Abstract
A novel MICA allele, MICA*070, was defined by sequencing. The new allele differs from the MICA*008:04 sequence in exon 2, encoding a C instead of G corresponding to cDNA nucleotide position 183. This nucleotide substitution is predicted to encode serine instead of arginine at residue 38 of the α1 domain of the MICA molecule.
Keywords: Major histocompatibility complex class I chain-related A genotype, Sequence-based typing, Phasing
The major histocompatibility complex (MHC) class I chain-related A (MICA) gene resides 46 kb centromeric to HLA-B, and plays a major role in the innate immune response [1, 2]. MICA is a highly polymorphic gene, encoding 80 unique alleles that give rise to 63 proteins and 2 null proteins (IMGT/HLA Database v.3.7.0, http://www.ebi.ac.uk/imgt/hla/) [3]. Previous studies have identified strong positive linkage disequilibrium of MICA and HLA-B alleles in ethnically diverse populations [4].
MICA is a ligand for the activating NKG2D receptor. Its role in hematopoietic cell transplantation has been explored in two models: recipient homozygosity and donor-recipient mismatching [5–7]. Patients homozygous for the residue 129 valine were found to be at higher risk for chronic GVHD after HLA-matched sibling HCT compared to patients homozygous for the methionine at this position, independent of acute GVHD [5]. The role of donor-recipient MICA mismatching has been evaluated by two recent studies [6, 7]. MICA mismatching was associated with an increased risk of grades II–IV acute GVHD but not grades III–IV acute GVHD in both HLA 10/10 matched and HLA-mismatched transplants [6]. The organ system most significantly involved in acute GVHD was the gastrointestinal tract and the association was independent of HLA-B and C mismatches. These data suggested that expression of MICA on the surface of intestinal cells may interact with NK cells through the NKG2D receptor as well as with γδ and αβ T cells. In a second study of patients receiving HLA 12/12 matched unrelated donor transplants, there was no evidence for an association between MICA mismatching and risk of GVHD [7]. Further examination of an independent cohort of 1676 HLA 8/8 matched unrelated donor transplants showed no differences in GVHD risk based on linkage of HLA-B alleles to MICA.
To provide a comprehensive genetic strategy for examining the MICA locus, we developed a sequence-based method that permits the physical linkage of polymorphisms across exons 1–5 of MICA. Our DNA-based approach is an alternative method to traditional cloning. We describe a novel MICA*070 allele in an individual of Caucasian background with the genotype HLA-A*02:01/09/43N, 02:01/09/43N; B*08:01/04, 40:01/07; Cw*07:01/06, 03:04; DRB1*03:01, 01:01; DQB1:02:01/02, 05:01. DNA samples from family members were not available. This study was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (FHCRC).
The sequencing protocol was developed to co-amplify and sequence both alleles of the MICA gene. Genomic DNA was prepared by the salting out method [8]. Oligonucleotide sequences and locations of primers used in this study are presented in Table 1 and Figure 1. A 1131 bp fragment containing exons 2 and 3 was PCR-amplified using primers a and b. A 783 bp fragment containing exons 4 and 5 was amplified using primers c and d. PCR reactions were conducted in a 12 µl volume containing 35–100 ng of genomic DNA, 1X Apex Hot Start Master Mix (Genesee Scientific, San Diego, CA) and 0.42 µM of reverse and forward primers (Table 1, Figure 1). PCR amplification was performed using the ABI GeneAmp®9700 PCR system (Applied Biosystems, Foster City, CA) with the following cycling parameters: activation of the Apex Taq polymerase at 95°C for 15 min; denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and extension at 72°C for 2 min for 30 cycles; a final extension at 72°C for 7 min, and a 4°C hold. Five microliters of the amplified product was electrophoresed on a 2% agarose gel at 125 V for 45 minutes to verify the expected size of the product. Prior to sequencing, 5 µl of the amplified product was purified from the excess dNTPs and PCR primers by adding 2 µl of ExpSAP-IT (USB, Cleveland, OH). The mixture was incubated in an ABI GeneAmp®9700 PCR system for 20 minutes at 37 °C followed by 15 minutes at 80 °C and a 4 °C hold.
Table 1.
