Abstract
The adrenal medullary tissue contributes to maintain body homeostasis in reaction to stressful environmental changes via the release of catecholamines into the blood circulation in response to splanchnic nerve activation. Accordingly, chromaffin cell stimulus-secretion coupling undergoes temporally restricted periods of anatomo-functional remodeling in response to prevailing hormonal requirements of the organism. The postnatal development of the adrenal medulla and response to stress are remarkable physiological situations in which the stimulus-secretion coupling is critically affected. Catecholamine secretion from rat chromaffin cells is under a dual control involving an incoming initial command arising from the sympathetic nervous system that releases acetylcholine at the splanchnic nerve terminal-chromaffin cell synapses and a local gap junction-mediated intercellular communication. Interestingly, these two communication pathways are functionally interconnected within the gland and exhibit coordinated plasticity mechanisms. This present article reviews the physiological and molecular evidence that the adrenal medullary tissue displays anatomical and functional adaptative remodeling of cell-cell communications upon physiological (postnatal development) and/or physiopathological (stress) situations associated with specific needs in circulating catecholamine levels.
Keywords: Adaptation, Physiological; Adrenal Medulla; cytology; growth & development; Animals; Cell Communication; Gap Junctions; metabolism; Humans; Stress, Physiological; Synaptic Transmission
Keywords: Gap junctions, cholinergic synaptic neurotransmission, adrenal medulla, chromaffin cells, stimulus-secretion coupling, development, stress, physiopathology
Introduction
In mammals, catecholamine secretion from adrenal chromaffin cells represents an ubiquitous mechanism helping the organism to cope with stressful situations. Once delivered into the blood circulation, these stress mediators exert multiple actions in particular on the cardiovascular system, leading to appropriate adjustments of blood pressure and cardiac rhythm, and on the energy metabolism, enabling the organism to cope with a threat for its survival.
Adrenal catecholamine release results from the coordination of two input pathways. The initial incoming neurogenic command comes from the sympathetic nervous system that releases acetylcholine at splanchnic nerve terminals synapsing onto chromaffin cells (Douglas, 1968; Wakade, 1981; reviewed in de Diego et al., 2008). This traditional view of stimulus-secretion coupling in adrenal chromaffin cells prevailed during many decades, but has been recently revisited in the light of new data. Thus, gap junctional coupling between chromaffin cells has entered the fray as a novel protagonist involved in catecholamine release. Indeed, although electrical discharges invading the splanchnic nerve endings are the major physiological stimulus triggering catecholamine release in vivo, compelling evidence coming from studies in acute adrenal slices indicates that a local communication mediated by gap junctions between chromaffin cells represents a functional route by which biological signals (electrical activity, changes in intracellular Ca2+ concentration, …) propagate between adjacent cells and subsequently contribute to catecholamine release (Martin et al., 2001; reviewed in Colomer et al., 2009). The present review focuses on the anatomical and functional remodeling of these two cell-cell communication pathways in response to changing environmental conditions.
Remodeling of adrenal gap junctional and synaptic communication during postnatal development
In the adrenal medulla, the neonatal period is marked by a switch from non-neurogenic to neurogenic control of catecholamine secretion. During this critical period, the medullary tissue undergoes persistent anatomical and functional remodeling that is crucial to the acquisition of the neurogenic control. Synaptogenesis in the adrenal medulla, which occurs during the first postnatal week (Slotkin, 1986), requires a finely tuned coordination between the gap junction-mediated electrical coupling and synaptic neurotransmission (Martin et al., 2005), as is also observed in the developing central nervous system (Kandler and Katz, 1995).
Report on gap junction-mediated intercellular coupling
Consistent with the fact that gap junction-coupled neuronal assemblies often precede the formation of synaptically connected neuronal networks, gap junction-mediated electrotonic and metabolic coupling is widespread among neonate adrenal chromaffin cells (Martin et al., 2003). From a functional point of view, this results in synchronized signal propagation between nearby chromaffin cells, likely involved in the triggering of catecholamine release in neonates. Coinciding with the establishment of functional chemical synapses, the number of gap junctions between chromaffin cells decreases during postnatal development (~65% of coupled cells in newborn rats versus 40% and 20% in female and male adult rats, respectively, Martin et al., 2001, 2003, Colomer et al., 2009). With regards to connexins, the basic building blocks of gap junctions, adult rat chromaffin cells express at least two connexins, Cx36 and Cx43 (Martin et al., 2001; Colomer et al., 2008a, reviewed in Colomer et al., 2009). Whether the expression pattern of connexins in neonate chromaffin cells differs from adults remains to be investigated.
