Abstract
STUDY QUESTION
Is there an association between oral contraceptive (OC) use (age at the start of use, duration of use, ethinylestradiol dose and generation) and time to pregnancy (TTP)?
SUMMARY ANSWER
Although OC use was associated with a transient delay in the return of fertility, we found no evidence that long-term OC use deleteriously affects fecundability.
WHAT IS KNOWN ALREADY
Studies using retrospective data on TTP have reported a short-term delay in the return of fertility after OC use. However, little is known about the long-term OC use and TTP.
STUDY DESIGN, SIZE, DURATION
Data were derived from ‘Snart Gravid.dk’, a prospective cohort study that enrolled participants from 1 June 2007 to 31 May 2010. The final study population consisted of 3727 women.
PARTICIPANTS, SETTING, METHODS
Eligible women were Danish pregnancy planners, aged 18–40 years, who completed a baseline questionnaire and bimonthly follow-up questionnaires until conception or for 12 months, whichever came first. Cohort retention was 80%. We used proportional probability regression models to estimate fecundability ratios (FRs) and 95% confidence intervals (CIs), with adjustment for potential confounders.
MAIN RESULTS AND ROLE OF CHANCE
Compared with barrier methods, the use of OCs as the last contraception method before attempting to conceive was associated with a short-term delay in return of fertility (FR = 0.87, 95% CI: 0.79–0.96). Longer term OC use was associated with higher fecundability: compared with OC use for less than 2 years; FRs were 0.98 (95% CI: 0.83–1.15) for 2–3 years, 1.16 (95% CI: 0.98–1.37) for 4–5 years, 1.10 (95% CI: 0.93–1.29) for 6–7 years, 1.17 (95% CI: 0.99–1.38) for 8–9 years, 1.23 (95% CI: 1.04–1.46) for 10–11 years and 1.28 (95% CI: 1.07–1.53) for ≥12 years of OC use.
LIMITATIONS, REASONS FOR CAUTION
Because this was a non-experimental study, where study volunteers provided information about their history of contraceptive use at baseline and were followed prospectively to assess their waiting times to pregnancy, there was some potential for error in the reporting of OC use and TTP. Nevertheless, participants reported data on OC use before the occurrence of pregnancy, thereby reducing the potential for systematic bias.
WIDER IMPLICATIONS OF THE FINDINGS
Women who have used OCs for many years should be reassured as there was no evidence that long-term OC use has a deleterious affects on fecundability. Both short- and long-term OC users are likely to experience a transient delay in conception compared with those discontinuing barrier methods.
STUDY FUNDING
This study was supported by the National Institute of Child Health and Human Development (R21050264) and the Danish Medical Research Council (271-07-0338). The authors declare that there are no conflicts of interest.
Keywords: oral contraceptives, fecundability, time to pregnancy
Introduction
In Western countries, 50–89% of women use oral contraceptives (OCs) at some point in their lifetime (Skouby, 2004; Mosher and Jones, 2010). OCs inhibit ovulation, thicken the cervical mucus and thin the endometrial lining, thereby preventing fertilization and implantation (Dickey, 2007). Several studies using retrospective data on time to pregnancy (TTP) have reported a short-term delay of 2–6 months in the return of fertility after OC use compared with other contraceptives (Vessey et al., 1978; Linn et al., 1982; Hassan and Killick, 2004; Kaplan et al., 2005; Axmon et al., 2006 ). However, the effect of long-term OC use on TTP has been evaluated prospectively in only two studies, reporting no association of OC use with fecundability (Wiegratz et al., 2006; Cronin et al., 2009). One of these studies also examined the type of OC and TTP but found little association (Cronin et al., 2009). No study has prospectively evaluated TTP in relation to age at the start of OC use.
Increasing proportions of Western women are delaying childbearing until their careers and relationships are well established. This trend has been accompanied by a longer duration of OC use before the first attempt to conceive (Martin, 2000; Petersen et al., 2009). Furthermore, an earlier sexual debut may lead to a younger age at first OC use, increasing the total duration of OC use. Given the high prevalence of OC use, any association with TTP may have a major public health impact. In the current study, we evaluated the effect of former OC use on TTP according to the type of OC [ethinylestradiol (EE) dose and progestin type], age at first use and duration of use, among women enrolled in a Danish web-based pregnancy planning study.
Materials and Methods
The soon pregnant study
Data for this study were collected as part of the Danish web-based pregnancy planning study (‘Snart-Gravid.dk’), described in detail elsewhere (Mikkelsen et al., 2009; Wise et al., 2010). Briefly, participants were enrolled via the study website (www.snart-gravid.dk). Potential participants were asked to read a consent form and complete a screening questionnaire to confirm eligibility. Eligible women were invited to complete a baseline questionnaire and bimonthly questionnaires until conception or for 12 months if no conception occurred.
The web-based baseline questionnaire included questions on socio-demographic background, reproductive and medical history and lifestyle and behavioral factors. The follow-up questionnaires collected information on pregnancy status, frequency and timing of intercourse and other lifestyle and behavioral exposures, such as alcohol intake, which may change over time.
Study population
The study included women who met the following criteria: aged 18–40 years, Danish residents, in a stable relationship with a male partner, attempting to conceive, and not receiving fertility treatment. A total of 5644 women were eligible and enrolled from 1 June 2007 to 31 May 2010. In the analyses, we excluded women who had tried to conceive for more than six cycles at study entry (n = 1104), women who did not complete at least one follow-up questionnaire (n = 534) and women who provided insufficient or implausible information about the date of their last menstrual period (LMP) or the date of their first pregnancy attempt (n = 279). Thus, the final study population comprised 3727 women.
