Abstract
Although the highest numbers of studies linking an infectious agent with schizophrenia has involved the parasite Toxoplasma gondii, the mechanistic underpinnings of this correlation has remained unaddressed. Incidentally, CD8 T cells, which play a pivotal role in mediating long-term immunity to Toxoplasma, are downregulated in schizophrenia patients. Recent studies have demonstrated that CD8 response is also impaired during chronic toxoplasmosis in murine models. In light of these new findings, in this article, we discuss the potential role of CD8 T cells in causing altered mental status in Toxoplasma seropositive schizophrenia patients.
Key words: Toxoplasma, CD8 T cells, exhaustion, PD-1, schizophrenia
Introduction
Toxoplasmosis is caused by Toxoplasma gondii, an obligate intracellular protozoan well known as the causative agent of Toxoplasmic encephalitis, which occurs due to reactivation of chronic Toxoplasma infection in immunosuppressed patients. Prevalence rates of Toxoplasma are dependent on the geographical location, among other factors, and can range upto 80% of a population.1 This combined with the fact that this pathogen has a broad host range2 makes it one of the most successful protozoan parasites. In warm-blooded intermediate hosts including humans, the parasite initially undergoes stage conversion to the highly motile and rapidly proliferating tachyzoite stage that is responsible for acute toxoplasmosis. This phase of infection in immunocompetent individuals is usually asymptomatic or manifested by mild flu-like symptoms. However, under immune pressure, majority of tachyzoites are eliminated, and the residual parasites persist life long in quiescent form as slowly replicating encysted bradyzoites in immune-privileged sites such as neurons and muscles. Till recently, the later phase of infection referred to as chronic phase was considered asymptomatic.3 However, a growing body of convincing evidence suggests a strong association between Toxoplasma infection and altered mental status including schizophrenia, dementia, personality changes, and suicidal tendencies among others even in immunocompetent individuals.3–5 Similarly, behavioral changes such as reduced neophobia6 and increased attraction to feline odors7 have been noted in rodent models of infection. Considering that genome-wide association studies of schizophrenia have shown weak-effect correlations,8 exploration of how nongenetic risk factors such as infectious disease agents interact with schizophrenia predisposing genes has gained importance.4 Significantly, the largest number of studies associating an infectious disease agent to schizophrenia has involved Toxoplasma.4 The correlation between Toxoplasma seropositivity and schizophrenia is further strengthened by the observation that several antipsychotic drugs commonly used for treating these patients also inhibit Toxoplasma replication in vitro.9 While these reports strongly correlate T. gondii seroprevalence with altered mental status, the mechanistic underpinnings of this association has remained unaddressed. In this article, we examine the possibility of immune factors namely CD8 T cells and their potential role in causing altered mental status in individuals seropositive for Toxoplasma.
Toxoplasmosis and Schizophrenia—The CD8 Connection
Recent studies of schizophrenic patients have revealed dampened CD8 T-cell immunity in such subjects.10–13 Similarly, patients with Alzheimer’s disease14 exhibit reduced CD8 T cell numbers, a condition that shares similarities in terms of molecular pathogenic mechanisms with schizophrenia.15 Interestingly, a recent study has demonstrated that vaccination approaches that enhance CD8 immunity helps to mitigate disease progression in a murine model of Alzheimer’s disease.16 Incidentally, CD8 T cells, primarily via production of the cytokine IFNγ, play a pivotal role in keeping chronic Toxoplasma infection under control.2 Depletion of these cells in chronically infected immunocompetent mice results in reconversion of encysted quiescent parasites to the acute stage-associated form, concomitant with disease recrudescence.17 Memory CD8 T cells are believed to persist life long, and reencounter with the pathogen can mediate protective responses in self-resolving infectious diseases.18 However, recent studies from our laboratory have reported progressive loss of CD8 T cell functionality (dysfunction in terms of proliferation, cytotoxicity, cytokine production, and survival) during chronic toxoplasmosis in certain strains of mice.19 This phenomenon referred to as “exhaustion” raises an important question: Does CD8 exhaustion contribute to altered mental status (as well as severity) in Toxoplasma seropositive patients? While this paradigm of CD8 exhaustion has been extensively explored in chronic infectious diseases and cancer models,20 its role in mediating (or exacerbating) mental disorders has remained almost entirely unaddressed. As CD8 immunity is reduced in schizophrenic patients,10,11 it is conceivable that a partial loss of CD8 functionality could result in periodic reactivation of some of the quiescent T. gondii parasites, resulting in focal necrosis and localized inflammation (contributed by innate immune cell-produced cytokines). Such accrued damage of nervous system potentially over time could result in some of the various forms of mental impairment noted above.
