Abstract
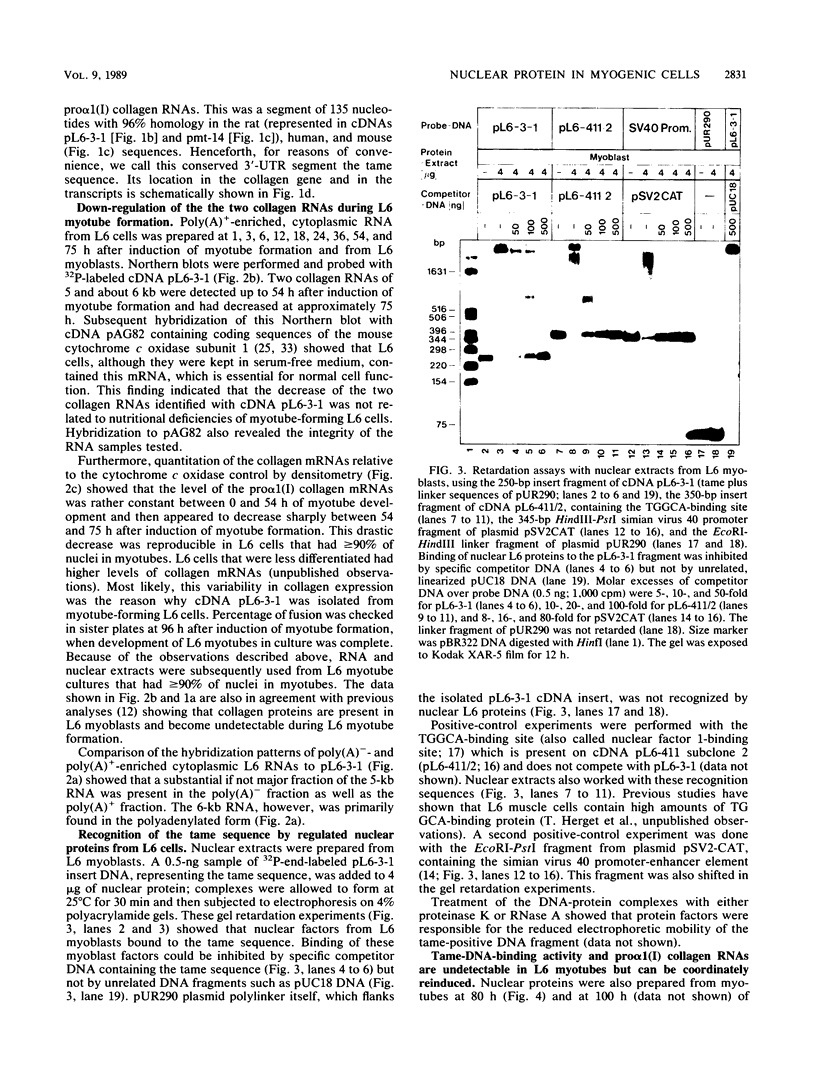
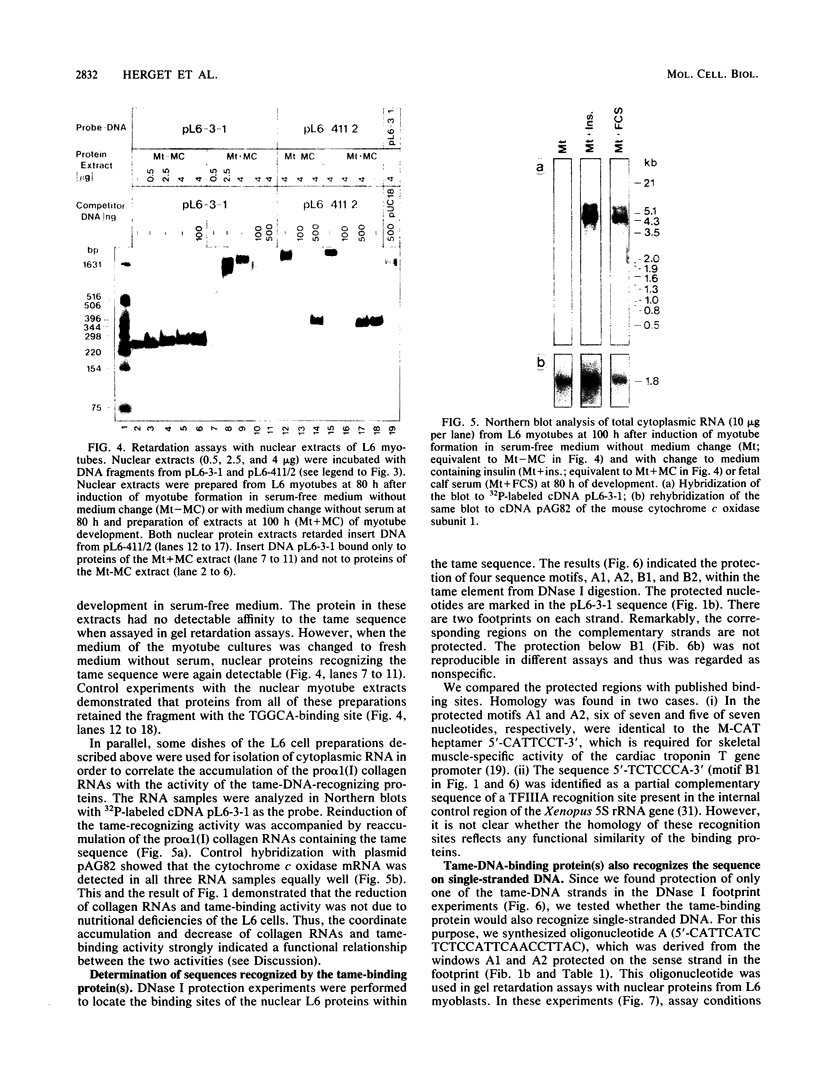
We describe the identification and DNA-binding properties of nuclear proteins from rat L6 myoblasts which recognize an interspecies conserved 3' untranslated segment of pro alpha 1 (I) collagen cDNA. Levels of the two pro alpha 1 (I) collagen RNAs, present in L6 myoblasts, decreased drastically between 54 and 75 h after induction of myotube formation in serum-free medium. Both mRNAs contained a conserved sequence segment of 135 nucleotides (termed tame sequence) in the 3' untranslated region that had 96% homology to the human and murine pro alpha 1 (I) collagen genes. The cDNA of this tame sequence was specifically recognized by nuclear protein(s) from L6 myoblasts, as judged by gel retardation assays and DNase I footprints. The tame-binding protein(s) was able to recognize its target sequence on double-stranded DNA but bound also to the appropriate single-stranded oligonucleotide. Protein that bound to the tame sequence was undetectable in nuclear extracts of L6 myotubes that did not accumulate the two collagen mRNAs. Therefore, the activity of this nuclear protein seems to be linked to accumulation of the sequences that it recognizes in vitro. The collagen RNAs and the nuclear tame-binding proteins reappeared after a change of medium, which further suggests that the RNAs and the protein(s) are coordinately regulated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M. P., Chu M. L., Myers J. C., Ramirez F., Eikenberry E. F., Prockop D. J. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983 Oct 25;22(22):5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- Buonanno A., Mudd J., Shah V., Merlie J. P. A universal oligonucleotide probe for acetylcholine receptor genes. Selection and sequencing of cDNA clones for the mouse muscle beta subunit. J Biol Chem. 1986 Dec 15;261(35):16451–16458. [PubMed] [Google Scholar]
- Carlin B. E., Lawrence J. C., Jr, Lindstrom J. M., Merlie J. P. Inhibition of acetylcholine receptor assembly by activity in primary cultures of embryonic rat muscle cells. J Biol Chem. 1986 Apr 15;261(11):5180–5186. [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., de Wet W., Bernard M., Ramirez F. Fine structural analysis of the human pro-alpha 1 (I) collagen gene. Promoter structure, AluI repeats, and polymorphic transcripts. J Biol Chem. 1985 Feb 25;260(4):2315–2320. [PubMed] [Google Scholar]