Report on cholinergic synaptic transmission and cholinergic receptors
Although pre-and post-synaptic elements are already present prenatally (Daikoku et al., 1977), synaptic transmission between the splanchnic nerve terminals and chromaffin cells is not fully competent at birth. A functional neuronal connection is observed on postnatal day 2 (Parker et al., 1988) and it completely matures during the first postnatal week (Slotkin, 1986). In rat, maturation of cholinergic synaptic transmission results in a 2-fold increase in synaptic current amplitude without significant change in synaptic current frequency (Martin et al., 2005). At rat splanchnic nerve-chromaffin cell synapses, postsynaptic nicotinic receptors (nAChRs) dominantly contribute to acetylcholine-mediated excitatory responses and ensuing secretion (Wakade and Wakade, 1983). Although neonate chromaffin cells express various nAChR subunits (α3, α5, α7, β2, and β4, Souvannakitti et al., 2010), the full blockade of excitatory postsynaptic currents (EPSCs) by hexamethonium, an antagonist of neuronal α3-containing nAChRs (Xiao et al., 1998) strongly suggests that only α3 subunit-containing nAChRs are activated in response to synaptically released acetylcholine (Martin et al., 2003). This differs from adult chromaffin cells in which EPSCs are supported by the co-activation of both α3 (Barbara and Takeda, 1996), α7 (Martin et al., 2003, 2005) and α9-containing nAChRs (Colomer et al., 2010). From a developmental point of view, a nicotinic receptor-mediated signaling pathway appears first in development, as evidenced by nicotine-evoked rise in cytosolic free Ca2+ concentration ([Ca2+]i) in chromaffin cells at embryonic day 19 (Oomori et al., 1998). At birth, chromaffin cells display nicotine responses that are similar to those in adults.
Besides nicotinic cholinergic receptors, adrenal chromaffin cells also express muscarinic receptors (reviewed in Olivos and Artalejo, 2008). Muscarinic stimulus-secretion coupling contributes to the fine tuning of the catecholamine secretory response, at least in adults. Muscarinic regulation of stimulus-secretion coupling occcurs both pre-and post-synaptically. Indeed, it has been proposed that activation of post-synaptic muscarinic receptors (likely m3 and m4 subtypes) facilitates catecholamine secretion by inducing a strong and long-lasting excitation of chromaffin cells (Barbara et al., 1998), thus favouring a tonic discharge of action potentials. As recently reported in rat adrenal medullary cells, this likely involves the inhibition of TWIK-related acid sensitive K (TASK)1-like channels (Inoue et al., 2008). By contrast, activation of pre-synaptic muscarinic receptors would reduce acetylcholine release, as evidenced by muscarine-induced decreased frequency and amplitude of synaptic currents (Barbara et al., 1998). By uncoupling synaptic inputs from chromaffin cells and because they appear to be activated upon intense synaptic activity (Barbara et al., 1998), these presynaptic autoreceptors may act as a protectory feedback mechanism in response to large amounts of synaptically released ACh. What about muscarinic receptors in neonates? Adrenal chromaffin cells become sensitive to muscarinic stimulation from postnatal day 0 but exhibit only a low-to-moderate rise in [Ca2+]i in response to muscarinic receptor agonists, that gradually completes by the first postnatal week (Oomori et al., 1998). This indicates that expression of adrenal muscarinic receptors parallels the maturation of neurogenic control. In addition, the expression level of muscarinic receptor transcripts seems to be unaffected by hypoxia (Ducsay et al., 2007), consistent with a minor contribution of muscarinic receptors at birth, as compared to the dominant contribution of nAChRs.
Developmental switch from electrical coupling to chemical neurotransmission: the key role of agrin
As a common finding, the critical phase of synapse formation is accompanied by a switch from a predominant gap junction-mediated electrotonic coupling to a fully mature synaptic neurotransmission. The cellular mechanisms underlying this developmental switch in the adrenal medulla are not yet fully elucidated. Among possible candidates, the heparan sulfate proteoglycan agrin has been reported to play a pivotal role by modulating dualistically electrical and chemical synapses (Martin et al., 2005). Indeed, agrin operates a rapid switch from gap junction-mediated electrical communication to synaptic transmission, thus promoting the acquisition of the neurogenic control of the stimulus-secretion coupling (Figure 1). In neonates, an acute agrin application first reduces by half the amplitude of junctional currents flowing between chromaffin cells within a few minutes, and second, it increases EPSC amplitude. This renders neonate chromaffin cells readily responsive to synaptically-released neurotransmitters, while reducing information transfer through gap junctions. Although agrin-activated intracellular transduction mechanisms still remain to be investigated in the adrenal medulla, Martin and colleagues (2005) proposed that activation of Src kinases by agrin may cause tyrosine-phosphorylation of Cx43, leading thus to a decrease in junctional communication (Lau et al., 1996) and may also tyrosine-phosphorylate the β subunit of α3-containing nAChRs (Wallace et al., 1991), resulting in their clustering. Agrin-triggered α3 nAChR clustering could thereafter activate an intracellular signaling cascade that would in turn regulate other nAChRs such as α7-built nAChRs (Brumwell et al., 2002), which is determinant for the negative control of the non-neurogenic response in neonates (Buttigieg et al., 2009). To date, the involvement of other factors in the adrenal medulla developmental switch fom electrical to chemical coupling has not yet been reported.