Because Toxoplasma seropositivity can be as high as 80% in certain regions, it raises the question of why only a minor subset of Toxoplasma seropositive individuals develop serious mental impairments? Apart from being underdiagnosed, multiple genetic factors and environmental cues, both immune system dependent and immune system independent, could be involved. For the purpose of this review, we will exclusively focus on CD8 T cell-related factors that can result in exacerbated dysfunction of these cells. The signal transduction pathway responsible for CD8 exhaustion in chronic Toxoplasma-infected mice is PD1-PD-L1. PD-1, an inhibitory molecule is transiently expressed on activated CD8 T cells during acute viral, bacterial, and parasitic infections, and once resolved, its expression is downregulated.21 Interaction of this molecule with its receptor PD-L1 restrains T-cell immunity and prevents pathology during acute response.21 However, during certain chronic infections, the PD1-PD-L1 pathway is usurped by pathogens such as human immunodeficiency virus and others.21 As a result, PD-1 is not downregulated on CD8 T cells even after resolution of acute-phase Toxoplasma infection, and its expression is further upregulated progressively during the chronic phase. This results in graded loss of function and elevated apoptosis of CD8 T cells in a PD-1-dependent manner over time.19 Cats and other felines constitute the definitive host for Toxoplasma,2 and individuals who are constantly exposed to these animals are at risk for repeated high dose of infection. In agreement with this notion, preliminary experiments from our laboratory reveal that initial infective dose and early control of acute-phase infection are strong predictors of the degree of PD-1 expression and CD8 exhaustion in murine models19 (unpublished data).
Dopamine Signaling and the CD8 Connection
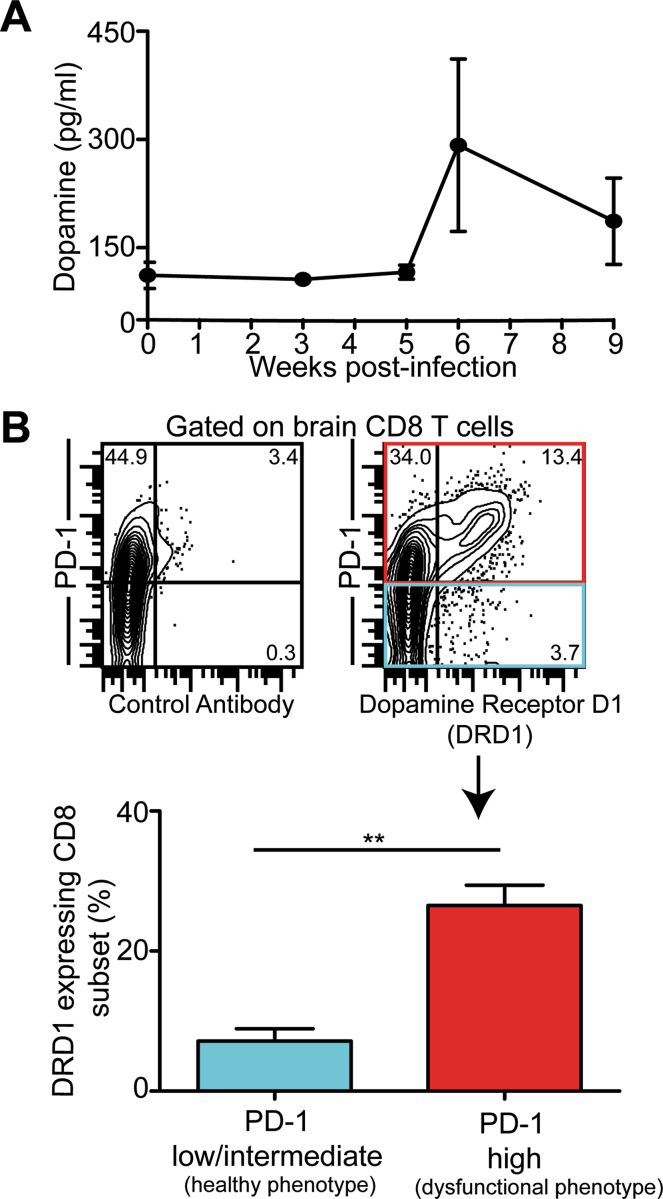
