Abstract
Objectives.
To characterize the trajectories of laboratory- and real world-based speed of processing (SOP) over 5 years using finite latent growth mixture modeling, and to explore associated baseline individual-level predictors and functional outcomes in 2,802 community-dwelling older adults from the Advanced Cognitive Training for Independent and Vital Elderly cohort.
Method.
Laboratory- and real world-based SOP and functional outcomes were assessed over 5 years, and candidate individual-level predictors were collected at baseline.
Results.
After controlling for intervention assignment and demographic information, 4 distinct trajectories were identified: 4.6% of older adults had poor laboratory-based SOP and very poor real world-based SOP that both declined substantially over time; 17.9% had poor laboratory- and real world-based SOP that declined moderately; 38.7% had neutral laboratory- and real world-based SOP that maintained stable; and 37.9% had good laboratory- and real world-based SOP that declined slightly. Non-White, depression, subjective memory complaints, and vascular factors predicted the trajectories. The trajectories significantly differed in the rate of decline in basic activities of daily living, instrumental activities of daily living, and grip strength over time.
Discussion.
Heterogeneous trajectories of SOP exist in old age. Future interventions addressing SOP should target the vulnerable group with poor SOP over time.
Key Words: Activities of daily living functioning, Grip strength, Speed of processing
Background
Speed of processing (SOP), defined as “the rate at which information, once made available to the senses, is processed and understood at the cognitive level,” is a fundamental brain process related to temporary information manipulation (Ball & Vance, 2007; Salthouse, 1996). SOP is also a sentinel brain function and among the first cognitive abilities to decline in normal aging as well as in many health conditions, such as neurodegenerative diseases, psychiatric disorders, and vascular diseases (Awad, Gagnon, & Messier, 2004; Ball, Edwards, & Ross, 2007; Twamley, Ropacki, & Bondi, 2006). According to a recent review, SOP reflects the integrity of multiple neural networks involved in most higher-order cognitive functions (e.g., memory, reasoning, and language; Eckert, 2011). Despite the pervasiveness of decline in SOP, the rate of decline in SOP highly varies across individuals. Some individuals experience only subtle declines in SOP, which do not interfere with daily activities, whereas other individuals show substantial decline in SOP, which significantly affects daily activities and may even herald progression to dementia (Han et al., 2011; Sylvain-Roy, Bherer, & Belleville, 2011). To understand the heterogeneity of SOP decline with age, the type and trajectory of SOP need to be examined at the individual level.
Most experimental assessments of cognitive abilities take place in the laboratory, an environment that allows for strict control of experimental conditions and is hoped to produce more reliable assessments of the constructs being examined. Similarly, SOP has traditionally been evaluated using laboratory-based tests in which the time and accuracy of performance on some repeated and abstract tasks are recorded, such as Useful Field of View (UFOV; Owsley, Ball, Sloane, Roenker, & Bruni, 1991), or a trail making test called the Connections Test (Salthouse et al., 2000). These tasks emphasize rapid processing of visual stimuli. However, processing speed is not a unitary construct; it relies on the dynamic coordination of multiple neural systems (Eckert, 2011). Laboratory-based SOP tests often fail to capture the complex operations involved in performing different real-world everyday tasks. For example, driving requires one to rapidly process different visual stimuli in order to navigate and operate the vehicle safely, whereas grocery shopping places different demands on flexible processing of simultaneous verbal and spatial stimuli. Assessments that directly reflect real world-based demands on processing speed can enhance the clinical significance of SOP assessment. More recently, ecologically validated cognitive tests, such as Road Sign Test (Ball, Beard, Roenker, Miller, & Griggs, 2000), and timed instrumental activities of daily living (TIADL; Owsley, Sloane, McGwin, & Ball, 2002), have been developed to assess SOP involved in everyday tasks (driving, grocery shopping, managing medication, etc.). SOP assessed in more traditional laboratory-based tasks may not be equivalent to SOP assessed in more ecologically valid real-world tasks given the different types and numbers of neural systems involved (Kliegel, Martin, McDaniel, & Phillips, 2007). Accordingly, dissociable patterns of laboratory- and real world-based SOP may predict dissociable functional outcomes.
On the other hand, SOP performance assessed using laboratory- and real world-based measures may not always show similar unidirectional decline in terms of longitudinal trajectory (Owsley, McGwin, Sloane, Stalvey, & Wells, 2001). Recent studies suggest that older adults who have substantial decline as measured by real-world SOP tasks often have functional decline in activities of daily living and may be at risk for dementia, whereas those with poor SOP based on laboratory assessments do not have the same functional decline and are at less risk of developing dementia (Koehler et al., 2012; Sternang, Wahlin, & Nilsson, 2008). These findings underscore the heterogeneity of SOP decline at the individual level and the notion that laboratory-based and real world-based assessment of SOP may differ with respect to functional outcomes and risk for dementia.
The use of growth mixture modeling (GMM) in longitudinal cognitive aging research emphasizes interindividual differences in intraindividual change (Hagenaars & McCutcheon, 2002). However, the traditional GMM approaches can only examine the trajectory of one dimension of cognitive domain, such as laboratory-based SOP. As stated, SOP is a multidimensional phenomenon that includes heterogeneous patterns of laboratory- and real world-based SOP. A common alternative using the traditional GMM is to combine the laboratory- and real world-based SOP (by using a composite score) into one variable of SOP. However, such approach may still obscure important patterns of SOP. For example, it would be hard to distinguish moderate impairments of laboratory- and real world-based SOP (indicating potential mild cognitive impairment) from relatively low level of laboratory-based SOP but high-level real world-based SOP (indicating normal aging process). Moreover, laboratory- and real world-based SOP may change in different rates. In the current study, a relatively new approach of GMM, called “finite mixture model,” was applied to model the growth heterogeneity of laboratory- and real world-based SOP. This approach identifies discrete classes by adding a latent categorical variable where each latent class has its own model of growth. The unreliability of classification and within-class variance and covariance is taken account to estimate the probability of membership in each class for each individual (Muthen & Shedden, 1999).
Further, the change of cognitive abilities can be viewed as the result of an individual’s genetic, behavioral, and environmental characteristics that are combined to promote or suppress brain or neural plasticity, in addition to the influence of two major demographic factors—age and education (Daffner, 2011). Different trajectories of laboratory- and real world-based SOP may reflect the distinct contributions of underlying pathology as well as psychological and behavioral changes. Because SOP trainings have successfully improved or maintained laboratory- and real world-based SOP abilities in the general elderly population (Ball et al., 2002), to determine the group that is most vulnerable to the SOP decline will orient future SOP training to more dedicated targeted population given the cost-effectiveness consideration. Further, older adults with different patterns of laboratory- and real world-based SOP may experience different functional outcomes, which have not been explored.