Assessment of OC use
At baseline, participants were asked to report all contraceptive methods ever used, as well as the last method used before their current attempt to become pregnant. For analytic purposes, the most recent contraceptive method was categorized into four groups: OCs (all types), intrauterine device [IUD (hormone and copper)], barrier methods (condom, foam, gel, cream, and suppository) and other methods (injectables, patch, withdrawal, calendar, body temperature, and unspecified).
Women who reported ever taking OCs were asked to provide the number of OC brands they had used, as well as the age at the start of use and duration of use for each brand. To enhance recall, the questionnaire included a list of all OC brand names available in Denmark. We categorized OCs according to the content of EE: none, low dose (20 or 30 µg) and high dose (35 µg or bi/triphasic OCs with amounts varying between 30 and 40 µg). We then further categorized OCs into generations according to progestin content: second generation (norgestrel and levonorgestrel), third generation (desogestrel and gestodene) and fourth generation (drospirenone). We estimated the total duration of OC use for each participant by summing duration (months and years) of OC use across different brands. At baseline, participants were asked if they intentionally had delayed their attempt to become pregnant after discontinuing OC use.
Assessment of pregnancy and cycles at risk
The event of interest was the first reported pregnancy during the follow-up period, regardless of pregnancy outcome. The follow-up questionnaires thus included questions on date of LMP, current pregnancy status and other pregnancy outcomes since the date of the last completed questionnaire. TTP was calculated as the number of days a woman had tried to conceive divided by cycle length. TTP included time before and after the study entry. Total cycles at risk were calculated as follows: (days of trying to achieve pregnancy at study entry/cycle length) + [(LMP date from most recent follow-up questionnaire − date of baseline questionnaire completion)/cycle length) + 1] (Wise et al., 2011).
Assessment of covariates
Weight, height, physical activity and smoking history were reported at baseline and allowed the calculation of body mass index (BMI), total metabolic equivalents (METs) and pack-years of smoking. Total METS were estimated by summing the METs from moderate and vigorous physical activity, i.e. hours per week multiplied by 3.5 and hours per week multiplied by 7.0, respectively (Ainsworth et al., 2000).
Data analysis
At baseline, the proportion of missing data for most covariates was <1% and a few variables had missing data for 1–8% of subjects. We performed multiple imputation using all gathered information including outcome variables to impute missing covariate values (Zhou et al., 2001). We examined the associations between TTP, last method of contraception and OC exposure (age at the start of OC use, duration of use, EE dose and generation) by calculating: (i) cycle-specific pregnancy probabilities, (ii) cumulative probability of pregnancy, using Kaplan–Meier curves and (iii) fecundability ratios (FRs) and 95% confidence intervals (CIs) using a proportional probabilities regression model (Weinberg et al., 1989). The FR represents the cycle-specific probability of conception among exposed women divided by that among unexposed women. A FR below 1 indicates reduced fertility among exposed women relative to unexposed women (Weinberg et al., 1989; Baird et al., 1994). At study entry, participants had been trying to conceive for a varying number of cycles, ranging from zero to six. To account for this variation, we based risk sets only on observed cycles at risk, i.e. a woman contributed risk (attempt) time only while participating in the study (Wise et al., 2010). Right-censoring occurred when a participant was lost to follow-up, started fertility treatment, stopped trying to conceive or reached the end of the observation period (12 cycles).
In multivariate analyses (including the Kaplan–Meier curves), we adjusted for potential confounders (age, parity, partner's age, education, cycle regularity, heaviness of menstrual flow, BMI, physical activity, smoking, alcohol intake, intercourse frequency, intentional delay between OC cessation and initiation of pregnancy attempt and history of sexually transmitted diseases) selected on the basis of the literature and clinical relevance. Time-varying exposures were updated using data reported on follow-up questionnaires.
In sub-analyses, we stratified data according to gravidity, parity and age at study entry. Finally, we used restricted cubic splines to explore the possibility of a nonlinear relation between the duration of OC use and fecundability (Durrleman and Simon, 1989). SAS statistical software (version 9.2, SAS Institute, Cary, NC) was used for all analyses.
Ethical approval
The study protocol was approved by the Danish Data Protection Agency, CVR no. 11-88-37-29 and the Boston University Medical Center.
Results
Of the 3727 participants in this analysis, 1894 (51%) achieved a pregnancy within six cycles of follow-up. Within 12 cycles of follow-up, 2546 (68%) achieved a pregnancy, 431 (12%) did not become pregnant, 171 (4.6%) were no longer trying to become pregnant, 259 (6.9%) started fertility treatment and 320 (8.6%) did not complete the study. Overall, the study retention was 80%. We found little difference in age, partner's age, last method of contraception, age at menarche, cycle length, BMI or TTP at study entry between women who completed the study and those lost to follow-up.
