Abstract
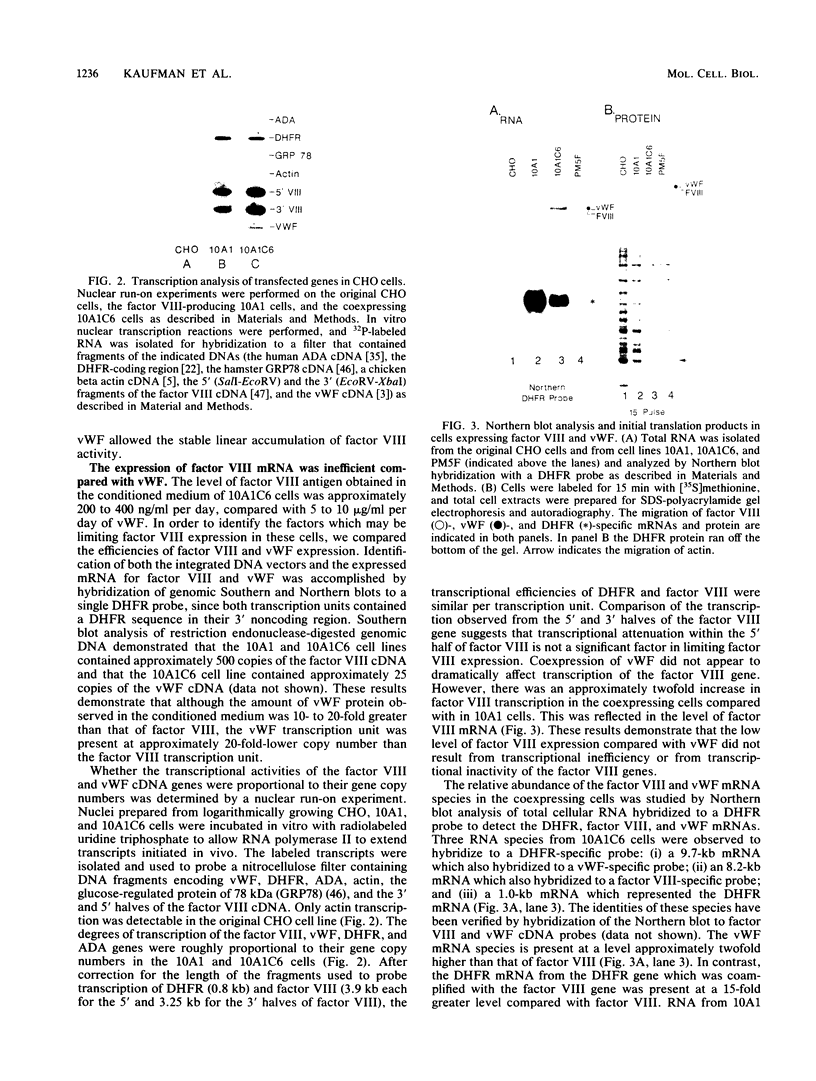
In plasma, antihemophilic factor (factor VIII) exists as a 200-kilodalton heavy-chain polypeptide in a metal ion association with an 80-kilodalton light-chain polypeptide. This complex is bound by hydrophobic and hydrophilic interactions to a large multimeric glycoprotein, von Willebrand factor (vWF). Accumulation of secreted human factor VIII activity expressed in Chinese hamster ovary cells requires the addition of serum in the growth medium, which provides vWF. Here we report that coexpression of vWF with factor VIII in Chinese hamster ovary cells resulted in increased stable accumulation of factor VIII activity in the absence of serum in the growth medium. In the coexpressing cells, the vWF cDNA transcription unit was transcribed to yield mRNA which was efficiently translated. vWF was properly processed and secreted to yield disulfide-bonded high-molecular-weight multimers similar to those observed in vWF secreted from human endothelial cells. Nuclear run-on assays showed that the factor VIII gene was transcribed at a level similar to that of the vWF gene, but the mRNA did not accumulate to high levels in the cytoplasm. In addition, although the translation efficiency of the factor VIII mRNA was similar to that of vWF, the processing and secretion of the factor VIII primary translation product was dramatically reduced compared with vWF. These results demonstrate that in Chinese hamster ovary cells both factor VIII mRNA accumulation and the processing and secretion of the primary factor VIII translation product are inefficient processes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempo F. A., Lewis J. H., Gorenc T. J., Spero J. A., Ragni M. V., Scott J. P., Starzl T. E. Liver transplantation in hemophilia A. Blood. 1987 Jun;69(6):1721–1724. [PMC free article] [PubMed] [Google Scholar]
- Bonthron D. T., Handin R. I., Kaufman R. J., Wasley L. C., Orr E. C., Mitsock L. M., Ewenstein B., Loscalzo J., Ginsburg D., Orkin S. H. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986 Nov 20;324(6094):270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- Brinkhous K. M., Sandberg H., Garris J. B., Mattsson C., Palm M., Griggs T., Read M. S. Purified human factor VIII procoagulant protein: comparative hemostatic response after infusions into hemophilic and von Willebrand disease dogs. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8752–8756. doi: 10.1073/pnas.82.24.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- DOUGLAS A. S. Antihemophilic globulin assay following plasma infusions in hemophilia. J Lab Clin Med. 1958 Jun;51(6):850–859. [PubMed] [Google Scholar]
- Dorner A. J., Bole D. G., Kaufman R. J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987 Dec;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Krane M. G., Kaufman R. J. Reduction of endogenous GRP78 levels improves secretion of a heterologous protein in CHO cells. Mol Cell Biol. 1988 Oct;8(10):4063–4070. doi: 10.1128/mcb.8.10.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D., Rodriguez H., Vehar G. A. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986 Jan 28;25(2):505–512. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- Ewenstein B. M., Warhol M. J., Handin R. I., Pober J. S. Composition of the von Willebrand factor storage organelle (Weibel-Palade body) isolated from cultured human umbilical vein endothelial cells. J Cell Biol. 1987 May;104(5):1423–1433. doi: 10.1083/jcb.104.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. J. Reconstitution of human factor VIII from isolated subunits. Arch Biochem Biophys. 1988 May 1;262(2):525–531. doi: 10.1016/0003-9861(88)90404-3. [DOI] [PubMed] [Google Scholar]
