Abstract
Gas-phase transformation of synthetic phosphatidylcholine (PC) monocations to structurally informative anions is demonstrated via ion/ion reactions with doubly deprotonated 1,4-phenylenedipropionic acid (PDPA). Two synthetic PC isomers, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (PC16:0/18:1) and 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphocholine (PC18:1/16:0) were subjected to this ion/ion chemistry. The product of the ion/ion reaction is a negatively charged complex, [PC+PDPA-H]−. Collisional activation of the long-lived complex causes transfer of a proton and methyl cation to PDPA, generating [PC-CH3]−. Subsequent collisional activation of the demethylated PC anions produces abundant fatty acid carboxylate anions and low abundance acyl neutral losses as free acids and ketenes. Product ion spectra of [PC-CH3]− suggest favorable cleavage at the sn-2 position over the sn-1 due to distinct differences in the relative abundances. In contrast, collisional activation of PC cations is absent of abundant fatty acid chain-related product ions and typically indicates only the lipid class via formation of the phosphocholine cation. A solution phase method to produce the gas-phase adducted PC anion is also demonstrated. Product ion spectra derived from the solution phase method are similar to the results generated via ion/ion chemistry. This work demonstrates a gas-phase means to increase structural characterization of phosphatidylcholines via ion/ion chemistry.
Keywords: Ion/ion, phosphatidylcholines, charge inversion, lipids, lipidomics
INTRODUCTION
Mass spectrometry (MS) has become an essential analytical tool for the identification and structural characterization of biological macromolecules.1, 2, 3 In tandem MS (MS/MS or MSn), the polarity in which the analyte ion is most efficiently formed does not always provide sufficient structural information upon collision-induced dissociation (CID). For instance, phosphatidylcholines (PC), a subclass of glycerophospholipids, are efficiently ionized in the positive polarity, but their cationic versions generate limited structural information upon CID. PCs compose thirty percent of cell membranes in mammalian cells4 and can act as secondary messengers in metabolic signaling/pathways.5 PCs are composed of a glycerol backbone with two fatty acid chains attached at the sn-1 and sn-2 positions while a phosphocholine moiety is attached at the sn-3 position. Due to the presence of a fixed positive charge quaternary amine, PCs readily generate gas-phase cations in positive ion mode electrospray ionization (ESI).6 However, collisional activation of PC cations generally yields the phosphocholine moiety (184 m/z) as the dominant and often exclusive product. The phosphocholine fragment ion provides insight on only the phospholipid class and does not provide structural information about the individual acyl chains.7, 8 Elucidating the composition of the individual fatty acid chains is important due to the diverse physical properties of PCs (i.e., the number of carbons and extent of unsaturation) as these properties relate to their lipid biochemistry.9, 10, 11 With the presence of the choline moiety, PCs cannot be ionized in the negative polarity by deprotonation6 and therefore structural information of the individual fatty acid chains cannot be easily accessed without solution phase additives (see below). Traditionally this might have been addressed by gas chromatographic analysis of the hydrolyzed and derivatized fatty acids arising from the PC fraction of the lipid extract, but this process results in a pooling of all fatty acids and a loss of molecular information.12
Efforts to better characterize PCs by tandem MS have included CID studies of adducted PCs in both the positive13 and negative ion modes.8, 14, 15, 16 Gas-phase lithium adducts of PCs generated by positive ESI of solution phase PC mixtures have shown neutral losses of lithiated fatty acid chains (i.e., RCO2Li, where R is the alkyl chain) upon CID. The difference in m/z between the product and precursor ion corresponds to the degree of unsaturation and chain length. However, the structurally informative peaks were of low relative abundance and the spectra were complicated by additional neutral losses.13 In the negative ion mode approach, millimolar concentrations of salts were added to a PC solution to produce gas-phase adducted PC anions (e.g., [PC+Cl]− and [PC+CH3CO2]−). Collisional activation of the adducted PC anions produced a neutral loss comprised of the counter anion and a methyl cation from the quaternary ammonium (i.e., CH3Cl and CH3CO2CH3), yielding [PC-CH3]−. Subsequent CID of [PC-CH3]− generated fatty acid carboxylate anions (RCO2−) and neutral losses associated with free acids (RCO2H) or ketene derivatives (RCHCO), which are all indicative of the composition of the individual fatty acid chains.8, 14–16 In both of the solution phase methods, the high salt concentrations can lead to poor ionization efficiency, spectral complexity upon ionization, and incompatibilities with liquid chromatography (LC) solvent systems.
