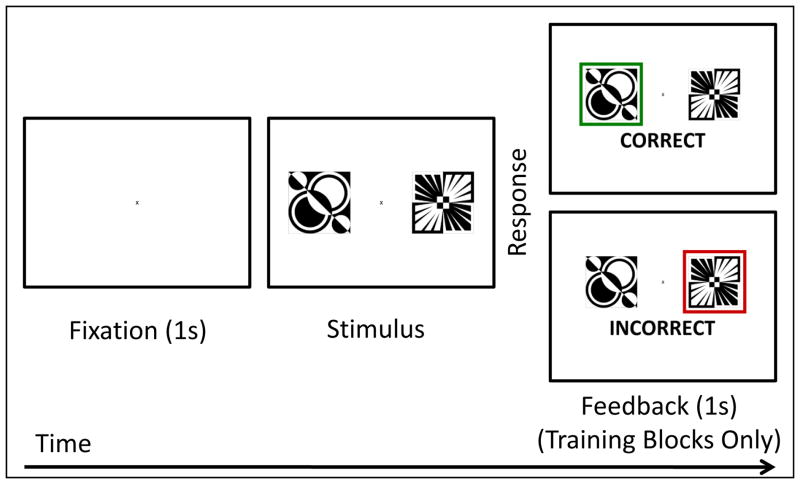
To investigate the ability to use transitive inference, we designed a task similar to that used by Acuna and colleagues (2002). Participants viewed black and white patterns and were asked to make button presses in response to what they saw. They were presented with two objects at a time, otherwise they were asked to maintain fixation on a centrally located cross. Participants had two response buttons, one that corresponded to the pattern to the left of fixation and the other that corresponded to the pattern to the right of fixation. The task was divided into four blocks. The first two blocks were training blocks and consisted of 48 trials each. The final two blocks were test blocks and consisted of 42 trials each.
Participants were given the following instructions:
“In this task you will see a series of black and white patterns. These patterns are arranged in an order of how correct they are. Your task is to figure out the order of the patterns. You will see two of these patterns at a time and your job is to determine which of the patterns is correct. If you think the pattern on the left is correct, press the button on the left. If you think the pattern on the right is correct, press the button on the right. You will play through four sets of items. On the first two sets you will be told if your answer is correct or incorrect. On the last two sets of stimuli you will not be told if you are correct or incorrect though you should still try to answer them correctly.”
During the training blocks, each trial began with a black fixation cross on a white background for 1 second. Then, a pair of black and white patterns was presented equidistant to the left and right of fixation and remained on the screen until the participant made a response. Once the participant made their response, they were given feedback. If the participant answered correctly, a green square surrounded the pattern they selected and “Correct” appeared beneath it. If the participant answered incorrectly, a red square surrounded the pattern the selected and “Incorrect” appeared beneath it. This feedback remained visible for 1 second, after which the trials repeated. In between blocks, participants were given a short break. Testing blocks were identical to training blocks, except that feedback was absent (see Figure 2). There were no pre-determined criteria, in terms of number of items answered correctly in the training blocks, before continuing on to the test blocks.
Figure 2.
Transitive Inference Task Trials. This figure depicts the sequence of events for each trial during the training phase. A fixation cross was presented for 1s, followed by the stimulus which remained on the screen until the person made a response. During training blocks, participants received feedback, either correct or incorrect, which remained on the screen for 1s. No feedback was given during test blocks.
The patterns used in the task were designed to be memorable but not recognizable as ordinary objects. The seven patterns we used were arranged in an arbitrary, linear sequence of ‘correctness’ (see Figure 3), (A>B>C>D>E>F>G). Training blocks consisted of pairs of patterns that are adjacent in this hierarchy of correctness (A & B, B & C, C & D, D & E, E & F, F & G). Testing blocks consisted of all possible pairings of patterns. This results in three types of test trials: 1. Learned items, consisting of trials of adjacent patterns that were previously used in the training phase; 2. Non-transitive items, where pairings contained either pattern A, which is always correct, or pattern G, which is always incorrect, requiring application of a static rule (e.g., A & C|D|E|F|G, G & B|C|D|E); and 3. Transitive items, where pairings of non-adjacent patterns were presented (e.g., B & D|E|F, C & E|F, D & F) (see Figure 4). Using 7 items makes the middle transitive pairs difficult to solve by relying on conditioning (Van Elzakker, O’Reilly, & Rudy, 2003), indeed we have classified ‘non-transitive’ items this way since they can be solved by applying a simpler rule rather than transitive per se although the relationships are also transitive in a strict sense. On the transitive items, to solve the problem accurately, participants would have to recall the correct pairings of items (this recall need not be explicit, and could occur outside of awareness) and extrapolate from these relationships using transitivity the correct responses.
Figure 3.

Patterns Order. This figure displays the patterns used in the task arranged in the predetermined, random order of correctness that the participants were instructed to infer from the training trials.
Figure 4.
Test Trial Types. A schematic representation of trial types, where pairs of patterns (in rows and columns) are either previously learned (black); do not require transitive inference to answer correctly, i.e., non-transitive (gray); or require transitive inference (white). Crossed out squares represent pairings not used in the study.
Statistical Analyses
For the primary analysis to test our main objective, we compared vmPFC, BDC, and NC groups using one-way analysis of variance (ANOVA) procedures for performance on learned, transitive, and non-transitive items. We planned to use Tukey’s post hoc test to pinpoint any significant group differences in all analyses. Given that we did observe small but statistically significant differences in education between groups we explored the possibility that this difference affected our results by first examining any potential correlations with education and our dependent measures both in our sample as a whole and within each group. Second we utilized an analysis of covariance (ANCOVA) with education as a covariate to rule out the possibility that education was driving any observed effects.
