Abstract
Background
The prevalence in the United States of dietary supplement use that may be harmful to those with chronic kidney disease (CKD) is unknown. We sought to characterize potentially harmful supplement use by individual CKD status.
Study Design
Cross-sectional national survey (National Health and Nutrition Examination Survey, 1999-2008)
Setting & Participants
Community-based survey of 21,169 non-pregnant, non-institutionalized U.S. civilian adults (≥20 years)
Predictor
CKD status (no CKD, at risk for CKD [presence of diabetes, hypertension and/or cardiovascular disease], stage 1/2 [albuminuria only (albumin-creatinine ratio ≥30 mg/g)], or stage 3/4 [estimated glomerular filtration rate of 15-59 ml/min/1.73 m2]).
Outcome
Self-reported use of dietary supplements containing any of 37 herbs the National Kidney Foundation identified as potentially harmful in the setting of CKD.
Measurements
Albuminuria and estimated glomerular filtration rate assessed from urine and blood samples; demographics and comorbid conditions assessed by standardized questionnaire.
Results
An estimated 8.0% of U.S. adults reported potentially harmful supplement use within the last 30 days. Lower crude estimated prevalence of potentially harmful supplement use was associated with higher CKD severity (no CKD, 8.5%; at risk, 8.0%; stage 1/2, 6.1%; and stage 3/4, 6.2%; p<0.001). However, after adjustment for confounders, those with or at risk for CKD were as likely to use a potentially harmful supplement as those without CKD: at-risk OR, 0.93 (95% CI, 0.79 -1.09); stage 1/2 OR, 0.83 (95% CI, 0.64 -1.08); stage 3/4 OR, 0.87 (95% CI, 0.63 -1.18); all vs. no CKD.
Limitations
Herb content was not available and the list of potentially harmful supplements examined is unlikely to be exhaustive.
Conclusions
The use of dietary supplements potentially harmful to people with CKD is common, regardless of CKD status. Healthcare providers should discuss the use and potential risks of supplements with patients with and at risk for CKD.
Chronic kidney disease (CKD) in the United States is common, affecting an estimated 14% of the general adult population who are aged 20 years or older.1 Currently, over 600,000 Americans have progressed to chronic kidney failure requiring renal replacement therapy, a condition that is associated with excess morbidity and mortality.2 Therefore, it is critically important to identify possible risks for and measures to decrease CKD progression.
Avoidance of substances that may be harmful to the kidney is one important method of reducing CKD progression. Although many herbs commonly found in dietary supplements can cause acute kidney injury and other forms of kidney injury,3 they are not subject to rigorous governmental standards for content or safety for the general population4 or for individuals with CKD, in whom the consequences could be particularly deleterious. Because roughly half of US adults report using dietary supplements,5,6 these products may be an important source of adverse renal effects that are under-recognized by both patients and providers.
To our knowledge, only a few small studies7-9 have described the use of dietary supplements among patients with CKD, or more specifically end-stage renal disease, and none have focused on supplements that may be harmful in the setting of kidney disease. In a nationally representative sample, we sought to characterize the extent of dietary supplement use that may have harmful consequences for persons with or at risk for CKD.
METHODS
Study Population
The study population was drawn from the National Health and Nutrition Examination Survey (NHANES).10 NHANES is a well-established representative survey of non-institutionalized civilian residents in the United States conducted by the National Center for Health Statistics of the U.S. Centers for Disease Control and Prevention. It consists of a standardized in-home interview, followed by a physical examination and blood and urine collection at a mobile examination center. All participants provide written informed consent. The protocol was approved by the National Center for Health Statistics Research Ethics Review Board. We included 21,169 non-pregnant, adult participants from NHANES 1999-2008 who met our study criteria. From a denominator of 24,693 adults aged ≥20 years, we excluded 9 with missing dietary supplement use data; an additional 2,262 with missing kidney function data; and finally 1,253 more with estimated glomerular filtration rate (eGFR) below 15 ml/min/1.73 m2. We excluded those with very low eGFR because our goal was to focus on individuals who would most benefit from identifying behaviors that may predispose to nephrotoxicity or CKD progression. After these exclusions, there were no pregnant participants remaining.
Measurements
Serum creatinine concentration was measured by the modified kinetic method of Jaffe using different analyzers in different survey years. Creatinine levels were calibrated as specified in NHANES documentation.11,12 Random spot urine samples were obtained; and urine albumin was measured using solid-phase fluorescence immunoassay and urine creatinine was measured using the modified Jaffe kinetic method in the same laboratory11 on frozen samples.
Definitions
Participants who responded “yes” to the question “Have you used or taken any vitamins, minerals, or other dietary supplements in the past month?” were asked to provide bottles for the individual supplements they took. Each provided supplement was classified as either potentially harmful in the setting of CKD or “other.” A supplement was considered potentially harmful if it contained at least 1 of 37 distinct herbs identified from literature review and expert opinion by the Council on Renal Nutrition for the National Kidney Foundation (NKF).13 We reviewed supplement ingredients using the variable dsdingr (ingredient name) in the Dietary Supplement Database-File 4, to determine the presence of any of the 37 potentially toxic herbs. For ingredients noted as “proprietary blends,” we located the actual product label to identify ingredients. In the event the product label could not be found, the variable dsdbcnam (blend component name, also in File 4) was queried. Participants were classified as taking any potentially harmful supplement, taking only “other” supplements, or taking no supplements.