The type of ion subjected to tandem MS is usually determined by the ionization method and conditions. An alternative approach is to transform in the gas-phase an ion-type that is readily generated by an ionization method to another ion-type that more readily yields structural information of interest, preferably mass-selection. Gas-phase ion/ion reactions have been shown to be particularly useful for many ion transformations,17, 18 particularly when conducted within the context of MSn, whereby the analyte and reagent ions are each mass-selected prior to reaction. The use of ion/ion reactions within the context of an MSn experiment allows the definition of the ion-type to be de-coupled from the initial ionization conditions.19 As a result, the ion-type subjected to activation can be independently optimized to generate the most informative fragmentation. Ion/ion reactions have been utilized in acid/base reactions (i.e., proton transfer), 20, 21 reduction/oxidation reactions (i.e., electron transfer), 22, 23 and covalent24, 25, 26, 27 and/or electrostatic modification.28, 29 Previous studies have demonstrated the significance of ion-type transformation and its relation to increased sequence coverage.30, 31, 32 For instance, MALDI-derived tryptic peptide monocations covalently and electrostatically modified with benzene disulfonic acid dianions via ion/ion reactions demonstrated increased sequence coverage upon collisional activation, especially in the form of b-type ions.33
In the case of PC ions, an example of gas-phase ion-type transformation via ion/ion chemistry has been demonstrated in the electron transfer dissociation (ETD) of doubly sodiated PC cations, [PC+2Na]2+ using azobenzene radical anions as the ETD reagent. ETD induced cleavages at each ester linkage (i.e., neutral loss of a fatty acid chain) with additional neutral loss of the quaternary nitrogen moiety. CID of the charged reduced electron transfer product that did not undergo dissociation (i.e., the ETnoD ion), [M+2Na]+•, generated results similar to the electron transfer reaction. Similar to the lithiated PC cation study, this method provided information on the number of carbons and the degree of unsaturation for each fatty acid chain via neutral losses.34
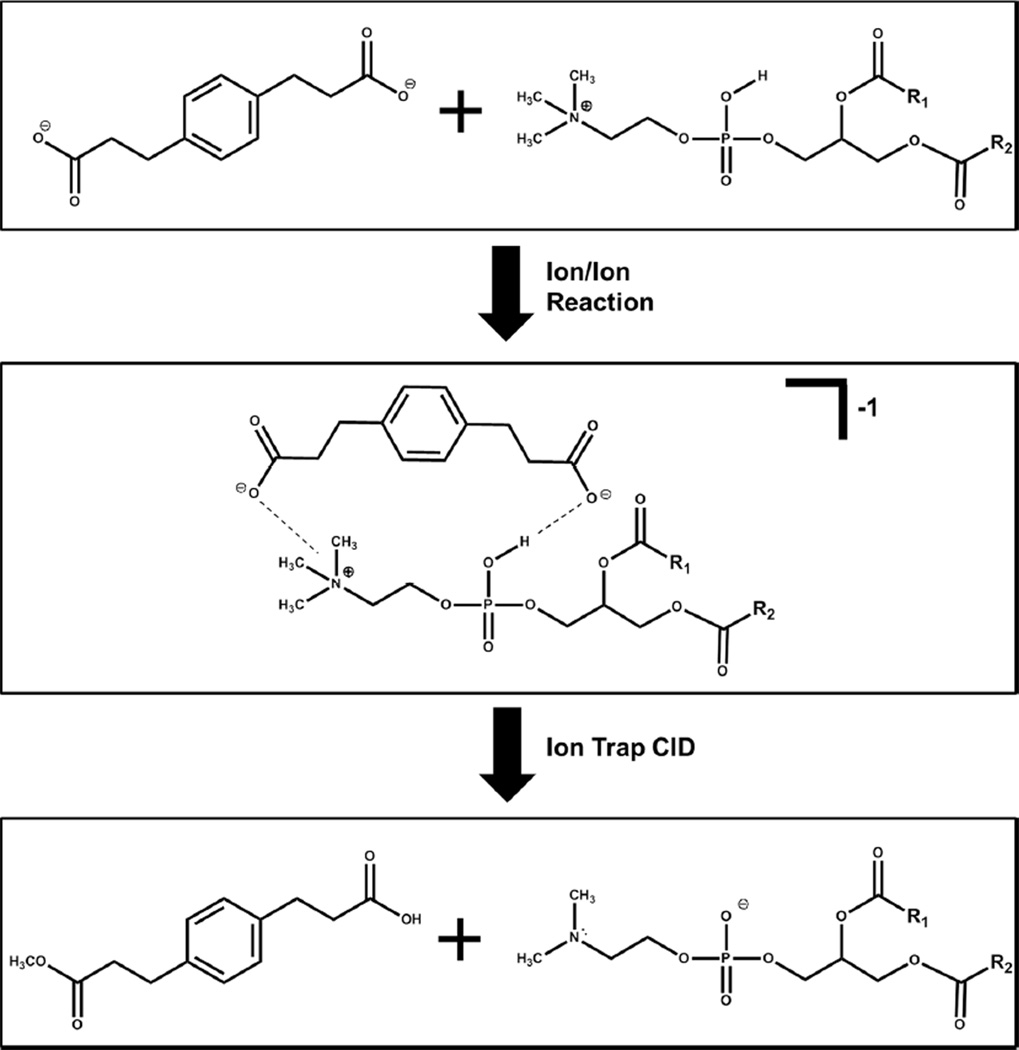
In this study, we demonstrate the gas phase transformation of PC monocations into demethylated PC anions (i.e., [PC-CH3]−) via ion/ion reactions, where pre- and post-transformation structural characterization is compared. PC monocations are subjected to ion/ion reactions with doubly deprotonated 1,4-phenylenedipropionic acid (PDPA). The product of the ion/ion reaction is a negatively charged complex, comprised of PDPA and PC, i.e. [PC+PDPA-H]−. Collisional activation of the complex generates [PC-CH3]− as seen in process (1):
| (1) |
Gas-phase ion/ion reactions have been performed on synthetic PC isomers, 1-palmitoyl-2-oleoylsn-glycero-3-phosphocholine (16:0/18:1 PC) and 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphocholine (18:1/16:0 PC). Collisional activation of [PC-CH3]− generates large relative contributions from fatty acid carboxylate anions (RCO2−) and less abundant neutral losses of fatty acids (RCO2H) and ketene derivatives (RCHCO), whereas collisional activation of [PC+H]+ produces exclusively the phosphocholine cation. Transformation of PC monocations via ion/ion reactions facilitates the production of structurally informative anions and the identification of fatty acid composition without recourse to solution phase salt additives.