To examine our secondary objective, we compared the MTL, BDC*, and NC groups using an identical statistical approach to our procedure for examining the vmPFC group, that is using separate ANOVAs for learned, transitive, and non-transitive items. If education was found to significantly impact the results in our primary analysis, we utilized an ANCOVA with education as a covariate instead.
Next, we planned three follow-up analyses to eliminate alternative explanations for any potential deficits in transitive inference. It is possible that there are group differences in the acquisition of relationships during the training phase of this study. We thus planned a factorial repeated measures analysis of variance (ANOVA) for learning effects during the training phase if we observed group differences in our main outcome variables above. We planned to use an 8 (8 phases of learning) x 3 (groups) design. The 8 phases of learning represent percentage of items answered correctly during sequential quartiles of trials (i.e., trials 1 – 12, trials 13 – 24, trials 25 – 36, and trials 37 – 48) for both blocks. We performed an identical repeated measures ANOVA to examine changes in response time as a function of learning, as a decrease in response time will reflect familiarity and learning of the relationships. In addition, we examined possible correlations between learning performance (performance on the second training block and previously learned items from the test blocks) and performance on transitive and non-transitive items to see if differential learning affected performance. Since transitive relationships differed in the number of relationships needed to make the inference (e.g., B > C & C > D, for B vs. D requires two steps, where B > C & C > D & D > E, for B vs. E requires three steps), we planned a factorial repeated measures ANOVA to examine whether there were differences in performance based on difficulty or ‘transitive distance’ between groups. For simplicity we will refer to transitive distance defined as the number of relationships needed to make the inference. In our study this required between transitive distances of 2, 3 and 4, thus resulting in a 3 (transitive distance) × 3 (group) design. Lastly, we sought to ensure that the groups did not differ in terms of differential reinforcement of particular items over others, which may create conditions where simple conditioned learning could better explain our results. We planned a 5 (Reinforcement/Punishment ratios for Patterns B – F) × 3 (group) repeated measures ANOVA, and predicted that there should be no differences between groups or between patterns. If we did observe differences between the patterns, we planned to compare performance between patterns for transitive items using a 5 (Percent correct on Patterns B – F) × 3 (group) factorial repeated measures ANOVA. Essentially, this follow-up analysis will examine whether the various patterns were reinforced differentially over the course of the experiment, and if so, whether there were differences in performance for particular patterns as a result of differential reinforcement. If group differences in performance on transitive items are indeed due to a deficit in transitive inference and not in response to conditioning, then there should be no differences in performance by pattern and the group effect should remain.
Given that sex and affected hemisphere are potential mediators of outcome following damage to limbic related brain regions (Koscik, Bechara, & Tranel, 2010; Tranel, Damasio, Denburg, & Bechara, 2005), it was important to ascertain whether these factors played a role in the results. Hence, we examined potential effects of sex and hemisphere of lesion. To examine sex, we planned a 3 (vmPFC, BDC, and NC groups) by 2 (sex) ANOVA. To examine hemisphere of damage, we planned to use a 2 (vmPFC and BDC groups) by 3 (bilateral, left and right hemispheres) ANOVA. Likewise, we planned similar analyses of sex and laterality effects in our secondary analysis of the MTL and BDC* groups. The only difference in the models is a 2 (MTL and BDC* groups) by 2 (left or right hemisphere) ANOVA, since there were no bilateral cases of MTL damage in our sample.
Results
vmPFC
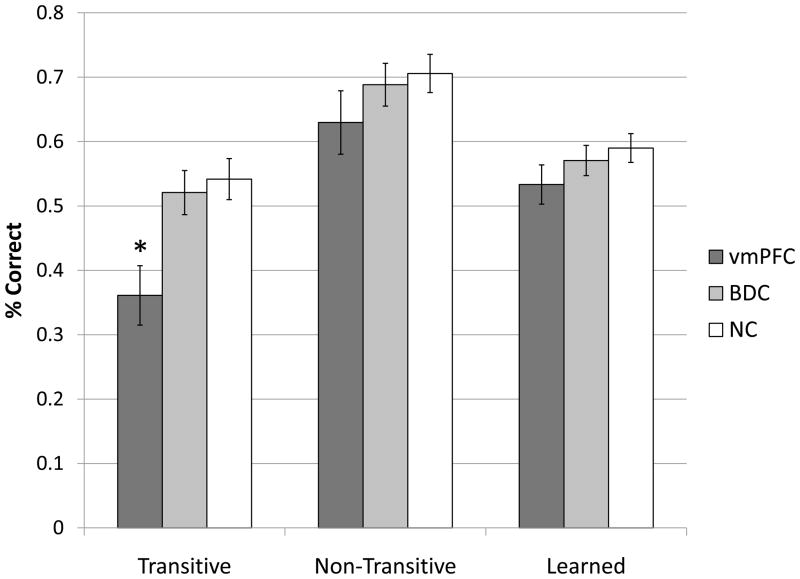
Our primary analysis comparing participants with vmPFC damage to the BDC and NC groups revealed significant group differences in the percentage of transitive items answered correctly (F(2,94) = 4.520, p = 0.013); vmPFC group (M = 0.36, SD = 0.18), BDC group (M = 0.52, SD = 0.21), and NC group (M = 0.54, SD = 0.21) (see Figure 5). Tukey’s post hoc test revealed that the vmPFC group answered significantly fewer transitive items correctly than the BDC group (mean difference = −0.16, p = 0.034) and the NC group (mean difference = −0.18, p = 0.011). These group differences were specific to transitive items. There were no differences between the vmPFC group (M = 0.53, SD = 0.12), the BDC group (M = 0.57, SD = 0.14), and the NC (M = 0.59, SD = 0.15) group for previously learned items (F(2,94) = 0.911, p = 0.406). Likewise, there were no significant differences between the vmPFC group (M = 0.63, SD = 0.19), the BDC group (M = 0.69, SD = 0.20), and the NC group (M = 0.71, SD = 0.20) for performance on non-transitive items (F(2,94) = 0.839, p = 0.436). If we exclude all participants whose performance on the last training block did not exceed chance performance, our results are unchanged but are weakened to trend-level significance (p = 0.061) for transitive items, which is to be expected given the smaller N when excluding subjects.