Figure 1. Postnatal developmental changes in the electrical and chemical synapses in the adrenal medulla.
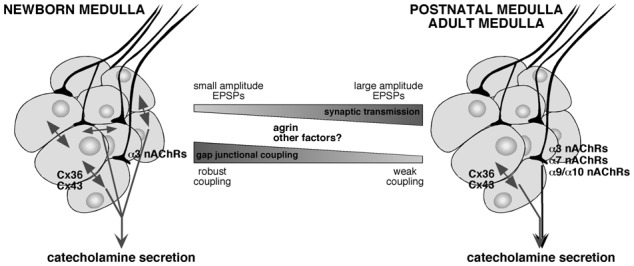
In the adrenal medulla of newborn rats, a robust gap junction-mediated chromaffin cell coupling co-exists with an immature cholinergic synaptic transmission. This contrasts with the weak junctional coupling and robust synaptic transmission in adult animals. This developmental switch from a predominant gap junctional communication to a primarily chemical neurotransmission contributes to the postnatal acquisition of the neurogenic control of catecholamine secretion. Note that it is accompagnied by a change in nAChR channels contributing to excitatory post-synaptic potentials, i.e. α3 nAChRs only in neonates versus a mixture of α3, α7 and α9/α10 nAChRs in adults. Agrin -and likely other factors- is significantly involved in this developmental switch. (EPSPs, excitatory post-synaptic potentials).
Remodeling of synaptic and gap junctional communication: adaptation to a variety of stressful situations?
Remodeling at birth: a lesson from hypoxic stress
During the transition from intrauterine to air-breathing life, neonate rats are subjected to various stressors, including hypoxia, hypoglycemia or glycopenia. During this period, the adrenal medulla exerts a very specific protective function enabling newborn mammals to survive, in particular against hypoxia. This involves a variety of respiratory, cardiovascular and metabolic adjustments that are achieved by a massive release of equal amounts of adrenaline and noradrenaline from the adrenal gland (Seidler and Slotkin, 1985). In the absence of functional synapses originating from the splanchnic nerve in neonates (Slotkin, 1986), this function is handled directly by chromaffin cells. Accordingly, neonatal chromaffin cells display a direct hypoxia-sensing mechanism, likely involving the mitochondrial electron transport chain (Nurse et al., 2006), thus confering on chromaffin cell the ability to respond directly to hypoxia by a non-neurogenic vital catecholamine surge at birth. Consistent with a non-neurogenic control of catecholamine secretion, neonatal chromaffin cell response to hypoxia is not blocked by an acute injection of a cholinergic antagonist, indicating that it relies on a direct sensitivity of chromaffin cells to hypoxia (Seidler and Slotkin, 1985). More recently, a direct activation of neonate chromaffin cells by CO2 has been reported (Muñoz-Cabello et al., 2005). With regards to intracellular signal transduction, hypoxia decreases potassium currents and induces a depolarization in a majority of chromaffin cells in culture, leading to calcium-dependent catecholamines secretion (Mochizuki-Oda et al., 1997; Thompson et al., 1997). Another striking feature of neonate chromaffin cells when compared to adult cells refers to the expression of T-type Ca2+ channels. Interestingly, the expression of α1H gene (encoding T-type Ca2+ channels) is induced by hypoxia (del Toro et al., 2003), thus arguing for a role for T-type Ca2+ channels as regulators of chromaffin cell responsiveness to hypoxia by modulating cell excitability, calcium influx and subsequent catecholamine release (Carabelli et al., 2007; Levitsky and López-Barneo, 2009).