There were three specific aims in this study: (a) to characterize the trajectories of laboratory- and real world-based SOP over 5 years using latent class modeling; (b) to explore the baseline individual-level profile that can predict the trajectories; and (c) to compare the changes of functional outcomes over time by the trajectories.
Method
Design
A secondary data analysis was performed using data collected from the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial (Ball et al., 2002). The ACTIVE trial is a prospective, randomized, controlled trial designed to evaluate three types of cognitive training intervention on cognitive abilities in community-dwelling older adults. The ACTIVE trial enrolled 2,832 community-dwelling older adults (>65 years old at baseline) without dementia (as screened using Mini-Mental State Examination ≥ 23). Participants were excluded from the study if they had substantial decline in basic activities of daily living (BADL) function, certain life-threatening medical conditions (e.g., cancer), or severe sensory loss or communicative problem at baseline. Participants were recruited from six metropolitan areas in the United States. The recruitment strategies for each site differed and details on these and other aspects of the ACTIVE trial are available elsewhere (Jobe et al., 2001). A sample of 2,802 participants were randomized to one of the three cognitive training groups or a no-contact control group and were included in this secondary analysis. The training interventions consisted of memory training, reasoning training, and SOP training. There were 10 original training sessions. A subset of participants in the three training groups also attended four booster training sessions 11 and 35 months after the original training sessions. Institution-specific institutional review boards approved the ACTIVE protocol and consent was obtained for each participant prior to participation. Latent class modeling allowed us to utilize the whole sample from the ACTIVE trial in the analysis because variables related to intervention assignment (i.e., group assignment, attendance of booster sessions, and recruitment site) were controlled in the analysis.
Measurement
Laboratory- and real world-based SOP measures were administered at baseline, and at 1-, 2-, 3-, and 5-year follow-up visits per the ACTIVE protocol (Willis et al., 2006). The data collection did not occur at 4-year follow-up. Laboratory-based SOP was measured using the UFOV (Owsley et al., 1991), a computerized measure of visual processing speed and attention. This test requires the participant to respond via button press to visual stimuli presented on a computer monitor. Reaction times for responses to four increasingly complex subtests were recorded. For each subtest, a double staircase method was used to determine the optimal presentation speed in which participants correctly complete the task 75% of the time. The optimal presentation speed for all four subtests was combined; fewer milliseconds to correctly perceive the target reflected a faster visual SOP.
A more ecologically valid approach to real world-based SOP assessment involved two timed tasks: The Road Sign Test (Ball et al., 2000) and the TIADL (Owsley et al., 2002). The tasks simulate the speed required by stimuli relevant to real-world activities of daily living. The Road Sign Test included 12 computerized test trials. Each trial required rapid processing of visual information from road signs with and without red slashes displayed in computer screening. Participants were instructed to ignore the signs with red slashes and to react to the signs without red slashes. For bicycle or pedestrian signs without a red slash, participants were required to click one of the mouse buttons as quickly as possible, whereas for the left or right turn arrow signs without a red slash, participants were required to move the mouse in the direction that the arrow pointed as quickly as possible. TIADL measured the speed and accuracy of performance on five everyday tasks. Interviewers provided standardized instruction about how to complete each task using stimulus materials. For each task, there was a required completion time in seconds (recorded using a stopwatch) and an error code. Z-transformation was performed on laboratory-based SOP. A composite score for the real world-based SOP was calculated using the mean and standard deviation of the original sample (n = 2,802) in the following procedure: Z-transformation was firstly performed on the raw score of each test, and then the mean score (composite score) of Z-scores of those tests was calculated. Higher composite scores indicated poorer levels of performance on each SOP measure. The Z-scores for laboratory- and real world-based SOP tasks allowed comparison of the two types of SOP.
Individual-level predictors of SOP performance included race, depression, subjective memory complaints, and history of vascular health. All data were collected at baseline. Race was collected by self-report, and categorized into White versus other racial groups. Depression was measured by 12 items from the Center for Epidemiological Studies Depression (CES-D) scale (Radloff, 1977), a widely applied psychometric instrument for assessing depression. A sum score was computed with higher scores indicating higher levels of depression. Internal consistency for this measure was 0.80 in this study. Subjective memory complaints were measured using 19 items from five domains of the Memory Functioning Questionnaire. Mean scores of items belonging to the same domain were calculated, and the mean of all mean scores from each domain was calculated with higher scores indicating lower levels of subjective memory complaints. The Memory Functioning Questionnaire has shown high internal consistency (0.83–0.94) and concurrent validity with standardized laboratory memory tests in elderly samples (Zelinski, Gilewski, & Anthony-Bergstone, 1990). In this study, internal consistency ranged from 0.86 to 0.91 across domains. History of vascular health included history of vascular diseases and cardiovascular disease risk factors (CVDRFs). History of heart disease, congestive heart failure (CHF), and stroke were collected using a single question “Has a doctor or a nurse ever told you that you have (heart disease, CHF, or stroke)?” Smoking was identified by a single question: “Do you smoke now?” Presence of other CVDRFs was obtained using the question: “Has a doctor or a nurse ever told you that you have (hypertension, diabetes, or high cholesterol)?” Data on height and weight were used in calculating body-mass index (BMI), and obesity was identified using BMI ≥ 30kg/m2.
