Abstract
Perinatal insults program sex differences in blood pressure, with males more susceptible than females. Aging may augment developmental programming of chronic disease, but the mechanisms involved are not clear. We previously reported that female growth-restricted offspring are normotensive after puberty. Therefore, we tested the hypothesis that age increases susceptibility to hypertension in female growth-restricted offspring. Blood pressure remained similar at 6 months of age; however, blood pressure was significantly elevated in female growth-restricted offspring relative to control by 12 months of age (137±3 versus 117±4 mmHg, P<0.01; respectively). Body weight did not differ at 6 or 12 months of age; however, total fat mass and visceral fat were significantly increased at 12 months in female growth-restricted offspring (P<0.05 versus control). Glomerular filtration rate remained normal, yet renal vascular resistance was increased at 12 months of age in female growth-restricted offspring (P<0.05 versus control). Plasma leptin, which can increase sympathetic nerve activity, did not differ at 6 months, but was increased at 12 months of age in female growth-restricted offspring (P<0.05 versus control). Due to the age-dependent increase in leptin, we hypothesized that the renal nerves may contribute to the age-dependent increase in blood pressure. Bilateral renal denervation abolished the elevated blood pressure in female growth-restricted offspring normalizing it relative to denervated female control offspring. Thus, these data indicate that age induces an increase in visceral fat and circulating leptin associated with a significant increase in blood pressure in female growth-restricted offspring with the renal nerves serving as an underlying mechanism.
Keywords: low birth weight, aging, cardiovascular risk, renal nerves, women’s health
Hypertension is the major cause of death worldwide and it is a multifactorial disorder affected by both genetic and environmental factors. The Barker hypothesis suggests that events during fetal life impact adult health and program increased cardiovascular (CV) risk and hypertension (1). Adverse events such as fetal undernutrition are hypothesized to program adult chronic disease due to metabolic and endocrine changes in addition to physiological and structural changes that occur in the undernourished organs (1). Low birth weight (LBW) serves as a crude marker indicative of undernutrition during fetal life and numerous epidemiological and experimental studies demonstrate a clear relationship between birth weight and blood pressure (2, 3).
It is well established that blood pressure increases with age in men and women with men exhibiting a higher blood pressure than women until post-menopause (4, 5). Higher blood pressures are also observed in LBW boys relative to LBW girls during childhood (6) with men demonstrating a strong association between birth weight and blood pressure in young adulthood (7). The association between birth weight and blood pressure amplifies with age (8) and hypertension is reported in LBW women by age 60 (9). Thus, age may serve as a secondary influence on the impact of poor fetal growth. Experimental models of fetal insult indicate that female offspring may be protected against programmed CV risk (10–12) or exhibit a delay in the development of adverse CV function (13). Yet, whether birth weight indicative of a poor fetal environment impacts chronic health in later life in LBW women has not been extensively studied; moreover, the mechanisms involved have not yet been elucidated.
Our laboratory utilizes a rodent model of intrauterine growth restriction (IUGR) induced by placental insufficiency that results in a decrease in birth weight of offspring from the reduced uterine perfusion dams relative to control offspring from the sham operated dams (12). Previously, we reported that prior to puberty at 8 weeks of age male and female growth-restricted offspring have a significant increase in mean arterial pressure relative to same-sex, age-matched controls (12). However, only male growth-restricted offspring demonstrate an increase in blood pressure after puberty, whereas blood pressure is normalized after puberty in female growth-restricted offspring relative to age-matched female controls (12). Mean arterial pressure remains normotensive in the female growth-restricted offspring at 4 months of age (14); yet, the impact of age-dependent changes on blood pressure in female growth-restricted offspring remains unknown.
It is well established that aging is a critical mediator of increased risk of hypertension (15). Aging is generally associated with a reduction in lean body mass and an increase in adipose tissue, particularly central body fat (16). Insulin resistance, dyslipidemia, and enhanced sympathetic activity result from an elevation in the adipose-derived chemokine leptin (17, 18). Moreover, the sympathetic nervous system is thought to contribute to obesity-related hypertension (19). A diminution in kidney function such as a decreased ability of kidney to excrete sodium and progressive decrease in functioning nephron units may also contribute to age-related increases in blood pressure (20). We previously reported that the ovarian hormones play a protective role against the development of high blood pressure in young adult female growth-restricted offspring (14). Age-related changes in CV risk in women are often associated with passage into menopause; yet, whether the increase in CV risk after menopause in women is due to aging per se, or to menopause itself, is unknown. Thus, this study tested the hypothesis that age-dependent changes, up to one year of age, serves as a secondary hit to program an increase in blood pressure in female growth-restricted offspring. Moreover, this study examined whether age-induced changes in adiposity accompanied the increase in blood pressure in female growth-restricted offspring and if the renal nerves, indicative of sympathetic involvement in obesity-related hypertension, served as a potential underlying mechanism.
METHODS
Please see Supplement for extended Methods.