Multiple studies suggest that increased dopamine signaling may contribute to schizophrenia.22 Incidentally, brain dopamine activity declines with age.23 This may perhaps explain why despite increased seroprevalence of T. gondii with age in the United States,24 the younger population remains highly susceptible to incidence of schizophrenia (http://www.nimh.nih.gov/health/publications/the-numbers-count-mental-disorders-in-america/index.shtml#RobinsEpi). Significantly, tyrosine hydroxylase, a rate-limiting enzyme in dopamine biosynthesis is also produced by Toxoplasma.25 Thus, it is hardly surprising that Toxoplasma-infected cells produce high amounts of dopamine.25 Interestingly, dopamine by itself has been shown to activate dopaminergic receptors (D1–D5) on human T cells26 and inhibit cytotoxicity and proliferation of CD8 T cells.27 Preliminary studies from our laboratory reveal that concomitant with progressive CD8 exhaustion, plasma dopamine levels are consistently elevated (although not significantly) in mice chronically infected with Toxoplasma (figure 1A). Additionally, CD8 T cells exhibiting high levels of the exhaustion marker PD-1, also coexpress augmented levels of dopamine receptor D1 (figure 1B). While admittedly this evidence is not definitive, it strongly suggests a close association among dopamine signaling, CD8 T-cell exhaustion, and schizophrenia, which needs to be further investigated. Carefully controlled studies in human and animal models are needed to interpret broader significance of these findings.
Fig. 1.
Exhausted CD8 T cells expressed elevated levels of Dopamine Receptor D1 (DRD1). (A) Dopamine levels were assessed by enzyme-linked immuno sorbent assay in plasma collected from C57BL/6 mice infected orally with 10 cysts of Toxoplasma gondii (ME49 strain). (B) PD-1 and DRD1 expressions were evaluated by polychromatic flow cytometry on brain CD8 T cells from chronically infected mice (week 6). The top panel depicts the percentage of CD8 T cells that stain for PD-1 and DRD1 (or control antibody) as contour plots. The bottom panel represents as bar graphs the percentage of DRD1 expressing CD8 T cells in healthy (PD-1intermediate/low) and exhausted (PD-1high) CD8 subsets. The data is representative of 2 experiments with at least 4 mice per condition. Error bars represent SE throughout.