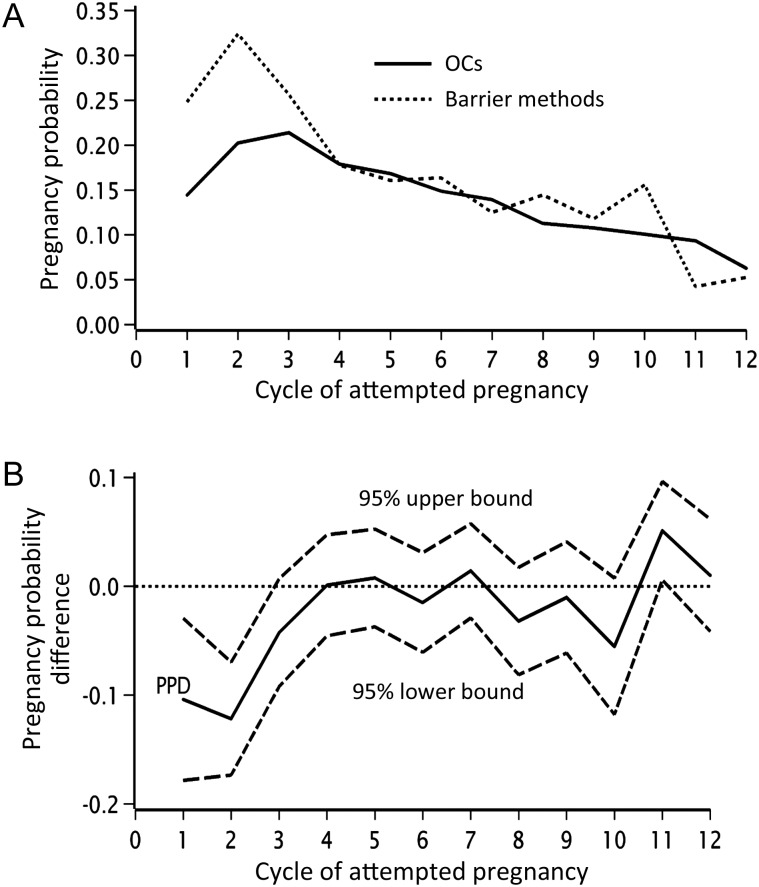
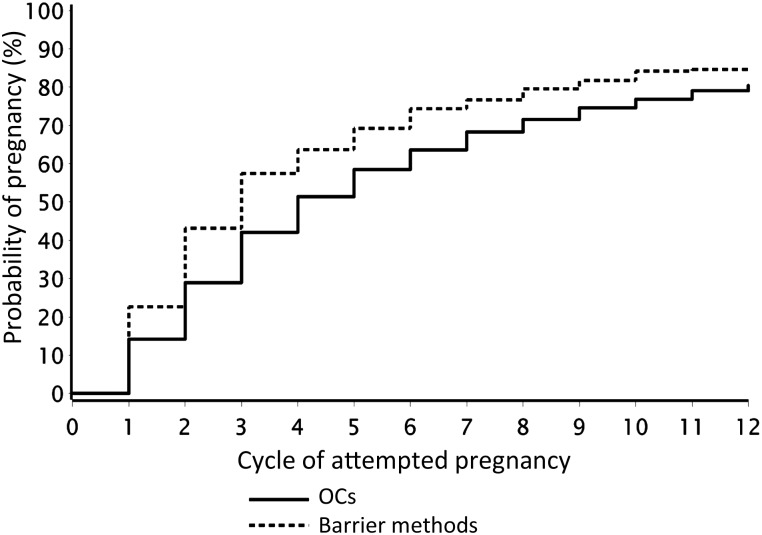
Overall, 3603 (97%) had ever used OCs. The median age at first OC use was 17.0 years (mean 17.4) and median duration of OC use was 7.0 years. Among ever users of OCs, 33, 38, 19 and 10% had used 1, 2, 3 or ≥4 different types of OCs, respectively, and the median duration of OC use was 84, 96, 82 and 60 months, respectively. In total, 2299 (62%) of the women reported OCs and 769 (21%) reported barrier methods as their most recent contraceptive method (Table I). Figure 1 illustrates cycle-specific pregnancy probabilities and differences in pregnancy probabilities with 95% CIs, for women whose last method of contraception was OCs compared with those who last used barrier methods. OC users had lower fecundability than users of barrier methods in the first three unprotected cycles. Thereafter, cycle-specific pregnancy rates decreased equally for those using OC and barrier methods, from ∼17% at cycle 4 to 10% at cycle 12. The adjusted Kaplan–Meier curve (Fig. 2) shows that the 25th, 50th and 75th percentiles for TTP were 2, 3 and 7 cycles, respectively, among women who had used barrier methods and 2, 4 and 9 cycles, respectively, among women who used OCs as their last method. Overall, the adjusted FRs for women using OCs, IUDs or other methods as their last type of contraception were 0.87 (95% CI: 0.79–0.96), 0.95 (95% CI: 0.82–1.11) and 0.82 (95% CI: 0.72–0.93), respectively, compared with those using barrier methods. In a sub-analysis, we restricted the ‘other method’ group to women reporting withdrawal, calendar or body temperature methods, because these less reliable methods may be associated with a history of fertility problems. This restriction did not change the overall FRs (data not shown). In total, 1239 (33%) participants had had a previous live birth. We stratified the analysis by gravidity (gravid and nulligravid), parity (parous and nulliparous) and age (<30 and ≥30 years) at study entry, because gravid and younger women may have increased fecundability regardless of their last method of contraception used. Adjusted FRs for OC users relative to users of barrier methods were 0.79 (95% CI: 0.69–0.92) for parous women and 0.91 (95% CI: 0.80–1.04) for nulliparous women (Table II); the findings were similar for gravid and nulligravid women (data not shown). When stratifying by age, the adjusted FRs for OC users relative to users of barrier methods were 0.84 (95% CI: 0.74–0.96) for women aged <30 and 0.88 (95% CI: 0.76–1.03) for women aged ≥30 years.