Hypoxia and cholinergic synaptic transmission
Rat chromaffin cells are most sensitive to hypoxia in the perinatal period (Thompson et al., 1997). The non-neurogenic response of the adrenal medulla, and the direct sensitivity of chromaffin cells to hypoxia is gradually lost (Garcia-Fernandez et al., 2007), as cholinergic innervation becomes fully competent, that is during the first post-natal week (Slotkin, 1986). This also parallels a decrease in T-type Ca2+ channels density expressed at the plasma membrane (Levitsky and López-Barneo, 2009). The tight correlation between chromaffin cell innervation and oxygen sensitivity is emphasized by the observation that both chromaffin cell sensitivity to hypoxia and the expression of T-type Ca2+ channels reappear after adrenal gland denervation, suggesting that i) these Ca2+ channels are essential for the chromaffin cell response to hypoxia and ii) adrenal innervation by cholinergic splanchnic nerve fibers critically contributes to down-regulate expression of T-type Ca2+ channels (Levitsky and López-Barneo, 2009). The sensitivity decrease to hypoxia is known to result from the activation of nAChRs since it can be accelerated by a treatment with nicotine (Nurse et al., 2009). Studies conducted in vitro indicate that the mechanisms involve the activation of α7-built nAChRs and, via a CaMkinase/PKC pathway, overexpression of KATP potassium channels preventing hypoxia-induced depolarization and blunting catecholamine release (Buttigieg et al., 2008, 2009). Regarding expression of nAChRs, it has been recently reported that neonatal/fetal hypoxia down-regulates transcripts encoding nAChR subunits, including α3, α5, α7, β2, and β4 mRNAs in rat and α1, α7, β1 and β2 mRNAs in sheep (Ducsay et al., 2007), through mechanisms involving reactive oxygen species signaling (Souvannakitti et al., 2010). As long as hypoxia persits, neurogenic catecholamine secretion is impaired, consistent with an altered nAChR function in hypoxic chromaffin cells (Lee et al., 1995). Interestingly, unlike nAChRs, expression of muscarinic receptors seems to be unaffected by hypoxia (Ducsay et al., 2007).
Hypoxia and gap junctional coupling
A striking feature of neonate adrenal medulla is the predominant gap junction-mediated communication between chromaffin cells (Martin et al., 2003, 2005). Whether gap junctional coupling is involved in hypoxic stress-induced chromaffin cell response remains to be investigated. However, it is noteworthy that Ca2+ ions, a messenger that is diffusible through gap junctions (Spray and Bennett, 1985), are involved in the non-neurogenic response to hypoxia (Mochizuki-Oda et al., 1997) and may therefore regulate gap junctional communication and related physiological function such as catecholamine secretion. Because the molecular determinants of hypoxia chemotransduction in neonatal adrenal chromaffin cells are not fully understood (Nurse et al., 2009), the involvement of other factors is highly plausible. Reactive oxygen species (ROS), messengers that can also diffuse through gap junctions (Upham and Trosko, 2009), could be one of these factors, as recently proposed (Thompson et al., 2007), although whether hypoxia increases or decreases ROS cell content is still controversial. Since only a proportion of chromaffin cells are sensitive to hypoxia in late embryos and neonates (Garcia-Fernandez et al., 2007), it is reasonable to propose that gap junctional coupling, that is predominant in the newborn medulla, facilitates the spreading of the response from hypoxia-sensitive cells to the whole population of chromaffin cells, bringing all the cells to participate in the massive catecholamine release that is necessary to cope with hypoxia at birth. Accordingly, gap junctions would play a major role in the extent of the response of the adrenal medulla to the birth-related hypoxic stress.
Remodeling during adulthood
Studies in stressed animals unveils that both gap junctional coupling and cholinergic synaptic neurotransmission undergo functional plasticity in adults. This is particularly obvious in response to chronic stress, a situation in which the organism demand in catecholamines becomes higher (Kvetnansky et al., 1978). To ensure an appropriate catecholamine secretion, adrenal chromaffin cells adapt their stimulus-secretion coupling to become more efficient. In the case of a 5-day cold stress for example, this is achieved by a simultaneous up-regulation of both gap junction-mediated communication and synaptic activity (Colomer et al., 2008a, 2008b, 2009). In addition, chromaffin cells excitability is increased, as evidenced by an increased mean spontaneous action potential discharge frequency (Colomer et al., 2008b). Regarding gap junctional communication, cold stress induces the appearance of a robust electrical coupling between chromaffin cells that allows the transmission of action potentials between coupled cells. This enhancement of gap junctional communication parallels an increase in expression levels of Cx36 and Cx43 proteins, and Cx36 transcripts (Colomer et al., 2008a). Cholinergic synaptic neurotransmission is also remodeled in stressed rats. First, the density of nerve fibers innervating the adrenal medulla is higher as compared to unstressed animals. This is consistent with the increased frequency of spontaneous EPSCs (Colomer et al., 2008b). Second, the pharmacological profile of synaptic currents is modified. Consistent with a dominant expression of α3/β4 nAChRs in chromaffin cells (reviewed in Sala et al., 2008), α3 subunit-containing nAChRs are the main contributing channels to synaptic events in unstressed rats. By contrast, in cold exposed rats, α9-containing nAChRs dominantly contribute to acetylcholine-induced current (Colomer et al., 2010), α3-containing nAChRs staying involved, but to a lesser extent. The dominant contribution of α9-containing nAChRs in cold stressed rats is associated with an over-expression of α9 nAChR transcripts and a preferential distribution of the α9 nAChRs at synaptic sites (Colomer et al., 2010). To date, the contribution of muscarinic receptors in stress-induced remodeling of cholinergic synaptic transmission has not yet been reported.