We defined CKD status as no CKD; at risk only, by presence of strong CKD risk factors (including diabetes, hypertension or cardiovascular disease); stage 1/2 CKD, as albuminuria only (urine albumin-creatinine ratio, ≥30 mg/g) with eGFR ≥60 ml/min/1.73 m2; or stage 3/4 CKD, as eGFR 15-59 ml/min/1.73 m2, regardless of albuminuria. Estimated GFR was calculated using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: GFR = 141 × min(Scr/κ,1)α × max(Scr/κ,1)−1.209 × 0.993age × 1.018 [if female] × 1.159 [if black], where Scr is serum creatinine (mg/dL), κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1.14
We defined diabetes by participant self-report. Hypertension was defined by self-report or an average of second and third blood pressure readings ≥140 mmHg systolic or ≥90 mmHg diastolic. Cardiovascular disease included self-report of coronary artery disease, stroke, heart attack, congestive heart failure, or angina.
We categorized age into three groups (20-44, 45-64, and ≥65 years) and used NHANES categories of self-reported race/ethnicity as non-Hispanic white, non-Hispanic black, Mexican-American, or other. Educational attainment was categorized as more than high school, high school or high school equivalent, and less than high school. We categorized income using the U.S. Census Bureau’s poverty index ratio (the ratio of family income to federal poverty level, where ≤1.00 is considered below the poverty level) into three groups (poverty index ratio ≤1.00, >1-<3, or ≥3). We included self-reported arthritis and cancer as co-morbid conditions ascertained uniformly in our study population that may prompt individuals to take dietary supplements as a means of prevention or treatment. 15
Tobacco use (no/past versus ongoing) was defined by significant lifetime use of cigarettes (≥100), snuff (≥20 times), and/or chewing tobacco (≥20 times). Current alcohol use was categorized as none/moderate versus heavy (>7 drinks/wk for women or >14 drinks/wk for men). We defined healthcare utilization as the number of healthcare visits within the last 12 months as a continuous variable to examine the effect of encounters with healthcare providers on supplement use.
Statistical Analysis
We calculated the proportion of all reported supplements containing at least one NKF-identified herb, and the proportion of potentially harmful supplements containing each specific NKF-identified herb. We used ordinal logistic regression to test whether supplement use varied by survey year. Among U.S. adults aged 20 years or older, we estimated the prevalent use of potentially harmful or only other supplements overall and by CKD status within groups defined by demographic characteristics, comorbid conditions, health-related behaviors, and healthcare visits. Among the subpopulation of U.S. adults who took any supplement, we used chi-square analysis to test whether potentially harmful supplement use varied by CKD status within each of these groups. We estimated the frequency and duration of any potentially harmful supplement use and used chi-square analysis to test whether these estimates were associated with CKD status. Finally, we used multivariable logistic regression to assess the presence, direction, strength, and independence of the association between taking a potentially harmful supplement and CKD status, among those taking any supplement. We added covariates to the model sequentially to examine their incremental effects on the likelihood of taking a potentially harmful supplement. We performed sensitivity analyses with CKD defined by GFR estimated according to the isotope-dilution mass spectrometry–traceable 4-variable Modification of Diet in Renal Disease (MDRD) Study equation.16 All analyses used recommended sampling weights11 and were performed using the “SVY” commands in STATA v. 12.0 (StataCorp LP, College Station, TX) to account for the study design.
RESULTS
Participants in our study reported use of 5,280 distinct supplements, of which 14.3% (n=757 unique supplements) were potentially harmful. Of the 37 NKF-identified herbs, 18 were found among supplement ingredients (Table S1, available as online supplementary material). Potential adverse renal effects of herbs found in reported dietary supplements are shown in Table 1.17 Ginseng was the most commonly found NKF-identified herb, contained in an estimated 36.5% of potentially harmful dietary supplements, followed by ginger (23.6%), alfalfa (19.6%), capsicum (14.9%), licorice (14.8%), dandelion (10.3%), aloe (9.3%), ma huang (7.4%), nettle (7.4%), horsetail (6.0%), yohimbe (2.6%), rhubarb (2.3%), cascara (2.0%), noni (1.0%), senna (1.0%); and broom, wormwood, bayberry, and buckthorn (<1.0% each). The most commonly (>1%) reported potentially harmful supplements, including several multivitamin formulations, and their NKF-identified herbal ingredients are listed in Table 2.
Table 1.
Herbs from NKF list found in reported dietary supplements and associated adverse renal effects
| Herb | Nephrotoxic | Aggravates CKD risk factor |
Risky in CKD |
|---|---|---|---|
| Alfalfa | Triggers lupus | - | - |
| Aloe | Albuminuria, acute or progressive kidney injury |
- | Hypovolemia |
| Bayberry | -- | - | Hypovolemia |
| Broom | - | - | - |
| Buckthorn | Albuminuria | - | Hypovolemia |
| Capsicum | - | - | Hypovolemia |
| Cascara | Albuminuria | - | Hypovolemia |
| Dandelion | - | - | Hypovolemia |
| Ginger | - | - | Hypoglycemia |
| Ginseng | - | - | Hypoglycemia |
| Horsetail | - | - | Hypoglycemia |
| Licorice | - | High BP | - |
| Ma huang | - | Hyperglycemia, high BP, kidney stones |
Hypovolemia |
| Nettle | Acute or progressive kidney injury | Hyperglycemia | - |
| Noni | - | - | Hyperkalemia |
| Pokeroot | - | - | Hypovolemia |
| Rhubarb | - | - | Hypovolemia |
| Senna | Acute or progressive kidney injury | - | Hypovolemia |
| Wormwood | Acute or progressive kidney injury, rhabdomyolysis |
- | Hypovolemia |
| Yohimbe | Acute or progressive kidney injury, triggers lupus |
- | - |
Note: hypovolemia due to diarrhea and/or vomiting.