Table I.
Baseline characteristics of 3727 women by last contraceptive method used before attempting to conceive.
| Characteristic | Last method of contraception |
|||
|---|---|---|---|---|
| OC | IUDa | Barrierb | Otherc | |
| No. of women | 2299 | 259 | 769 | 400 |
| Age, years (mean) | 27.9 | 30.3 | 28.8 | 29.5 |
| Partner's age, years (mean) | 30.5 | 32.5 | 30.7 | 31.9 |
| Age at menarche, years (mean) | 13.0 | 13.0 | 12.9 | 12.9 |
| Irregular cycles, yes (%) | 25.1 | 17.4 | 22.9 | 23.0 |
| Cycle length, days (median) | 29.0 | 28.0 | 29.0 | 29.0 |
| Parous, ever had live birth (%) | 26.5 | 73.0 | 37.0 | 39.5 |
| Body mass index, kg/m2 (median) | 22.9 | 23.2 | 23.0 | 22.3 |
| Physical activity, MET h/week (median) | 21.0 | 21.0 | 21.0 | 21.0 |
| Vocational training (%) | ||||
| Short (none, semi-skilled, <3 years) | 44.6 | 37.5 | 37.8 | 32.3 |
| Medium (3–4 years) | 32.6 | 37.5 | 37.8 | 37.3 |
| Long (>4 years) | 22.9 | 25.1 | 24.3 | 30.5 |
| Pack-years of ever smoking (mean) | 2.1 | 2.3 | 1.9 | 2.4 |
| Alcohol intake, drinks/week (mean) | 2.9 | 2.5 | 2.9 | 2.8 |
| Time in steady relationship, years (mean) | 4.6 | 6.0 | 5.6 | 5.3 |
| Frequency of intercourse, ≥4 times/week (%) | 20.7 | 22.8 | 15.2 | 16.3 |
| Attempt time before study entry, cycles (%) | ||||
| 0–1 | 52.3 | 56.8 | 56.2 | 57.5 |
| 2–3 | 26.3 | 24.7 | 23.5 | 25.3 |
| 4–6 | 21.4 | 18.5 | 20.3 | 17.2 |
| Hypertension, yes (%) | 4.1 | 6.2 | 3.8 | 3.3 |
| Sexually transmitted infection, yes (%)d | 31.8 | 35.1 | 25.4 | 31.8 |
OC, oral contraceptive; MET, total metabolic equivalents.
aIntrauterine device (hormone and copper).
bBarrier (condom, foam, gel, cream and suppository).
cOther [injectables (N = 12), patch (N = 16), withdrawal (N = 261), calendar or body temperature methods (N = 83) and unspecified (28)].
dChlamydia, gonorrhea, genital herpes and unspecified.
Figure 1.
Cycle-specific pregnancy probability by last contraceptive method (A) and difference (B) in pregnancy probability among last contraceptive method, N = 3068.
Figure 2.
Kaplan–Meier, pregnancy probability curves by last contraception method. The curves are adjusted for age, partner's age, educational level, cycle regularity, heaviness of flow, BMI, physical activity, smoking, alcohol intake, intercourse frequency, intentional delay, sexually transmitted disease history and total duration of OC use in months. N= 3068.
Table II.
Fecundability for parous and nulliparous women by last contraceptive method used (N = 3671).
| Parity | Contraception method | Pregnancies | TTP, cycles | Unadjusted model |
Adjusted modela |
||
|---|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | ||||
| Parous | Barrier | 232 | 938 | 1.00 | Ref. | 1.00 | Ref. |
| OC | 450 | 2365 | 0.78 | 0.68–0.90 | 0.79 | 0.69–0.92 | |
| IUD | 145 | 658 | 0.90 | 0.76–1.08 | 0.91 | 0.76–1.09 | |
| Otherb | 117 | 606 | 0.76 | 0.63–0.93 | 0.76 | 0.63–0.93 | |
| Nulli-parous | Barrier | 327 | 2 085 | 1.00 | Ref. | 1.00 | Ref. |
| OC | 1089 | 7619 | 0.91 | 0.81–1.02 | 0.91 | 0.80–1.04 | |
| IUD | 44 | 313 | 0.93 | 0.69–0.99 | 0.95 | 0.70–1.28 | |
| Otherb | 142 | 1114 | 0.82 | 0.72–1.05 | 0.85 | 0.70–1.02 | |
FR, fecundability ratio; CI, confidence interval.
aAdjusted for age, partner's age, educational level, cycle regularity, heaviness of flow, BMI, physical activity, smoking, alcohol intake, intercourse frequency, intentional delay, sexually transmitted disease history and total duration of OC use in months.
bWomen who used hormone injections, hormone patch and unspecified methods are excluded.
The adjusted cumulative 25th, 50th and 75th percentiles of TTP were 4, 7 and >12 cycles, for women who started OCs at age <16 years and 3, 6 and >12 cycles, for women who started OCs at age 22 years (Kaplan–Meir curve not shown). Compared with the first OC use at age ≥21 years, the first OC use at ages <16, 16–17 or 18–21 years was associated with reduced fecundability, with adjusted FRs of 0.81 (95% CI: 0.69–0.94), 0.88 (95% CI: 0.76–1.02) and 0.90 (95% CI: 0.79–1.04), respectively (Table III).