Do gap junctional and synaptic communication remodel in response to other stressors? We previously reported that they are both up-regulated in the adrenal medulla of rats exposed to a restraint stress, suggesting that the reshapes may occur in response to several stressors (Colomer et al., 2008b). But because the intensity of stimulation of the adrenomedullary hormonal system is stressor-specific (Goldstein and Kopin, 2007), it is likely that other adaptative mechanisms take place in the adrenal medulla, also enabling the organism to cope with stress. Regarding this, the critical role played by the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) as a sympathoadrenal co-neurotransmitter involved in the stimulus-secretion-synthesis coupling supporting stress responses is particularly relevant. PACAP not only crucially contributes to maintain epinephrine secretion upon prolonged metabolic stress in vivo (Hamelink et al., 2002) but also stimulates catecholamine release under elevated splanchnic nerve firing (Kuri et al., 2009) and catecholamine biosynthetic enzymes in response to stress (Stroth and Eiden, 2010). Remodeling of gene expression is also an adaptive mechanism whereby the adrenal medullary tissue copes with stress. This is particularly well illustrated by the contribution of glucocorticoids in the gene induction of catecholamine biosynthesis-related enzymes (reviewed in Kvetnansky et al., 2009). Of a particular physiological relevance, expression of phenylethanolamine N-methyltransferase, the enzyme responsible for epinephrine biosynthesis, is glucocorticoid-dependent (Tai et al., 2002). Altogether, the appropriate orchestration of adrenergic response that is critical to challenge with stress implies a finely tuned coordination between both molecular, cellular and tissular mechanisms.
Cross-talk between synaptic transmission and gap junctional communication: synergistic or opposite action?
Functional interactions between gap junctions and synaptic transmission occur in the adrenal medulla, like in the central nervous system where cross-talk between gap junctions and synapses is so pervasive that it is no longer tenable to investigate the mechanisms of neuronal signal integration without considering the intricate interactions between both systems of intercellular contacts. Obviously, adrenal gap junctional coupling undergoes a long-lasting up-regulation in physiological/pathological situations associated with impaired or immature synaptic transmission, indicating that under normal neurotransmission, synaptic activity exerts a tonic inhibitory control on gap junctional communication (Martin et al., 2003). This crosstalk is not restricted to the adrenal medulla, since it also occurs in the nervous system, both in response to pathological situations and during postnatal development. In particular, gap junction-mediated intercellular communication between motor neurons undergoes sustained upregulation in response to peripheral nerve injury (Chang and Balice-Gordon, 2000). In developing motor neurons, gap junctional connections decline during postnatal development, likely due to an increase in glutamatergic synaptic activity, as evidenced by the finding that early postnatal blockade of the NMDA subtype of glutamate receptors arrest the developmental decrease in electrotonic and dye coupling during the first postnatal week (Mentis et al., 2002). It is noteworthy that gap junctional communication and synaptic activity are not always regulated in an opposite manner. Interestingly and counter to expectations, in stressful conditions, the tonic inhibitory control is removed, allowing synaptic transmission and gap junctional communication to act in synergy to ensure a secretory response appropriate to the organism demand. To date, the cellular mechanisms shutting down this inhibitory control remain to be elucidated. As hypothetized by Colomer and colleagues (2009), in case of a high nerve firing frequency, gap junctional communication would not act in synergy with synaptic activity, but conversely would counteract synaptic transmission and have a buffering and filtering action on signal propagation, to avoid a huge catecholamine release potentially harmful for the organism or even lethal.