Functional outcomes included BADL, instrumental activities of daily living (IADL), grip strength, and two domains of health-related quality of life (HRQOL), physical and mental functioning. Self-report BADL and IADL were measured by items from the Minimum Data Set-Home Care interview at baseline, 1-, 2-, 3-, and 5-year follow-up. BADL performance was assessed with questions such as, “In the last 7 days, how much of the activity (e.g., combing/brushing hair) did you do on your own?” using a response scale from 1 (independent) to 5 (total dependence). IADL performance was assessed with questions involving seven activities (e.g., planning meals, handling money and checks, and keeping track of doctor appointments) using a scale from 1 (did all on own) to 4 (fully performed by others). Sum scores of items from BADL and IADL were computed, respectively, with higher scores indicating lower levels of BADL or IADL functioning. Grip strength was included as a measure of general physical robustness and was assessed using a dynamometer (Lafayette Instruments, Layfayette, IN) at baseline, 3-year, and 5-year follow-up. Participants were allowed to make their maximal effort with the dominant hand as instructed in the trial. One minute of rest was taken between two trials. The mean of the scores from the two trials were computed. Higher scores indicated greater grip strength. Participants who had recent worsening of pain or of arthritis in their wrists, tendonitis, or surgery on their hands or arms within past 3 months were waived from the grip strength test. Two domains of HRQOL, physical and mental functioning were measured by the Physical and Mental Component Summary Score from the Medical Outcomes Study SF-36 version 1, a widely used valid and reliable measure in older adults (McHorney, Ware, & Raczek, 1993; Ware, 2000; Ware & Sherbourne, 1992). Data were collected at baseline, 3-year, and 5-year follow-up. The two summary component scores were calculated by an algorithm and results in standardized scores ranging from 0 to 100 with higher scores indicating a higher level of functioning (McHorney et al., 1993).
Participant’s age, sex, and years of education were also collected at baseline. Each participant’s codes on group assignment (one of three training groups or control group), attendance of booster sessions, and recruitment sites were also identified. All these variables were controlled in the latent class modeling.
Data Analysis
Latent class analysis was conducted using R, whereas all other analyses were conducted using IBM SPSS 19.0. Before conducting latent class analysis, correlation between laboratory- and real world-based SOP measures at each visit was calculated using linear regression model taking real world-based SOP as the dependent variable and laboratory-based SOP as the predictor. Age, gender, years of education, group assignment, attendance of booster sessions, and recruitment site were controlled. If the two types of SOP were highly correlated, the advantage of finite mixture model would be less impactful.
Step 1: Bivariate latent class analysis. Data on laboratory- and real world-based SOP measures were jointly modeled as a bivariate GMM using the finite mixture method developed by Leisch (2004) and implemented in R package “FlexMix.” Age, gender, years of education, group assignment, attendance of booster sessions, and recruitment site were controlled for longitudinal performance of SOP measures in the modeling analysis. The researchers did not control these covariates for the class designation, which might interfere with the understanding of the natural course of SOP in old age, and might accidently assign participants who attended SOP training during ACTIVE trial into the same class. The choice of best fitting model was based on the following criteria: the Akaike Information criterion (AIC), the Bayesian Information criterion (BIC), and the negative Log-likelihood. With the selected best model, the posterior probabilities are used to segment data from participants to the classes with maximum posterior probability. Each class should have more than 1% of the total sample (Jung & Wickrama, 2008). As an additional analysis for the association between the covariates (i.e., age, gender, years of education, group assignment, attendance of booster sessions, and recruitment site) and the class, analysis of variance was applied to compare the continuous covariates by the class, and chi-square test was applied to compare the categorical covariates by the class.
Step 2: Predictors of class membership were determined using a multinomial logistic regression model. The variable of four latent classes emerged in Step 1 was taken as the dependent variable using class 4 (i.e., the least impaired group) as the referent group and potential predictors (race, depression, subjective memory complaints, and history of vascular health) as independent variables.
Step 3: Changes of functional outcomes over time by latent class were examined using a series of generalized estimating equations (GEE) with unstructured working correlation matrix (Zeger, Liang, & Albert, 1988). The four latent classes were considered as a categorical variable taking class 4 (i.e., the least impaired group) as the referent group and time considered as a continuous variable in the GEE models. Each health outcome was used as a dependent variable, and latent class, time, and an interaction between latent class and time were included as predictors. Any significant main effects of latent class would indicate a difference in health outcomes across different latent classes, whereas a significant interaction term involving time would indicate different rates of change in health outcome over time as a function of the latent class.
Results
As shown in Table 1, participants at baseline were on average 73.6 years old and were predominately White (72.4%) and women (75.9%). For the rest of the 27.6% non-White participants, the majority was Black or African American (26.0%). The average years of education were 13.5.
Table 1.
Baseline Demographic and Health Characteristics (N = 2,802)
| Age, mean (SD) | 73.63 (5.91) |
| Men, n (%) | 676 (24.1%) |
| White, n (%) | 2,028 (72.4%) |
| Years of education, mean (SD) | 13.53 (2.70) |
| Depression, mean (SD) | 5.02 (5.28) |
| Subjective memory complaint, mean (SD) | 4.64 (0.91) |
| History of vascular health, n (%) | |
| Heart disease | 421 (15.0%) |
| CHF | 138 (4.9%) |
| Stroke | 195 (7.0%) |
| Smoke | 208 (7.4%) |
| Obesity | 1,114 (39.8%) |
| Hypertension | 1,428 (51.0%) |
| Diabetes | 358 (12.8%) |
| Hypercholesterolemia | 1,226 (43.8%) |
Notes. CHF = congestive heart failure; SD = standard deviation.
Correlation Between Laboratory- and Real World-Based SOP
After controlling for age, gender, years of education, group assignment, attendance of booster sessions, and recruitment site, only 10%–16% of variances in real world-based SOP were explained by the laboratory-based SOP across visit (all p < .001).
Trajectories of Laboratory- and Real World-Based SOP
The most parsimonious model (one-class model) was followed by sequentially increasing the number of latent classes up to five latent classes. After controlling for age, gender, years of education, group assignment, booster sessions, and recruitment site, the four-class bivariate latent class model demonstrated the best fit as indicated by the lowest AIC, BIC, and negative Log-likelihood (Table 2).
Table 2.
Model Fit for the Class
| Model fit indicators | One-class model | Two-class model | Three-class model | Four-class model | Five-class model |
|---|---|---|---|---|---|
| AIC | 36895.91 | 27992.58 | 26328.11 | 25839.57 | 26327.70 |
| BIC | 37156.26 | 28520.51 | 27123.63 | 26902.67 | 27123.22 |
| Negative Log-likelihood | 18411.96 | 13923.29 | 13054.05 | 12772.78 | 13053.85 |
Notes. AIC = Akaike Information criterion; BIC = Bayesian Information criterion.