Table III.
Fecundability by age of first OC use and duration of OC use (N = 3603).
| Pregnancies | Cycles | Unadjusted model |
Adjusted modela,b |
|||
|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | |||
| Age at first OC use (years) | ||||||
| <16 | 638 | 4305 | 0.88 | 0.77–1.01 | 0.81 | 0.69–0.94 |
| 16–17 | 835 | 4945 | 0.98 | 0.86–1.12 | 0.88 | 0.76–1.02 |
| 18–20 | 779 | 4567 | 1.01 | 0.88–1.15 | 0.90 | 0.79–1.04 |
| ≥20 | 222 | 1299 | 1.00 | Ref. | 1.00 | Ref. |
| Total duration of OC use (years) | ||||||
| <2 | 229 | 1508 | 1.00 | Ref. | 1.00 | Ref. |
| 2–3 | 311 | 2102 | 0.95 | 0.81–1.12 | 0.98 | 0.83–1.15 |
| 4–5 | 402 | 2340 | 1.13 | 0.95–1.33 | 1.16 | 0.98–1.37 |
| 6–7 | 367 | 2400 | 1.04 | 0.88–1.22 | 1.10 | 0.93–1.29 |
| 8–9 | 401 | 2328 | 1.10 | 0.94–1.29 | 1.17 | 0.99–1.38 |
| 10–11 | 356 | 1988 | 1.15 | 0.98–1.35 | 1.23 | 1.04–1.46 |
| ≥12 | 408 | 2450 | 1.12 | 0.95–1.31 | 1.28 | 1.07–1.53 |
FR, fecundability ratio; CI, confidence interval.
aAge at first OC use adjusted for age, partner's age, educational level, cycle regularity, heaviness of flow, BMI, physical activity, smoking, alcohol intake, parity, intercourse frequency, intentional delay, sexually transmitted disease history, total duration of OC use, last type of contraception used, and months since recent (last) OC use.
bTotal duration of OC use adjusted for age, partner's age, educational level, cycle regularity, heaviness of flow, BMI, physical activity, smoking, alcohol intake, parity, intercourse frequency, intentional delay, sexually transmitted disease history, age at first OC use and last type of contraception used.
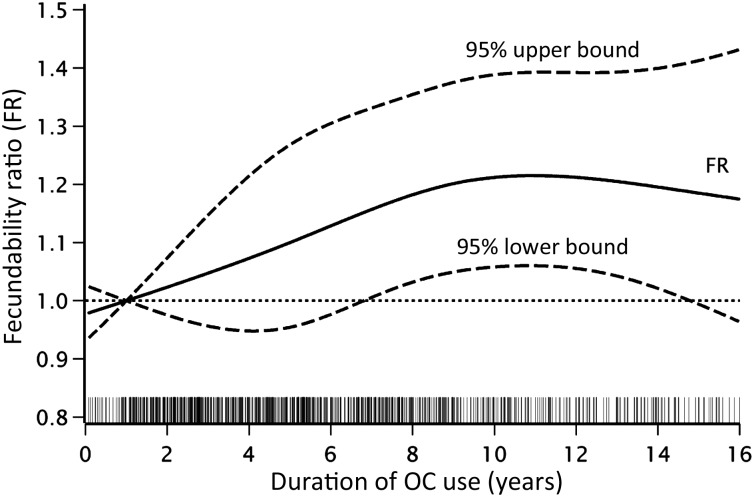
After adjusting for covariates, including age, parity, last method of contraception and age at first OC use, we found a dose–response relation between the total duration of OC use and increased fecundability. Compared with the OC use <2 years, the FRs were 0.98 (95% CI: 0.83–1.15) for 2–3 years of OC use, 1.16 (95% CI: 0.98–1.37) for 4–5 years, 1.10 (95% CI: 0.93–1.29) for 6–7 years, 1.17 (95% CI: 0.99–1.38) for 8–9 years, 1.23 (95% CI: 1.04–1.46) for 10–11 years and 1.28 (95% CI: 1.07–1.53) for ≥12 years (Table III). Because younger women lacked the opportunity to use OCs as long as older women, we also stratified by age at study entry (18–24 versus >24 years). This analysis yielded similar, but less precise results (data not shown). The adjusted cumulative 25th, 50th and 75th percentiles of TTP were 4, 7 and >11 cycles, respectively, for OC use of <2 years and 3, 6 and >12 cycles, respectively, for OC use ≥12 years (Kaplan–Meir curve not shown). Figure 3 depicts a pattern of increasing fecundability with increasing duration of OC use, from 1 year of use until the peak at ∼11 years of use.
Figure 3.
Relation between the duration of OC use and fecundability, fitted by restricted cubic splines. The dashed curves indicate the 95% CI. The reference level is 1 year of OC use. The curves are adjusted for age, partner's age, educational level, cycle regularity, heaviness of flow, BMI, physical activity, smoking, alcohol intake, parity, intercourse frequency, intentional delay, sexually transmitted disease history, age at the first OC use and last type of contraception used. Four knots were located at 1, 5, 9 and 14.5 years of use.The grey spikes show the distribution of duration of OC use. N= 3603.
Among women using OCs as their last method of contraception, 1575 (69%) used OCs containing low-dose EE (20 or 30 µg), 354 (15%) used high-dose OCs (35 µg or bi/triphasic with 30–40 µg) and 368 (16%) used OCs containing no EE or an unknown amount of EE. High-dose OC use was associated with a shorter TTP (FR = 1.18, 95% CI: 1.05–1.34) (Table IV).