BP, blood pressure; NKF, National Kidney Foundation; CKD, chronic kidney disease
Table 2.
Estimated prevalence of most commonly used potentially harmful supplements
| Supplement | Estimated prevalence (%) |
NKF-identified herb(s) in supplement |
|---|---|---|
| Centrum Advanced Formula Carb Assist Complete Multivitamin/ Multimineral From A to Zinc |
3.4 | ginseng |
| GNC Men’s Timed Release Senior Formula | 1.7 | ginseng, nettle |
| Centrum Performance Complete Multivitamin Specially Formulated With Ginseng, Ginkgo, and Higher Levels Of 5 Essential B |
1.6 | ginseng |
| One Source Complete Women’s With Ester-C Calcium 500mg Cranberry EGCG (Green Tea Extract) Multivitamin Mineral & Herb |
1.6 | ginseng |
| GNC Men’s Ultra Saw Palmetto Formula | 1.4 | ginseng |
| One Source Complete Women’s Multivitamin/ Multimineral/ Herbs with EGCG Green Tea Extract 27mg & Cranberry 50mg |
1.4 | ginseng |
| Member’s Mark Advanced Multi Performance Multivitamin for Adults | 1.3 | ginseng |
| Member’s Mark Advanced Multi with Herbs | 1.1 | ginseng |
| Metabolic Cleansing System 343 Advocare | 1.1 | aloe |
| One Source Pure Performance The Advanced Formula Multivitamin Multimineral Herbs Formulated for Active Adults Complete |
1.1 | ginseng |
Note: n=1,421, , among US adults aged >20 years taking potentially harmful supplement, NHANES 19992008.
NHANES, National Health and Nutrition Examination Survey; NKF, National Kidney Foundation
GNC = General Nutrition Centers
Among U.S. adults aged 20 years or older, supplement use did not vary across survey years (p=0.3 by chi-square). An estimated 8.0% used a potentially harmful supplement(s) and an additional 44.5% used other supplement(s) (Table 3). These estimates were similar among participants who were excluded due to lack of kidney function data (p=0.8 by chi-square). The overall prevalence of potentially harmful supplement use was lower with higher CKD status and age, but that of other supplement use was higher with these characteristics. This pattern was similar by CKD status. Supplement use (potentially harmful and other) was most common among non-Hispanic whites, both overall and within each CKD status stratum. Overall, the prevalence of potentially harmful and other supplement use was higher among persons with higher income, educational attainment, and number of healthcare visits. A similar pattern was found in analyses within each CKD status stratum.
Table 3.
Point-estimated prevalence of dietary supplement use among US adults aged ≥20 years by characteristic and CKD status
| Characteristic | Column Percent |
Overall | No CKD (46.3% of total) |
At risk for CKD (39.0% of total) |
CKD stage ½ (8.0% of total) |
CKD stage ¾ (6.6% of total) |
p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Potentially Harmful suppl |
Other suppl |
Any Potentially Harmful suppl |
Other suppl |
Any Potentially Harmful suppl |
Other suppl |
Any Potentially Harmful suppl |
Other suppl |
Any Potentially Harmful suppl |
Other suppl |
|||
| All | 8.0 | 44.5 | 8.5 | 40.4 | 8.0 | 46.9 | 6.1 | 44.1 | 6.2 | 58.9 | <0.001 | |
| Age group | <0.001 | |||||||||||
| 20-44 y | 49.0 | 7.6 | 35.1 | 8.0 | 34.6 | 7.1 | 36.4 | 5.4 | 32.4 | 9.2 | 47.7 | |
| 45-64 y | 33.8 | 9.5 | 48.7 | 9.7 | 48.9 | 9.6 | 49.0 | 7.7 | 44.7 | 9.6 | 50.7 | |
| ≥65 y | 17.2 | 6.0 | 62.9 | 6.3 | 66.0 | 6.6 | 64.5 | 5.1 | 58.3 | 5.3 | 61.3 | |
| Sex | 0.07 | |||||||||||
| Male | 50.2 | 7.7 | 38.7 | 7.4 | 35.4 | 8.4 | 41.0 | 6.0 | 39.0 | 6.7 | 50.1 | |