Animals
All experimental procedures were in accordance with National Institutes of Health guidelines with approval by the Animal Care and Use Committee at the University of Mississippi Medical Center. Timed pregnant Sprague Dawley (SD) rats were purchased from Harlan Inc (Indianapolis, IN). Offspring from 24 control (sham) pregnant and 27 offspring reduced uterine perfusion pregnant rats were randomly assigned into groups studied at 6 or 12 months of age. The experimental protocol included: One group subjected to body composition measurements and 24 hour metabolic studies conducted one week prior to the experimental end point at 6 months of age. The experimental end point involved measurement of renal and systemic hemodynamic parameters and harvest of plasma, serum and tissues at 6 months of age. A second group followed the same experimental protocol, but was studied at 12 months of age. A third group was studied at 12 months of age, but was subjected to either sham or bilateral renal denervation as described below. In the third group, two weeks after sham denervation or bilateral renal denervation mean arterial pressure was measured via telemetry in a subgroup and via carotid catheter in all animals in the conscious state.
Reduced uterine perfusion in the pregnant rat
Reduced utero-placental perfusion was utilized for induction of intrauterine growth restriction (IUGR) at day 14 of gestation as previously described (12).
Body Composition
Total fat mass and total lean mass were determined in conscious animals using an Echo-MRI-700 (Echo Medical Systems, Houston TX). Results are expressed as gram weight.
Bilateral renal denervation
Bilateral renal denervation was performed as previously described (21).
Measurement of mean arterial pressure and heart rate by radiotelemetry
Implantation of the radio-telemetry probe and collection of data was performed as previously described (21).
Measurement of blood pressure and renal hemodynamics
Mean arterial pressure (MAP), glomerular filtration rate (GFR), effective renal plasma flow (eRPF), and calculation of renal vascular resistance (RVR), renal blood flow (RBF) and filtration fraction (FF) were performed in conscious animals as previously described (12).
Leptin
Plasma leptin levels were measured using a commercially available kit (Leptin Quantikine ELISA Kit, R&D system, MN).
Measure of renal catecholamine content
Renal norepinephrine was measured by HPLC via electrochemical detection.
Statistics
Data are presented as mean values ± SEM with n representing the number of female offspring from different mothers per group. Data were analyzed for significance using the Student t-test for comparison between two groups for birth weight and renal hemodynamic data (Figure 1a and 4). Pearson R correlation coefficient was used to calculate the relationship between body weight and fat mass. Two-way randomized ANOVA was used for the remainder of studied data (Figure 1c, 2, 3, 5 and renal norepinephrine as well as food intake). Post hoc testing was performed using the Bonferroni test (GraphPad Prism 5.0). Differences were reported as significant only when P < 0.05.
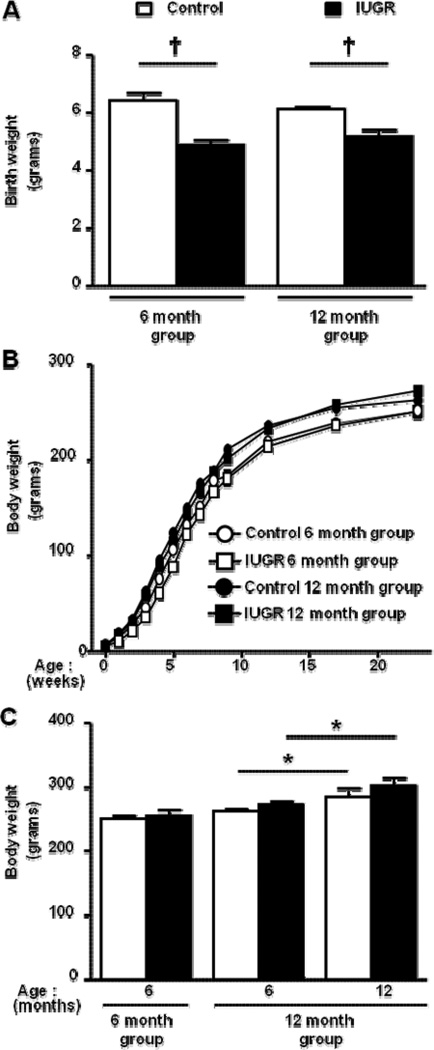
Figure 1.
Birth weight (a), growth curve (b), and body weight (c) in female control and female growth-restricted (IUGR) offspring, *P<0.05 and †P<0.001. Data values represent mean±SE.