Table IV.
Fecundability for women using OCs as their last method of contraception by type of OC. N = 2299.
| Number | Pregnancies | Cycles | Unadjusted model |
Adjusted modela |
|||
|---|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | ||||
| EE dose in last OC usedb | |||||||
| Low (20–30 µg) | 1575 | 1031 | 6959 | 1.00 | Ref. | 1.00 | Ref. |
| High (>30 µg)c | 354 | 259 | 1421 | 1.21 | 1.07–1.36 | 1.18 | 1.05–1.34 |
| Generation of last OC usedd | |||||||
| Second | 333 | 240 | 1354 | 1.00 | Ref. | 1.00 | Ref. |
| Third | 1285 | 858 | 5627 | 0.87 | 0.76–0.99 | 0.87 | 0.76–0.99 |
| Fourth | 344 | 218 | 1535 | 0.79 | 0.67–0.94 | 0.81 | 0.68–0.96 |
| Uncertain | 335 | 221 | 1464 | 0.92 | 0.76–1.10 | 0.94 | 0.78–1.13 |
FR, fecundability ratio; CI, confidence interval.
aAdjusted for age, partner's age, educational level, cycle regularity, heaviness of flow, BMI, physical activity, smoking, alcohol intake, parity, intercourse frequency, intentional delay and total duration of OC use.
bParticipants using OCs containing no EE (64) or an unknown amount of EE (304) were excluded from the analysis.
cOCs containing 35 µg EE or bi/triphasic OCs with EE amounts varying between 30 and 40 µg.
dSecond generation (norgestrel and levonorgestrel), third generation (desogestrel and gestoden) and fourth generation (drospirenone).
Second, third and fourth generation OCs were used by 333 (14%), 1285 (56%) and 344 (15%) women, respectively. Table IV shows FRs for women using third and fourth generation OCs compared with women using second generation. The estimates indicate longer TTPs for users of third and fourth generation OCs, with adjusted FRs of 0.87 (95% CI: 0.76–0.99) and 0.81 (95% CI: 0.68–0.96), respectively.
Discussion
Our prospectively collected data on TTP confirm previous findings from retrospective studies indicating a short-term delay in return of fertility after OC use when compared with barrier methods (Vessey et al., 1978; Linn et al., 1982; Hassan and Killick, 2004; Kaplan et al., 2005; Axmon et al., 2006). Among ever users of OCs, we found that OC use for longer time periods was associated with increased fecundability and young age at the first OC use was associated with reduced fecundability compared with initiation of OC use after the age of 21. Also, the use of third and fourth generation OCs was associated with longer TTP compared with the use of second generation OCs.
Some methodological explanations for our findings should be considered. Selection bias could occur if the choice of contraceptive method or choice of OC type is related to underlying fertility. For instance, highly fertile women might prefer more reliable OCs over other contraceptives. If that were that the case, the observed short-term delay in conception might be an underestimate. However, the results from our sub-analysis of nulligravid women, who presumably had no knowledge about their fertility, did not lend support to this hypothesis. In addition, as our study comprises women planning a pregnancy, it may overestimate TTP because unplanned pregnancies are more likely to occur among highly fertile women. We tried to address this problem by restricting the study population to women who had attempted pregnancy for six or less cycles at study entry. TTP was measured prospectively and cycles were used as the time metric, as each cycle offers a single ovulatory opportunity (Weinberg et al., 1994). However, because we collected TTP data bimonthly, some misclassification may have occurred, but it is unlikely to be related to exposure. In addition, data on former OC exposure were reported retrospectively and covered a long time period; thus, potential for underreporting exists. However, systematic bias in reporting of OC use is unlikely because participants reported OC brands and duration of OC use at baseline, before the occurrence of pregnancy. Cohort retention in our study was 80% (women enrolled 1 June 2007 to 31 May 2010), similar to that of other prospective cohort studies (Olsen et al., 2001; Russell et al., 2001), and we found no major differences in baseline characteristics between women who completed the study and women who were lost to follow-up. We adjusted for a large number of potential confounders, but some residual confounding is still possible because we were not able to account for type of OCs used previously, polycystic ovarian syndrome (PCOS), menstrual pain and intolerance of OCs, which may be related both to last the contraception used and fertility. Finally, the study population consists of self-selected volunteers who were recruited via the Internet. Because all our comparisons are made within the population of our study subjects, and not between our study subjects and others who did not participate in the study, and because women who volunteered for the study did so before the occurrence of the outcome (pregnancy), the internal validity of the study is not affected by differences between the study population and the general population. On the other hand, if the biologic relations between OC use and TTP differ for study volunteers and others, the generalizability of our study findings might be limited, but is seems unlikely that the effect of OCs on TTP would differ for study volunteers and others.