EXPERIMENTAL SECTION
Materials
Methanol, chloroform, and ammonium hydroxide were purchased from Mallinckrodt (Phillipsburg, NJ). Acetonitrile and 1,4-phenylenedipropionic acid were purchased from Sigma-Aldrich (St. Louis, MO). 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine and 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphocholine were purchased from Avanti Polar Lipids, Inc (Alabaster, AL). PC analytes were prepared at a concentration of ~30 μM in a solution of 50/50 (v/v) chloroform/methanol prior to positive nanoelectrospray ionization (nESI). The reagent anion, PDPA, was prepared at a concentration of ~ 220 μM in a solution of 49.5/49.5/1 (v/v/v) acetonitrile/methanol/ammonium hydroxide prior to negative nESI.
Solution Phase PDPA Spiking
A solution mixture of PC (i.e., PC16:0/18:1 or PC18:1/16:0) and PDPA was prepared at concentrations of 10 μM and 140 μM, respectively. The solution was subjected to negative mode nESI, where MSn analyses and subsequent mass analysis were performed in Q3.
Mass Spectrometry
All experiments were performed on a QTRAP 4000 triple quadrupole/linear ion trap mass spectrometer (AB SCIEX, Concord, ON, Canada), which has been modified to perform ion/ion reactions.35 Alternately pulsed nESI emitters sequentially inject mass-selected anions and cations into the q2 reaction cell.36, 37 Doubly deprotonated PDPA anions were first ionized and accumulated in the q2 reaction cell. Next, the singly charged PC cations were ionized and transferred to the q2 cell to undergo a mutual storage reaction for 1000 milliseconds. Following accumulation of the doubly deprotonated PDPA anions and throughout the ion/ion reaction period in the transformation efficiency study, any singly charged PDPA anions that were formed from ion/molecule reactions due to adventitious basic vapors or ion/ion reactions were subjected to continuous resonance ejection to minimize the possibility of singly charged anions reacting with the singly charged cations. Product anions resulting from the ion/ion reaction were transferred to Q3 to perform MSn analyses and subsequent mass analysis via mass-selective axial ejection (MSAE).38
RESULTS AND DISCUSSION
Transformation of PC cations
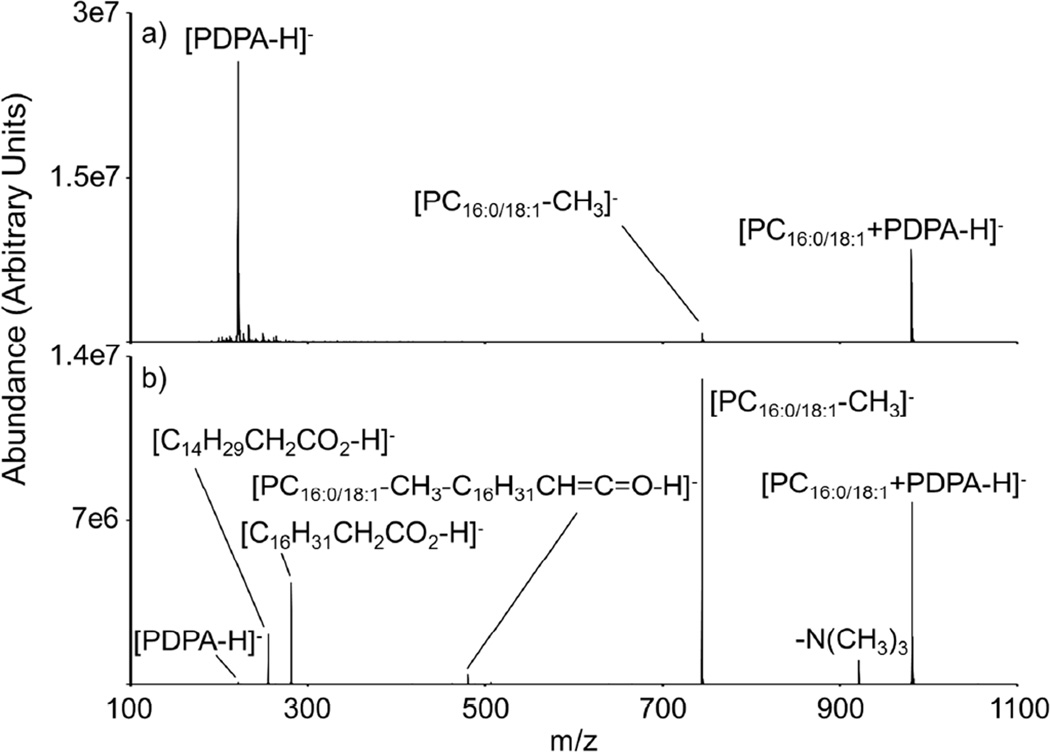
Two PC isomers, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (16:0/18:1 PC) and 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphocholine (18:1/16:0 PC), have been subjected to ion/ion reactions with doubly deprotonated PDPA. The ion/ion reaction produces a negatively charged complex (i.e., [PC+PDPA-H]−), which is stabilized by a strong electrostatic interaction between the fixed charge quaternary ammonium group of PC and a carboxylate of PDPA (Scheme 1).25 Figure 1 (a) illustrates the product ion spectrum following an ion/ion reaction between [PDPA-2H]2− anions and [PC16:0/18:1+H]+ cations. The post ion/ion reaction spectrum displays product ions corresponding to [PC16:0/18:1+PDPA-H]− and a charge reduced reagent anion (i.e., [PDPA-H]−). Proton transfer to the reagent dianion can occur via two pathways: a fly-by mechanism or charge transfer upon separation of the long-lived complex.39
Scheme 1.