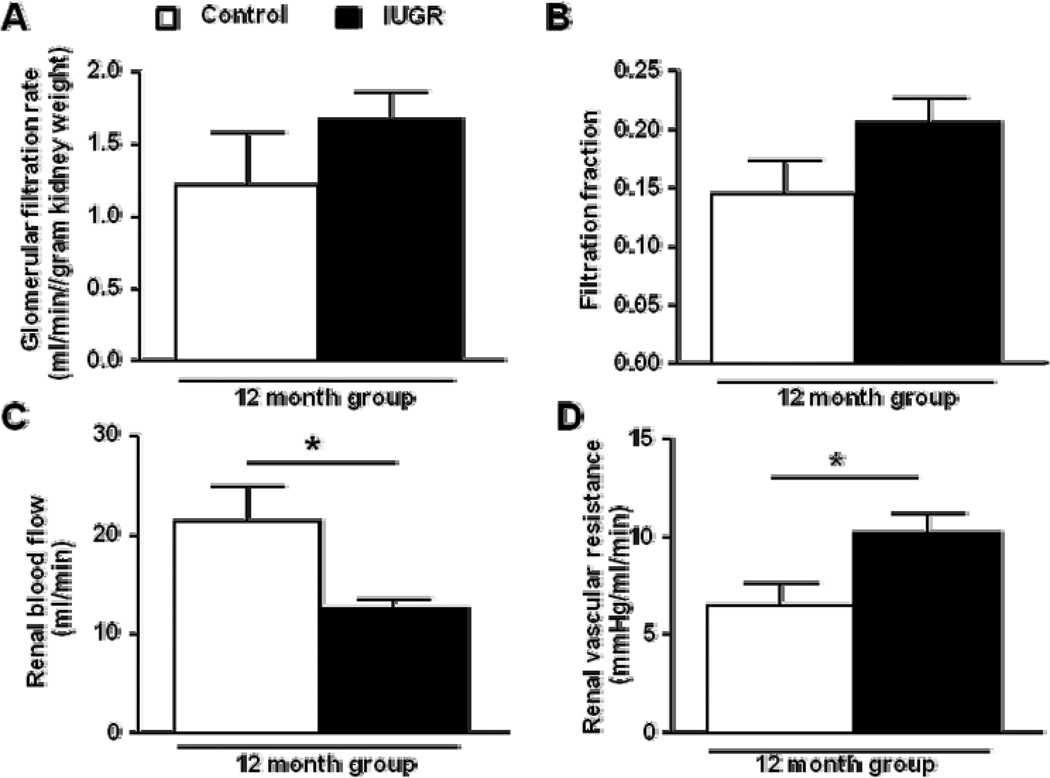
Figure 4.
Glomerular filtration rate adjusted to kidney weight (a), filtration fraction (b), renal blood flow (c) and renal vascular resistance (d) measured in conscious, chronically instrumented animals at 12 months of age (control n=5 and IUGR n=6). *P<0.05 versus age-matched control counterpart. Data values represent mean±SE.
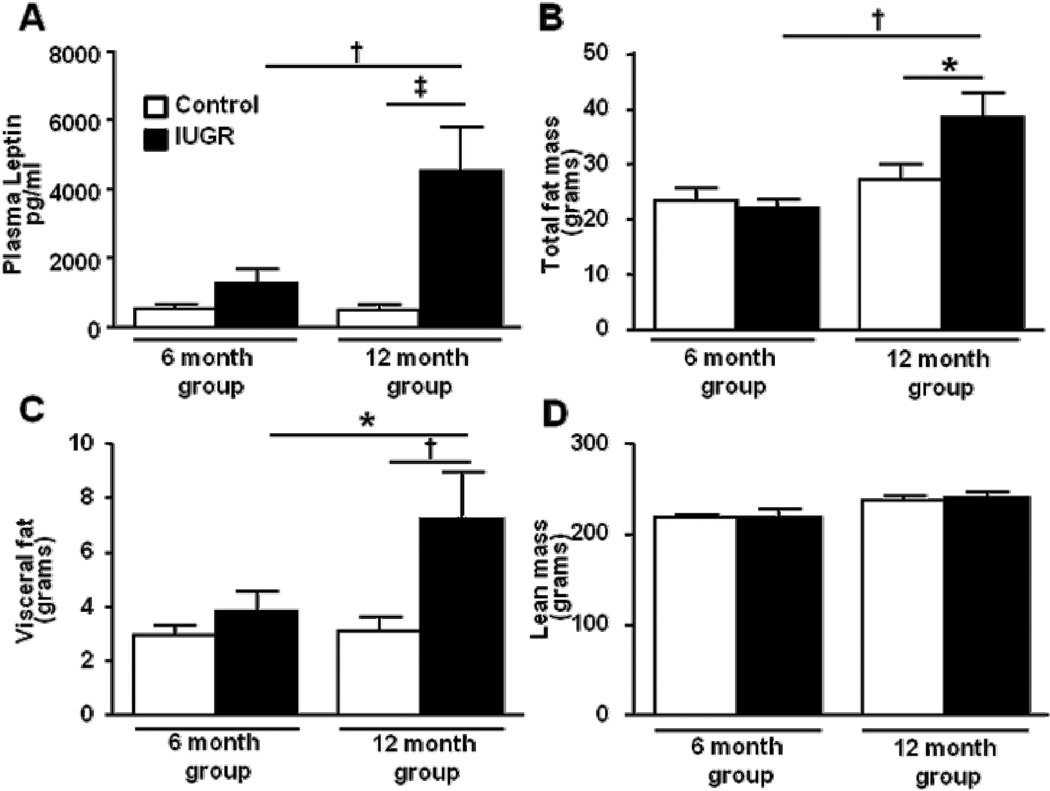
Figure 2.