- Foster P. A., Fulcher C. A., Marti T., Titani K., Zimmerman T. S. A major factor VIII binding domain resides within the amino-terminal 272 amino acid residues of von Willebrand factor. J Biol Chem. 1987 Jun 25;262(18):8443–8446. [PubMed] [Google Scholar]
- Fulcher C. A., Gardiner J. E., Griffin J. H., Zimmerman T. S. Proteolytic inactivation of human factor VIII procoagulant protein by activated human protein C and its analogy with factor V. Blood. 1984 Feb;63(2):486–489. [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Girma J. P., Meyer D., Verweij C. L., Pannekoek H., Sixma J. J. Structure-function relationship of human von Willebrand factor. Blood. 1987 Sep;70(3):605–611. [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Goralka T. M., Wion K. L., Chen E. Y., Eaton D. H., Vehar G. A., Capon D. J., Lawn R. M. Characterization of the human factor VIII gene. Nature. 1984 Nov 22;312(5992):326–330. doi: 10.1038/312326a0. [DOI] [PubMed] [Google Scholar]
- Haynes J., Weissmann C. Constitutive, long-term production of human interferons by hamster cells containing multiple copies of a cloned interferon gene. Nucleic Acids Res. 1983 Feb 11;11(3):687–706. doi: 10.1093/nar/11.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer L. W., Trabold N. C. The effect of thrombin on human factor VIII. Cleavage of the factor VIII procoagulant protein during activation. J Lab Clin Med. 1981 Jan;97(1):50–64. [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P., Davies M. V. Translational efficiency of polycistronic mRNAs and their utilization to express heterologous genes in mammalian cells. EMBO J. 1987 Jan;6(1):187–193. doi: 10.1002/j.1460-2075.1987.tb04737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P., Ingolia D. E., Yeung C. Y., Kellems R. E. Selection and amplification of heterologous genes encoding adenosine deaminase in mammalian cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3136–3140. doi: 10.1073/pnas.83.10.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary dna gene. J Mol Biol. 1982 Aug 25;159(4):601–621. doi: 10.1016/0022-2836(82)90103-6. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Construction of a modular dihydrofolate reductase cDNA gene: analysis of signals utilized for efficient expression. Mol Cell Biol. 1982 Nov;2(11):1304–1319. doi: 10.1128/mcb.2.11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Dorner A. J. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem. 1988 May 5;263(13):6352–6362. [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Furie B. C., Furie B., Shoemaker C. B. Expression, purification, and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cells. J Biol Chem. 1986 Jul 25;261(21):9622–9628. [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Spiliotes A. J., Gossels S. D., Latt S. A., Larsen G. R., Kay R. M. Coamplification and coexpression of human tissue-type plasminogen activator and murine dihydrofolate reductase sequences in Chinese hamster ovary cells. Mol Cell Biol. 1985 Jul;5(7):1750–1759. doi: 10.1128/mcb.5.7.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. A., Summerfield J. A., Tuddenham E. G. Localization of factor VIIIC: antigen in guinea-pig tissues and isolated liver cell fractions. Br J Haematol. 1984 Apr;56(4):535–543. doi: 10.1111/j.1365-2141.1984.tb02178.x. [DOI] [PubMed] [Google Scholar]