The sequence of generating [PC-CH3]− via ion/ion reactions between PDPA dianions and PC monocations. Top, mass-selected precursor ions; middle, electrostatic complex generated via a mutual storage ion/ion reaction; bottom, the main products following collisional activation of the negative complex.
Figure 1.
(a) Product ion spectrum following ion/ion reactions between PDPA dianions and 16:0/18:1 PC monocations, (b) Ion trap CID of the long-lived complex, [PC16:0/18:1+PDPA-H]−
Subsequent isolation and collisional activation of the long-lived complex, [PC16:0/18:1+PDPA-H]−, produces a highly abundant [PC16:0/18:1-CH3]− ion along with consecutive fragments consistent with charged and neutral losses of the fatty acid chains (Figure 1(b)). Observation of the demethylated PC anions indicates methyl cation and proton transfers to the anionic reagent within dissociation of the complex, thus producing a neutralized PDPA reagent (see Scheme 1). The observation of [PC-CH3]− mirrors the results of solution phase acetate and chloride salt experiments, where collisional activation of [PC+CH3CO2H]− or [PC+Cl]− generateda demethylated PC anion.8, 14–16 Gas-phase methyl cation transfer between carboxylates and quaternary ammonium groups has been observed previously within the context of an ion/ion reaction.27 Alkyl (i.e., ethyl, propyl, butyl, isobutyl) cation transfer has also been observed upon CID of ESI-generated complexes composed of sulfophenyl benzoic acid dianions and tetraalkylammonium monocations.40 Collisional activation of the gas-phase anionic complex produced either proton or alkyl cation transfer to the sulfophenyl benzoic acid dianions, as similarly seen in this work with collisional activation of PDPA-adducted PCs. The low relative abundance of the proton transfer product (i.e., [PDPA-H]−) in the dissociation spectrum is particularly noteworthy (Figure 1(b)). This low contribution of [PDPA-H]− compared to [PC16:0/18:1-CH3]− suggests that transfer of a proton and methyl cation upon collisional activation is a dominant pathway, while sole proton transfer without methyl cation transfer is a less favorable pathway. The other PC isomer, 18:1/16:0 PC, yields similar results to the 16:0/18:1 compound for both the ion/ion reaction and subsequent collisional activation of the long-lived complex (Supporting Information, S-1).
The analytical utility of the gas-phase transformation of PC cations to demethylated PC anions is dependent upon the value of the information gained from changing one ion-type to another, as discussed below, as well as the reaction efficiency. A lower limit to the efficiency of converting PC16:0/18:1 cations to the anion complex [PC16:0/18:1-CH3]− can be derived from the spectrum of Figure 1(a) from the ratio [PDPA-H]−/([PDPA-H]−+[PC16:0/18:1-CH3]−). This yields an efficiency of roughly 25%. However, a significant fraction of the observed [PDPA-H]− signal in Figure 1(a) arises from ion/molecule proton transfer from polar vapors present in the vacuum system. Furthermore, the accumulation of [PDPA-H]− due to ion/ion and ion/molecule reactions leads to an increase in the neutralization of the PC cations from ion/ion reactions with the [PDPA-H]− anions. We therefore chose to measure an “operational” efficiency of transforming PC16:0/18:1 cations to demethylated PC anions, which is defined as:41, 42
where Σ[PC-CH3-H] is the abundance of demethylated PC anions produced upon CID of the long-lived complex ([PC+PDPA-H]−), Σ(ConFragments) is the abundance of consecutive fragments from [PC-CH3]− following CID of the long-lived complex, ΣPCi is the abundance of [PC+H]+ after isolation and before ion/ion transformation, and ΣPCf is the abundance of residual [PC+H]+ after transformation. A resonance ejection voltage was applied throughout the reaction period to eject [PDPA-H]− ions generated by ion/ion and ion/molecule reactions to minimize any neutralization of the PC cations associated with ion/ion reactions with [PDPA-H]−. By subtracting PCi from PCf, the transformation efficiency is limited to the fraction of [PC+H]+ that undergoes an ion/ion reaction with [PDPA-2H]2−. The transformation efficiency determined in this way represents the maximum possible efficiency for conversion of [PC+H]+ to [PC-CH3]− because it represents the value that would be obtained if all reactant cations undergo an ion/ion reaction. Complete depletion of analyte ions is achievable provided there is an excess of reagent ions, there is good overlap in the ion populations, and the reaction time is sufficient to allow for the reaction to go to completion. It is also emphasized here that the measurement yields an “operational” efficiency rather than an absolute efficiency due to possible differences in detection efficiencies for positive ions and negative ions. The absolute values for voltages applied to the conversion dynode and multiplier were the same in both detection polarities with the only difference being the polarity of the conversion dynode. In this study, the transformation efficiency of PC cations to demethylated PC anions was measured to be 49 ± 7%. The major loss process is expected to be neutralization of the cation by [PDPA-2H]2− as there are no major competing charge inversion channels and little resonance ejection of the [PC+PDPA-H]− complex is expected under the collisional activation conditions used to dissociate it. Overall, the results demonstrate that the ion/ion charge inversion/CID process used to generate the [PC-CH3]− ions is clearly efficient enough for analytical use.