Numerous evidences have been accumulated that the adrenal medullla stimulus-secretion coupling is finely tuned by environmental conditions. Strikingly, depending on the nature of the environment changes (i.e. birth, situations associated with impaired synaptic transmission, chronic stress,…), adaptative responses carried out by the organism at the adrenal medullary level involve either opposite or synergistic remodeling of the cholinergic synaptic neurotransmission and gap junctional communication between chromaffin cells. The subtile combination with other mechanisms not reviewed here, such as paracrine or endocrine communication, enables in fine an organism to respond effectively to a threat for its survival.
Acknowledgments
The authors thank the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Ministère de l’Enseignement Supérieur et de la Recherche, Fondation pour la Recherche Médicale, Association pour la Recherche sur le Cancer and Région Languedoc-Roussillon.
Footnotes
Conflict of interest statement: no conflict declared
Contributor Information
Nathalie C. Guérineau, Biologie Neurovasculaire Intégrée CNRS : UMR6214, INSERM : U771, Université d'Angers, FR.
Michel G. Desarménien, IGF, Institut de génomique fonctionnelle CNRS : UMR5203, INSERM : U661, Université Montpellier I, Université Montpellier II, 141, Rue de la Cardonille 34094 MONTPELLIER CEDEX 5,FR
References
- Barbara JG, Takeda K. Quantal release at a neuronal nicotinic synapse from rat adrenal gland. Proc Natl Acad Sci U S A. 1996;93:9905–9909. doi: 10.1073/pnas.93.18.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara JG, Lemos VS, Takeda K. Pre- and post-synaptic muscarinic receptors in thin slices of rat adrenal gland. Eur J Neurosci. 1998;10:3535–3545. doi: 10.1046/j.1460-9568.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- Brumwell CL, Johnson JL, Jacob MH. Extrasynaptic alpha 7-nicotinic acetylcholine receptor expression in developing neurons is regulated by inputs, targets, and activity. J Neurosci. 2002;22:8101–8109. doi: 10.1523/JNEUROSCI.22-18-08101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttigieg J, Brown S, Zhang M, Lowe M, Holloway AC, Nurse CA. Chronic nicotine in utero selectively suppresses hypoxic sensitivity in neonatal rat adrenal chromaffin cells. FASEB J. 2008;22:1317–1326. doi: 10.1096/fj.07-9194com. [DOI] [PubMed] [Google Scholar]
- Buttigieg J, Brown S, Holloway AC, Nurse CA. Chronic nicotine blunts hypoxic sensitivity in perinatal rat adrenal chromaffin cells via upregulation of KATP channels: role of alpha7 nicotinic acetylcholine receptor and hypoxia-inducible factor-2alpha. J Neurosci. 2009;29:7137–7147. doi: 10.1523/JNEUROSCI.0544-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli V, Marcantoni A, Comunanza V, de Luca A, Díaz J, Borges R, Carbone E. Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol. 2007;584:149–165. doi: 10.1113/jphysiol.2007.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Balice-Gordon RJ. Gap junctional communication among developing and injured motor neurons. Brain Res Brain Res Rev. 2000;32:242–249. doi: 10.1016/s0165-0173(99)00085-5. [DOI] [PubMed] [Google Scholar]
- Colomer C, Olivos-Oré LA, Coutry N, Mathieu MN, Arthaud S, Fontanaud P, Iankova I, Macari F, Thouënnon E, Yon L, Anouar Y, Guérineau NC. Functional remodeling of gap junction-mediated electrical communication between adrenal chromaffin cells in stressed rats. J Neurosci. 2008a;28:6616–6626. doi: 10.1523/JNEUROSCI.5597-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer C, Lafont C, Guérineau NC. Stress-induced intercellular communication remodeling in the rat adrenal medulla. Ann N Y Acad Sci. 2008b;1148:106–111. doi: 10.1196/annals.1410.040. [DOI] [PubMed] [Google Scholar]
- Colomer C, Desarménien MG, Guérineau NC. Revisiting the stimulus-secretion coupling in the adrenal medulla: role of gap junction-mediated intercellular communication. Mol Neurobiol. 2009;40:87–100. doi: 10.1007/s12035-009-8073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer C, Olivos-Oré LA, Vincent A, McIntosh JM, Artalejo AR, Guérineau NC. Functional characterization of alpha9-containing cholinergic nicotinic receptors in the rat adrenal medulla: implication in stress-induced functional plasticity. J Neurosci. 2010;30:6732–6742. doi: 10.1523/JNEUROSCI.4997-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku S, Kinutani M, Sako M. Development of the adrenal medullary cells in rats with reference to synaptogenesis. Cell Tissue Res. 1977;179:77–86. doi: 10.1007/BF00278463. [DOI] [PubMed] [Google Scholar]