- Knutson G. J., Fass D. N. Porcine factor VIII:C prepared by affinity interaction with von Willebrand factor and heterologous antibodies: sodium dodecyl sulfate polyacrylamide gel analysis. Blood. 1982 Mar;59(3):615–624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. H., Bontempo F. A., Spero J. A., Ragni M. V., Starzl T. E. Liver transplantation in a hemophiliac. N Engl J Med. 1985 May 2;312(18):1189–1190. doi: 10.1056/NEJM198505023121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. C., Zimmerman T. S., Collins C. J., Brown M., Morin M. J., Ling E. H., Livingston D. M. Molecular cloning of cDNA for human von Willebrand factor: authentication by a new method. Cell. 1985 May;41(1):49–56. doi: 10.1016/0092-8674(85)90060-1. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Jenny R. J., Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988;57:915–956. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- Marchioro T. L., Hougie C., Ragde H., Epstein R. B., Thomas E. D. Hemophilia: role of organ homografts. Science. 1969 Jan 10;163(3863):188–190. doi: 10.1126/science.163.3863.188. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Kaufman R. J., Latt S. A., Weinberg R. A. Construction and use of a dominant, selectable marker: a Harvey sarcoma virus-dihydrofolate reductase chimera. Mol Cell Biol. 1983 Jan;3(1):32–43. doi: 10.1128/mcb.3.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C., Kelley W. N., Daddona P. E. Transient expression of human adenosine deaminase cDNAs: identification of a nonfunctional clone resulting from a single amino acid substitution. Mol Cell Biol. 1985 Apr;5(4):762–767. doi: 10.1128/mcb.5.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Over J., Sixma J. J., Bruïne M. H., Trieschnigg M. C., Vlooswijk R. A., Beeser-Visser N. H., Bouma B. N. Survival of 125iodine-labeled Factor VIII in normals and patients with classic hemophilia. Observations on the heterogeneity of human Factor VIII. J Clin Invest. 1978 Aug;62(2):223–234. doi: 10.1172/JCI109120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- RAPAPORT S. I., SCHIFFMAN S., PATCH M. J., AMES S. B. The importance of activation of antihemophilic globulin and proaccelerin by traces of thrombin in the generation of intrinsic prothrombinase activity. Blood. 1963 Feb;21:221–236. [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Rotblat F., O'Brien D. P., O'Brien F. J., Goodall A. H., Tuddenham E. G. Purification of human factor VIII:C and its characterization by Western blotting using monoclonal antibodies. Biochemistry. 1985 Jul 30;24(16):4294–4300. doi: 10.1021/bi00337a007. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. von Willebrand factor and von Willebrand disease. Blood. 1987 Oct;70(4):895–904. [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill S. J., Devos R., Van der Heyden J., Fiers W. Expression and characterization of the product of a human immune interferon cDNA gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4654–4658. doi: 10.1073/pnas.80.15.