Plasma leptin (a), total (b) and visceral (c) fat mass, and lean mass (d) in female control and female growth-restricted (IUGR) offspring at 6 months of age (control n=5 and IUGR n=6) and at 12 months of age (control n=14 and IUGR n=12). *P<0.05, †P<0.01, ‡P<0.001. Data values represent mean±SE.
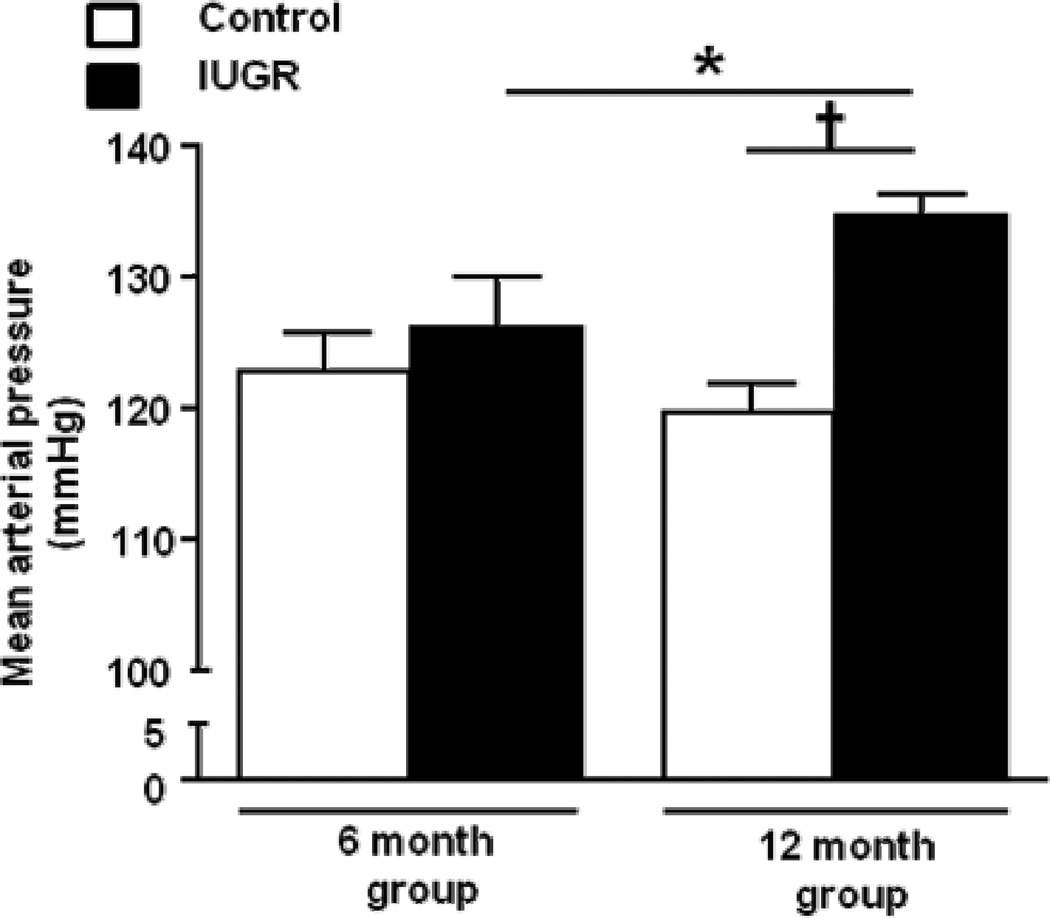
Figure 3.
Mean arterial pressure in female control and female growth-restricted (IUGR) offspring measured in conscious, chronically instrumented animals at 6 months of age (control n=5 and IUGR n= 6) and at 12 months of age (control n=14 and IUGR n=14). *P<0.05, †P<0.001. Data values represent mean±SE.
Figure 5.