- Cleveland D. W. Autoregulated instability of tubulin mRNAs: a novel eukaryotic regulatory mechanism. Trends Biochem Sci. 1988 Sep;13(9):339–343. doi: 10.1016/0968-0004(88)90103-x. [DOI] [PubMed] [Google Scholar]
- DePonti-Zilli L., Seiler-Tuyns A., Paterson B. M. A 40-base-pair sequence in the 3' end of the beta-actin gene regulates beta-actin mRNA transcription during myogenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1389–1393. doi: 10.1073/pnas.85.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garfinkel L. I., Periasamy M., Nadal-Ginard B. Cloning and characterization of cDNA sequences corresponding to myosin light chains 1, 2, and 3, troponin-C, troponin-T, alpha-tropomyosin, and alpha-actin. J Biol Chem. 1982 Sep 25;257(18):11078–11086. [PubMed] [Google Scholar]
- Garrels J. I. Changes in protein synthesis during myogenesis in a clonal cell line. Dev Biol. 1979 Nov;73(1):134–152. doi: 10.1016/0012-1606(79)90143-x. [DOI] [PubMed] [Google Scholar]
- Ginsberg A. M., King B. O., Roeder R. G. Xenopus 5S gene transcription factor, TFIIIA: characterization of a cDNA clone and measurement of RNA levels throughout development. Cell. 1984 Dec;39(3 Pt 2):479–489. doi: 10.1016/0092-8674(84)90455-0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Mohun T., Ng S. Y., Ponte P., Kedes L. Evolution of the human sarcomeric-actin genes: evidence for units of selection within the 3' untranslated regions of the mRNAs. J Mol Evol. 1984;20(3-4):202–214. doi: 10.1007/BF02104727. [DOI] [PubMed] [Google Scholar]
- Herget T., Reich M., Stüber K., Starzinski-Powitz A. Regulated expression of repetitive sequences including the identifier sequence during myotube formation in culture. EMBO J. 1986 Apr;5(4):659–664. doi: 10.1002/j.1460-2075.1986.tb04264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegwater P. A., van der Vliet P. C., Rupp R. A., Nowock J., Sippel A. E. Functional homology between the sequence-specific DNA-binding proteins nuclear factor I from HeLa cells and the TGGCA protein from chicken liver. EMBO J. 1986 Feb;5(2):381–386. doi: 10.1002/j.1460-2075.1986.tb04223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar J. H., Ordahl C. P. A conserved CATTCCT motif is required for skeletal muscle-specific activity of the cardiac troponin T gene promoter. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6404–6408. doi: 10.1073/pnas.85.17.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J., Simon M. P., Lone Y. C., Cognet M., Kahn A. Tissue-specific heterogeneity of the 3'-untranslated region of L-type pyruvate kinase mRNAs. Eur J Biochem. 1986 Jul 1;158(1):33–41. doi: 10.1111/j.1432-1033.1986.tb09717.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Borgmeyer U., Nowock J. Interaction of the TGGCA-binding protein with upstream sequences is required for efficient transcription of mouse mammary tumor virus. EMBO J. 1987 May;6(5):1355–1360. doi: 10.1002/j.1460-2075.1987.tb02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooslehner K., Harbers K. Two mRNAs of mouse pro alpha 1(I) collagen gene differ in the size of the 3'-untranslated region. Nucleic Acids Res. 1988 Jan 25;16(2):773–773. doi: 10.1093/nar/16.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D., Brickell P. M., Latchman D. S., Willison K., Rigby P. W. Transcripts regulated during normal embryonic development and oncogenic transformation share a repetitive element. Cell. 1983 Dec;35(3 Pt 2):865–871. doi: 10.1016/0092-8674(83)90119-8. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Nowock J., Sippel A. E. Specific protein-DNA interaction at four sites flanking the chicken lysozyme gene. Cell. 1982 Sep;30(2):607–615. doi: 10.1016/0092-8674(82)90257-4. [DOI] [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinset C., Whalen R. G. Induction of myogenic differentiation in serum-free medium does not require DNA synthesis. Dev Biol. 1985 Apr;108(2):284–289. doi: 10.1016/0012-1606(85)90032-6. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakonju S., Brown D. D. Contact points between a positive transcription factor and the Xenopus 5S RNA gene. Cell. 1982 Dec;31(2 Pt 1):395–405. doi: 10.1016/0092-8674(82)90133-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Jackson I. J., Brown D. D. Domains of the positive transcription factor specific for the Xenopus 5S RNA gene. Cell. 1984 Jun;37(2):645–652. doi: 10.1016/0092-8674(84)90396-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Deschamps J., Van Beveren C., Sassone-Corsi P. Human fos gene. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):949–958. doi: 10.1101/sqb.1986.051.01.108. [DOI] [PubMed] [Google Scholar]