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J., Wooden S. K., Kriz R., Kelleher K., Kaufman R. J., Lee A. S. The nucleotide sequence encoding the hamster 78-kDa glucose-regulated protein (GRP78) and its conservation between hamster and rat. Gene. 1987;55(1):147–152. doi: 10.1016/0378-1119(87)90258-7. [DOI] [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Tuddenham E. G., Lane R. S., Rotblat F., Johnson A. J., Snape T. J., Middleton S., Kernoff P. B. Response to infusions of polyelectrolyte fractionated human factor VIII concentrate in human haemophilia A and von Willebrand's disease. Br J Haematol. 1982 Oct;52(2):259–267. doi: 10.1111/j.1365-2141.1982.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Preparation and properties of bovine factor VIII (antihemophilic factor). Biochemistry. 1980 Feb 5;19(3):401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Keyt B., Eaton D., Rodriguez H., O'Brien D. P., Rotblat F., Oppermann H., Keck R., Wood W. I., Harkins R. N. Structure of human factor VIII. Nature. 1984 Nov 22;312(5992):337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Hart M., Pannekoek H. Expression of variant von Willebrand factor (vWF) cDNA in heterologous cells: requirement of the pro-polypeptide in vWF multimer formation. EMBO J. 1987 Oct;6(10):2885–2890. doi: 10.1002/j.1460-2075.1987.tb02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Fay P. J., Sporn L. A., Sinha S., Lawrence S. O., Marder V. J. Divergent fates of von Willebrand factor and its propolypeptide (von Willebrand antigen II) after secretion from endothelial cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1955–1959. doi: 10.1073/pnas.84.7.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasley L. C., Atha D. H., Bauer K. A., Kaufman R. J. Expression and characterization of human antithrombin III synthesized in mammalian cells. J Biol Chem. 1987 Oct 25;262(30):14766–14772. [PubMed] [Google Scholar]
- Webster W. P., Zukoski C. F., Hutchin P., Reddick R. L., Mandel S. R., Penick G. D. Plasma factor VIII synthesis and control as revealed by canine organ transplantation. Am J Physiol. 1971 May;220(5):1147–1154. doi: 10.1152/ajplegacy.1971.220.5.1147. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Sussman I. I., Hoyer L. W. Stabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand's disease. J Clin Invest. 1977 Aug;60(2):390–404. doi: 10.1172/JCI108788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wion K. L., Kelly D., Summerfield J. A., Tuddenham E. G., Lawn R. M. Distribution of factor VIII mRNA and antigen in human liver and other tissues. Nature. 1985 Oct 24;317(6039):726–729. doi: 10.1038/317726a0. [DOI] [PubMed] [Google Scholar]
- Wise R. J., Pittman D. D., Handin R. I., Kaufman R. J., Orkin S. H. The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell. 1988 Jan 29;52(2):229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Capon D. J., Simonsen C. C., Eaton D. L., Gitschier J., Keyt B., Seeburg P. H., Smith D. H., Hollingshead P., Wion K. L. Expression of active human factor VIII from recombinant DNA clones. Nature. 1984 Nov 22;312(5992):330–337. doi: 10.1038/312330a0. [DOI] [PubMed] [Google Scholar]
- Zelechowska M. G., van Mourik J. A., Brodniewicz-Proba T. Ultrastructural localization of factor VIII procoagulant antigen in human liver hepatocytes. Nature. 1985 Oct 24;317(6039):729–730. doi: 10.1038/317729a0. [DOI] [PubMed] [Google Scholar]
- van Dieijen G., Tans G., Rosing J., Hemker H. C. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981 Apr 10;256(7):3433–3442. [PubMed] [Google Scholar]