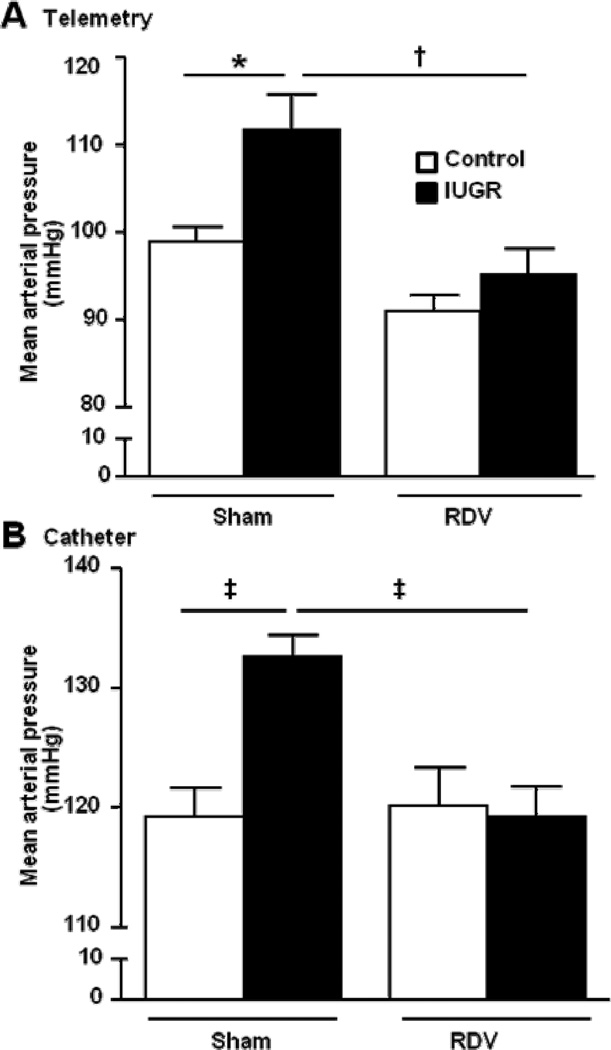
Mean arterial pressure in female control and growth-restricted (IUGR) offspring measured in freely moving animals chronically instrumented for radiotelemetry (a) or in conscious, chronically instrumented animals instrumented with carotid catheters (b) at 12 months of age 2 weeks post sham or bilateral renal denervation (control sham n=7, IUGR sham n=8, control RDV n=5, and IUGR RDV n=8). *P<0.05, †P<0.01, ‡P<0.001. Data values represent mean±SE.
RESULTS
Birth weight, body weight and growth rates
Birth weight was significantly reduced in female growth-restricted offspring compared to female control, P<0.05 (Figure 1a). However, female-growth-restricted offspring no longer exhibited a significant reduction in body weight relative to the age-matched control by two months of age (Figure 1b). Body weight did not differ at six months of age in animals studied at 6 months and 12 months of age (Figure 1c). Body weight remained similar at 12 months of age, but the body weight of female control and growth-restricted offspring was significantly greater at 12 months of age relative to the 6 month body weights (P<0.05) (Figure 1c).
Plasma leptin and body fat
No difference in plasma leptin was observed in the group studied at 6 months of age. However, circulating levels of leptin were significantly increased in female growth-restricted offspring compared to female control at 12 months of age (P<0.05) (Figure 2a). The increase in circulating leptin was associated with a marked increase in total body fat mass and visceral fat in the female growth-restricted offspring relative to female control (P<0.05) at 12 months of age (Figures 2b, 2c). Moreover, there was a positive correlation (Pearson r 0.7562; P<0.007) between body weight and fat mass in female growth-restricted offspring at 12 months of age relative to age-matched control counterparts. However, lean body mass (Figure 2d) did not differ upon comparison of female growth-restricted offspring relative to female control offspring at 12 months of age.
Mean arterial pressure and renal hemodynamic parameters
MAP when measured via arterial catheter in conscious, chronically instrumented rats was similar upon comparison of female growth-restricted offspring to female control offspring at 6 months of age. Yet, blood pressure was significantly elevated in female growth-restricted offspring relative to their age-matched controls at 12 months of age when measured via catheter (Figure 3). To determine the mechanism underlying the age-dependent increase in blood pressure in female growth-restricted offspring at 12 months of age, renal hemodynamic parameters were measured. GFR (2.18 ± 0.06 and 2.58 ± 0.28 ml/min, control versus IUGR, respectively) and GFR adjusted for kidney weight (Figure 4a) did not differ at 12 months of age (Figure 4a). FF was also not altered (Figure 4b). However, RBF (Figure 4c) and eRPF were significantly reduced in female growth-restricted offspring relative to female control (14.92 ± 03.17 and 8.14 ± 0.46 ml/min, control versus IUGR, P<0.05 respectively). In addition, the significant increase in blood pressure in female growth-restricted offspring at 12 months of age was associated with a marked increase in RVR (Figure 4d).
Effect of bilateral renal denervation on blood pressure, heart rate and renal catecholamine content
Bilateral renal denervation (RDV) initiated two weeks prior to 12 months of age in the 12 month study group abolished the age-dependent increase in blood pressure in female growth-restricted offspring normalizing it relative to MAP in the denervated female control offspring (P<0.05). This was evident regardless of whether MAP was measured via three days continuous collection by radiotelemetry (Figure 5a) or in conscious, chronically instrumented animals (P<0.05) (Figure 5b). Verification of renal denervation was confirmed by analysis of renal catecholamine levels. Renal norepinephrine content was significantly reduced by bilateral renal denervation in control and growth-restricted offspring relative to their sham denervated counterpart (213.9±18.9 versus 30.2±7.5 and 182.9±5.9 versus 29.9±7.3 pg/mg kidney tissue; control sham versus control RDV and IUGR sham versus IUGR RDV, respectively; P<0.05 sham versus RDV counterpart). Heart rate was not significantly different between female growth-restricted relative to age-matched female sham or female RDV (348.5± 9.5, 355.6±7.5, 359.2±6.5 and 359.1±7.0 beats per minute, control sham, control RDV, IUGR sham and IUGR RDV, respectively).