Oligonucleotide primers used for amplification and sequencing
| Primer name1 | Sequence 5’-3’ | Orientation | Location2 |
|---|---|---|---|
| Primer a | CCCCCTTCTTCTGTTCATCA | Forward | 10561–10580 |
| Primer b | CCAACAGGAAATGCCTTCAT | Reverse | 11673–11692 |
| Primer c | CCAGAGTGAGGACAGACTTGC | Forward | 12025–12045 |
| Primer d | CATGCCTATCTTTGCAGGAG | Reverse | 12744–12763 |
| Primer e | ATTTCCTGCCCCAGGAAGGTTGG | Forward | 10658–10680 |
| Primer f | TCGTGATTGGCCCTAAGTTC | Forward | 3714–3733 |
| Primer g | CCGAGGAGGACTGAAAAGTG | Reverse | 4092–4111 |
| Primer h | CCTGCTGAGTTCCACTGAC | Reverse | 11131–11152 |
Primers b, c, d, f and g were designed using the Primer3 program v. 0.4.0 (http://frodo.wi.mit.edu/) [9], primers a and h according to reference 10 and primer e according to reference 11.
Numbered according to NCBI reference of MICA sequence (http://www.ncbi.nlm.nih.gov/nuccore/NC_000006.11?report=genbank&from=31367561&to=31383090).
Fig. 1. MICA amplification and sequencing primers (not to scale).
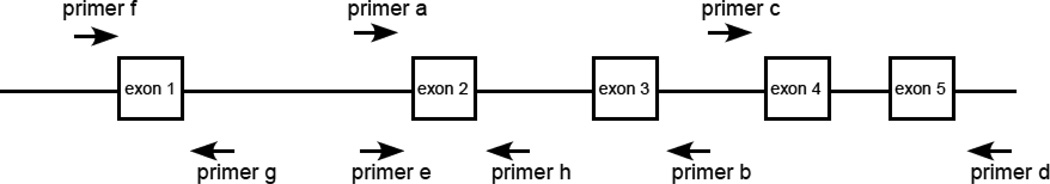
Purified products (1 µl) were sequenced using the ABI BigDye Terminator v. 3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA). Primer e was used to sequence exon 2 in the forward direction; primer b was used to sequence exon 3 in the reverse direction. Primer c was used to sequence exon 4 in the forward direction; primer d was used to sequence exon 5 in the reverse direction (Table 1, Figure 1). Sequenced products were purified using the BigDye XTerminator™ Purification Kit (Applied Biosystems, Foster City, CA) following the manufacturer’s protocol. Samples were electrophoresed for 2 hours using an ABI 3730XL DNA analyzer (Applied Biosystems, Foster City, CA).
Exon 2, 3 and 4 sequences were analyzed using Sequencher 5.0 software (Gene Codes Corporation, Ann Arbor, MI). Data from each exon were concatenated from variance tables into a nucleotide string containing all 52 known polymorphic positions of exons 2, 3 and 4 (IMGT/HLA Database v.3.7.0, http://www.ebi.ac.uk/imgt/hla/). The 52-nucleotide string from the sample was imported into a FileMaker Pro v10.0 program (FileMaker Inc., Santa Clara, CA) and cross-referenced against the pairwise combinations of known alleles (Table S1, Supporting Information). Allele combinations that show unique polymorphisms in exons 2, 3 and 4 can be definitively genotyped with this method with the exception of 16 alleles that have identical sequences in exons 2, 3 and 4 (Table S2). Four polymorphic positions and the GCT short tandem repeat (STR) in exon 5 permit delineation of 595 out of 1217 ambiguous allele combinations (Table S1, Table S2; IMGT/HLA Database v.3.7.0, http://www.ebi.ac.uk/imgt/hla/). The exon 5 sequence of the sample was manually compared to all possible pairwise combinations of the eight types of GCT repeats (modified from the approach described by Zou et al [11]; Table S3).