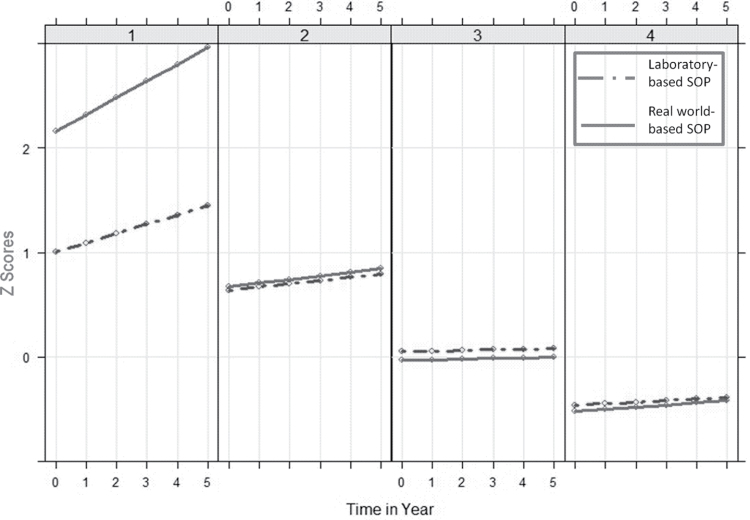
Table 3 shows the intercepts and slopes of laboratory- and real world-based SOP performance in each class. Figure 1 shows the trajectories of the four classes based on two types of SOP (laboratory- and real world-based). Participants in class 1 (4.6% of participants, n = 128) had the worst SOP performance of the four classes at baseline, and the level of real world-based SOP was worse than the laboratory-based SOP. In this class, both types of SOP declined fastest over time among the four classes, and real world-based SOP declined even faster than laboratory-based SOP (laboratory-based SOP: I = 1.00, S = 0.13; real world-based SOP: I = 2.20, S = 0.22). Participants in class 2 (17.9% of participants, n = 501) had similar poor levels of SOP at baseline. Both types of SOP declined moderately over time (laboratory-based SOP: I = 0.64, S = 0.06; real world-based SOP: I = 0.69, S = 0.04). Participants in class 3 (38.7% of participants, n = 1,084) had relatively neutral levels of SOP at baseline, which were close to zero. Both types of SOP stayed relatively stable or declined very slightly over time (laboratory-based SOP: I = 0.06, S = 0.02; real world-based SOP: I = −0.03, S = 0.01). Participants in class 4 (37.9% of participants, n = 1,062) had comparable positive levels of SOP at baseline. Both types of SOP declined very slightly over time (laboratory-based SOP: I = −0.47, S = 0.01; real world-based SOP: I = −0.52, S = 0.02). In addition, 27 participants (<1%) whose data on SOP measures were missing were excluded from the analysis.
Table 3.
Parameters of Latent Class of Laboratory- and Real World-Based SOP
| Laboratory-based SOP | Real world-based SOP | ||||
|---|---|---|---|---|---|
| Class | N (%)a | Intercept (SE) | Slope for time (SE) | Intercept (SE) | Slope for time (SE) |
| 1 | 128 (4.6%) | 0.9987 (0.0545)b | 0.1300 (0.0138)b | 2.1978 (0.0448)b | 0.2246 (0.0161)b |
| 2 | 501 (17.9%) | 0.6398 (0.0275)b | 0.0563 (0.0061)b | 0.6873 (0.0224)b | 0.0387 (0.0073)b |
| 3 | 1,084 (38.7%) | 0.0613 (0.0186)b | 0.0218 (0.0038)b | −0.0314 (0.0151)c | 0.0079 (0.0046) |
| 4 | 1,062 (37.9%) | −0.4680 (0.0187)b | 0.0123 (0.0035)b | −0.5190 (0.0151)b | 0.0205 (0.0044)b |
Notes. SE = standard error; SOP = speed of processing. Age, gender, years of education, group assignment, booster sessions, and recruitment site were controlled when generating latent class. Laboratory-based SOP: Z-score of Useful Field of View scores; real world-based SOP: means of Z-scores of the Road Sign Test and timed instrumental activities of daily living scores.
aMissing: n = 27.
b<.05.
c<.001.
Figure 1.
Growth trajectories of laboratory- and real world-based speed of processing for each latent class.
The comparison of covariates by the class is presented in the supplementary data. Age, years of education, and recruitment site, but not gender, group assignment, or attendance of booster sessions, significantly differed by the class.
Individual-Level Predictors of Membership in Latent Class
At least 97.3% of the data on potential predictors were available. Table 4 shows the individual predictors of membership in each latent class using multinomial logistic regression with class 4 as the referent group. Compared with non-White, White participants were less likely to be in classes 1, 2, and 3 than to be in class 4. Every one unit increase in the score of depression (i.e., more depressive symptoms) would increase the participant’s likelihood of being in classes 1, 2, and 3 than being in class 4. Every one unit decrease in the score of subjective memory complaint (i.e., more memory complaint) would increase the participant’s likelihood of being in classes 1, 2, and 3 than being in class 4. Compared with those without any history of vascular disease or CVDRFs, participants having heart disease, CHF, stroke, and diabetes were more likely to be in classes 1, 2, or/and 3 than to be in class 4. On the contrary, obese participants and those with hypercholesterolemia were less likely to be in classes 1 and 2 than to be in class 4.
Table 4.