Our data extend research from earlier studies in several ways. A prospective study of 2064 women planning pregnancy reported little difference in pregnancy rates for women who had used OCs for <24 months compared with those who had used OCs for ≥24 months (Cronin et al., 2009). The other prospective study of 746 women who discontinued OCs to conceive found that the preceding duration of OC use (mean 21.5 cycles) had little influence on cumulative 1-year pregnancy rates (Wiegratz et al., 2006). Similar to our findings, Farrow et al.'s study of 8497 pregnant women who reported TTP retrospectively in four broad categories (≤6 months; 7–12 months, 13–35 months and ≥36 months) showed that prolonged OC use (≥5 years) was associated with improved fecundity. The odds ratios for achieving a pregnancy within 12 months were 0.46 (95% CI: 0.33–0.65), 0.52 (95% CI: 0.39–0.70) and 0.71 (95% CI: 0.56–0.91) for OC use of <1, 1–2 and 3–4 years, respectively, compared with ≥5 years of OC use. No information was provided on intentional delay after OC use or frequency of intercourse (Farrow et al., 2002). In our study, we have not been able to compare long time OC users with never users because only 3% of our sample had never used OCs. Thus, we cannot rule out the possibility that fecundability is reduced after short-term OC use and improves thereafter, rather that fecundability is simply enhanced after long use.
During the reproductive development phase in adolescence, OCs are used to treat menstrual pain, cycle irregularities, PCOS, heavy menstrual flow and for contraception (Dickey, 2007). Only one earlier study, based on retrospective data from the Nurses' Health Study (NHS) II, has explored the possible association between age at first OC use and TTP later in life. It found no increased risk of impaired fertility when OC use was initiated at a young age (Chasan-Taber et al., 1997). That conflicts with our finding of longer TTPs in particular for women who started OC use before the age of 16. In the NHS II study, age at first OC use was categorized into 13–19 years, ≥20 years and never use. The mean age at first OC use was 20.1 years, compared with 17.4 years in our study. Thus, the majority of women in NHS II did not use OCs at a very young age. In addition, the use of broad age groups may mask a possible effect, explaining the discrepancy between the NHS II results and our results. A weakness of our study is the lack of information on reason for starting OC use before the age of 16. Although we adjusted for cycle irregularity and heaviness of flow, decreased fecundability could occur if young women used OCs for therapeutic reasons and these problems persisted after OC cessation.
Using data from 2064 women who participated in a phase IV surveillance study of both drospirenone-containing OCs and other progestin-containing OCs, Cronin et al. found that the progestin type and EE dose (20 versus 30 µg) did not greatly influence initial or 1-year pregnancy rates after OC cessation (Cronin et al., 2009). A cross-sectional study found that women discontinuing OCs containing ≥50 µg EE had greater conception delays than those discontinuing OCs with lower EE doses (Bracken et al., 1990). We found slightly shorter TTPs for women who discontinued high-dose OCs (35 µg or bi/triphasic OCs with 30–40 µg). However, it is difficult to compare findings across these studies because of differences in OC formulations and study designs. In contrast to Cronin et al., we found that progestin type was associated with TTP; users of third and fourth generation OCs had longer TTPs compared with second generation OC users.
Several biological mechanisms may explain our findings. OCs prevent ovulation by suppressing hypothalamic and pituitary secretion of hormones (Dickey, 2007), which may influence both the short-term delay in fecundability after OC discontinuation and improved fecundability after long-term OC use. A residual hormonal effect influencing ovulation after OC cessation could lead to the short-term delay in fecundability. A recent study documented that menstrual cycle biomarkers were altered for at least two cycles after discontinuation of OC use. Specifically, recent OC use was associated with lower cervical mucus quality, later estimated date of ovulation and decreased intensity of menstrual flow (a marker for quality of endometrial lining) (Nassaralla et al., 2011). A long-term use of OCs might increase fertility by inhibiting follicle depletion over a woman's reproductive life. This hypothesis seems plausible as levels of the anti-Mullerian hormone, a clinical marker of ovarian reserve, have been found to be stable with extended OC use (Somunkiran et al., 2007; Steiner et al., 2010). Moreover, some studies have shown that OCs postpone the depletion of the follicle pool and delay natural menopause (Gold et al., 2001; Palmer et al., 2003).
Women who have used OCs for 4 years or more should be reassured because we found no evidence that long-term OC use deleteriously affects fecundability. Like short-term users, long-term users appear to experience a short-term delay in getting pregnant compared with those discontinuing barrier methods. The association between young age at first OC use and reduced fecundability is notable, but may partially reflect early age at first use among women with cycle irregularities. We believe this issue merits further investigation, as does our finding of slightly reduced fecundability for users of third and fourth generation OCs.
Authors' roles
All authors contributed to the design of the study. E.M.M. wrote the first and successive drafts of the paper. A.H.R. and L.A.W. carried out the statistical analysis. All authors contributed to the interpretation of results, reviewed and approved the final manuscript.
Funding
The study was supported by the National Institute of Child Health and Human Development (R21050264) and the Danish Medical Research Council (271-07-0338).
Conflict of interest
None declared.
Acknowledgements
We are grateful to Tina Christensen for her support with data collection and media contacts, Donna Day Baird for her feedback on questionnaire development, Rose Radin's assistance with macro development, Kristen Hahn for assistance with data cleaning and Thomas Jensen for his assistance with website and questionnaire design.