| Female | 49.8 | 8.3 | 50.3 | 9.6 | 45.7 | 7.7 | 53.0 | 6.2 | 48.4 | 5.9 | 64.8 | |
| Race/ethnicity | 0.002 | |||||||||||
| Non-Hispanic White |
72.0 | 8.7 | 49.6 | 9.3 | 45.4 | 8.9 | 51.6 | 6.1 | 51.3 | 6.9 | 62.1 | |
| Non-Hispanic Black |
10.3 | 6.0 | 30.1 | 6.5 | 27.6 | 6.1 | 31.2 | 5.8 | 29.3 | 1.0 | 41.8 | |
| Mexican American |
7.6 | 5.4 | 25.2 | 5.4 | 22.6 | 5.8 | 27.2 | 4.6 | 30.2 | 2.5 | 43.0 | |
| Other | 10.1 | 6.4 | 37.2 | 6.9 | 32.4 | 5.4 | 43.5 | 7.7 | 34.4 | 4.9 | 44.4 | |
| Income | 0.005 | |||||||||||
| PIR≤1 | 12.2 | 3.6 | 30.0 | 3.0 | 25.4 | 4.8 | 32.6 | 2.9 | 30.9 | 2.0 | 47.6 | |
| PIR >1-<3 | 33.9 | 7.3 | 40.4 | 8.1 | 33.3 | 7.0 | 43.4 | 6.8 | 42.5 | 5.6 | 59.2 | |
| PIR≥3 | 53.9 | 9.3 | 50.3 | 9.8 | 47.6 | 9.3 | 52.0 | 6.9 | 50.8 | 8.0 | 61.4 | |
| Education | <0.001 | |||||||||||
| < High school | 19.8 | 4.2 | 31.3 | 4.1 | 22.9 | 3.8 | 34.1 | 4.9 | 33.1 | 4.9 | 49.6 | |
| High school | 25.6 | 6.5 | 42.6 | 7.2 | 36.0 | 6.8 | 45.8 | 2.9 | 43.7 | 4.6 | 63.4 | |
| > High school | 54.6 | 10.0 | 50.2 | 10.2 | 47.3 | 10.2 | 52.2 | 8.6 | 50.5 | 8.5 | 63.2 | |
| Diabetes | <0.001 | |||||||||||
| No | 92.5 | 8.1 | 44.2 | 8.5 | 40.4 | 8.2 | 46.5 | 6.5 | 44.9 | 6.5 | 60.2 | |
| Yes | 7.5 | 5.6 | 47.8 | - | - | 5.6 | 51.0 | 4.9 | 41.4 | 5.4 | 54.4 | |
| Hypertension | <0.001 | |||||||||||
| No | 51.4 | 8.3 | 41.0 | 8.5 | 40.4 | 8.0 | 46.7 | 4.8 | 42.9 | 8.6 | 56.8 | |
| Yes | 48.6 | 7.6 | 48.1 | - | - | 10.4 | 46.9 | 6.8 | 44.7 | 5.8 | 59.4 | |
| CV diseasec | <0.001 | |||||||||||
| No | 91.3 | 8.1 | 43.6 | 8.5 | 40.4 | 8.2 | 46.3 | 6.4 | 42.0 | 6.9 | 60.0 | |
| Yes | 8.7 | 6.0 | 53.0 | - | - | 6.7 | 50.4 | 4.8 | 56.4 | 5.0 | 57.1 | |
| Arthritis | <0.001 | |||||||||||
| No | 75.8 | 8.0 | 41.1 | 8.3 | 39.3 | 8.3 | 42.6 | 6.3 | 39.7 | 6.2 | 54.3 | |
| Yes | 24.2 | 7.7 | 54.9 | 9.6 | 47.1 | 7.4 | 57.5 | 5.8 | 53.0 | 6.3 | 62.9 | |
| Cancer | <0.001 | |||||||||||
| No | 91.7 | 7.9 | 42.9 | 8.4 | 39.4 | 7.9 | 45.3 | 6.3 | 42.4 | 6.2 | 57.6 | |
| Yes | 8.3 | 8.6 | 61.2 | 10.4 | 60.1 | 9.2 | 61.6 | 5.0 | 58.5 | 6.3 | 63.4 | |
| Significant tobacco used | 0.06 | |||||||||||
| No/Past | 74.6 | 8.8 | 48.3 | 9.4 | 44.1 | 9.0 | 50.6 | 6.6 | 47.6 | 6.4 | 61.2 | |
| Ongoing | 25.4 | 5.7 | 33.2 | 6.1 | 31.1 | 5.2 | 35.4 | 4.9 | 34.7 | 4.6 | 39.3 | |
| Heavy alcohol usee | 0.008 | |||||||||||
| No | 71.7 | 8.2 | 45.3 | 8.8 | 41.0 | 8.3 | 48.6 | 5.4 | 44.3 | 6.7 | 60.3 | |
| Yes | 28.3 | 7.2 | 42.4 | 7.4 | 38.7 | 7.4 | 43.1 | 7.7 | 43.8 | 5.3 | 56.4 | |
| No. of healthcare visits in past 12 mo | 0.001 | |||||||||||
| 0 | 16.5 | 6.7 | 30.0 | 6.0 | 30.8 | 8.1 | 28.1 | 5.1 | 27.3 | 10.1 | 44.0 | |
| 1-3 | 46.5 | 8.0 | 44.4 | 8.7 | 41.0 | 7.6 | 47.2 | 6.4 | 44.5 | 7.1 | 56.2 | |
| ≥4 | 36.9 | 8.5 | 51.1 | 10.0 | 47.0 | 8.5 | 52.2 | 6.2 | 48.5 | 5.6 | 61.1 | |
Note: “Column %” column shows the distribution of individuals within the listed categories. The remaining values are percentages of the given characteristic subgroup (ie, the row) who are using the indicated dietary supplement type overall and by CKD status as defined by CKD-EPI equation. N=21,169, NHANES 1999-2008 (age ≥20 years).
Chi-square test of association between potentially harmful supplement use and CKD status within characteristic, among those taking a dietary supplement.
Defined using the US Census Bureau’s PIR, the ratio of family income to federal poverty level, where ≤1.00 is considered below the poverty level.