MS/MS of [PC-CH3]− Anions
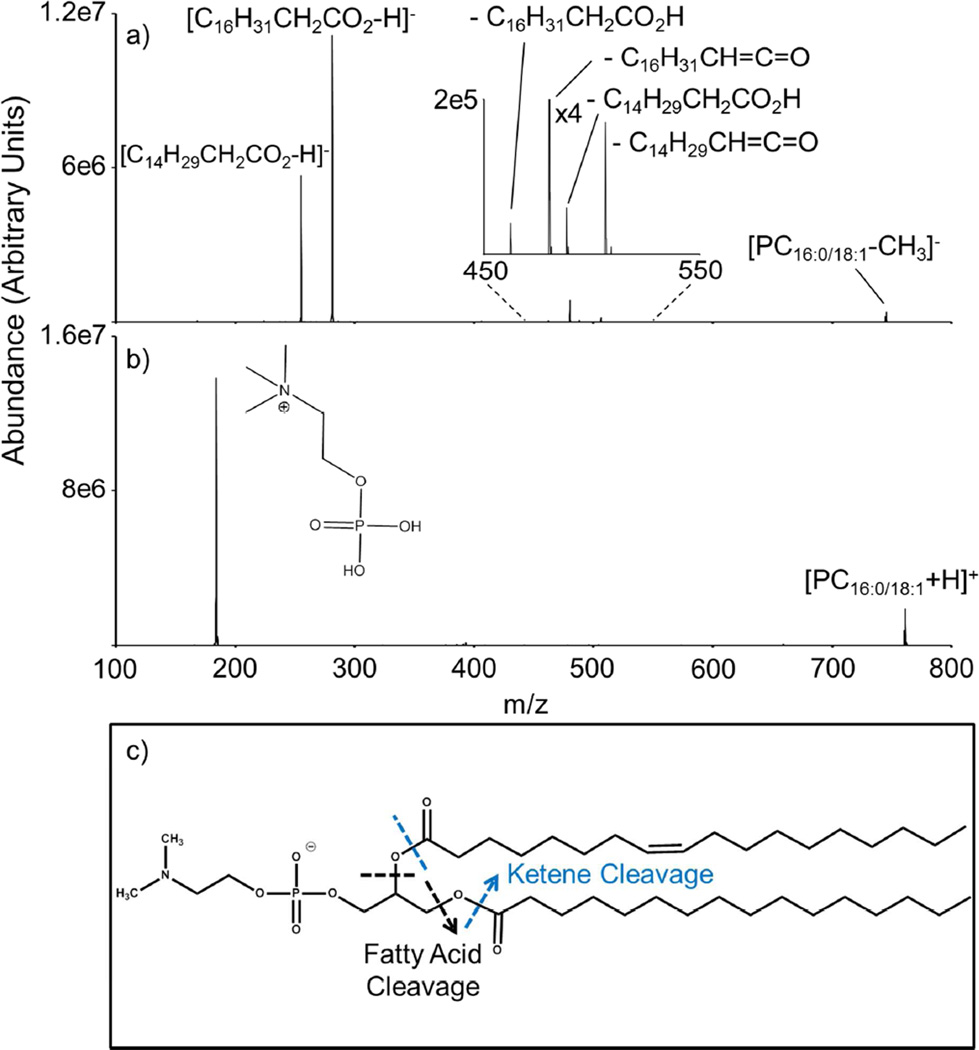
Ion trap CID of 16:0/18:1 PC prior to and after ion/ion transformation (i.e., [PC16:0/18:1+H]+ and [PC16:0/18:1-CH3]−) are compared in Figure 2. Collisional activation of [PC16:0/18:1-CH3]− produces a structurally informative product ion spectrum, where loss of neutral and charged fatty acid chains are observed (Figure 2 (a)). These fragment ions allow for the determination of chain length and the degree of unsaturation. The most favorable dissociation pathway appears to be the generation of a charged fatty acid chain from the sn-1 or sn-2 position, i.e., [C16H31CH2COO-H]− and [C14H29CH2COO-H]−. Neutral losses of fatty acids as ketenes (e.g., [PC16:0/18:1-CH3-C14H29CH=C=O-H]−) and free fatty acids (e.g., [PC16:0/18:1-CH3-C14H29CH2COOH-H]−) are also observed in low abundance. Similar dissociation behavior of demethylated PC anions8, 14–16 and other glycerophospholipids43, 44, 45, 46 has been noted previously in the literature. In contrast, CID of the [PC16:0/18:1+H]+ ion yields the phosphocholine cation as the strongly dominant product (Figure 2(b)). Figure 2(c) depicts the relationship between bond cleavage and product ion-type (e.g., cleavage of the ester bond leads to charged or neutral loss of a single fatty acid chain). In addition, a comparison of sn-cleavage position reveals a larger contribution from ions produced from the cleavage at the sn-2 position (i.e., [C16H31CH2COOH]− and [PC16:0/18:1-CH3-C16H31CH=C=O-H]−) compared to those produced from cleavage at the sn-1 position (i.e., [C14H29CH2COO-H]− and [PC16:0/18:1-CH3-C14H29CH=C=O-H]−). Ekroos et al. noted the relative favorability of sn-2 cleavage in synthetic PC isomers and biological samples;14 however, structural characterization via relative abundance comparisons can be misleading due to the likely isomeric mixtures present in synthetic and natural samples. Additionally, relative abundance ratios of the sn-1 and sn-2 position were previously demonstrated to be dependent on fatty acid chain length and degree of saturation, thus suggesting caution in assigning exact structures.47 Collisional activation of the remaining demethylated PC isomer, [PC18:1/16:0-CH3]−, generates fragment ions analogous to those generated from [PC16:0/18:1-CH3]−, as well as increased structural information compared to the protonated version (S-2). While caution should be exercised in the interpretation of these ion abundances, the fact that these low abundant spectral features can be detected following charge inversion presents a significant advantage over analysis in the positive ion mode alone.
