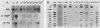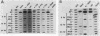Abstract
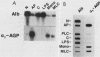
Seven simian virus 40 (SV40)-hepatocyte cell lines were characterized with respect to the ability to express eight liver acute-phase genes. cDNA clones corresponding to albumin, serum amyloid A, alpha 1-acid glycoprotein, haptoglobin, alpha-, beta-, and gamma-fibrinogen, and alpha 1-major-acute-phase protein mRNAs were used in Northern (RNA) or slot blot analyses. In the noninduced state, six of the seven cell lines showed significant (i.e., liverlike) levels of constitutive expression of all genes examined except that expression of haptoglobin mRNA was considerable lower than in the normal liver. To examine whether these immortalized liver cells can respond appropriately to inflammatory mediators, cells were treated with conditioned medium from activated human monocytes or mixed lymphocyte cultures. Results showed that these SV40-hepatocyte cell lines responded to the conditioned media in culture by down-regulating albumin gene expression and up-regulating other acute-phase genes in a time- and dose-dependent manner. These results indicate that the SV40-hepatocytes retained not only the ability to express a number of acute-phase genes but also the ability to respond to external stimuli. The usefulness of these cell lines for analysis of the molecular mechanisms involved in the regulation of these acute-phase genes is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Geiger T., Hirano T., Kishimoto T., Tran-Thi T. A., Decker K., Heinrich P. C. Regulation of synthesis and secretion of major rat acute-phase proteins by recombinant human interleukin-6 (BSF-2/IL-6) in hepatocyte primary cultures. Eur J Biochem. 1988 Apr 15;173(2):287–293. doi: 10.1111/j.1432-1033.1988.tb13997.x. [DOI] [PubMed] [Google Scholar]
- Andus T., Geiger T., Hirano T., Northoff H., Ganter U., Bauer J., Kishimoto T., Heinrich P. C. Recombinant human B cell stimulatory factor 2 (BSF-2/IFN-beta 2) regulates beta-fibrinogen and albumin mRNA levels in Fao-9 cells. FEBS Lett. 1987 Aug 31;221(1):18–22. doi: 10.1016/0014-5793(87)80344-7. [DOI] [PubMed] [Google Scholar]
- Arcone R., Gualandi G., Ciliberto G. Identification of sequences responsible for acute-phase induction of human C-reactive protein. Nucleic Acids Res. 1988 Apr 25;16(8):3195–3207. doi: 10.1093/nar/16.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Baumann H., Onorato V., Gauldie J., Jahreis G. P. Distinct sets of acute phase plasma proteins are stimulated by separate human hepatocyte-stimulating factors and monokines in rat hepatoma cells. J Biol Chem. 1987 Jul 15;262(20):9756–9768. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuscher H. U., Fallon R. J., Colten H. R. Macrophage membrane interleukin 1 regulates the expression of acute phase proteins in human hepatoma Hep 3B cells. J Immunol. 1987 Sep 15;139(6):1896–1901. [PubMed] [Google Scholar]
- Braciak T. A., Northemann W., Hudson G. O., Shiels B. R., Gehring M. R., Fey G. H. Sequence and acute phase regulation of rat alpha 1-inhibitor III messenger RNA. J Biol Chem. 1988 Mar 15;263(8):3999–4012. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Courtois G. M., Kilian P. L., Fuller G. M., Crabtree G. R. Induction of fibrinogen and a subset of acute phase response genes involves a novel monokine which is mimicked by phorbol esters. J Biol Chem. 1987 Aug 5;262(22):10850–10854. [PubMed] [Google Scholar]
- Feldhoff R. C., Taylor J. M., Jefferson L. S. Synthesis and secretion of rat albumin in vivo, in perfused liver, and in isolated hepatocytes. Effects of hypophysectomy and growth hormone treatment. J Biol Chem. 1977 Jun 10;252(11):3611–3616. [PubMed] [Google Scholar]
- Fey G. H., Fuller G. M. Regulation of acute phase gene expression by inflammatory mediators. Mol Biol Med. 1987 Dec;4(6):323–338. [PubMed] [Google Scholar]
- Gasser C. S., Simonsen C. C., Schilling J. W., Schimke R. T. Expression of abbreviated mouse dihydrofolate reductase genes in cultured hamster cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6522–6526. doi: 10.1073/pnas.79.21.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Sauder D. N., McAdam K. P., Dinarello C. A. Purified interleukin-1 (IL-1) from human monocytes stimulates acute-phase protein synthesis by rodent hepatocytes in vitro. Immunology. 1987 Feb;60(2):203–207. [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Andus T., Klapproth J., Northoff H., Heinrich P. C. Induction of alpha 1-acid glycoprotein by recombinant human interleukin-1 in rat hepatoma cells. J Biol Chem. 1988 May 25;263(15):7141–7146. [PubMed] [Google Scholar]
