Abstract
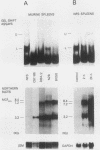
Using in vitro protein binding and in vivo functional studies, we have identified novel regulatory sequences near the 5' end of murine leukemia virus (MuLV) long terminal repeats (LTRs). These sequences are highly conserved in all MuLV LTRs as well as in feline leukemia virus and gibbon ape leukemia virus LTRs. In this upstream conserved region (UCR), gel retardation assays detected two overlapping but distinct binding sites (UCR-U and UCR-L) for nuclear proteins (UCRF-U and UCRF-L). Three lines of evidence suggest a negative regulatory role for the UCR in viral transcription: (i) an inverse correlation was found between MuLV transcripts and nuclear proteins binding the UCR in the spleens of five different mouse strains; (ii) in vivo treatment of NFS mice with lipopolysaccharide resulted in the induction of splenic viral transcripts and the concomitant disappearance of UCR-binding proteins; and (iii) in mouse L cells transfected with an MuLV LTR linked to the chloramphenicol acetyltransferase (CAT) gene, cotransfected UCR oligonucleotides increased CAT expression, presumably by competing for inhibitory trans-acting factors.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barklis E., Mulligan R. C., Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986 Nov 7;47(3):391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Etzerodt M., Mikkelsen T., Pedersen F. S., Kjeldgaard N. O., Jørgensen P. The nucleotide sequence of the Akv murine leukemia virus genome. Virology. 1984 Apr 15;134(1):196–207. doi: 10.1016/0042-6822(84)90285-x. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Phillips S. M., Stephenson J. R., Aaronson S. A. Induction of mouse type-C RNA virus by lipopolysaccharide. J Immunol. 1975 Jul;115(1):317–320. [PubMed] [Google Scholar]
- Harbers K., Schnieke A., Stuhlmann H., Jähner D., Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Hyman R., Ralph P., Sarkar S. Cell-specific antigens and immunoglobulin synthesis of murine myeloma cells and their variants. J Natl Cancer Inst. 1972 Jan;48(1):173–184. [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kelly M., Holland C. A., Lung M. L., Chattopadhyay S. K., Lowy D. R., Hopkins N. H. Nucleotide sequence of the 3' end of MCF 247 murine leukemia virus. J Virol. 1983 Jan;45(1):291–298. doi: 10.1128/jvi.45.1.291-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Laigret F., Rodi C. P. Expression of mink cell focus-forming murine leukemia virus-related transcripts in AKR mice. J Virol. 1987 Mar;61(3):876–882. doi: 10.1128/jvi.61.3.876-882.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Martin M. A. Endogenous murine leukemia proviral long terminal repeats contain a unique 190-base-pair insert. Proc Natl Acad Sci U S A. 1983 May;80(9):2699–2703. doi: 10.1073/pnas.80.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Khan A. S., Steinberg A. D. Multiple endogenous xenotropic and mink cell focus-forming murine leukemia virus-related transcripts are induced by polyclonal immune activators. J Virol. 1988 Oct;62(10):3545–3550. doi: 10.1128/jvi.62.10.3545-3550.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A. M., Steinberg A. D., Khan A. S. Increased expression of novel full-length endogenous mink cell focus-forming-related transcripts in autoimmune mouse strains. Virology. 1988 Jan;162(1):274–276. doi: 10.1016/0042-6822(88)90422-9. [DOI] [PubMed] [Google Scholar]
- Laigret F., Repaske R., Boulukos K., Rabson A. B., Khan A. S. Potential progenitor sequences of mink cell focus-forming (MCF) murine leukemia viruses: ecotropic, xenotropic, and MCF-related viral RNAs are detected concurrently in thymus tissues of AKR mice. J Virol. 1988 Feb;62(2):376–386. doi: 10.1128/jvi.62.2.376-386.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Lerner R. A., Wilson M. C. The Gv-1 locus coordinately regulates the expression of multiple endogenous murine retroviruses. Cell. 1985 May;41(1):289–299. doi: 10.1016/0092-8674(85)90082-0. [DOI] [PubMed] [Google Scholar]
- Max E. E., Korsmeyer S. J. Human J chain gene. Structure and expression in B lymphoid cells. J Exp Med. 1985 Apr 1;161(4):832–849. doi: 10.1084/jem.161.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moroni C., Schumann G. Lipopolysaccharide induces C-type virus in short term cultures of BALB/c spleen cells. Nature. 1975 Mar 6;254(5495):60–61. doi: 10.1038/254060a0. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Pitha P. M. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Williams M. E., Innis M. A. Nucleotide sequences of the envelope genes of two isolates of feline leukemia virus subgroup B. J Virol. 1984 Feb;49(2):629–632. doi: 10.1128/jvi.49.2.629-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. M., Stephenson J. R., Aaronson S. A. Genetic factors infuencing mouse type-C RNA virus induction by naturally occurring B cell mitogens. J Immunol. 1977 Feb;118(2):662–666. [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Moore M. A., Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976 Jun 1;143(6):1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando R. F., Pellett P. E., Luciw P. A., Bohan C. A., Srinivasan A. Transactivation of human immunodeficiency virus by herpesviruses. Oncogene. 1987 Mar;1(1):13–18. [PubMed] [Google Scholar]
- Ricciardi-Castagnoli P., Robbiati F., Barbanti E., Doria G., Adorini L. Establishment of functional, antigen-specific T cell lines by radLV-induced transformation of murine T lymphocytes. Curr Top Microbiol Immunol. 1982;100:89–96. doi: 10.1007/978-3-642-68586-6_10. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Silverman T., Rein A., Orrison B., Langloss J., Bratthauer G., Miyazaki J., Ozato K. Establishment of cell lines from somite stage mouse embryos and expression of major histocompatibility class I genes in these cells. J Immunol. 1988 Jun 15;140(12):4378–4387. [PubMed] [Google Scholar]
- Speck N. A., Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987 Mar;7(3):1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornell A., Hallberg B., Grundström T. Differential protein binding in lymphocytes to a sequence in the enhancer of the mouse retrovirus SL3-3. Mol Cell Biol. 1988 Apr;8(4):1625–1637. doi: 10.1128/mcb.8.4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor C. D., Scott M. L., Josephs S. F., Fry K. E., Reitz M. S., Jr Nucleotide sequence of the large terminal repeat of two different strains of gibbon ape leukemia virus. Virology. 1984 Aug;137(1):201–205. doi: 10.1016/0042-6822(84)90025-4. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Rands E., Chattopadhyay S. K., Lowy D. R., Verma I. M. Long terminal repeat of murine retroviral DNAs: sequence analysis, host-proviral junctions, and preintegration site. J Virol. 1982 Feb;41(2):542–556. doi: 10.1128/jvi.41.2.542-556.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]