Figure 5.
VMPC Performance. Dark gray bars indicate percentage of items answered correctly by the vmPFC group. The vmPFC group does significantly poorer for transitive items but not non-transitive or learned items compared to the BDC group (p = 0.033) and the NC group (p = 0.011).
Education
When examining the possible effects of education on our dependent measures, there were small and non-significant correlations between years of education and performance on learned items (r = 0.169, p = 0.101), transitive items (r = 0.060, p = 0.562), or non-transitive items (r = 0.054, p = 0.604). Within groups, there was a significant correlation between years of education and learned items within the NC group (r = 0.313, p =0.038); all other correlations were non-significant (all ps > 0.400). When including education as a covariate in an ANCOVA, the results were unchanged. For previously learned items, we did not observe significant effects of education (F(1,91) = 1.795, p = 0.184) or of group (F(2,91) = 0.462, p = 0.632). Similarly for non-transitive items, we did not observe significant effects of education (F(1,91) = 0.042, p = 0.838) or group (F(2,91) = 0.716, p = 0.491). For transitive items we did not observe a significant effect of education (F(1,91) = 0.008, p = 0.929), and our group effect was essentially unchanged (F(2,91) = 4.294, p = 0.017).
Learning
In our follow-up analyses, we compared vmPFC, BDC and NC groups on learning, transitive distance, and reinforcement. In our analysis of learning during the training phase, we observed a significant effect of learning over trials (F(7,644) = 7.568, p < 0.0005) (see Figure 6). However, there were no significant group differences in learning, i.e., all groups were able to learn the relationships between items (F(2,92) = 1.927, p = 0.151); nor was there a significant block by group interaction (F(14,644) = 0.821, p = 0.646). Given that we did not have established performance criteria for continuing to test portions of the procedure, it was important to demonstrate that groups did not differ in how well they learned the items as above. Moreover, all groups displayed a similar decrease in response time as a function of learning across training blocks, where there is a significant decrease in response time over trials (F(7,644)=7.568, p<0.0005), but no group difference (F(2,92)=1.927, p=0.151) nor a learning by group interaction (F(14,644)=0.821, p=0.646). Additionally, our correlation analysis reveals positive relationships between learning (both performance in later training trials and in previously learned items) with performance on learned items. However, the relationship between learning and performance on transitive items is inconsistent and if present much weaker (see Table 2). In fact the relationship between learning and transitive items is not present in the vmPFC and NC groups and only in the BDC (and BDC*) groups.
Figure 6.
MTL Performance. Dark gray bars indicate the percentage of items of each type answered correctly on average for participants with medial temporal lobe damage, light gray represents the BDC* group and white the NC group. There are no group differences for any measure.
Table 2.
Correlations: Learning and Performance
| Group | Transitive | Non-Transitive | ||
|---|---|---|---|---|
| Training | Learned | Training | Learned | |
| vmPFC | −0.131 (0.849) | 0.159 (0.572) | 0.440 (0.101) | 0.455 (0.089) |
| BDC | 0.337 (0.044)* | 0.437 (0.008)* | 0.679 (<0.0005)* | 0.664 (<0.0005)* |
| NC | 0.188 (0.222) | 0.190 (0.216) | 0.644 (<0.0005)* | 0.629 (<0.0005)* |
|
|
||||
| MTL | 0102 (0.697) | 0.138 (0.598) | 0.638 (0.006)* | 0.755 (<0.0005)* |
| BDC* | 0.558 (0.013)* | 0.616 (0.005)* | 0.703 (0.001)* | 0.609 (0.006)* |
significant correlations
Both measures of learning (% correct during the last training block and % correct of learned items during the testing phase) are highly correlated with performance on non-transitive items, though this relationship is weak in the vmPFC group. Learning is not consistently related to performance on transitive items, except in BDC groups. This might suggest that performance on transitive items does not normally depend on learning per se and may indeed reflect inferential processing.