- de Diego AM, Gandía L, García AG. A physiological view of the central and peripheral mechanisms that regulate the release of catecholamines at the adrenal medulla. Acta Physiol. 2008;192:287–301. doi: 10.1111/j.1748-1716.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- del Toro R, Levitsky KL, López-Barneo J, Chiara MD. Induction of T-type calcium channel gene expression by chronic hypoxia. J Biol Chem. 2003;278:22316–22324. doi: 10.1074/jbc.M212576200. [DOI] [PubMed] [Google Scholar]
- Douglas WW. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968;34:451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducsay CA, Hyatt K, Mlynarczyk M, Root BK, Kaushal KM, Myers DA. Long-term hypoxia modulates expression of key genes regulating adrenomedullary function in the late gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1997–R2005. doi: 10.1152/ajpregu.00313.2007. [DOI] [PubMed] [Google Scholar]
- García-Fernández M, Mejías R, López-Barneo J. Developmental changes of chromaffin cell secretory response to hypoxia studied in thin adrenal slices. Pflügers Arch. 2007;454:93–100. doi: 10.1007/s00424-006-0186-y. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ. Evolution of concepts of stress. Stress. 2007;10:109–120. doi: 10.1080/10253890701288935. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, Eiden LE. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Harada K, Matsuoka H, Sata T, Warashina A. Inhibition of TASK1-like channels by muscarinic receptor stimulation in rat adrenal medullary cells. J Neurochem. 2008;106:1804–1814. doi: 10.1111/j.1471-4159.2008.05521.x. [DOI] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Neuronal coupling and uncoupling in the developing nervous system. Curr Opin Neurobiol. 1995;5:98–105. doi: 10.1016/0959-4388(95)80093-x. [DOI] [PubMed] [Google Scholar]
- Kuri BA, Chan SA, Smith CB. PACAP regulates immediate catecholamine release from adrenal chromaffin cells in an activity-dependent manner through a protein kinase C-dependent pathway. J Neurochem. 2009;110:1214–1225. doi: 10.1111/j.1471-4159.2009.06206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetnansky R, Sun CL, Lake CR, Thoa N, Torda T, Kopin IJ. Effect of handling and forced immobilization on rat plasma levels of epinephrine, norepinephrine, and dopamine-beta-hydroxylase. Endocrinology. 1978;103:1868–1874. doi: 10.1210/endo-103-5-1868. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- Lau AF, Kurata WE, Kanemitsu MY, Loo LW, Warn-Cramer BJ, Eckhart W, Lampe PD. Regulation of connexin43 function by activated tyrosine protein kinases. J Bioenerg Biomembr. 1996;28:359–668. doi: 10.1007/BF02110112. [DOI] [PubMed] [Google Scholar]
- Lee K, Ito A, Koshimura K, Ohue T, Takagi Y, Miwa S. Differential effects of hypoxia on ligand binding properties of nicotinic and muscarinic acetylcholine receptors on cultured bovine adrenal chromaffin cells. J Neurochem. 1995;64:874–882. doi: 10.1046/j.1471-4159.1995.64020874.x. [DOI] [PubMed] [Google Scholar]
- Levitsky KL, López-Barneo J. Developmental change of T-type Ca2+ channel expression and its role in rat chromaffin cell responsiveness to acute hypoxia. J Physiol. 2009;587:1917–1929. doi: 10.1113/jphysiol.2009.168989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AO, Mathieu MN, Chevillard C, Guérineau NC. Gap junctions mediate electrical signaling and ensuing cytosolic Ca2+ increases between chromaffin cells in adrenal slices: a role in catecholamine release. J Neurosci. 2001;21:5397–5405. doi: 10.1523/JNEUROSCI.21-15-05397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AO, Mathieu MN, Guérineau NC. Evidence for long-lasting cholinergic control of gap junctional communication between adrenal chromaffin cells. J Neurosci. 2003;23:3669–3678. doi: 10.1523/JNEUROSCI.23-09-03669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AO, Alonso G, Guérineau NC. Agrin mediates a rapid switch from electrical coupling to chemical neurotransmission during synaptogenesis. J Cell Biol. 2005;169:503–514. doi: 10.1083/jcb.200411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Diaz E, Moran LB, Navarrete R. Increased incidence of gap junctional coupling between spinal motoneurones following transient blockade of NMDA receptors in neonatal rats. J Physiol. 