Figure 2.
(a) Ion trap CID of [PC16:0/18:1-CH3]−. (b) Ion trap CID of [PC16:0/18:1+H]+. (c) Relating bond cleavage and product ion type for [PC16:0/18:1-CH3]−.
Solution Phase PDPA Mixture
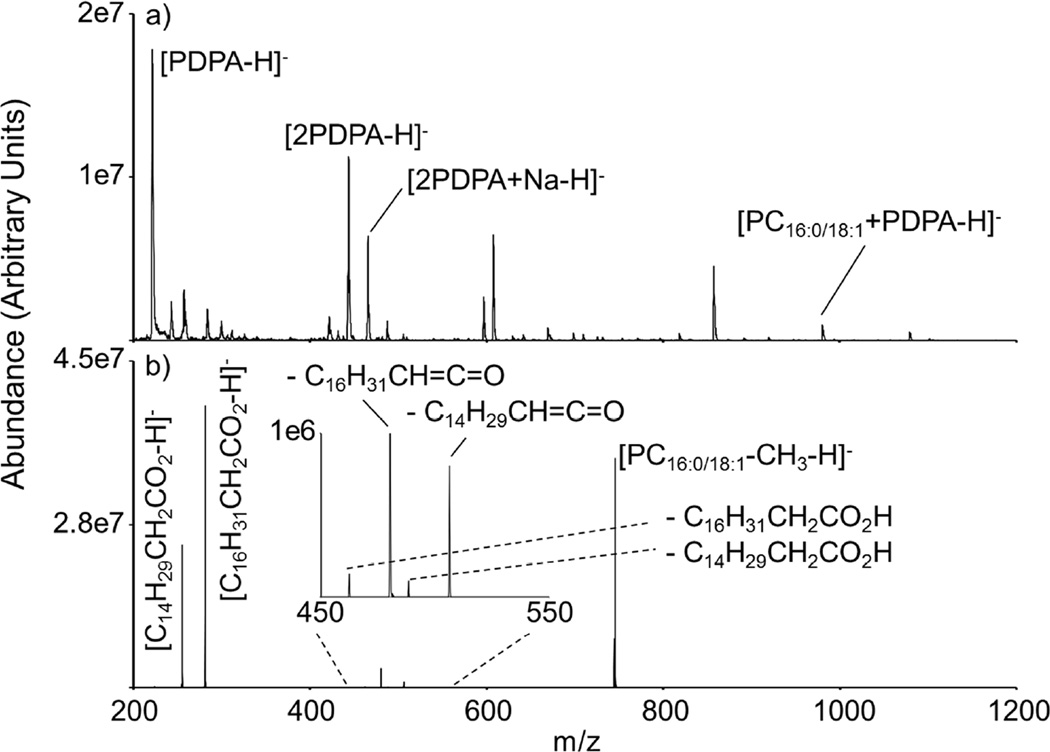
The preceding results demonstrate the formation of a [PC+PDPA-H]− complex, which dissociates to the [PC-CH3]− ion that can yield structurally informative products upon CID in the gas-phase. We also sought to generate such a complex directly from solution for two reasons. First, generation of the complex directly from solution would enable access to the precursor ion of interest without recourse to gas-phase ion/ion reactions, in analogy with the work of Ekroos et al.14 Of course, such an approach might also require a separate positive ion experiment to confirm the phospholipid class information. Second, we were interested to see if a complex formed in solution would differ in its CID behavior from that of the complex formed via ion/ion reaction. Direct ionization of a solution phase mixture containing PDPA and 16:0/18:1 PC via negative mode nESI is illustrated in Figure 3(a). Ionization of the mixture produces low abundance PDPA-adducted PC anions (i.e., [PC16:0/18:1+PDPA-H]−) among dimers of PDPA and substantial chemical noise. Collisional activation of the complex produces the demethylated PC anion (data not shown). Ion trap CID of the demethylated PC anion (Figure 3(b)) generates similar dissociation behavior compared to the analogous [PC16:0/18:1-CH3]− produced via ion/ion reactions (Figure 2(a)). The solution phase method clearly exhibits spectral complexity as well as limited ion signal from the adducted PC anion upon ionization, which could lead to complications in complex mixture analysis. The main advantages of the ion/ion chemistry are the high degree of control over the identities of the reactants via mass-selection prior to reaction, the avoidance of spectral complexity that can arise in solution phase reaction mixtures, the avoidance of ion suppression in the ionization step arising from the high salt concentration in the sample, and ready access to the original [PC+H]+ ions and the product [PC-CH3]− ion from a single solution and set of ionization conditions. Solution phase experiments with 18:0/16:0 PC (S-3) produced results similar to those observed with 16:0/18:1 PC. The dissociation behavior of [PC18:0/16:0-CH3]− derived from the solution phase is also similar to that observed with the ion/ion chemistry.