CV disease includes angina, heart attack, stroke, coronary artery disease, or congestive heart failure.
Lifetime use of cigarettes (≥100), snuff (≥20 times), and/or chewing tobacco (≥20 times).
>7 drinks/wk for women or >14 drinks/wk for men.
CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Dsiease Epidemiology Collaboration; CV, cardiovascular; PIR, poverty index ratio; NHANES, National Health and Nutrition Examination Survey; suppl, supplement
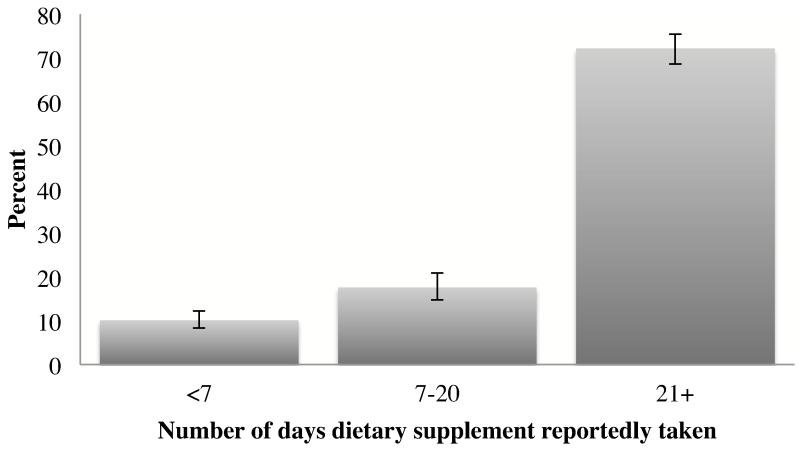
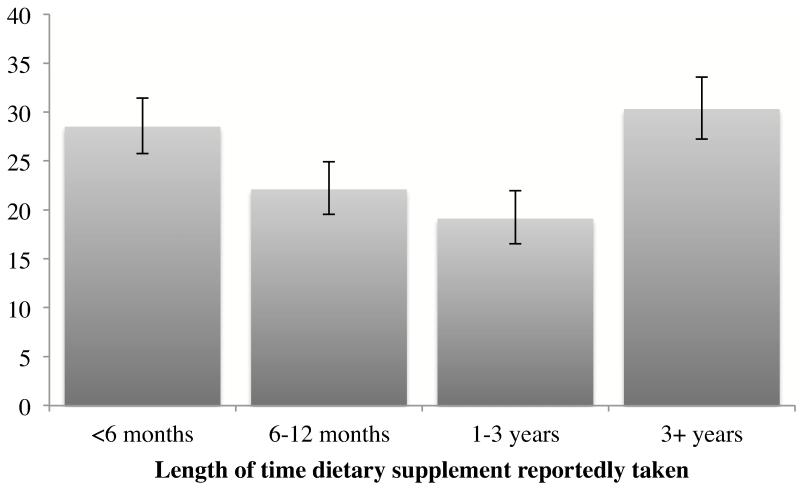
The vast majority of participants taking a potentially harmful supplement had done so nearly every day within the past month (Figure 1) and the frequency of use was not significantly different by CKD status (as defined by CKD-EPI equation, p=0.3; or by MDRD Study equation, p=0.1). Nearly one-third of study participants taking potentially harmful supplements reported doing so for more than 3 years (Figure 2), which was increasingly common with greater CKD severity (as defined by CKD-EPI equation: 24.8% for no CKD, 32.9% for at risk, 40.1% for stage 1/2, and 50.5% for stage 3/4 [p=0.002]; or by MDRD Study equation: 24.7% for no CKD, 32.9% for at risk, 40.8% for stage 1/2, and 45.1% for stage 3/4 [p=0.008]).
Figure 1.
Frequency of supplement use in the last 30 days, among U.S. adults aged ≥20 years reporting potentially harmful supplement use, NHANES 1999-2008
Figure 2.
Duration of supplement use, among U.S. adults aged ≥20 years reporting potentially harmful supplement use, NHANES 1999-2008
Among supplement users (n=10,224), the unadjusted model indicated that persons with or at risk for CKD had a lower likelihood of taking a potentially harmful supplement as compared to those without CKD (Table 4). This finding was attenuated after adjustment for age. Additional adjustment for gender, race/ethnicity, poverty index ratio, educational attainment, co-morbid conditions, tobacco and alcohol use, or number of healthcare visits did not significantly change these findings. Of note, the number of healthcare visits was not a significant independent predictor of taking a potentially harmful supplement. Results were similar when CKD status was defined by the MDRD Study equation (Tables S2-S3).
Table 4.