Conclusions
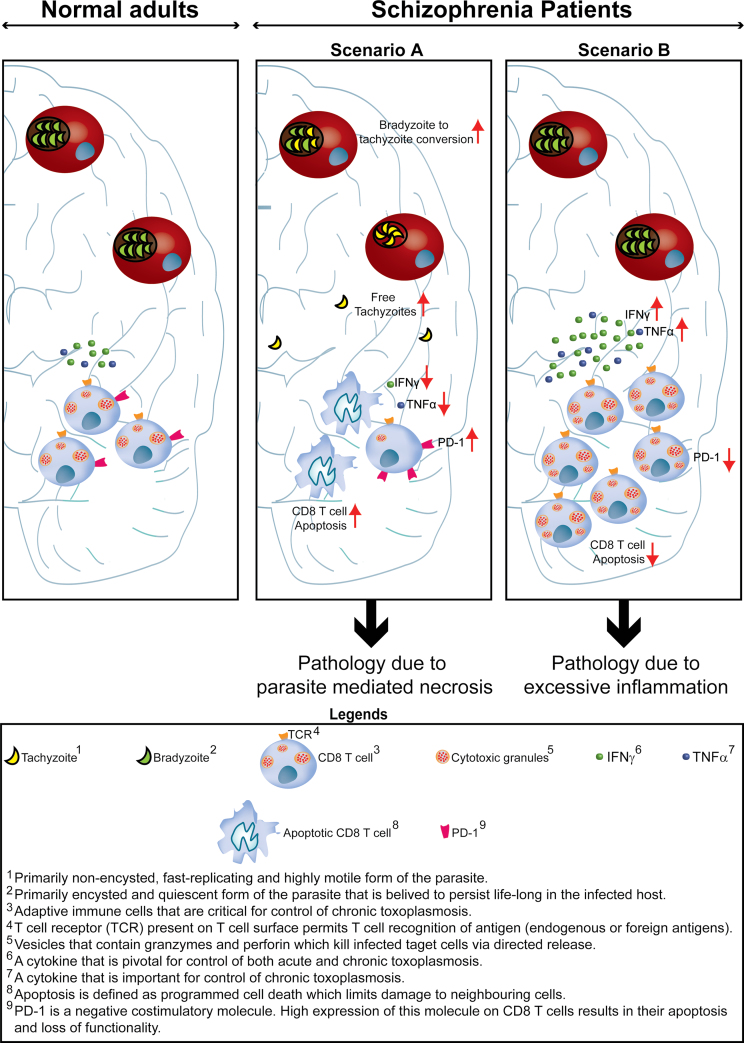
Based on evidence pointing toward downregulated T-cell response in schizophrenia patients and the overarching role played by this subset in mediating long-term protection against Toxoplasma, in this article, we have emphasized the putative role of attenuated CD8 T-cell response in mediating (or augmenting) schizophrenia in Toxoplasma seropositive individuals. Although based on current evidence it is less likely, the potential role of pathology caused by hyperactivated CD8 T cells needs to be considered as well (figure 2). Incidentally deficient PD-1 signaling as a result of PD-1 gene polymorphisms in humans has been associated with various autoimmune diseases.21 Combined, this article creates a strong case for dissecting CD8 T-cell biology in Toxoplasma seropositive schizophrenia patients and in seropositive asymptomatic individuals and Toxoplasma seronegative schizophrenia patients. Data gleaned from such studies in combination with animals models (where behavioral changes are noted as well) may provide more definitive evidence on the potential role of CD8 T cells in mediating schizophrenia in Toxoplasma seropositive individuals and create a rational basis for developing novel therapeutic approaches.
Fig. 2.
Altered mental status in Toxoplasma seropositive individuals and CD8 T cells. In normal adults chronically infected with Toxoplasma, CD8 T cells primarily via production of IFNγ ensure that parasites remain quiescent. We postulate that altered mental status in Toxoplasma seropositive adults is a result of inappropriate CD8 T-cell response. This may be due CD8 T-cell exhaustion (Scenario A) ie, initially functional CD8 T cells lose their functionality over time (IFNγ, TNFα, and cytotoxicity) and undergo apoptosis due to high expression of the inhibitory molecule PD-1. Attrition of CD8 response can result in partial reactivation of parasites involving stage conversion of the pathogen from quiescent encysted stage (bradyzoites) to fast-replicating and highly motile form (tachyzoites). This results in direct parasite-mediated pathology of brain tissue and pathology induced by inflammation, leading to altered mental status. Alternatively (Scenario B), this may be a result of augmented CD8 T-cell response due to downregulation of negative regulators of T-cell response such as PD-1. Excessive inflammation can result in pathology that may lead to altered behavior and cognition in Toxoplasma seropositive patients.