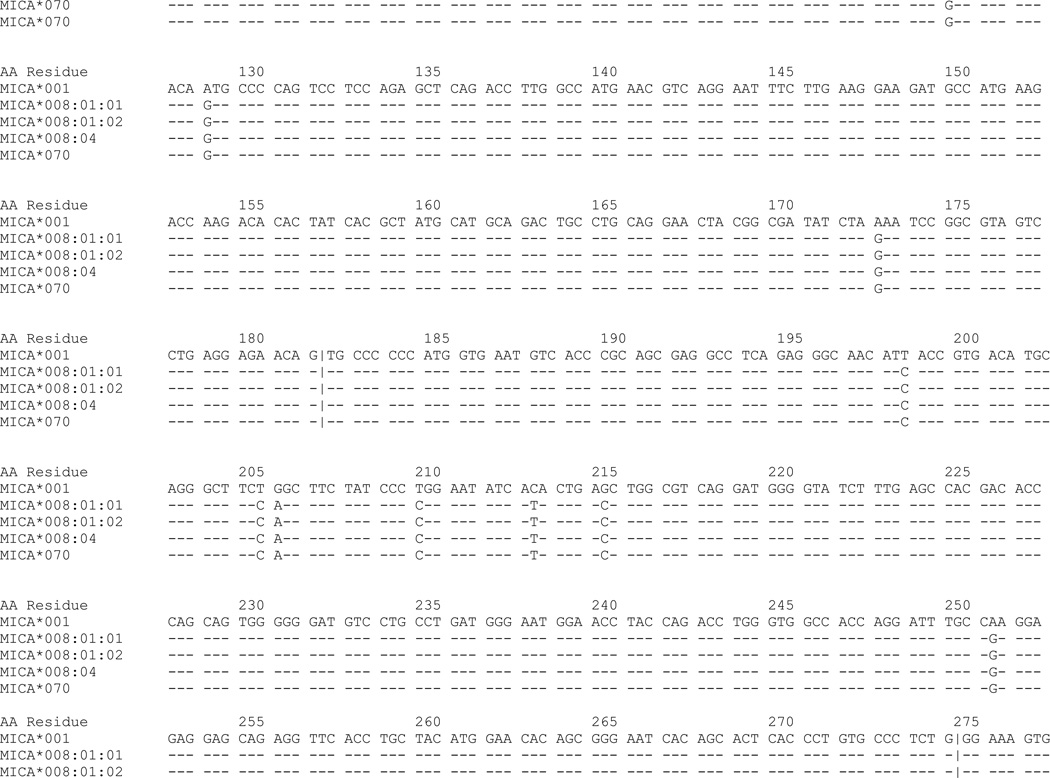
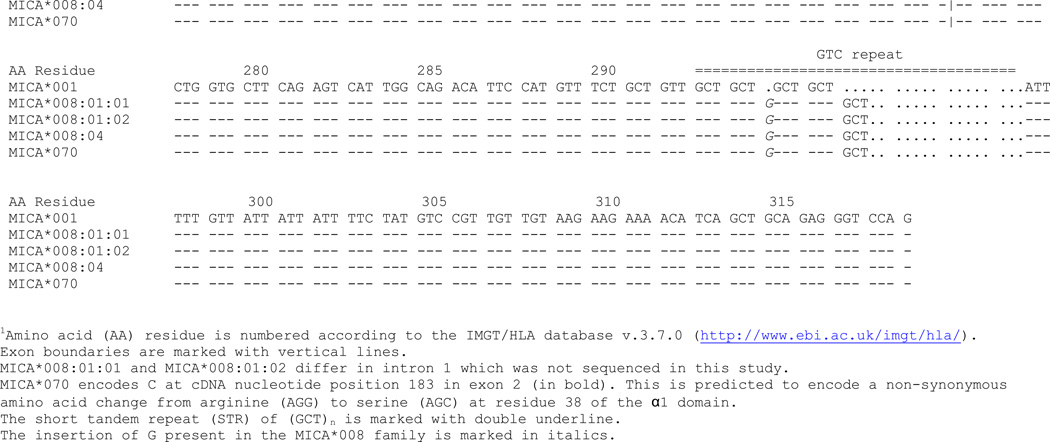
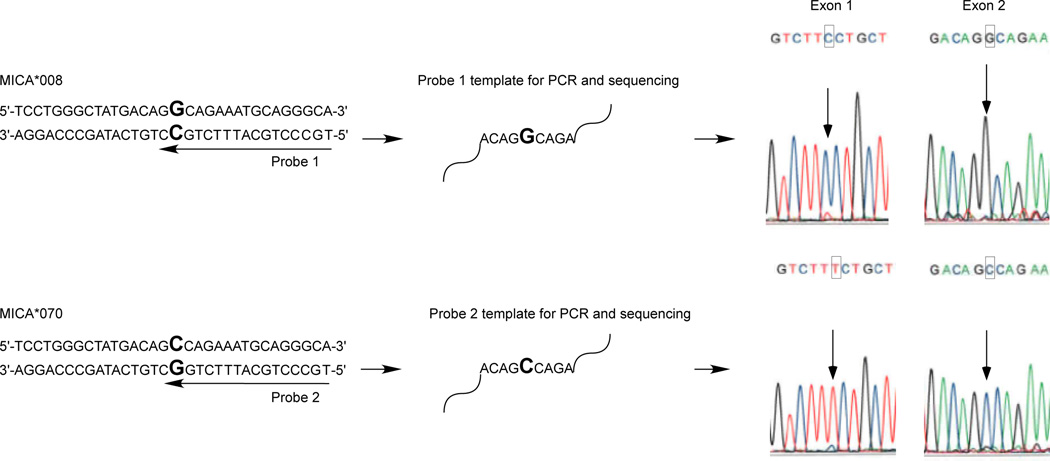
The exon 2, 3, 4 and 5 sequences of the MICA*070 allele were consistent with MICA*008:01 and/or MICA*008:04 with a new substitution of G/C corresponding to cDNA nucleotide position 183 in exon 2, which is predicted to encode a non-synonymous amino acid change from arginine (AGG) to serine (AGC) at residue 38 of the α1 domain (Figure 2). Three approaches were taken to verify the substitution and to determine the most likely allele. First, to confirm the presence of the nucleotide substitution, exon 2 was resequenced in forward and reverse directions (amplified with primers a and b, and sequenced with primers e and h; Table 1, Figure 1) from two independent DNA preparations. Second, exon 1 was sequenced to determine whether the sample was homozygous MICA*008:01, homozygous MICA*008:04, or heterozygous. Exon 1 was amplified and sequenced using primers f and g. The sample displayed heterozygous incorporation of both C and T at nucleotide position 21, consistent with MICA*008:01 and 008:04. Finally, to determine whether the novel allele is more closely related to MICA*008:01 or 008:04, DNA was extracted from a lymphoblastoid cell line using the EZ1® DNA tissue kit with the EZ1® Advanced Robot (Qiagen, Valencia, CA). Two oligonucleotide probes were designed