Odds ratios for taking potentially harmful supplement (vs any other supplement), among dietary supplement users by CKD status
| Model | At risk of CKD (14.7%)* |
CKD stage ½ (12.3%)* |
CKD stage ¾ (9.6%)* |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| unadjusted | 0.82 (0.70-0.95) | 0.01 | 0.67 | 0.51, 0.87 | 0.003 | 0.50 | 0.39, 0.66 | <0.001 | |
| + age group | 0.92 | 0.79, 1.08 | 0.3 | 0.81 | 0.62, 1.05 | 0.1 | 0.84 | 0.63, 1.13 | 0.3 |
| + Sex, race/ethnicity | 0.92 | 0.79, 1.08 | 0.3 | 0.81 | 0.62, 1.06 | 0.1 | 0.85 | 0.63, 1.15 | 0.3 |
| + education, income | 0.93 | 0.79, 1.09 | 0.3 | 0.82 | 0.63, 1.08 | 0.2 | 0.87 | 0.64, 1.18 | 0.4 |
| + arthritis, cancer, tobacco and alcohol use, healthcare visits | 0.93 | 0.79, 1.09 | 0.4 | 0.83 | 0.64, 1.08 | 0.2 | 0.87 | 0.63, 1.18 | 0.4 |
Note: n=10,224; based on NHANES 1999-2008. CKD status defined by CKD Epidemiology Collaboration equation. Reference group is no CKD (which, among dietary supplement users, had a 17.4% point-estimated prevalence of potentially harmful supplement use).
percentage refers to the point-estimated prevalence of potentially harmful supplement use among dietary supplement users in this CKD category.
CKD=chronic kidney disease; OR=odds ratio. CI, confidence interval; NHANES, National Health and Nutrition Examination Survey
DISCUSSION
Dietary supplements are widely used in the United States, despite their potential for harmful effects.18 Individuals with or at risk for CKD may be particularly vulnerable to harmful effects of supplement use through direct nephrotoxicity and other renal complications, as well as decreased clearance of substances, resulting in adverse product accumulation. To our knowledge, our study is the first to describe the use of dietary supplements potentially harmful in persons with and at risk for CKD in a nationally representative sample. Similar to the findings in other studies,5,6 we found that more than half of U.S. adults (age ≥20 years) reported taking any supplement within the 30 days prior to the survey. We show that roughly 1 in 12 U.S. adults is taking at least one supplement that is potentially harmful in persons with kidney disease and that those with or at risk for CKD have a similar likelihood of taking such supplements when compared to those without CKD, after accounting for important confounders. Certainly, our findings that potentially harmful supplements are frequently marketed under seemingly benign product names (such as multivitamins) and by trusted manufacturers, as well as that most individuals report taking them nearly every day and for prolonged periods of time, underscore the scope of this issue.
The lack of variability by CKD status in taking potentially harmful supplements may in part be due to unawareness of CKD. An estimated 80%-90% of individuals with substantially decreased kidney function are unaware of their CKD.19,20 Further, most individuals with CKD may be unaware they are at increased risk of harm, given that consumers often assume “natural” products are safe and beneficial to health.21 Unawareness regarding potential harm of supplements may also be attributed to the lack of rigorous pre-marketing regulation and safety testing. With the passage of the Dietary Supplement Health and Education Act in 1994 (which classified supplements as a subcategory of food, rather than a drug) 22 manufacturers were permitted to market supplement products directly to consumers without submitting proof of safety or efficacy to the U.S. Food and Drug Administration. Consequently, marketing of these products often includes information that is inaccurate and possibly deceptive.23-25 Furthermore, products are often not available in reliable or consistent potencies and dosages, making research on safety or efficacy extremely difficult.26
While aristolochic acid nephropathy, a rapidly progressive interstitial fibrosis of the kidneys frequently leading to end-stage renal disease and urothelial carcinomas, is one of the most dramatic and highly cited examples of herb-induced nephrotoxicity,27,28 the ingestion of more common herbs contained in supplements may be an underappreciated source of nephrotoxicity or other adverse effects of particular concern in those at risk for or with advanced CKD. For example, dietary supplements containing herbs that increase blood pressure or worsen glycemic control may indirectly lead to or worsen existing CKD. Dietary supplements containing herbs that induce hypoglycemia or hyperkalemia may be of particular risk for those with advanced CKD. Older individuals and those with concomitant medication use may be particularly vulnerable.3 Similarly, dietary supplements containing herbs that lead to diarrhea and vomiting may cause decreased kidney perfusion that results in acute kidney injury, an established CKD risk factor.29,30 Since patients may be less likely to attribute harmful effects to dietary supplements,21 there may be delays in diagnosing the etiology of such complications.
Interestingly, we found that the prevalence of both potentially harmful and other supplement use was higher with higher number of healthcare visits. We expected that those with more healthcare visits would have a lower prevalence of potentially harmful supplement use because of more opportunities for healthcare providers to assess and advise against potentially harmful ingestions. This contradictory finding may be explained by people with chronic conditions having more frequent healthcare encounters and higher likelihood of complementary and alternative medicine use than those without a chronic condition15 but may also be a reflection of provider recommendations supporting the patient’s use of generally accepted supplements (e.g., vitamin D and calcium) but lack of awareness regarding the patient’s use of potentially harmful supplements or CKD status. Provider unawareness may be due to a failure to ascertain whether the patient is taking other supplements, coupled with provider unawareness of dietary supplement safety31 and purposeful patient non-disclosure. This assertion is supported by our finding that the number of healthcare visits did not affect the likelihood of taking a potentially harmful supplement but was an independent predictor for taking other dietary supplements (data not shown). In a national health survey of supplement users, only 33% of individuals---and 51% of individuals with chronic conditions---reported disclosing this use to their primary healthcare provider,32 possibly due to skepticism of provider knowledge and attitude toward supplements.21
Prior research has shown that those with higher educational attainment and income are more likely to use supplements, possibly because these individuals are more knowledgeable regarding purported supplement indications and have greater disposable income for such purchases than less well-educated or affluent Americans.33 In our study, the finding that prevalence of supplement use potentially harmful in kidney disease was also higher among adults with higher educational attainment and income may suggest a general lack of awareness of the risk these supplements may impose.