Membership in Latent Classes as a Function of Individual-Level Characteristicsa
| Class 1 | Class 2 | Class 3 | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable (analytic sample sizeb) | OR | 95% CI | Wald χ2 | p Value | OR | 95% CI | Wald χ2 | p Value | OR | 95% CI | Wald χ2 | p Value | ||||||||||||
| White (2,775) | 0.31 | 0.20, 0.47 | 29.44 | <.001 | 0.30 | 0.23, 0.39 | 88.52 | <.001 | 0.56 | 0.45, 0.70 | 27.27 | <.001 | ||||||||||||
| Depression (2,745) | 3.32 | 2.28, 4.84 | 38.97 | <.001 | 2.68 | 2.09, 3.45 | 59.58 | <.001 | 1.93 | 1.56, 2.40 | 35.53 | <.001 | ||||||||||||
| Subjective memory complaints (2,739) | 0.67 | 0.60, 0.74 | 52.73 | <.001 | 0.76 | 0.70, 0.82 | 44.27 | <.001 | 0.91 | 0.85, 0.98 | 6.18 | .013 | ||||||||||||
| Heart disease (2,750) | 1.83 | 1.08, 3.10 | 5.02 | .025 | 1.07 | 0.76, 1.51 | 0.16 | .693 | 1.44 | 1.11, 1.87 | 7.56 | .006 | ||||||||||||
| CHF (2,751) | 1.43 | 0.63, 3.25 | 0.74 | .390 | 1.98 | 1.19, 2.74 | 6.90 | .009 | 1.04 | 0.66, 1.65 | .026 | .872 | ||||||||||||
| Stroke (2,757) | 2.05 | 1.05, 3.98 | 4.56 | .033 | 1.79 | 1.17, 2.74 | 7.19 | .007 | 1.28 | 0.89, 1.85 | 1.78 | .183 | ||||||||||||
| Smoke (2,774) | 0.70 | 0.31, 1.60 | 0.72 | .397 | 0.90 | 0.57, 1.40 | 0.24 | .627 | 1.35 | 0.96, 1.89 | 2.96 | .086 | ||||||||||||
| Obesity (2,775) | 0.60 | 0.40, 0.91 | 5.85 | .016 | 0.77 | 0.61, 0.97 | 4.90 | .027 | 0.95 | 0.79, 1.14 | 0.30 | .586 | ||||||||||||
| Hypertension (2,775) | 0.81 | 0.54, 1.21 | 1.09 | .297 | 0.96 | 0.76, 1.21 | 0.14 | .710 | 1.06 | 0.88, 1.27 | 0.40 | .528 | ||||||||||||
| Diabetes (2,772) | 2.18 | 1.29, 3.68 | 8.42 | .004 | 1.80 | 1.28, 2.51 | 11.67 | .001 | 1.36 | 1.02, 1.82 | 4.25 | .037 | ||||||||||||
| Hypercholesterolemia (2,727) | 0.56 | 0.37, 0.85 | 7.56 | .006 | 0.88 | 0.70, 1.11 | 1.12 | .290 | 0.97 | 0.81, 1.16 | 0.11 | .741 | ||||||||||||
Notes. Bold indicates significant p value. CI = confidence interval; CHF = congestive heart failure; OR = odds ratio.
aClass 4 is the referent group.
bBased on the total sample, not the classes.
Changes of Functional Outcomes Over Time by Latent Class
Table 5 shows the changes of functional outcomes over time by latent class using GEE models with class 4 as the referent group. After controlling for all potential covariates (age, gender, years of education, group assignment, booster sessions, recruitment site, non-White, depression, heart disease, CHF, stroke, smoke, diabetes, hypertension, and hypercholesterolemia), participants in the four latent classes did not differ in the baseline levels of functional outcomes. However, compared with class 4, classes 1, 2, and 3 all declined significantly faster in IADL (range: 0.202–1.273 units per visit) and grip strength (range: 0.572–2.147 units per visit) over time. Compared with class 4, class 1 declined significantly faster in BADL (0.994 units per visit) over time.
Table 5.
Parameter Estimate (β ± SE) of Health Outcomes Over Time by SOP Latent Classa
| Variable | Time | Class | Class × Time | Analytical sample size | ||
|---|---|---|---|---|---|---|
| BADL | 0.002±0.0109 | 1 | −1.372±0.8563 | 0.994±0.4613 * | 2,465 | |
| 2 | 0.142±0.1250 | −0.011±0.0301 | ||||
| 3 | −0.018±0.0635 | −0.008±0.0164 | ||||
| IADL | −0.221±0.0363 | 1 | −1.268±1.4905 | 1.273±0.5756 * | 2,465 | |
| 2 | −1.074±0.5125 | 0.407±0.1157 *** | ||||
| 3 | −0.428±0.3576 | 0.202±0.0770 ** | ||||
| Grip strength | −0.080±0.1621 | 1 | 1.118±1.5797 | −2.147±0.9095 * | 1,668 | |
| 2 | 0.989±0.9043 | −1.219±0.3810 ** | ||||
| 3 | 0.085±0.6161 | −0.572±0.2462 * | ||||
| Physical functioning | −0.604±0.1366 | 1 | 2.399±2.8647 | −0.749±1.4346 | 2,306 | |
| 2 | −3.098±1.3223 | 0.014±0.3102 | ||||
| 3 | −1.380±0.7824 | 0.023±0.1792 | ||||
| Mental functioning | −0.184±0.0987 | 1 | −5.795±4.1045 | 1.293±1.2155 | 2,306 | |
| 2 | 0.282±0.9589 | −0.293±0.2691 | ||||
| 3 | −0.704±0.5972 | 0.060±0.1546 | ||||
Notes. Bold indicates significant p value. BADL = basic activities of daily living; IADL = instrumental activities of daily living; SE = standard error; SOP = speed of processing. Controlling for age, gender, years of education, group assignment, booster sessions, recruitment site, race, depression, heart disease, congestive heart failure, stroke, smoke, diabetes, hypertension, and hypercholesterolemia.
aClass 4 is the referent group.
*p < .05. **p < .01. ***p < .001.
For GEE models, participants who completed at least two waves visit were included in the analysis. Because participants who had comorbid conditions within past 3 months were waived from the grip strength test, a relatively large proportion of data on grip strength were missing (40.5%). The researchers compared the demographic and health characteristics between the participants with and without data on grip strength, and found that participants without data on grip strength were older (M = 74.79 vs. 72.85, t = 8.66, p < .001), had lower levels of education (M = 13.67 vs. 13.32, t = −3.42, p = .001), had poorer IADL (M = 3.00 vs. 2.90, t = 7.27, p < .001) and BADL (M = 15.56 vs. 15.39, t = 2.49, p = .013) functioning, and more concentrated in class 1 (7.2% vs. 2.8%, χ2 = 110.54, p < .001) compared with participants with data on grip strength. For the other functional outcome measures, 12.0%–17.8% of the data were missing. The patterns of differences in demographic and health variables between participants with and without those data were similar to the pattern in grip strength (data not shown).
Discussion
In this secondary data analysis, support was found for the hypothesis of extensive heterogeneity in both the type and trajectory of SOP with aging. Four distinct patterns emerged: 4.6% of older adults had poor laboratory-based SOP and very poor real world-based SOP and both declined substantially over time (class 1); 17.9% had relatively poor laboratory- and real world-based SOP that declined moderately over time (class 2); 38.7% had relatively neutral laboratory- and real world-based SOP that remained relatively stable over time (class 3); and 37.9% had relatively good laboratory- and real world-based SOP that declined slightly over time (class 4). Second, non-White race, depression, subjective memory complaints, and a history of vascular disease and/or CVDRFs were found to predict membership of the trajectories. Finally, the researchers report that although individuals in these groups did not differ in baseline levels of functional outcomes, they did differ significantly in the decline rate of BADL, IADL, and grip strength over time.
