Abstract
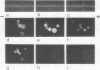
Preparations of purified immunoglobulins, light chains, and unrelated proteins (used as controls) were covalently linked to agarose beads and used to study the specificity of fluorescein isothiocyanate conjugates. The data demonstrate how these beads can be used to detect immunological and non-immunological reactivity in conjugates. Commercial conjugates, conjugates prepared in this laboratory, and fluorescein-labeled normal immunoglobulins demonstrated high reactivity with control beads unless chromatographed on diethylaminoethyl-cellulose to select for the proper fluorescein-protein ratio. Undesirable immunological reactivity could be demonstrated in commercial conjugates and was shown to be due to anti-light-chain antibody.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bergquist N. R., Kreisler M. E. The use of polymerized immunoglobulin particles for specificity control of fluorescent conjugates. J Immunol Methods. 1974 Mar;4(2):195–202. doi: 10.1016/0022-1759(74)90061-1. [DOI] [PubMed] [Google Scholar]
- CHAPLIN H., COHEN S., PRESS E. M. PREPARATION AND PROPERTIES OF THE PEPTIDE CHAINS OF NORMAL HUMAN 19 S GAMMA-GLOBULIN (IGM). Biochem J. 1965 Apr;95:256–261. doi: 10.1042/bj0950256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno J. V., Sweeney M. J., Oels H. C. Rapid batch method for the preparation of fluorescein isothiocyanate-conjugated antibody. Infect Immun. 1973 Mar;7(3):366–369. doi: 10.1128/iai.7.3.366-369.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case J. B., Lussier L. M., Mannik M. Evaluation of immunological specificity of flurescein-conjugated antisera with agarose-antigen sections. Infect Immun. 1975 Feb;11(2):415–416. doi: 10.1128/iai.11.2.415-416.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry W. B., Reimer C. B. Standardization of diagnostic materials. 4. Diagnostic immunofluorescence. Bull World Health Organ. 1973 Jun;48(6):737–746. [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- GOLDSTEIN G., SLIZYS I. S., CHASE M. W. Studies on fluorescent antibody staining. I. Non-specific fluorescence with fluorescein-coupled sheep anti-rabbit globulins. J Exp Med. 1961 Jul 1;114:89–110. doi: 10.1084/jem.114.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb R. W., Normansell D., Stanworth D. R. A structural study of human exocrine IgA globulin. J Immunol. 1968 Nov;101(5):905–914. [PubMed] [Google Scholar]
- van Dalen J. P., Knapp W., Ploem J. S. Microfluorometry on antigen-antibody interaction in immunofluorescence using antigens covalently bound to agarose beads. J Immunol Methods. 1973 Jun;2(4):383–392. doi: 10.1016/0022-1759(73)90004-5. [DOI] [PubMed] [Google Scholar]