We recognize limitations of our study. First, because the NKF website is a prominent resource for public health education about kidney health, we chose to investigate only the herbs listed on the website as it may have served as a possible deterrent for potentially harmful supplement use for those with CKD. However, this list is unlikely to be exhaustive. For example, the list did not include acai berry, an herb touted for weight loss but has COX-1 and COX-2 inhibitory action34 like certain non-steroidal anti-inflammatory drugs, which are associated with acute kidney injury in the general population35 and with disease progression among those with CKD.36 Further, the NHANES question to ascertain supplement use does not specifically include teas, which may be important but under-recognized sources of potentially harmful herbs. Therefore, our finding that roughly 14% of reported supplements are potentially harmful in the setting of CKD is likely conservative. A more comprehensive list of herbs may have resulted in even higher estimates. On the other hand, of herbs included on the NKF list and reported among our study population, broom was the only herb for which we found no associated literature for adverse renal effects. Therefore, our estimate of prevalence is unlikely to be an underestimation of prevalent use of potentially harmful supplements.
Similarly, we acknowledge that some herbs included in the NKF list may have potential benefits. Senna, for example, is widely prescribed for constipation and a recent uncontrolled pilot trial found wormwood significantly reduced proteinuria in 10 patients with IgA nephropathy.37 However given the lack of consistency between products,26,38 the lack of rigorous safety or efficacy testing, and tendency for patients to believe people are rarely or never harmed by supplements,21 it is important to raise awareness of potential harm.
Moreover, we did not examine dosages of supplements taken and, therefore, cannot conclude that individuals taking potentially harmful supplements are taking enough to do significant harm. Given the wide variety of documented discrepancies in product label claims and actual content in addition to possible undisclosed contaminations,38,39 true quantitation and comparison of dosages would be impossible without direct product analysis. Regardless, it is important to note that a substantial proportion of our study population reported taking supplements over many years, possibly placing themselves at risk of cumulative effects. Consistent with this possibility, we found that individuals with CKD were more likely to report long-term supplement use than those with preserved kidney function. While we are unable to determine causality due to study design, this association highlights the need for further examination of the longitudinal relationship between supplement use and kidney function.
In conclusion, the use of dietary supplements potentially harmful in the setting of CKD is common regardless of CKD status, even after accounting for confounders. Further study of a more comprehensive list of potentially harmful herbs and repeated measures to determine the actual risk to kidney function or other organ systems resulting from supplement use is needed. Nevertheless, this study supports the recommendation that providers vigilantly ask patients about all ingestions and appropriately advise patients about potential risks within the context of their CKD status.
Supplementary Material
Acknowledgements
The Centers for Disease Control and Prevention (CDC) CKD Surveillance Team consists of members groups led by University of California, San Francisco (Neil Powe [PI], Laura Plantinga, Chi-yuan Hsu, Kirsten Bibbins-Domingo, Deidra Crews, Vanessa Grubbs, Delphine Tuot, Tanushree Banerjee, Annie Rein-Weston), University of Michigan (Rajiv Saran [PI], Elizabeth Hedgeman, Brenda Gillespie, William Herman, Friedrich Port, Bruce Robinson, Vahakn Shahinian, Jerry Yee, Eric Young, William McClellan, Ann O’Hare, Anca Tilea), and CDC (Desmond Williams [Technical Advisor], Nilka Ríos Burrows, Mark Eberhardt, Paul Eggers, Nicole Flowers, Linda Geiss, Susan Hailpern, Regina Jordan, Juanita Mondeshire, Bernice Moore, Gary Myers, Meda Pavkov, Deborah Rolka, Sharon Saydah, Anton Schoolwerth, Rodolfo Valdez, Larry Waller).
We thank the participants and staff of the NHANES survey.
Support: This project was supported under a cooperative agreement from the CDC, grant number 1U58DP003839-01. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Dr Powe was partially supported by grant K24DK02643 from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). Dr Grubbs was also supported by the NIDDK through a Diversity Supplement to grant R01 DK70939 and by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation. Dr Tuot was supported by award number KL2RR024130 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Supplementary Material
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System . USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and EndStage Renal Disease in the United States. Bethesda: MD2010. [Google Scholar]
- 3.Luyckx VA, Naicker S. Acute kidney injury associated with the use of traditional medicines. Nat Clin Pract Nephrol. 2008 Dec;4(12):664–671. doi: 10.1038/ncpneph0970. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration [Accessed 3/12/12];Dietary Supplements. 2011 http://www.fda.gov/Food/DietarySupplements/default.htm.
- 5.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011 Feb;141(2):261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA : the journal of the American Medical Association. 2002 Jan 16;287(3):337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 7.Akyol AD, Yildirim Y, Toker E, Yavuz B. The use of complementary and alternative medicine among chronic renal failure patients. J Clin Nurs. 2011 Apr;20(7-8):1035–1043. doi: 10.1111/j.1365-2702.2010.03498.x. [DOI] [PubMed] [Google Scholar]
- 8.Nowack R, Balle C, Birnkammer F, Koch W, Sessler R, Birck R. Complementary and alternative medications consumed by renal patients in southern Germany. J Ren Nutr. 2009 May;19(3):211–219. doi: 10.1053/j.jrn.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Duncan HJ, Pittman S, Govil A, et al. Alternative medicine use in dialysis patients: potential for good and bad! Nephron Clin Pract. 2007;105(3):c108–113. doi: 10.1159/000097986. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 1999-2008. National Health and Nutrition Examination Survey Data. [Google Scholar]
- 11.National Health and Nutrition Examination Survey . Analytic and Reporting Guidelines. Centers for Disease Control and Prevention. National Center for Health Statistics; Hyattsville, MD: 2005. [Google Scholar]
- 12.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007 Dec;50(6):918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation [Accessed March 4, 2011];Use of Herbal Supplements in Chronic Kidney Disease. A to Z Health Guide. 2002 http://www.kidney.org/atoz/content/herbalsupp.cfm.