to be specific for the novel exon 2 substitution: 5’-TGCCCTGCATTTCTGGC-3’ and 5’-TGCCCTGCATTTCTGCC-3’ (Figure 3). The two alleles were separated using the EZ1® HaploPrep kit following the manufacturer’s protocol. Exons 1 and 2 of each allele were amplified and sequenced as described above. One allele carried C corresponding to cDNA nucleotide position 21 in exon 1 and G corresponding to cDNA nucleotide position 183 in exon 2, and hence is the MICA*008:01 allele. The second allele carried T corresponding to cDNA nucleotide position 21 in exon 1 which is observed in MICA*008:04 and the novel C substitution corresponding to cDNA nucleotide position 183 in exon 2 (Figure 2). Hence, MICA*070 is a variant of MICA*008:04. The MICA genotype for this individual is MICA*008:01, 070. MICA*070 has been assigned the GenBank JQ522969 accession number. Sequence data for exon 1 in addition to data for exons 2, 3, 4 and 5 permit delineation of 808 of 1217 allele combinations.
Fig. 2. Alignment of MICA*008:01, MICA*008:04 and MICA*070.
Fig. 3. Phasing of the new allele.
Two oligonucleotide probes were designed to separate MICA*008 from MICA*070 using the Qiagen HaploPrep kit. Separated alleles were amplified and sequenced to determine the linkage of exon 1 to exon 2. Original chromatograms depict the novel exon 2 substitution at cDNA nucleotide position 183 in MICA*070 compared to MICA*008.