Figure 3.
(a) Ionization of PDPA and 16:0/18:1 solution phase mixture via negative mode nESI (b) Ion trap CID of [PC16:0/18:1-CH3]−
CONCLUSIONS
Synthetic phosphatidylcholine monocations have been subjected to ion/ion reactions with doubly deprotonated PDPA. Collisional activation of the long-lived complex, comprised of PDPA and PC, generates a demethylated PC anion (i.e., [PC-CH3]−), indicating transfer of a proton from the phosphate group and methyl cation from the quaternary amine group to the carboxylate groups of PDPA. Ion trap CID of [PC-CH3]− generates product ions consistent with charged fatty acid chains and neutral losses of the acyl chains from the PC analyte as free acids and ketenes. Fragmentation spectra generate structurally informative product ions, which allows for the elucidation of fatty acid composition (i.e., the number of carbons and degree of unsaturation in each acyl chain). The dissociation behavior of demethylated PC anions also exhibits preferential cleavages at the sn-2 over the sn-1 position as reflected in the relative abundances. The dissociation behaviors in this study are consistent with previous solution phase experiments reported in the literature. Solution phase studies demonstrated here, where a mixture of PC and PDPA are directly ionized via negative mode nESI, show that the complex that leads to demethylated PC anions can also be formed directly in solution, although with relatively low abundance. The ion/ion reaction approach is attractive in that a single set of ionization conditions in one polarity, which can be optimized for [PC+H]+ formation, can be used to generate two ion-types, the [PC+H]+ and [PC-CH3]− ions, that provide complementary information upon CID.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by AB Sciex and the National Institutes of Health under Grant GM 45372. S.J.B. Also acknowledged support from the Australian Research Council through the Centres of Excellence (CE0561607) and Discovery (DP120102922) Programs.
References
- 1.Hunt DF, Yates JR, Shabanowitz J, Winston S, Hauer CR. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6233–6237. doi: 10.1073/pnas.83.17.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biemann K, Martin SA. Mass Spectrom. Rev. 1987;6:1–75. [Google Scholar]
- 3.Aebersold R, Mann M. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 4.Kawai K, Fujita M, Nakao M. Biochim. Acta. 1974;369:222–233. [PubMed] [Google Scholar]
- 5.Zeisel SH, Blusztajn JK. Annu. Rev. Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 6.Han X, Gross R. Proc. Natl. Acad. Sci. USA. 1994;91:10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Proc. Natl. Acad. Sci. USA. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han X, Gross R. J. Am. Soc. Mass Spectrom. 1995;6:1202–1210. doi: 10.1016/1044-0305(95)00568-4. [DOI] [PubMed] [Google Scholar]
- 9.Farooqui AA, Horrocks LA. Cell. Mol. Neurobiol. 1998;18:599–608. doi: 10.1023/a:1020625717298. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell TW, Pham H, Thomas MC, Blanksby SJ. J. Chromatog. B. 2009;877:2722–2735. doi: 10.1016/j.jchromb.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Brown SHJ, Mitchell TW, Oakley AJ, Pham HT, Blanksby SJ. J. Am. Soc. Mass Spectrom. 2012;23:1441–1449. doi: 10.1007/s13361-012-0410-2. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell TW, Pham H, Thomas MC, Blanksby S. J. J Chromatogr. B. 2009;877:2722–2735. doi: 10.1016/j.jchromb.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Hsu FF, Bohrer A, Turk J. J. Am. Soc. Mass Spectrom. 1998;9:516–526. doi: 10.1016/S1044-0305(98)00012-9. [DOI] [PubMed] [Google Scholar]
- 14.Ekroos K, Ejsing C, Bahr U, Karas M, Simons K, Shevchenko A. J. Lipid Res. 2003:2181–2192. doi: 10.1194/jlr.D300020-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Harrison KA, Murphy RC. J. Mass Spectrom. 1995;30:1772–1773. [Google Scholar]
- 16.Kerwin JL, Tuininga AR, Ericsson LH. J. Lipid Res. 1994;35:1102–1114. [PubMed] [Google Scholar]