2002;544:757–764. doi: 10.1113/jphysiol.2002.028159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki-Oda N, Takeuchi Y, Matsumura K, Oosawa Y, Watanabe Y. Hypoxia-induced catecholamine release and intracellular Ca2+ increase via suppression of K+ channels in cultured rat adrenal chromaffin cells. J Neurochem. 1997;69:377–387. doi: 10.1046/j.1471-4159.1997.69010377.x. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cabello AM, Toledo-Aral JJ, López-Barneo J, Echevarría M. Rat adrenal chromaffin cells are neonatal CO2 sensors. J Neurosci. 2005;25:6631–6640. doi: 10.1523/JNEUROSCI.1139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse CA, Buttigieg J, Thompson R, Zhang M, Cutz E. Oxygen sensing in neuroepithelial and adrenal chromaffin cells. Novartis Found Symp. 2006;272:106–114. doi: 10.1002/9780470035009.ch9. [DOI] [PubMed] [Google Scholar]
- Nurse CA, Buttigieg J, Brown S, Holloway AC. Regulation of oxygen sensitivity in adrenal chromaffin cells. Ann N Y Acad Sci. 2009;1177:132–139. doi: 10.1111/j.1749-6632.2009.05031.x. [DOI] [PubMed] [Google Scholar]
- Olivos L, Artalejo AR. Muscarinic excitation-secretion coupling in chromaffin cells. Acta Physiol. 2008;192:213–220. doi: 10.1111/j.1748-1716.2007.01816.x. [DOI] [PubMed] [Google Scholar]
- Oomori Y, Habara Y, Kanno T. Muscarinic and nicotinic receptor-mediated Ca2+ dynamics in rat adrenal chromaffin cells during development. Cell Tissue Res. 1998;294:109–123. doi: 10.1007/s004410051161. [DOI] [PubMed] [Google Scholar]
- Parker TL, Kesse WK, Tomlinson A, Coupland RE. Ontogenesis of preganglionic sympathetic innervation of rat adrenal chromaffin cells. In: Liss AR, editor. Progress in Catecholamine Research, part A: Basic aspects and peripheral mechanisms. 1988. pp. 227–232. [Google Scholar]
- Sala F, Nistri A, Criado M. Nicotinic acetylcholine receptors of adrenal chromaffin cells. Acta Physiol. 2008;192:203–212. doi: 10.1111/j.1748-1716.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Adrenomedullary function in the neonatal rat: responses to acute hypoxia. J Physiol. 1985;358:1–16. doi: 10.1113/jphysiol.1985.sp015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Development of the sympathoadrenal axis. In: Gootman PM, editor. Developmental Neurobiology of the Autonomic Nervous System. Humana Press; Totowa: 1986. pp. 69–96. [Google Scholar]
- Souvannakitti D, Kuri B, Yuan G, Pawar A, Kumar GK, Smith C, Fox AP, Prabhakar NR. Neonatal intermittent hypoxia impairs neuronal nicotinic receptor expression and function in adrenal chromaffin cells. Am J Physiol Cell Physiol. 2010;299:C381–C388. doi: 10.1152/ajpcell.00530.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Bennett MV. Physiology and pharmacology of gap junctions. Annu Rev Physiol. 1985;47:281–303. doi: 10.1146/annurev.ph.47.030185.001433. [DOI] [PubMed] [Google Scholar]
- Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–1030. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai TC, Claycomb R, Her S, Bloom AK, Wong DL. Glucocorticoid responsiveness of the rat phenylethanolamine N-methyltransferase gene. Mol Pharmacol. 2002;61:1385–1392. doi: 10.1124/mol.61.6.1385. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Jackson A, Nurse CA. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J Physiol. 1997;498:503–510. doi: 10.1113/jphysiol.1997.sp021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Buttigieg J, Zhang M, Nurse CA. A rotenone-sensitive site and H2O2 are key components of hypoxia-sensing in neonatal rat adrenomedullary chromaffin cells. Neuroscience. 2007;145:130–141. doi: 10.1016/j.neuroscience.2006.11.040. [DOI] [PubMed] [Google Scholar]
- Upham BL, Trosko JE. Oxidative-dependent integration of signal transduction with intercellular gap junctional communication in the control of gene expression. Antioxid Redox Signal. 2009;11:297–307. doi: 10.1089/ars.2008.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade AR, Wakade TD. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 1983;10:973–978. doi: 10.1016/0306-4522(83)90235-x. [DOI] [PubMed] [Google Scholar]
- Wallace BG, Qu Z, Huganir RL. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron. 1991;6:869–878. doi: 10.1016/0896-6273(91)90227-q. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol. 1998;54:322–333. doi: 10.1124/mol.54.2.322. [DOI] [PubMed] [Google Scholar]