Our phased sequencing method provides an approach for investigating the role of MICA in disease and complements the techniques are currently available [10, 11]. MICA*070 provides evidence for the diversity of the MICA genetic locus. Although a family study was not available to assign the HLA-B haplotype to MICA*070, our data suggest that complete characterization of exons 1, 2, 3, 4 and 5 is required to definitively type the majority of the alleles of this locus. MICA*070 furthermore sheds light on potential functional consequences of sequence variation in the α1 domain. The 63 known MICA proteins to date all encode arginine at residue 38 of the α1 domain. The MICA*070 allele is the first reported sequence to have a substitution at this residue. Interestingly, crystallographic studies indicate a role for residue 38 in interactions with the NKG2D receptor [12]. Future studies are indicated to define the significance of the residue 38 serine substitution in ligand-receptor interactions.
Supplementary Material
Acknowledgments
EWP designed the study and procured funding. DM designed the reagents and performed genotyping. All authors contributed to data analysis and preparation of the manuscript. This work was supported by grants CA100019 and CA18029 (EWP, MM, DM) from the US National Institutes of Health, and Research on Allergic Disease and Immunology (Health, and Labor Science Research Grant), the Ministry of Health, Labour and Welfare of Japan (SM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors have competing interests.
Supporting Information
The following supporting information is available for this manuscript:
Table S1 All possible 52 nucleotide string combinations descriptive of known pairwise MICA allele combinations according to IMGT/HLA release v3.7.0.
Table S2 Further characterization of Exon 5 for groups of alleles with identical sequences in exon 2, 3 and 4.
Table S3 All possible pairwise sequence combinations descriptive of the heterozygous short tandem repeat (STR).
References
- 1.Bahram S, Bresnahan M, Geraghty DE, Spies T. A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci USA. 1994;91:6259–6263. doi: 10.1073/pnas.91.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González S, Groh V, Spies T. Immunobiology of human NKG2D and its ligands. Curr Top Microbiol Immunol. 2006;298:121–138. doi: 10.1007/3-540-27743-9_6. [DOI] [PubMed] [Google Scholar]
- 3.Robinson J, Malik A, Parham P, Bodmer JG, Marsh SG. IMGT/HLA database--a sequence database for the human major histocompatibility complex. Tissue Antigens. 2000;55:280–287. doi: 10.1034/j.1399-0039.2000.550314.x. [DOI] [PubMed] [Google Scholar]
- 4.Fodil N, Pellet P, Laloux L, Hauptmann G, Theodorou I, Bahram S. MICA haplotypic diversity. Immunogenetics. 1999;49:557–560. doi: 10.1007/s002510050536. [DOI] [PubMed] [Google Scholar]
- 5.Boukouaci W, Busson M, Peffault de Latour R, et al. MICA-129 genotype, soluble MICA, and anti-MICA antibodies as biomarkers of chronic graft-versus-host disease. Blood. 2009;114:5216–5224. doi: 10.1182/blood-2009-04-217430. [DOI] [PubMed] [Google Scholar]
- 6.Parmar S, Del Lima M, Zou Y, et al. Donor-recipient mismatches in MHC class I chain-related gene A in unrelated donor transplantation lead to increased incidence of acute graft-versus-host disease. Blood. 2009;114:2884–2887. doi: 10.1182/blood-2009-05-223172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson E, Grzywacz B, Wang H, et al. Limited role of MHC class I chain-related gene A (MICA) typing in assessing graft-versus-host disease risk after fully human leukocyte antigen-matched unrelated donor transplantation. Blood. 2009;114:4753–4754. doi: 10.1182/blood-2009-08-239301. [DOI] [PubMed] [Google Scholar]
- 8.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozen Steve, Skaletsky Helen J. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 10.Field SF, Nejentsev S, Walker NM, et al. Sequencing-based genotyping and association analysis of the MICA and MICB genes in type 1 diabetes. Diabetes. 2008;57:1753–1756. doi: 10.2337/db07-1402. [DOI] [PubMed] [Google Scholar]
- 11.Zou Y, Han M, Wang Z, Stastny P. MICA allele-level typing by sequence-based typing with computerized assignment of polymorphic sites and short tandem repeats within the transmembrane region. Hum Immunol. 2006;67:145–151. doi: 10.1016/j.humimm.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Li P, Morris DL, Willcox BE, Steinle A, Spies T, Strong RK. Complex structure of the activating immunoreceptor NKG2D and its MHC class I-like ligand MICA. Nat Immunol. 2001;2:443–451. doi: 10.1038/87757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.