Transitive distance
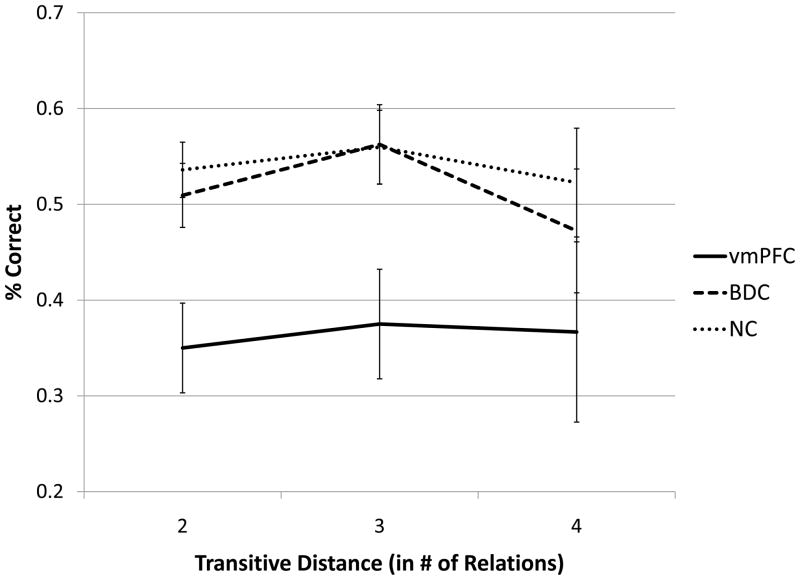
In our analysis of transitive distance, we found no effect of distance (Greenhouse-Geisser F(1.543,141.979) = 1.086, p = 0.327), nor a distance by group interaction (Greenhouse-Geisser F(3.087,141.979) = 0.303, p = 0.828). There was a main effect of group, whereby the vmPFC group answered fewer items correctly (F(2,92) = 3.336, p = 0.040) (see Figure 7).
Figure 7.
Learning. Lines represent the percentage of items answered correctly for different blocks of training trials. Each block consists of 12 trials. For all groups there is a clear trend toward increased percentage correct across trials, showing that all groups were able to learn the relationships between items.
Reinforcement
In our analysis of reinforcement/punishment ratios for each pattern, we did not observe any group differences (F(2,82) = 0.479, p = 0.621), nor a group by reinforcement interaction (Greenhouse-Geisser F(4.607,188.906) = 1.262, p = 0.284). We did observe significant differences between patterns (Greenhouse-Geisser F(2.304, 188.906) = 11.401, p < 0.0005) (see Figure 9). The reinforcement/punishment ratio for Pattern B was higher than for the other patterns (B – C = 1.351, p < 0.0005; B – D = 0.813, p = 0.013; B – E = 1.132, p < 0.0005; B – F = 1.061, p < 0.0005). The reinforcement/punishment ratio for Pattern C was significantly higher than Pattern B, (C – B = 0.538, p = 0.002). All other differences between reinforcement/punishment ratios were non-significant at p < 0.05 (see Figure 8). Since there were some differences between the patterns in terms of their reinforcement/punishment ratios, we conducted a final analysis to examine whether or not performance differed on transitive items as a function of the presence or absence of a particular pattern. If performance on transitive items is a product of conditioning, then we expected that items containing more highly rewarded patterns would be answered correctly more often. Our analysis revealed no differences in percentage of transitive items answered correctly between patterns (Greenhouse-Geisser F(2.393,220.134) = 1.828, p = 0.155), nor a pattern by group interaction (Greenhouse-Geisser F(4.786,220.134) = 0.982, p = 0.428) (see Figure 9). Our effect of group remains (F(2,92) = 4.916, p = 0.009), where the vmPFC group answered fewer transitive items correctly compared to BDCs (mean difference = −0.166, p = 0.008) and NCs (mean difference = −0.182, p = 0.003).
Figure 9.
Reinforcement/Punishment Ratio. Bars represent the ratio of reinforcement to punishment for each group for each pattern. Indicative of learning, participants receive more reinforcement for pattern B, the ‘most correct’ pattern in this group. Patterns A and G are excluded as pattern A is always correct and G always incorrect. We observed no group differences in reinforcement.
Figure 8.
Transitive distance. Transitive distance provides a measure of transitive relationships, i.e., it represents the number of direct relationships that need to be utilized to draw the inference. We observed no effect of transitive distance overall or within each group. The vmPFC group was significantly worse for all transitive items regardless of distance.
Taken together, our follow-up analyses rule out alternative explanations for the observed deficit in transitive inference. Our data suggest that the deficit in transitive inference observed in the vmPFC group was not due to deficits in learning the relationships between patterns, was not influenced by the difficulty of the inferences, and was not due to differential reinforcement of patterns between groups.
Sex and Laterality
When comparing vmPFC, BDC, and NC groups, we observed no effect of sex or group by sex interaction for learned items (sex: F(1,89) = 0.284, p = 0.596; group by sex: F(2,89) = 0.421, p = 0.658), non-transitive items (sex: F(1,89) = 0.405, p = 0.526; group by sex: F(2,89) = 0.047, p = 0.954), or transitive items (sex: F(1,89) = 0.349, p = 0.556; group by sex: F(2,89) = 0.375, p = 0.688). Likewise, we observed no effect of hemisphere of damage or group by hemisphere interaction when comparing vmPFC and BDC groups for learned items (hemisphere: F(2,89) = 1.150, p = 0.326; group by hemisphere: F(2,89) = 0.534, p = 0.590), non-transitive items (hemisphere: F(2,89) = 0.655, p = 0.524; group by hemisphere: F(2,89) = 0.418, p = 0.661), or transitive items (hemisphere: F(2,89) = 0.195, p = 0.824; group by hemisphere: F(2,89) = 1.090, p = 0.345).
MTL Results
Our secondary analysis revealed no significant differences between MTL, BDC*, and NC groups for learned (F(2,79) = 0.934, p = 0.397), transitive (F(2,79) = 0.124, p = 0.884), and non-transitive items (F(2,79) = 0.671, p = 0.514) (see Figure 10).
Figure 10.