Effect of age on uterine weight
Uterine weight was not altered in female growth-restricted rats at 6 (1.69 ± 0.20 and 1.78 ± 0.20 grams/grams body weight; control versus IUGR, respectively) or 12 (2.83 ± 0.43 and 2.34 ± 0.22 grams/grams body weight; control versus IUGR, respectively) months of age.
DISCUSSION
This study reported numerous novel findings. First, we reported that IUGR leads to age-dependent changes at 12 months of age that were associated with an increase in total body fat mass, circulating levels of leptin and increased blood pressure in female growth-restricted offspring relative to female control. Second, we demonstrated that the increase in blood pressure in female growth-restricted offspring at 12 months of age was not associated with a marked reduction in GFR or changes in the 24-hour excretion of urinary sodium. However, RBF was significantly decreased and RVR was increased at 12 months of age in female growth-restricted offspring. Third, bilateral renal denervation abolished the increase in blood pressure in the female growth-restricted offspring normalizing blood pressure relative to renal denervated female control offspring indicating a role for the renal nerves as an underlying mechanism in the age-dependent increase in blood pressure in female growth-restricted offspring.
Sex-specific programming of high blood pressure following fetal insult is observed in many models of developmental programming (10, 11, 12, 22). Specifically, male offspring of late gestation diabetic dams (22), moderate protein restricted dams (11), and dams that undergo uteroplacental insufficiency via total ligation of the uterine vessels at day 18 gestation (10) or reduced uterine perfusion at day 14 gestation (12) exhibit a marked increase in blood pressure while female littermates remain normotensive. Previously our laboratory demonstrated a protective role for estradiol at four months of age against programmed increases in blood pressure in female growth-restricted offspring of reduced uterine perfusion dams (14). The importance of estradiol is also demonstrated in female offspring exposed to a severe (6%) reduction in maternal protein intake during fetal life (23). Characteristic changes in rodent ovarian hormonal cycles indicative of perimenopause occur around 18 months of age (24). In females rats exposed to uteroplacental insufficiency induced by bilateral uterine ligation at day 18 of gestation, no significant increase in blood pressure is observed at 18 months of age (10). However, the method for collection of arterial pressure in the aforementioned study involved tail cuff and tail arterial catheter (10). Whether an increase in adiposity occurs in this study is not reported (10). Thus, this model of uteroplacental insufficiency differs in the timing and severity of fetal insult and may be protected against age-dependent increases in arterial pressure.
As previously reported, female growth-restricted offspring are normotensive at 4 months of age (14). In the current study we report that blood pressure remained normotensive in female growth-restricted offspring relative to age-matched female control offspring at 6 months of age with hypertension present by 12 months. These findings indicate that the age-dependent increase in blood pressure in female growth-restricted offspring at 12 months of age did not develop as a direct consequence of placental insufficiency per se, but rather evolved in response to an additional influence(s) that impacted the effect of IUGR in female offspring. Hypertension can be induced in young adult female growth-restricted offspring by removal of the ovarian hormones (14). However, the aims of this study were to examine the effect of age prior to the onset of changes in ovarian hormone status, which is present by 18 months of age (24). Uterine weight, a crude indicator of ovarian hormone status (25, 26), was not altered in female growth-restricted offspring relative to age-matched female control at 12 months of age. Thus, these findings suggest that a change in ovarian status may not be a causative factor that contributes to the age-dependent increase in blood pressure in female growth-restricted offspring. Obesity is well recognized as a major risk factor for increased blood pressure (17); recent studies also implicate the importance of adiposity (27). Programming of increased adiposity is reported in models of maternal undernutrition (28, 29). In the current study, hypertension in female growth-restricted offspring was associated with an increase in total and visceral fat mass at 12 months of age. Thus, age-dependent increases in adiposity may be a causative factor in the development of hypertension in female growth-restricted offspring at 12 months of age.
Adiposity has a significant influence on blood pressure with leptin indicated to be an important mediator acting through the sympathetic nervous system (19). Increased body fat is is associated with an increase in plasma leptin in women (30). Obesity is also linked to an increase in sympathetic neural discharge in obese women (31, 32). Moreover, age can impact sympathetic neural activity and blood pressure in women independent of ovarian hormonal status (33). Although hypertension may be programmed by events that occur during fetal life (34), sympathetic overactivity may be influenced by an increase in adiposity (35) and plasma leptin (34) that develop with age. In female growth-restricted offspring at one year of age, significant increases in visceral adiposity were associated with an increase in circulating levels of leptin. Leptin, a circulating hormone produced by adipose tissue, acts on the hypothalamus to increase renal sympathetic nerve activity and blood pressure (36). Thus, one aim of this study was to delineate the importance of the renal nerves in mediating the age-dependent increase in blood pressure in female growth-restricted offspring. Bilateral renal denervation normalized blood pressure in the female growth-restricted offspring relative to renal denervated female control offspring resulting in a decrease in mean arterial pressure. Thus, findings from this study indicate that the renal nerves contribute to the development of age-dependent hypertension following IUGR in the female rat.