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saydah SH, Eberhardt MS. Use of complementary and alternative medicine among adults with chronic diseases: United States 2002. J Altern Complement Med. 2006 Oct;12(8):805–812. doi: 10.1089/acm.2006.12.805. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007 Apr;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 17.Jellin JM, PJ G. Natural Medicines: Comprehensive Database 2013. 13 ed. Therapeutic Research Faculty; Stockton: 2012. [Google Scholar]
- 18.De Smet PA. Herbal remedies. The New England journal of medicine. 2002 Dec 19;347(25):2046–2056. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 19.Flessner MF, Wyatt SB, Akylbekova EL, et al. Prevalence and awareness of CKD among African Americans: the Jackson Heart Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009 Feb;53(2):238–247. doi: 10.1053/j.ajkd.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plantinga LC, Boulware LE, Coresh J, et al. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med. 2008 Nov 10;168(20):2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blendon RJ, DesRoches CM, Benson JM, Brodie M, Altman DE. Americans’ views on the use and regulation of dietary supplements. Arch Intern Med. 2001 Mar 26;161(6):805–810. doi: 10.1001/archinte.161.6.805. [DOI] [PubMed] [Google Scholar]
- 22.DSHEA (Dietary Supplement Health and Education Act of 1994) Public Law No. 103-417, 108 Stat. 432s, 21 U.S.C. ss. 301 et seq. 19941994.
- 23.Denham BE. Dietary supplements--regulatory issues and implications for public health. JAMA : the journal of the American Medical Association. 2011 Jul 27;306(4):428–429. doi: 10.1001/jama.2011.982. [DOI] [PubMed] [Google Scholar]
- 24.Morris CA, Avorn J. Internet marketing of herbal products. JAMA : the journal of the American Medical Association. 2003 Sep 17;290(11):1505–1509. doi: 10.1001/jama.290.11.1505. [DOI] [PubMed] [Google Scholar]
- 25.Temple NJ. The marketing of dietary supplements in North America: the emperor is (almost) naked. J Altern Complement Med. 2010 Jul;16(7):803–806. doi: 10.1089/acm.2009.0176. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine . Dietary Supplements. Complementary Alternative Medicine in the United States. The National Academies Press; Washington, D.C.: 2005. [Google Scholar]
- 27.Vanherweghem JL, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993 Feb 13;341(8842):387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 28.Depierreux M, Van Damme B, Vanden Houte K, Vanherweghem JL. Pathologic aspects of a newly described nephropathy related to the prolonged use of Chinese herbs. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1994 Aug;24(2):172–180. doi: 10.1016/s0272-6386(12)80178-8. [DOI] [PubMed] [Google Scholar]
- 29.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney international. 2009 Oct;76(8):893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA : the journal of the American Medical Association. 2009 Sep 16;302(11):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 31.Ashar BH, Rice TN, Sisson SD. Medical residents’ knowledge of dietary supplements. South Med J. 2008 Oct;101(10):996–1000. doi: 10.1097/SMJ.0b013e31817cf79e. [DOI] [PubMed] [Google Scholar]
- 32.Mehta DH, Gardiner PM, Phillips RS, McCarthy EP. Herbal and dietary supplement disclosure to health care providers by individuals with chronic conditions. J Altern Complement Med. 2008 Dec;14(10):1263–1269. doi: 10.1089/acm.2008.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bardia A, Nisly N, Zimmerman M, Gryzlak B, Wallace R. Use of herbs among adults based on evidence-based indications: findings from the National Health Interview Survey. Mayo Clin Proc. 2007;82(5):561–566. doi: 10.4065/82.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schauss AG, Wu X, Prior RL, et al. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae mart. (acai) J Agric Food Chem. 2006 Nov 1;54(22):8604–8610. doi: 10.1021/jf0609779. [DOI] [PubMed] [Google Scholar]
- 35.Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005 Mar;45(3):531–539. doi: 10.1053/j.ajkd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Gooch K, Culleton B, Manns B, et al. NSAID use and progression of chronic kidney disease. Am J Med. 2007;120(3):280.e281–287. doi: 10.1016/j.amjmed.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Krebs S, Omer B, Omer TN, Fliser D. Wormwood (Artemisia absinthium) for poorly responsive early-stage IgA nephropathy: a pilot uncontrolled trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010 Dec;56(6):1095–1099. doi: 10.1053/j.ajkd.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Harkey MR, Henderson GL, Gershwin ME, Stern JS, Hackman RM. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr. 2001 Jun;73(6):1101–1106. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- 39.Cole MR, Fetrow CW. Adulteration of dietary supplements. Am J Health Syst Pharm. 2003 Aug 1;60(15):1576–1580. doi: 10.1093/ajhp/60.15.1576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.