Transitive Inference by Pattern. Bars represent the proportion of transitive items answered correctly for each pattern. We found no effect of individual pattern, i.e., the vmPFC group performed poorly for all transitive items irrespective of pattern, therefore differences in reinforcement between patterns did not affect transitive inference performance.
In our analysis of learning during the training phase, we observed a significant effect of learning over trials (F(7,539) = 9.386, p < 0.0005). However, there were no significant differences between MTL, BDC*, and NC groups in learning the relationships between items (F(2,77) = 1.202, p = 0.306); nor was there a significant block by group interaction (F(14,539) = 0.364, p = 0.984).
Finally, our analyses of sex and laterality in our secondary groupings of MTL and BDC* groups, we observed no effect of sex or group by sex interaction for learned items (sex: F(1,74) = 0.432, p = 0.513; group by sex: F(2,74) = 0.143, p = 0.867), transitive items (sex: F(1,74) = 0.536, p = 0.466; group by sex: F(2,74) = 0.285, p = 0.753), or non-transitive items (sex: F(1,74) = 1.076, p = 0.303; group by sex: F(2,74) = 0.009, p = 0.991). In our analysis of laterality we observed significant effect of side of damage on transitive items (F(1,30) = 5.324, p = 0.028), where right hemisphere damage (M = 0.43, SD = 0.16) resulted in poorer performance than left hemisphere damage (M = 0.59, SD = 0.21). Though there was no effect of group (F(1,30) > 0.0005, p = 0.987) or group by side interaction (F(1,30) = 0.055, p = 0.816). For learned and non-transitive items we found no effects of group, side or group by side interactions (all ps > 0.168).
Discussion
In support of our main prediction, we found that damage to the vmPFC resulted in a deficit in the ability to use transitive inference. Unilateral damage to the MTL (including hippocampus) did not result in such a deficit. The deficit we found in the vmPFC patients cannot be attributed to brain damage per se, as patients with damage throughout various other parts of the telencephalon (as represented in the BDC group) performed no differently from neurologically normal individuals on the transitive inference task. However, other than the MTL, brain regions covered in the BDC group are not sufficiently sampled to draw strong conclusions concerning non-involvement of these regions on a case-by-case basis. The deficit observed in relation to vmPFC damage is not due to deficient learning of relationships between items, as the patients in the vmPFC group performed no differently than comparison groups for previously learned items and displayed normal acquisition of these relationships during the training phase. In addition, learning is no correlated with transitive inference performance in the vmPFC and NC groups (though there is a positive relationship in the BDC groups), which suggests that learning per se is not responsible for transitive inference, instead these data are consistent with a view that inferential processes are at least somewhat distinct from learning processes. The deficit is not attributable to extrapolating to novel pairings in general, as the vmPFC group performed normally for non-transitive pairings. Furthermore, the deficit is not attributable to differences in reinforcement and punishment conditions during the training phase.
A recent study suggested that vmPFC damage does not affect performance on the Matrix Reasoning test from the WAIS battery (Tranel, Manzel, & Anderson, 2008). This task requires the participant to draw inferences, as missing patterns need to be inferred from the stimuli that are given. However, the inferences required by Matrix Reasoning are not transitive per se (Lezak, Howieson, Bigler, & Tranel, 2011), so it is possible that vmPFC damage does not impair an individual’s ability to draw inferences in general--rather, our evidence suggests that their deficit may be limited to transitive inference in particular.
Inferential abilities have been studied with other tasks, as well, and there are important differences among tasks that might account for some differences in findings across various studies. For example, the task utilized by Wendelken and Bunge (Wendelken & Bunge, 2010) is similar to the Matrix Reasoning test in that both involve reasoning or manipulation of information that is maintained in full view. In essence, these tasks require deliberate reasoning in order to draw explicit inferences, possibly through the application of a logical rule to single items. Ours and similar tasks (e.g., Acuna et al., 2002), by contrast, do not involve on-line reasoning from visible stimuli. Instead, they require drawing inferences by applying previously acquired knowledge, and this process could happen implicitly and without explicit knowledge of having drawn the inference. This is somewhat similar to procedural forms of memory, where knowledge is acquired over time and across multiple learning epochs, and is deployed implicitly and without deliberate (or conscious) guidance. Another way of thinking about this distinction is that some reasoning tasks involve application of the transitivity rule to solve a problem, whereas ours and similar tasks require extrapolating transitive relationships from multiple, asynchronous encounters with stimuli. It is reasonable to predict that explicit versus implicit applications of transitive inference may rely on at least partially distinct neural networks. It may be that the deficit observed in participants with vmPFC damage occurs when drawing implicit inferences from relationships, perhaps stored in memory, but not when performing explicit reasoning, which may be related to more dorsolateral PFC regions. This remains an open question that can be tested in future research.
An interesting corollary can be drawn with the observation that the profound social deficits observed in the real-world social behaviour of individuals with vmPFC damage occur in the face of intact social knowledge per se (Saver & Damasio, 1991). The deficits observed following vmPFC damage may not be in recalling previously acquired knowledge (either social knowledge or the relationships between patterns in the task used here), and as explained above, the deficits do not appear to be due to reasoning about information that remains on-line (such as in Matrix Reasoning). Instead, it appears that the deficits observed following vmPFC damage become manifest when there is a requirement for integrating multiple elements of previously acquired knowledge in novel situations. This is particularly relevant in the social domain, where comprehending relationships between individuals is paramount, but where the social value of individuals must be extracted over time and flexibly applied in subsequent novel situations.