References
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr., Schmitz KH, Emplaincourt PO, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Axmon A, Rylander L, Albin M, Hagmar L. Factors affecting time to pregnancy. Hum Reprod. 2006;21:279–1284. doi: 10.1093/humrep/dei469. [DOI] [PubMed] [Google Scholar]
- Baird DD, Weinberg CR, Schwingl P, Wilcox AJ. Selection bias associated with contraceptive practice in time-to-pregnancy studies. Ann N Y Acad Sci. 1994;709:156–164. doi: 10.1111/j.1749-6632.1994.tb30395.x. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Hellenbrand KG, Holford TR. Conception delay after oral contraceptive use: the effect of estrogen dose. Fertil Steril. 1990;53:21–27. [PubMed] [Google Scholar]
- Chasan-Taber L, Willett WC, Stampfer MJ, Spiegelman D, Rosner BA, Hunter DJ, Colditz GA, Manson JE. Oral contraceptives and ovulatory causes of delayed fertility. Am J Epidemiol. 1997;146:258–265. doi: 10.1093/oxfordjournals.aje.a009261. [DOI] [PubMed] [Google Scholar]
- Cronin M, Schellschmidt I, Dinger J. Rate of pregnancy after using drospirenone and other progestin-containing oral contraceptives. Obstet Gynecol. 2009;114:616–622. doi: 10.1097/AOG.0b013e3181b46f54. [DOI] [PubMed] [Google Scholar]
- Dickey RP. Managing Contraceptive Pill Patients. 13th edn. Dallas, TX, USA: EMIS Medical Publishers; 2007. [Google Scholar]
- Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- Farrow A, Hull MG, Northstone K, Taylor H, Ford WC, Golding J. Prolonged use of oral contraception before a planned pregnancy is associated with a decreased risk of delayed conception. Hum Reprod. 2002;17:2754–2761. doi: 10.1093/humrep/17.10.2754. [DOI] [PubMed] [Google Scholar]
- Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004;81:384–392. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- Kaplan B, Nahum R, Yairi Y, Hirsch M, Pardo J, Yogev Y, Orvieto R. Use of various contraceptive methods and time of conception in a community-based population. Eur J Obstet Gynecol Reprod Biol. 2005;123:72–76. doi: 10.1016/j.ejogrb.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Linn S, Schoenbaum SC, Monson RR, Rosner B, Ryan KJ. Delay in conception for former ‘pill’ users. JAMA. 1982;247:629–632. [PubMed] [Google Scholar]
- Martin SP. Diverging fertility among U.S. women who delay childbearing past age 30. Demography. 2000;37:523–533. doi: 10.1353/dem.2000.0007. [DOI] [PubMed] [Google Scholar]
- Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort profile: the Danish Web-based Pregnancy Planning Study-‘Snart-Gravid. Int J Epidemiol. 2009;38:938–943. doi: 10.1093/ije/dyn191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher WD, Jones J. Use of contraception in the United States:1982–2008. Vital Health Stat. 2010;23:1–44. [PubMed] [Google Scholar]
- Nassaralla CL, Stanford JB, Daly KD, Schneider M, Schliep KC, Fehring RJ. Characteristics of the menstrual cycle after discontinuation of oral contraceptives. J Womens Health. 2011;20:169–177. doi: 10.1089/jwh.2010.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, Taxbol D, Hansen KD, Juhl M, Schow TB, et al. The Danish National Birth Cohort-its background, structure and aim. Scand J Public Health. 2001;29:300–307. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- Palmer JR, Rosenberg L, Wise LA, Horton NJ, Adams-Campbell LL. Onset of natural menopause in African American women. Am J Public Health. 2003;93:299–306. doi: 10.2105/ajph.93.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CB, Mortensen LH, Morgen CS, Madsen M, Schnor O, Arntzen A, Gissler M, Cnattingius S, Andersen AM. Socio-economic inequality in preterm birth: a comparative study of the Nordic countries from 1981 to 2000. Paediatr Perinat Epidemiol. 2009;23:66–75. doi: 10.1111/j.1365-3016.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- Russell C, Palmer JR, Adams-Campbell LL, Rosenberg L. Follow-up of a large cohort of Black women. Am J Epidemiol. 2001;154:845–853. doi: 10.1093/aje/154.9.845. [DOI] [PubMed] [Google Scholar]
- Skouby SO. Contraceptive use and behavior in the 21st century: a comprehensive study across five European countries. Eur J Contracept Reprod Health Care. 2004;9:57–68. doi: 10.1080/13625180410001715681. [DOI] [PubMed] [Google Scholar]
- Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196–201. doi: 10.1016/j.ejogrb.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Steiner AZ, Stanczyk FZ, Patel S, Edelman A. Antimullerian hormone and obesity: insights in oral contraceptive users. Contraception. 2010;81:245–248. doi: 10.1016/j.contraception.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey MP, Wright NH, McPherson K, Wiggins P. Fertility after stopping different methods of contraception. Br Med J. 1978;1:265–267. doi: 10.1136/bmj.1.6108.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129:1072–1078. doi: 10.1093/oxfordjournals.aje.a115211. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Baird DD, Wilcox AJ. Sources of bias in studies of time to pregnancy. Stat Med. 1994;13:671–681. doi: 10.1002/sim.4780130528. [DOI] [PubMed] [Google Scholar]
- Wiegratz I, Mittmann K, Dietrich H, Zimmermann T, Kuhl H. Fertility after discontinuation of treatment with an oral contraceptive containing 30 microg of ethinyl estradiol and 2 mg of dienogest. Fertil Steril. 2006;85:1812–1819. doi: 10.1016/j.fertnstert.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25:253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Mikkelsen EM, Rothman KJ, Riis AH, Sorensen HT, Huybrechts KF, Hatch EE. A prospective cohort study of menstrual characteristics and time to pregnancy. Am J Epidemiol. 2011;174:701–709. doi: 10.1093/aje/kwr130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20:1541–1549. doi: 10.1002/sim.689. [DOI] [PubMed] [Google Scholar]