- 17.McLuckey SA, Huang T-Y. Anal. Chem. 2009;81:8669–8676. doi: 10.1021/ac9014935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLuckey SA, Mentinova M. J. Am. Soc. Mass Spectrom. 2011;22:3–12. doi: 10.1007/s13361-010-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLuckey SA. Eur. J. Mass Spectrom. 2010;16:429–436. doi: 10.1255/ejms.1031. [DOI] [PubMed] [Google Scholar]
- 20.Stephenson JL, Jr, McLuckey SA. J. Am. Chem. Soc. 1996;118:7390–7397. [Google Scholar]
- 21.Stephenson JL, Jr, McLuckey SA. Anal. Chem. 1996;68:4026–4032. doi: 10.1021/ac9605657. [DOI] [PubMed] [Google Scholar]
- 22.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc. Natl. Acad. Sci. USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coon JJ, Shabanowitz J, Hunt DF, Syka JEP. J. Am. Soc. Mass Spectrom. 2005;16:880–882. doi: 10.1016/j.jasms.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Han H, McLuckey SA. J. Am. Chem. Soc. 2009;131:12884–12885. doi: 10.1021/ja904812d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mentinova M, McLuckey SA. J. Am. Chem. Soc. 2010;132:18248–18257. doi: 10.1021/ja107286p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGee WM, Mentinova M, McLuckey SA. J. Am. Chem. Soc. 2012;134:11412–11414. doi: 10.1021/ja304778j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prentice BM, Gilbert JD, Stutzman JR, Forrest WP, McLuckey SA. J. Am. Soc. Mass Spectrom. doi: 10.1007/s13361-012-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He M, Emory JF, McLuckey SA. Anal. Chem. 2005;77:3173–3182. doi: 10.1021/ac0482312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stutzman JR, Luongo CA, McLuckey SA. J. Mass Spectrom. 2012;47:669–675. doi: 10.1002/jms.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassell KM, Stutzman JR, McLuckey SA. Anal. Chem. 2010;82:1594–1597. doi: 10.1021/ac902732v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mentinova M, McLuckey SA. Int. J Mass Spectrom. 2011;308:133–136. doi: 10.1016/j.ijms.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stutzman JR, Hassel KM, McLuckey SA. Int. J Mass Spectrom. 2012;312:195–200. doi: 10.1016/j.ijms.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stutzman JR, McLuckey SA. Anal. Chem. 2012;84:10679–10685. doi: 10.1021/ac302374p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang X, Liu J, LeBlanc Y, Covey T, Ptak AC, Brenna JT, McLuckey SA. J. Am. Soc. Mass Spectrom. 2007;18:1783–1788. doi: 10.1016/j.jasms.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia Y, Wu J, Londry FA, Hager JW, McLuckey SA. J. Am. Soc. Mass Spectrom. 2005;16:71–81. doi: 10.1016/j.jasms.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Liang X, Xia Y, McLuckey SA. Anal. Chem. 2006;78:3208–3212. doi: 10.1021/ac052288m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang X, Han H, Xia Y, McLuckey SA. J. Am. Soc. Mass Spectrom. 2007;18:369–376. doi: 10.1016/j.jasms.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Londry FA, Hager JW. J. Am. Soc. Mass Spectrom. 2003;14:1130–1147. doi: 10.1016/S1044-0305(03)00446-X. [DOI] [PubMed] [Google Scholar]
- 39.Wells JM, Chrisman PA, McLuckey SA. J. Am. Chem. Soc. 2003;125:7238–7249. doi: 10.1021/ja035051l. [DOI] [PubMed] [Google Scholar]
- 40.Gronert S. Acc. Chem. Res. 2003;36:848–857. doi: 10.1021/ar020042n. [DOI] [PubMed] [Google Scholar]
- 41.Hassell KM, LeBlanc Y, McLuckey SA. Anal. Chem. 2011;83:3252–3255. doi: 10.1021/ac200439k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassell KM, Hilger RT, McLuckey SA. Phys. Chem. Chem. Phys. 2011;13:18418–18427. doi: 10.1039/c1cp21581g. [DOI] [PubMed] [Google Scholar]
- 43.Hsu FF, Turk J. J. Am. Soc. Mass Spectrom. 2000;11:797–803. doi: 10.1016/S1044-0305(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 44.Hsu FF, Turk J. J. Am. Soc. Mass Spectrom. 2000;11:892–899. doi: 10.1016/S1044-0305(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 45.Hsu FF, Turk J. J. Am. Soc. Mass Spectrom. 2000;11:986–999. doi: 10.1016/S1044-0305(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 46.Hsu FF, Turk J. J. Am. Soc. Mass Spectrom. 2000;11:1036–1043. doi: 10.1016/S1044-0305(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 47.Huang Z, Gage DA, Sweeley CC. J. Am. Soc. Mass Spectrom. 1992;3:71–78. doi: 10.1016/1044-0305(92)85020-K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.