We did not observe an effect of transitive distance, either a main effect or interaction with group. We had initially speculated that the greater number of relationships that needed to be recalled to make an inference, the more difficult the inference would be (as reflected, for example, in lower performance for items with greater distance). However, this is not apparently the case, at least for the types of stimuli and distances used in our study. It may be that increased transitive distance actually makes the inference easier as items with greater separation in the learned hierarchy may be considered more obviously different, which might thereby facilitate the comparison. However, the data do not directly match this prediction either as one would expect performance to improve with distance. It may actually be some combination of these two factors—both recall of individual relationships as well as some sort of storage of the hierarchy in its totality—that ultimately determines (along with other factors) the difficulty of transitive inference. Dissociating these possibilities would make an interesting target of future investigations.
A limitation of our study is that we are unable to pinpoint involvement of specific prefrontal regions beyond the classifications of dorsolateral and ventromedial PFC. We are confident that vmPFC lesions disrupt normal use of transitive inference. Our findings thus extend the results of neuroimaging work, which has pointed to a role for dorsolateral regions in transitive inference. Our BDC sample included four patients with lesions that covered large portions of dorsolateral and dorsomedial PFC. We observed no deficits in transitive inference in these four individuals. Indeed these cases of dorsolateral PFC damage averaged 50% correct for transitive items, which is 14% higher than the average for participants with vmPFC damage. Nonetheless, the limited sampling of the dorsolateral PFC in our study, as well as the different forms of transitive inference tasks employed across different studies, precludes any strong conclusions of dlPFC involvement based on our lesion work. It could be the case that different prefrontal regions are involved with different aspects of inference, and further research is needed to address this possibility.
Another potential limitation of our study concerns the effects of motivation and response to feedback. It is possible that our vmPFC subjects were simultaneously less motivated to complete our task successfully and were less responsive to the reward and punishment feedback that they received. Taken together, these factors could yield a pattern of performance similar to what we observed. We find this very unlikely, though, for a number of reasons. First, vmPFC participants display similar levels of effort and cooperation on tasks in our laboratory, compared to the other participants, both anecdotally and as exemplified by normal performance on effortful tasks such as the intelligence testing. In addition, the vmPFC participants exhibited normal learning of items and normal performance for non-transitive items, demonstrating that they were sufficiently responsive to feedback to learn the relationships. It seems unlikely that lack of effort would pertain only to transitive items. Moreover, we have evidence from other paradigms(e.g., the Iowa Gambling Task, see Bechara, Damasio, Tranel, & Anderson, 1998) that vmPFC patients have normal psychophysiological responses to reward and punishment.
Turning to the secondary objective of our study, the data suggest that unilateral MTL damage is insufficient to cause a deficit in transitive inference. Our findings are consistent with the idea that one intact side of the medial temporal lobe system is sufficient for normal function. It is possible that unilateral MTL damage may produce more subtle deficits beyond the fidelity of the current study, e.g., increased reaction times. Neuroimaging work has observed unilateral activity in association with transitive inference—for example, Zalesak and Heckers (2009) observed left hippocampal activation during a transitive inference task, but the authors did not offer an interpretation of the left-sided lateralization of the finding. One possibility explanation involves stimulus content, where verbal versions of inference tasks might rely on the left hippocampus and the visual versions on the right. For example, hippocampal activity during a transverse patterning task (where A>B, B>C, and C>A, which is not transitive) shows the left hippocampus is more activate for a verbal version and the right for a visual version (Hanlon et al., 2011). Given that the task we used is a visual task, these data would suggest that damage to the right hippocampus would result in poorer performance than damage to the left. We did observe poorer performance in individuals with right hemisphere damage however this was not specific to the hippocampus. Our data suggest several possibilities: 1) Unilateral hippocampal involvement (as suggested by functional imaging approaches) is an artifact of the particular paradigm, sample, or experimental design; 2) The unilateral lesions in our sample did not damage the hippocampus sufficiently to disrupt its function entirely; 3) Post-lesion plasticity might allow lateralized functions to be assumed by the intact homologous structure in the opposite hemisphere; or 4) Both hippocampi are involved in transitive inference in the neurologically normal brain, but one is sufficient for the function, i.e., there is some plasticity and duplication of function.
It is interesting that our secondary analysis of laterality of brain damage revealed a significant effect whereby right-sided brain damage was more likely to be associated with poor performance on transitive items only. A parsimonious account of this finding is that right hemisphere lesions are more likely to interfere with difficult nonverbal cognitive operations. If the task were to be constructed to be language based, we would predict accordingly that left hemisphere lesions would produce a larger deficit. These ideas remain empirical questions. Our main analysis of laterality, which included all brain-damaged participants, revealed no effect of laterality; however, this included patients with bilateral damage as well as strictly unilateral damage, making the test less sensitive to differences.
In conclusion, our findings support the idea that the vmPFC is necessary for normal use of transitive inference. The deficits in transitive inference observed following vmPFC damage could potentially underlie some of the social deficits observed in these patients, given that transitive relationships are particularly relevant among social agents. Our data are consistent with the predictions of the Inferential Brain Hypothesis. As an interesting future direction, we intend to explore how these deficits in transitive inference observed following vmPFC damage might interfere with inferring relationships in social hierarchies.