- Isom H. C. DNA synthesis in isolated hepatocytes infected with herpesviruses. Virology. 1980 May;103(1):199–216. doi: 10.1016/0042-6822(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Koj A., Gauldie J., Regoeczi E., Sauder D. N., Sweeney G. D. The acute-phase response of cultured rat hepatocytes. System characterization and the effect of human cytokines. Biochem J. 1984 Dec 1;224(2):505–514. doi: 10.1042/bj2240505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Liao W. S., Jefferson L. S., Taylor J. M. Changes in plasma albumin concentration, synthesis rate, and mRNA level during acute inflammation. Am J Physiol. 1986 Dec;251(6 Pt 1):C928–C934. doi: 10.1152/ajpcell.1986.251.6.C928. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Reuben J., Hersh E. M., Kilbourn R., Hester J. P., Bielski M., Talpaz M., Mavligit G. M. Comparative functional analysis of lymphocytes and monocytes from plateletapheresis. Transfusion. 1983 May-Jun;23(3):201–206. doi: 10.1046/j.1537-2995.1983.23383224895.x. [DOI] [PubMed] [Google Scholar]
- MacIntyre S. S., Schultz D., Kushner I. Synthesis and secretion of C-reactive protein by rabbit primary hepatocyte cultures. Biochem J. 1983 Mar 15;210(3):707–715. doi: 10.1042/bj2100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel A. L., Mehta S. R., Hauft S., Franzini D., Lachman L. B., Ford R. J. Human T lymphocyte/monocyte interaction in response to lectin: kinetics of entry into the S-phase. J Immunol. 1981 Sep;127(3):1058–1064. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metha K., Turpin J., Lopez-Berestein G. Induction of tissue transglutaminase in human peripheral blood monocytes by intracellular delivery of retinoids. J Leukoc Biol. 1987 Apr;41(4):341–348. doi: 10.1002/jlb.41.4.341. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Stearman R. S., Peltzman C. G., Potter D. A. Induction of hepatic synthesis of serum amyloid A protein and actin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4718–4722. doi: 10.1073/pnas.78.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero S., Morrone G., Cortese R. The human haptoglobin gene: transcriptional regulation during development and acute phase induction. EMBO J. 1987 Jul;6(7):1905–1912. doi: 10.1002/j.1460-2075.1987.tb02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto J. M., Grenett H. E., Fuller G. M. The coordinated regulation of fibrinogen gene transcription by hepatocyte-stimulating factor and dexamethasone. J Cell Biol. 1987 Sep;105(3):1067–1072. doi: 10.1083/jcb.105.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowse K. R., Baumann H. Hepatocyte-stimulating factor, beta 2 interferon, and interleukin-1 enhance expression of the rat alpha 1-acid glycoprotein gene via a distal upstream regulatory region. Mol Cell Biol. 1988 Jan;8(1):42–51. doi: 10.1128/mcb.8.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca G. A., Hamilton R. W., McLean J. W., Conn A., Kalinyak J. E., Taylor J. M. Rat alpha 1-acid glycoprotein mRNA. Cloning of double-stranded cDNA and kinetics of induction of mRNA levels following acute inflammation. J Biol Chem. 1981 Oct 25;256(20):10362–10368. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. An in vitro bioassay for leukocytic endogenous mediator(s) using cultured rat hepatocytes. Inflammation. 1981 Dec;5(4):275–287. doi: 10.1007/BF00911093. [DOI] [PubMed] [Google Scholar]
- Rupp R. G., Fuller G. M. The effects of leucocytic and serum factors on fibrinogen biosynthesis in cultured hepatocytes. Exp Cell Res. 1979 Jan;118(1):23–30. doi: 10.1016/0014-4827(79)90579-2. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Howlett G., Nagashima M., Millership A., Martin H., Urban J., Kotler L. The acute phase response of plasma protein synthesis during experimental inflammation. J Biol Chem. 1982 Sep 10;257(17):10271–10277. [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Woodworth C. D., Isom H. C. Regulation of albumin gene expression in a series of rat hepatocyte cell lines immortalized by simian virus 40 and maintained in chemically defined medium. Mol Cell Biol. 1987 Oct;7(10):3740–3748. doi: 10.1128/mcb.7.10.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Kreider J. W., Mengel L., Miller T., Meng Y. L., Isom H. C. Tumorigenicity of simian virus 40-hepatocyte cell lines: effect of in vitro and in vivo passage on expression of liver-specific genes and oncogenes. Mol Cell Biol. 1988 Oct;8(10):4492–4501. doi: 10.1128/mcb.8.10.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C., Secott T., Isom H. C. Transformation of rat hepatocytes by transfection with simian virus 40 DNA to yield proliferating differentiated cells. Cancer Res. 1986 Aug;46(8):4018–4026. [PubMed] [Google Scholar]