SOP performance was examined in two ways—an abstract laboratory test and two ecologically validated tests. This study identified several longitudinal patterns and trajectories of SOP. Although the classes with initial neutral or positive laboratory- and real world-based SOP showed statistically significant decline over time, such decline (0.0079–0.0218 unit per visit) was actually subtle when comparing with the range of SOP scores (classes 3 and 4). In contrast, the other two classes, which had initial poor or very poor laboratory- and real world-based SOP, demonstrated a relatively greater decline rate over time (0.0387–0.2246 unit per visit; classes 1 and 2). In addition, among the covariates of SOP, age appeared to be the only one having a theoretically meaningful and statistically significant relationship with the SOP classification. That is, participants with older age tended to perform with poorer SOP abilities.
It should also be noted that compared with the other three classes that had similar levels of laboratory- and real world-based SOP, the poorest class (class 1) had much worse real world-based than laboratory-based SOP performance at baseline, and real world-based SOP declined much faster over time. From the behavioral or cognitive standpoint, to complete real world-based SOP tasks may utilize compensatory strategies, such as common sense knowledge that maintained relatively intact in normal aging process (Reuter-Lorenz & Park, 2010). For example, when receiving the task of searching a telephone number in the yellow book, a cognitively healthy older adult may use his common sense immediately—to initiate the search using the alphabetical order of the family name, which may help save time on completing the task (Park & Reuter-Lorenz, 2009). The impairment in such compensatory mechanisms, which was possibly shown in class 1, may indicate the incidence of cognitive impairment. In fact, some investigators discretely examined laboratory- or real world-based SOP, suggesting the unique predictive value of real world- but not laboratory-based SOP on incident dementia (Koehler et al., 2012; Sternang, Wahlin, & Nilsson, 2008). It should also be noted that, class 2 also showed substantial but equal decline in laboratory- and real world-based SOP when comparing with classes 3 and 4. The researchers do not have data on clinical diagnosis of cognitive impairment in ACTIVE data set. However, the classes 1 and 2 do warrant the attention of different stages of impairment, and the relationship between the pattern and trajectory of laboratory- and real world-based SOP and incidence of different stages of dementia (e.g., preclinical dementia, mild cognitive impairment, or Alzheimer’s dementia) deserves future examination. As a future exploration, it will also be important to investigate the possible role of brain networks in explaining different patterns of SOP abilities. That is, laboratory- and real world-based SOP assessment may tap different functional networks (Eckert, 2011). The cognitive operations of laboratory-based SOP may rely heavily on prefrontal cortex, whereas cognitive operation of real world-based SOP may recruit broader brain networks (e.g., frontal cortical, parietal, or temporal lobe networks), as well as require additional compensatory engagement of frontal cortex to offset possible age-related changes in other brain networks (Eckert, 2011). Disruption of multiple brain networks and failure of the compensation may be revealed by deficits in and decline of SOP measured with real world-based tasks (as seen in class 1). In addition, given the small proportion of participants in class 1 (4.6%), to avoid the potential overexaction of the classes (Bauer & Curran, 2003), reproducing this class with the substantial difference in the trajectories from other classes is needed in other cohort studies.
Taken together, the results of this study paint a fairly detailed portrait of individuals at risk for SOP decline. Beyond the influence of age, sex, and education, non-White (especially Black or African American) older adults who have symptoms of depression, subjective memory loss, and a history of several vascular related conditions (heart disease, CHF, stroke, and diabetes) are more vulnerable to SOP decline and by extension, functional decline. Accumulative evidence supports the value of depression and subjective memory complaint in predicting cognitive decline, and the two risk factors significantly influence each other (Amariglio, Townsend, Grodstein, Sperling, & Rentz, 2012; McDermott & Ebmeier, 2009). This study confirmed the predictive value of the two modifiable risk factors in understanding the pattern and trajectory of laboratory- and real world-based SOP abilities in a community-dwelling elder cohort without dementia at baseline. Compared with the class with best SOP abilities over time (class 4), the predictive patterns of these potential risk factors appeared to be relatively consistent across other classes. However, being Black and having severe subjective memory complaints, depression, heart disease, stroke, and diabetes posed the highest likelihood of being in the class with poorest SOP abilities (class 1), which further support the unique clinical characteristics of class 1. However, two limitations to the study should be recognized, which temper the findings related to this individual portfolio. The first limitation is related to the vascular factors examined. In this study, participant self-report data was used to assess history of vascular disease and CVDRFs, and this may result in exposure misclassification. The onset (e.g., midlife or late-life) of these factors was not ascertained. Although most studies have demonstrated a consistent negative effect of vascular disease and some CVDRFs (e.g., diabetes) on cognition, the effects of other CVDRFs, such as obesity and cholesterol levels on cognition have been less consistent. For both obesity and hypercholesterolemia, midlife but not late-life onset may be risk factors for cognitive decline (Anstey, Lipnicki, & Low, 2008; Naderali, Ratcliffe, & Dale, 2009). Additionally, hypercholesterolemia is in need of further classification, because high total cholesterol but not high low-density cholesterol or low high-density cholesterol have been related to cognitive decline (Anstey et al., 2008; Naderali et al., 2009). Regardless, positive relationships between SOP and obesity and hypercholesterolemia were found, and the reasons for the associations in the current sample remain unclear and deserve further investigation in future studies. In addition, the researchers only examined single vascular diseases or CVDRFs in this study. Previous studies, including a report from the authors of this study, found that the influence of CVDRF appears to be additive, as concurrent CVDRFs predict cognitive decline to a greater extent than single risks (Lin, Friedman, Quinn, Chen, & Mapstone, 2012; Reitz et al., 2011). It will be interesting to explore the prospective relationship between the number of vascular diseases or CVDRFs and pattern and trajectory of SOP measures over time. Next, other potential predictors of cognitive trajectories, such as physical exercise, mental activities, and APOE 4 genotype (Middleton & Yaffe, 2010) were not included in this analysis, which should be considered in future studies.