Acknowledgments
Supported by NINDS P50 NS19632, NIDA R01 DA022549, and NSERC PGS-D
References
- Acuna B, Eliassen J, Donoghue J, Sanes J. Frontal and parietal lobe activation during transitive inference in humans. Cerebral Cortex. 2002;12(12):1312–1321. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- Adams M. Logical competence and transitive inference in young children. Journal of Experimental Child Psychology. 1978;25(3):477–489. [Google Scholar]
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy, White women. Health Psychology. 2000;19(6):586. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Anderson A, Christoff K, Stappen I, Panitz D, Ghahremani D, Glover G, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Anderson S, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. Journal of the International Neuropsychological Society. 2006;12(2):224–235. doi: 10.1017/s1355617706060346. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson S. Dissociation of working memory from decision making within the human prefrontal cortex. Journal of Neuroscience. 1998;18(1):428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Animal behaviour. 2003;65(3):479–487. [Google Scholar]
- Boysen ST, Berntson GG, Shreyer TA, Quigley KS. Processing of ordinality and transitivity by chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1993;107(2):208. doi: 10.1037/0735-7036.107.2.208. [DOI] [PubMed] [Google Scholar]
- Bryant P, Trabasso T. Transitive inferences and memory in young children. Nature. 1971 doi: 10.1038/232456a0. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT press; 1995. [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18(2):192. [PubMed] [Google Scholar]
- Damasio A. Descartes’ error: Emotion, reason, and the human brain. Putnam Adult; 1994. [Google Scholar]
- Damasio H, Frank R. Three-dimensional in vivo mapping of brain lesions in humans. Archives of Neurology. 1992;49(2):137. doi: 10.1001/archneur.1992.00530260037016. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Davis H. Transitive inference in rats (Rattus norvegicus) Journal of Comparative Psychology. 1992;106(4):342–349. doi: 10.1037/0735-7036.106.4.342. [DOI] [PubMed] [Google Scholar]
- DeVito LM, Kanter BR, Eichenbaum H. The hippocampus contributes to memory expression during transitive inference in mice. Hippocampus. 2010;20(1):208–217. doi: 10.1002/hipo.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewsbury DA. Dominance rank, copulatory behavior, and differential reproduction. The Quarterly Review of Biology. 1982;57(2):135–159. doi: 10.1086/412672. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. Oxford University Press; USA: 2004. [Google Scholar]
- Ellis L. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethology and Sociobiology. 1995;16(4):257–333. [Google Scholar]
- Evans A, Dai W, Collins L, Neelin P, Marrett S. Warping of a computerized 3-D atlas to match brain image volumes for quantitative neuroanatomical and functional analysis. Proceedings of the International Society of Optical Engineering (SPIE): Medical Imaging, V; 1991. p. 11. [Google Scholar]
- Frank M, O’Reilly R, Curran T. When memory fails, intuition reigns. Psychological Science. 2006;17:700. doi: 10.1111/j.1467-9280.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306(5703):1940. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Frank R, Damasio H, Grabowski T. Brainvox: An interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5(1):13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Gilad Y, Wiebe V, Przeworski M, Lancet D, Pääbo S. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol. 2004;2(1):e5. doi: 10.1371/journal.pbio.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan DJ. Reasoning in the chimpanzee: II. Transitive inference. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7(2):150. [Google Scholar]
- Gottfried J, Zald D. On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Research Reviews. 2005;50(2):287–304. doi: 10.1016/j.brainresrev.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Greene AJ, Gross WL, Elsinger CL, Rao SM. An FMRI analysis of the human hippocampus: inference, context, and task awareness. Journal of Cognitive Neuroscience. 2006;18(7):1156–1173. doi: 10.1162/jocn.2006.18.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick L, Clement T, Fernald R. Fish can infer social rank by observation alone. Nature. 2007;445(7126):429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Hanlon F, Houck J, Pyeatt C, Lundy S, Euler M, Weisend M, Tesche C. Bilateral hippocampal dysfunction in schizophrenia. Neuroimage. 2011;58(4):1158–1168. doi: 10.1016/j.neuroimage.2011.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Zalesak M, Weiss A, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14(2):153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- Johnson H, Zhao Y. BRAINSDemonWarp: An application to perform demons registration. 2009 from http://www.nitrc.org/projects/brainsdemonwarp/
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Frontiers in Human Neuroscience. 2008:2. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik T, Bechara A, Tranel D. Sex-related functional asymmetry in the limbic brain. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(1):340. doi: 10.1038/npp.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik T, Tranel D. Brain evolution and human neuropsychology: The Inferential Brain Hypothesis. Journal of the International Neuropsychological Society. doi: 10.1017/S1355617712000264. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuze N, Malim T, Kohshima S. Developmental changes in the facial morphology of the Borneo orangutan (Pongo pygmaeus): possible signals in visual communication. American Journal of Primatology. 2005;65(4):353–376. doi: 10.1002/ajp.20121. [DOI] [PubMed] [Google Scholar]
- Lazareva OF, Smirnova AA, Bagozkaja MS, Zorina ZA, Rayevsky VV, Wasserman EA. Transitive responding in hooded crows requires linearly ordered stimuli. Journal of the experimental analysis of behavior. 2004;82(1):1. doi: 10.1901/jeab.2004.82-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Bigler E, Tranel D, editors. Neuropsychological assessment. 5. New York: Oxford University Press; 2011. [Google Scholar]
- Lutkus A, Trabasso T. Transitive inferences by preoperational, retarded adolescents. American Journal of Mental Deficiency. 1974 [PubMed] [Google Scholar]
- MacLean E, Merritt D, Brannon E. Social complexity predicts transitive reasoning in prosimian primates. Animal behaviour. 2008;76(2):479–486. doi: 10.1016/j.anbehav.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovits H, Dumas C, Malfait N. Understanding transitivity of a spatial relationship: A developmental analysis. Journal of Experimental Child Psychology. 1995;59(1):124–141. [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Pike B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2001;356(1412):1293. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle B, Chalmers M. Monkeys are rational! The Quarterly Journal of Experimental Psychology Section B. 1992;45(3):189–228. [Google Scholar]
- Nagode JC, Pardo JV. Human hippocampal activation during transitive inference. NeuroReport. 2002;13(7):939. doi: 10.1097/00001756-200205240-00008. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Sallet J, Rudebeck PH, Buckley MJ, Rushworth MF. Does the medial orbitofrontal cortex have a role in social valuation? European Journal of Neuroscience. 2010;31(12):2341–2351. doi: 10.1111/j.1460-9568.2010.07271.x. [DOI] [PubMed] [Google Scholar]
- Paz-y-Miño C. Pinyon jays use transitive inference to predict social dominance. Papers in Behavior and Biological Sciences. 2004:25. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Phelps MT. Transitive Inference in Rats: A Test of the Spatial Coding Hypothesis. Psychological Science. 1994;5(6):368–374. [Google Scholar]
- Rorden C. MRIcroN. 2007 Retrieved from http://www.cabiatl.com/mricro/mricron/index.html.