Development of hypertension via the action of the renal nerves may involve alterations in tubular sodium reabsorption, RVR or renin release (37). Hypertension induced by prenatal exposure to glucocorticoids is associated with an increase in renal sodium transporter abundance in male offspring through a mechanism that involves the renal nerves (38). Despite the elevated blood pressure at 12 months of age in female growth-restricted offspring, 24-hour urinary excretion of sodium did not differ upon comparison to female control offspring (Table S1). These findings suggest that female growth-restricted offspring at 12 months of age are able to maintain sodium balance at the expense of an increase in blood pressure. The age-dependent increase in blood pressure in female growth-restricted offspring at 12 months of age was associated with significant increase in RVR. Whether the effect of bilateral renal denervation on blood pressure was due to a reduction in vasomotor tone leading to an overall decrease in total peripheral resistance is not yet known. Moreover, the importance of renin in the age-dependent development of hypertension in female growth-restricted offspring requires further investigation.
Perspectives
It is well established that women have lower blood pressures than age-matched men prior to menopause (4, 5). In addition, the increase in blood pressure that occurs with age in women may be influenced to a greater degree by changes in body mass than menopausal-related changes in hormones (33). Few epidemiological studies have examined sex differences in the developmental programming of cardiovascular risk. Fewer studies have studied the impact of aging. Findings from this study indicate that age impacts programmed cardiovascular risk in female growth-restricted offspring implicating a need for further investigation into the effects of age on cardiovascular health in low birth weight women. The influence of adiposity in middle-age following low birth weight may denote a potential confounding factor on later chronic health in low birth weight women. Moreover, the importance of the renal nerves in mediating the increase in blood pressure that accompanies the age-dependent increase in adiposity in female growth-restricted offspring in this study advocates investigation into the importance of a sympathetic component in regards to future therapeutic interventions in low birth weight women.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new: Few studies have investigated sex differences in programmed cardiovascular risk; fewer studies have investigated the age-dependent changes in programmed cardiovascular risk
What is relevant? Aging was associated with a significant increase in adiposity, circulating leptin, and increased blood pressure following intrauerine growth restriction in female rats. Age-dependent increases in blood pressure in female growth-restricted rats did not develop as a direct consequence of the fetal insult, but rather was due to an additional influence that did not impact cardiovascular health in female control counterparts. An age-related increase in adiposity leading to an increase in renal sympathetic nerve activity may serve as an underlying mechanism in the age-dependent increase in blood pressure that follows intrauterine growth restriction in female rats.
Summary: Age-dependent changes may serve as a secondary influence in the developmental programming of adult blood pressure and insight from this study highlights the importance for further studies investigating the impact of age on modulating cardiovascular risk in low birth weight women.
Acknowledgments
SOURCES OF FUNDING
Dr. Alexander is supported by NIH grants HL074927 and HL51971. Dr. Intapad is supported by an American Heart Association, Post-doctoral Fellowship grant, 12POST11980021. Dr. Ojeda is supported by Norman Siegel Research Scholar Grant from the American Society of Nephrology (ASN). The authors gratefully acknowledge the assistance of the Analytical and Assay Core Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES. None
REFERENCES
- 1.Barker DJ, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol. 2009;36:445–458. doi: 10.1080/03014460902980295. [DOI] [PubMed] [Google Scholar]
- 2.Lenfant C. Low birth weight and blood pressure. Metabolism. 2008;57:S32–S35. doi: 10.1016/j.metabol.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 3.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury S, Yarows SA, O'Brien TK, Sowers JR. Ambulatory blood pressure monitoring in a nonacademic setting. Effects of age and sex. Am J Hypertens. 1992;5:616–623. doi: 10.1093/ajh/5.9.616. [DOI] [PubMed] [Google Scholar]
- 5.Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 6.Jones A, Beda A, Osmond C, Godfrey KM, Simpson DM, Phillips DI. Sex-specific programming of cardiovascular physiology in children. Eur Heart J. 2008;29:2164–2170. doi: 10.1093/eurheartj/ehn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvelin MR, Sovio U, King V, Lauren L, Xu B, McCarthy MI, Hartikainen AL, Laitinen J, Zitting P, Rantakallio P, Elliott P. Early life factors and blood pressure at age 31 years in the 1966 northern Finland birth cohort. Hypertension. 2004;44:838–846. doi: 10.1161/01.HYP.0000148304.33869.ee. [DOI] [PubMed] [Google Scholar]