Finally, it is not surprising that participants across SOP classes appeared to have similar functional outcomes (e.g., BADL, IADL) at baseline given the inclusion/exclusion criteria of the original ACTIVE study. That is, older adults with impaired BADL and Mini-Mental State Examination < 23 at baseline were excluded from the ACTIVE study, which purposely included a group of older adults without wide variation in baseline functional outcomes. However, the results suggest that SOP may be useful in predicting the rate of functional decline in initially non-demented older adults. That is, participants with poorer SOP trajectories (classes 1–3) were found to decline more rapidly in IADL and grip strength than participants with better SOP (class 4). In addition, participants with the fastest SOP decline (class 1) also declined significantly faster in BADL than participants with better SOP (class 4). These findings highlight the importance of carefully characterizing both the type and trajectory of SOP for predicting functional outcomes. It might not come as a surprise that SOP decline and IADL impairments are linked in this manner. Rapid processing of external information is critical to many IADL’s such as cooking, finding food items during grocery shopping, and managing finances. Faster SOP also allows for more rapid responses to the environment. For example, rapid action is critical to quickly locate the hand rails and adjust body orientation when one trips and begins to fall (Vance, 2009). Participants who had recent comorbid condition (e.g., pain or of arthritis in their wrists) were waived from the grip strength test, which made a subgroup of participants with lower education, poorer IADL and BADL functioning, and poorer SOP abilities to be excluded from the GEE analysis of the relationship between SOP classes and grip strength. Interestingly, within the remaining participants, the results still revealed a significant decline in grip strength in the classes with poor SOP abilities that declined fast over time (classes 1 and 2). Grip strength is a commonly used measure of frailty in the elderly and is strongly linked to future disability, morbidity, and mortality (Syddall, Cooper, Martin, Briggs, & Aihie Sayer, 2003). The association between SOP and grip strength reinforces the relationship between cognitive and physical health and their effects on functional capacities. Further, this relationship suggests that SOP is a sentinel cognitive process, similar to grip strength for physical function that can predict future functional outcomes. In addition, it should also be noted that class 3 had significantly faster decline in IADL and grip strength than class 4 did, although participants in the class had relatively stable laboratory- and real world-based SOP over time. Such groups may represent targets for health promotion programs to maximize the older adults’ functional health.
Although this notion is provocative, this study failed to find a longitudinal relationship between SOP and HRQOL. The overall level of HRQOL remained stable over 5 years, indicating that older adults in this study perceived themselves to be functioning mentally and physically normally. In this study, change in SOP may not immediately and sensitively reflect older adults’ subjective functional state. Future studies may investigate whether intermediate functional outcomes (BADL, IADL, or grip strength) serve as mediators between longitudinal changes in SOP and HRQOL.
Changes in SOP in old age can be easily measured and importantly, may be amenable to interventions that may fundamentally change the underlying brain networks or provide compensatory coping mechanisms involved in SOP information. Future interventions that target SOP abilities should target the vulnerable profile reported here to prevent or slow potential functional decline. SOP training generally involves computer-based game-embedded non-verbal mental exercises designed to improve the broad capacity for fluid mental processing efficiency. The training is based on neuroplasticity theory, which promotes neurotrophic factor production and neuro-genesis in animals. In cognitively normal older adults, approximately 5–10hr of standardized SOP training resulted in improved laboratory-based SOP that was sustained up to several years post intervention, but the training failed to improve real world-based SOP (Willis et al., 2006). Similarly, in this study, the intervention assignment from the original ACTIVE trial did not influence the classification of SOP measures. It indicates that SOP training may not be particularly effective for real world-based SOP ability. Older adults who have similar rate and level of decline in laboratory- and real world-based SOP (classes 2 and 3) may benefit from SOP training. However, for older adults who showed much worse real world-based SOP ability (class 1), it is necessary to investigate other compensatory/rehabilitation strategies that can be combined with SOP training to slow the decline or compensate the impairment in older adults’ real world-based SOP ability.
Funding
The original ACTIVE study was supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew Senior Life (U01NR04507), Indiana University School of Medicine (U01 NR04508), Johns Hopkins University (U01AG14260), New England Research Institutes (U01AG14282), Pennsylvania State University (U01AG14263), the University of Alabama at Birmingham (U01 AG14289), and the University of Florida (U01AG14276).
Supplementary Material
Supplementary material can be found at: http://psychsocgerontology.oxfordjournals.org/
References
- Amariglio R. E., Townsend M. K., Grodstein F., Sperling R. A., Rentz D. M. (2012). Specific subjective memory complaints in older persons may indicate poor cognitive function. Journal of the American Geriatrics Society, 59, 1612–1617 doi:10.1111/j.1532-5415.2011.03543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey K. J., Lipnicki D. M., Low L. F. (2008). Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. The American Journal of Geriatric Psychiatry, 16, 343–354 doi:10.1097/JGP.0b013e31816b72d4 [DOI] [PubMed] [Google Scholar]
- Awad N., Gagnon M., Messier C. (2004). The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. Journal of Clinical and Experimental Neuropsychology, 26, 1044–1080 doi:10.1080/13803390490514875 [DOI] [PubMed] [Google Scholar]
- Ball K., Beard B., Roenker D., Miller R., Griggs D. (2000). Increasing mobility and reducing accidents of older drivers.In Schaie K., Pietrucha M. (Eds.), Mobility and transportation in the elderly (pp. 213–251). New York, NY: Springer Publishing; [Google Scholar]
- Ball K., Berch D. B., Helmers K. F., Jobe J. B., Leveck M. D., Marsiske M., … Willis S. L. (2002). Effects of cognitive training interventions with older adults: A randomized controlled trial. Journal of the American Medical Association, 288, 2271–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K., Edwards J. D., Ross L. A. (2007). The impact of speed of processing training on cognitive and everyday functions. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 62 Spec No 1, 19–31 doi:62/suppl_Special_Issue_1/19 [DOI] [PubMed] [Google Scholar]
- Ball K. K., Vance D. E. (2007). Everyday life applications and rehabilitation of processing speed deficits: Aging as a model for clinical populations. In DeLuca J., Kalmar J. H. (Eds.), Information processing speed in clinical population (pp. 243–263). New York, NY: Taylor & Francis; [Google Scholar]