- Rorden C. MRIcroGL. 2008 Retrieved from http://www.cabiatl.com/mricrogl/
- Ryan JD, Moses SN, Villate C. Impaired relational organization of propositions, but intact transitive inference, in aging: Implications for understanding underlying neural integrity. Neuropsychologia. 2009;47(2):338–353. doi: 10.1016/j.neuropsychologia.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29(12):1241–1249. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2008 doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S, Tomer R, Goldsher D, Berger B, Aharon-Peretz J. Impairment in cognitive and affective empathy in patients with brain lesions: anatomical and cognitive correlates. Journal of Clinical and Experimental Neuropsychology. 2004;26(8):1113–1127. doi: 10.1080/13803390490515531. [DOI] [PubMed] [Google Scholar]
- Smith C, Squire LR. Declarative memory, awareness, and transitive inference. The Journal of Neuroscience. 2005;25(44):10138. doi: 10.1523/JNEUROSCI.2731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steirn JN, Weaver JE, Zentall TR. Transitive inference in pigeons: Simplified procedures and a test of value transfer theory. Learning & Behavior. 1995;23(1):76–82. [Google Scholar]
- Thirion JP. Image matching as a diffusion process: an analogy with Maxwell’s demons. Medical Image Analysis. 1998;2(3):243–260. doi: 10.1016/s1361-8415(98)80022-4. [DOI] [PubMed] [Google Scholar]
- Titone D, Ditman T, Holzman PS, Eichenbaum H, Levy DL. Transitive inference in schizophrenia: impairments in relational memory organization. Schizophrenia research. 2004;68(2–3):235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- Tranel D. The Iowa-Benton school of neuropsychological assessment. Neuropsychological assessment of neuropsychiatric and neuromedical disorders. 2009:66–83. [Google Scholar]
- Tranel D, Damasio H, Denburg N, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Tranel D, Manzel K, Anderson S. Is the prefrontal cortex important for fluid intelligence? A neuropsychological study using matrix reasoning. Clinical Neuropsychologist. 2008;22(2):242–261. doi: 10.1080/13854040701218410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treichler FR, Van Tilburg D. Concurrent conditional discrimination tests of transitive inference by macaque monkeys: List linking. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22(1):105. [PubMed] [Google Scholar]
- Van Elzakker M, O’Reilly R, Rudy J. Transitivity, flexibility, conjunctive representations, and the hippocampus. I. An empirical analysis. Hippocampus. 2003;13(3):334–340. doi: 10.1002/hipo.10083. [DOI] [PubMed] [Google Scholar]
- von Fersen L, Wynne C, Delius JD, Staddon J. Transitive inference formation in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17(3):334. doi: 10.1037//0097-7403.17.3.281. [DOI] [PubMed] [Google Scholar]
- Waltz J, Knowlton B, Holyoak K, Boone K, Back-Madruga C, McPherson S, Miller B. Relational Integration and Executive Function in Alzheimer’s Disease. Neuropsychology. 2004;18(2):296. doi: 10.1037/0894-4105.18.2.296. [DOI] [PubMed] [Google Scholar]
- Waltz J, Knowlton B, Holyoak K, Boone K, Mishkin F, Santoa M, Miller B. A system for relational reasoning in human prefrontal cortex. Psychological Science. 1999;10(2):119–125. [Google Scholar]
- Weiss B, Kehmeier S, Schloegl C. Transitive inference in free-living greylag geese, Anser anser. Animal behaviour. 2010;79(6):1277–1283. [Google Scholar]
- Wendelken C, Bunge SA. Transitive inference: distinct contributions of rostrolateral prefrontal cortex and the hippocampus. Journal of Cognitive Neuroscience. 2010;22(5):837–847. doi: 10.1162/jocn.2009.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson RG. Health, hierarchy, and social anxiety. Annals of the New York Academy of Sciences. 1999;896(1):48–63. doi: 10.1111/j.1749-6632.1999.tb08104.x. [DOI] [PubMed] [Google Scholar]
- Zalesak M, Heckers S. The role of the hippocampus in transitive inference. Psychiatry Research: Neuroimaging. 2009;172(1):24–30. doi: 10.1016/j.pscychresns.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]