- 8.Davies AA, Smith GD, May MT, Ben-Shlomo Y. Association between birth weight and blood pressure is robust, amplifies with age, and may be underestimated. Hypertension. 2006;48:431–436. doi: 10.1161/01.HYP.0000236551.00191.61. [DOI] [PubMed] [Google Scholar]
- 9.Andersson SW, Lapidus L, Niklasson A, Hallberg L, Bengtsson C, Hulthen L. Blood pressure and hypertension in middle-aged women in relation to weight and length at birth: a follow-up study. J Hypertens. 2000;18:1753–1761. doi: 10.1097/00004872-200018120-00008. [DOI] [PubMed] [Google Scholar]
- 10.Moritz KM, Mazzuca MQ, Siebel AL, Mibus A, Arena D, Tare M, Owens JA, Wlodek ME. Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J Physiol. 2009;587:2635–2646. doi: 10.1113/jphysiol.2009.170407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1131–R1136. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 12.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virdis A, Bruno RM, Neves MF, Bernini G, Taddei S, Ghiadoni L. Hypertension in the elderly: an evidence-based review. Curr Pharm Des. 2011;17:3020–3031. doi: 10.2174/138161211798157711. [DOI] [PubMed] [Google Scholar]
- 16.Huffman DM, Barzilai N. Contribution of adipose tissue to health span and longevity. Interdiscip Top Gerontol. 2010;37:1–19. doi: 10.1159/000319991. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE, Jones DW, Kuo JJ, da Silva A, Tallam LS, Liu J. Impact of the obesity epidemic on hypertension and renal disease. Curr Hypertens Rep. 2003;5:386–392. doi: 10.1007/s11906-003-0084-z. [DOI] [PubMed] [Google Scholar]
- 18.Patel SB, Reams GP, Spear RM, Freeman RH, Villarreal D. Leptin: linking obesity, the metabolic syndrome, and cardiovascular disease. Curr Hypertens Rep. 2008;10:131–137. doi: 10.1007/s11906-008-0025-y. [DOI] [PubMed] [Google Scholar]
- 19.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26:1529–1533. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 21.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45:754–758. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- 22.Katkhuda R, Peterson ES, Roghair RD, Norris AW, Scholz TD, Segar JL. Sex-specific programming of hypertension in offspring of late-gestation diabetic rats. Pediatr Res. 2012;72:352–361. doi: 10.1038/pr.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension in adult female rat offspring--influence of oestradiol. Br J Nutr. 2012;107:665–673. doi: 10.1017/S0007114511003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durbin PW, Williams MH, Jeung N, Arnold JS. Development of spontaneous mammary tumors over the life-span of the female Charles River (Sprague-Dawley) rat: the influence of ovariectomy, thyroidectomy, and adrenalectomy-ovariectomy. Cancer Res. 1966;26:400–411. [PubMed] [Google Scholar]
- 25.Lopez-Belmonte J, Nieto C, Estevez J, Delgado JL, del Prado JM. Comparative uterine effects on ovariectomized rats after repeated treatment with different vaginal estrogen formulations. Maturitas. 2012;72:353–358. doi: 10.1016/j.maturitas.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Naciff JM, Overmann GJ, Torontali SM, Carr GJ, Khambatta ZS, Tiesman JP, Richardson BD, Daston GP. Uterine temporal response to acute exposure to 17alpha-ethinyl estradiol in the immature rat. Toxicol Sci. 2007;97:467–490. doi: 10.1093/toxsci/kfm046. [DOI] [PubMed] [Google Scholar]
- 27.Rheaume C, Leblanc ME, Poirier P. Adiposity assessment: explaining the association between obesity, hypertension and stroke. Expert Rev Cardiovasc Ther. 2011;9:1557–1564. doi: 10.1586/erc.11.167. [DOI] [PubMed] [Google Scholar]
- 28.Desai M, Gayle D, Han G, Ross MG. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci. 2007;14:329–337. doi: 10.1177/1933719107303983. [DOI] [PubMed] [Google Scholar]
- 29.Venu L, Harishankar N, Prasanna Krishna T, Raghunath M. Maternal dietary vitamin restriction increases body fat content but not insulin resistance in WNIN rat offspring up to 6 months of age. Diabetologia. 2004;47:1493–1501. doi: 10.1007/s00125-004-1506-4. [DOI] [PubMed] [Google Scholar]
- 30.Shah NR, Bravemann ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body, fat, and leptin. PLos One. 2012;7:e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, Howell-Stampley T, Vonpatanasin W, Victor Rg. Overweight and sympathetic overactivity in black Americans. Hypertension. 2001;38:379–383. doi: 10.1161/01.hyp.38.3.379. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro MM, Trombetta IC, Batalha LT, Rondon MU, Forjaz CL, Barretto AC, Villares SM, Negrão CE. Muscle sympathetic nerve activity and hemodynamic alterations in middle-aged obese women. Braz J Med Biol Res. 2001;34:475–478. doi: 10.1590/s0100-879x2001000400006. [DOI] [PubMed] [Google Scholar]
- 33.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Genderselective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 34.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 35.Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JM, Coen CW, Poston L, Taylor PD. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55:76–82. doi: 10.1161/HYPERTENSIONAHA.109.139402. [DOI] [PubMed] [Google Scholar]
- 36.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53:375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 38.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol. 2008;295:F29–F34. doi: 10.1152/ajprenal.00123.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.