- Bauer D. J., Curran P. J. (2003). Distributional assumptions of growth mixture models: Implications for overextraction of latent trajectory classes. Psychological Methods, 8, 338–363 doi:10.1037/1082-989X.8.3.338 [DOI] [PubMed] [Google Scholar]
- Daffner K. R. (2011). Promoting successful cognitive aging: A comprehensive review. Journal of Alzheimer’s Disease, 19, 1101–1122 doi:10.3233/JAD-2010-1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M. A. (2011). Slowing down: Age-related neurobiological predictors of processing speed. Frontiers in Neuroscience, 5, 25 doi:10.3389/fnins.2011.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars J. A., McCutcheon A. L. (2002). Applied latent class analysis. Cambridge, UK: Cambridge University Press; [Google Scholar]
- Han S. D., Suzuki H., Jak A. J., Chang Y. L., Salmon D. P., Bondi M. W. (2011). Hierarchical cognitive and psychosocial predictors of amnestic mild cognitive impairment. Journal of the Inter national Neuropsychological Society, 16, 721– 729 doi:10.1017/S1355617710000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe J. B., Smith D. M., Ball K., Tennstedt S. L., Marsiske M., Willis S. L., … Kleinman K. (2001). ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials, 22, 453–479 doi:S0197-2456(01)00139-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T., Wickrama K. A. S. (2008). An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass, 2, 302– 317 [Google Scholar]
- Kliegel M., Martin M., McDaniel M. A., Phillips L. H. (2007). Adult age differences in errand planning: The role of task familiarity and cognitive resources. Experimental Aging Research, 33, 145–161 doi:10.1080/03610730601177395 [DOI] [PubMed] [Google Scholar]
- Koehler M., Kliegel M., Wiese B., Bickel H., Kaduszkiewicz H., van den Bussche H., Pentzek M. (2012). Malperformance in verbal fluency and delayed recall as cognitive risk factors for impairment in instrumental activities of daily living. Dementia and Geriatric Cognitive Disorders, 31, 81–88 doi:10.1159/000323315 [DOI] [PubMed] [Google Scholar]
- Leisch F. (2004). Flexmix: A general framework for finite mixture models and latent class regression in R. Journal of Statistical Software, 11, 1–18 [Google Scholar]
- Lin F., Friedman E., Quinn J., Chen D., Mapstone M. (2012). Effect of leisure activities on inflammation and cognitive function in an aging sample. Archives of Gerontology and Geriatrics. doi:dx.doi.org/10.1016/j.archger.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott L. M., Ebmeier K. P. (2009). A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders, 119, 1–8 doi:10.1016/j.jad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- McHorney C. A., Ware J. E., Jr., Raczek A. E. (1993). The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care, 31, 247–263 [DOI] [PubMed] [Google Scholar]
- Middleton L. E., Yaffe K. (2010). Targets for the prevention of dementia. Journal of Alzheimer’s Disease, 20, 915–924 doi:10.3233/JAD-2010-091657 [DOI] [PubMed] [Google Scholar]
- Muthen B., Shedden K. (1999). Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics, 55, 463–469 [DOI] [PubMed] [Google Scholar]
- Naderali E. K., Ratcliffe S. H., Dale M. C. (2009). Obesity and Alzheimer’s disease: A link between body weight and cognitive function in old age. American Journal of Alzheimer’s Disease and Other Dementias, 24, 445–449 doi:10.1177/1533317509348208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C., Ball K., Sloane M. E., Roenker D. L., Bruni J. R. (1991). Visual/cognitive correlates of vehicle accidents in older drivers. Psychology and Aging, 6, 403–415 [DOI] [PubMed] [Google Scholar]
- Owsley C., McGwin G., Jr., Sloane M. E., Stalvey B. T., Wells J. (2001). Timed instrumental activities of daily living tasks: Relationship to visual function in older adults. Optometry and Vision Science, 78, 350–359 [DOI] [PubMed] [Google Scholar]
- Owsley C., Sloane M., McGwin G., Jr., Ball K. (2002). Timed instrumental activities of daily living tasks: Relationship to cognitive function and everyday performance assessments in older adults. Gerontology, 48, 254–265 doi:58360 [DOI] [PubMed] [Google Scholar]
- Park D. C., Reuter-Lorenz P. (2009). The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology, 60, 173–196 doi:10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The ces-d scale: A self report depression scale for research in the general. Applied Psychological Measurement, 1, 385–401 [Google Scholar]
- Reitz C., Tang M. X., Schupf N., Manly J. J., Mayeux R., Luchsinger J. A. (2011). A summary risk score for the prediction of Alzheimer disease in elderly persons. Archives of Neurology, 67, 835–841. doi:10.1001/archneurol.2010.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P. A., Park D. C. (2010). Human neuroscience and the aging mind: a new look at old problems. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65, 405–415 doi:10.1093/geronb/gbq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. A. (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103, 403–428 [DOI] [PubMed] [Google Scholar]
- Salthouse T. A., Toth J., Daniels K., Parks C., Pak R., Wolbrette M., Hocking K. J. (2000). Effects of aging on efficiency of task switching in a variant of the trail making test. Neuropsychology, 14, 102–111 doi:10.1037/0894-4105.14.1.102 [PubMed] [Google Scholar]
- Sternang O., Wahlin A., Nilsson L. G. (2008). Examination of the processing speed account in a population-based longitudinal study with narrow age cohort design. Scandinavian Journal of Psychology, 49, 419–428 doi:10.1111/j.1467-9450.2008.00663.x [DOI] [PubMed] [Google Scholar]
- Syddall H., Cooper C., Martin F., Briggs R., Aihie Sayer A. (2003). Is grip strength a useful single marker of frailty? Age and Ageing, 32, 650–656 [DOI] [PubMed] [Google Scholar]
- Sylvain-Roy S., Bherer L., Belleville S. (2011). Contribution of temporal preparation and processing speed to simple reaction time in persons with Alzheimer’s disease and mild cognitive impairment. Brain and Cognition, 74, 255–261 doi:10.1016/j.bandc.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Twamley E. W., Ropacki S. A., Bondi M. W. (2006). Neuropsychological and neuroimaging changes in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Society, 12, 707–735 doi:10.1017/S1355617706060863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D. E. (2009). Speed of processing in older adults: A cognitive overview for nursing. Journal of Neuroscience Nursing, 41, 290–297 [DOI] [PubMed] [Google Scholar]
- Ware J. E., Jr (2000). SF-36 health survey update. Spine, 25, 3130–3139 [DOI] [PubMed] [Google Scholar]
- Ware J. E., Jr., Sherbourne C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care, 30, 473–483 [PubMed] [Google Scholar]
- Willis S. L., Tennstedt S. L., Marsiske M., Ball K., Elias J., Koepke K. M., … Wright E. (2006). Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association, 296, 2805–2814 doi:10.1001/jama.296.23.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger S. L., Liang K. Y., Albert P. S. (1988). Models for longitudinal data: A generalized estimating equation approach. Biometrics, 44, 1049–1060 [PubMed] [Google Scholar]
- Zelinski E. M., Gilewski M. J., Anthony-Bergstone C. R. (1990). Memory functioning questionnaire: Concurrent validity with memory performance and self-reported memory failures. Psychology and Aging, 5, 388–399 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.