Funding
National Institutes of Health (AI-33325 to I.A.K.).
Acknowledgments
The authors wish to thank Dr Paul Shepard for his advice and support with the current manuscript. We also want to thank Drs Farzana Ajmal (Geriatric Medicine Shady Grove Adventist Clinic) and Lubna Bukhari (Adolescent Psychiatrist Stuanton Clinic) for their critical comments.
References
- 1. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhadra R, Gigley JP, Khan IA. The CD8 T-cell road to immunotherapy of toxoplasmosis. Immunotherapy. 2011;3:789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flegr J. Effects of toxoplasma on human behavior. Schizophr Bull. 2007;33:757–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38:642–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pedersen MG, Mortensen PB, Norgaard-Pedersen B, Postolache TT. Toxoplasma gondii infection and self-directed violence in mothers. Arch Gen Psychiatry. 2012:1–8 [DOI] [PubMed] [Google Scholar]
- 6. Webster JP, Brunton CF, MacDonald DW. Effect of Toxoplasma gondii upon neophobic behaviour in wild brown rats, Rattus norvegicus. Parasitology. 1994;109 (Pt 1):37–43 [DOI] [PubMed] [Google Scholar]
- 7. Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci USA. 2007;104:6442–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gershon ES, Alliey-Rodriguez N, Liu C. After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry. 2011;168:253–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62:237–244 [DOI] [PubMed] [Google Scholar]
- 10. Steiner J, Jacobs R, Panteli B, et al. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci. 2010;260:509–518 [DOI] [PubMed] [Google Scholar]
- 11. Craddock RM, Lockstone HE, Rider DA, et al. Altered T-cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PLoS ONE. 2007;2:e692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riedel M, Spellmann I, Schwarz MJ, et al. Decreased T cellular immune response in schizophrenic patients. J Psychiatr Res. 2007;41:3–7 [DOI] [PubMed] [Google Scholar]
- 13. Matloubi H, Vodjgani M, Nasehi AA, et al. Decreased T cell response to mitogen and increased anti-cytoplasmic antibody in drug-free schizophrenic patients. Iran J Immunol. 2007;4:32–37 [PubMed] [Google Scholar]
- 14. Pirttilä T, Mattinen S, Frey H. The decrease of CD8-positive lymphocytes in Alzheimer’s disease. J Neurol Sci. 1992;107:160–165 [DOI] [PubMed] [Google Scholar]
- 15. Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci USA. 2008;105:5585–5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher Y, Nemirovsky A, Baron R, Monsonego A. T cells specifically targeted to amyloid plaques enhance plaque clearance in a mouse model of Alzheimer’s disease. PLoS ONE. 2010;5:e10830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180 [PubMed] [Google Scholar]
- 18. Ahmed R, Akondy RS. Insights into human CD8(+) T-cell memory using the yellow fever and smallpox vaccines. Immunol Cell Biol. 2011;89:340–345 [DOI] [PubMed] [Google Scholar]
- 19. Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci USA. 2011;108:9196–9201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gigley JP, Bhadra R, Moretto MM, Khan IA. T cell exhaustion in protozoan disease. Trends Parasitol. 2012;28:377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci USA. 2000;97:7673–7675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volkow ND, Logan J, Fowler JS, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry. 2000;157:75–80 [DOI] [PubMed] [Google Scholar]
- 24. Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol. 2001;154:357–365 [DOI] [PubMed] [Google Scholar]
- 25. Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS ONE. 2011;6:e23866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both. J Neuroimmunol. 2005;169:161–171 [DOI] [PubMed] [Google Scholar]
- 27. Saha B, Mondal AC, Basu S, Dasgupta PS. Circulating dopamine level, in lung carcinoma patients, inhibits proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1 dopamine receptors: an in vitro analysis. Int Immunopharmacol. 2001;1:1363–1374 [DOI] [PubMed] [Google Scholar]