Abstract
Homeodomain proteins are crucial transcription factors for cell differentiation, cell proliferation and organ development. Interestingly, their homeodomain signature structure is important for both their DNA-binding and their nucleocytoplasmic trafficking. The accurate nucleocytoplasmic distribution of these proteins is essential for their functions. We summarize information on a) the roles of karyopherins for import and export of homeoproteins, b) the regulation of their nuclear transport during development, and c) the corresponding complexity of homeoprotein nucleocytoplasmic transport signals.
Keywords: Homeodomain, homeoprotein, nuclear transport, importin α, karyopherin β, NLS, NES, development, regulation
Nucleocytoplasmic transport of macromolecules is essential in eukaryotes to regulate gene expression, signal transduction and cell cycle progression [1-7]. Nuclear import and export are signal-dependent. Proteins bearing nuclear localization signals (NLSs) or nuclear export signals (NESs) are recognized by receptors that relocate them from the cytoplasm into the nucleus (or vice versa) via nuclear pore complexes (NPCs). The receptors are in both cases members of the karyopherin β superfamily and are referred to as karyopherins. Nuclear localization signals are categorized into classical NLSs (cNLS) and nonclassical NLSs (ncNLS). cNLSs are characterized by either monopartite (e.g. PKKKRRV from SV40 large T antigen) or bipartite (e.g. KRPAATKKAGQAKKKK from nucleoplasmin) stretches of basic amino acids [8]. Shared characteristics of ncNLSs have not been identified. The best-known ncNLSs are the M9 sequence from heterogeneous nuclear ribonucleoprotein (hnRNP) A1 and the importin-β-binding domain (IBB) of importin α (a protein that is not related to the karyopherin β superfamily) [9]. The best-characterized NES is the so-called “leucine-rich” NES (e.g. LxxLxL). Transport cargoes interact with members of karyopherin β superfamily either directly or as complexes with adaptor proteins such as importin αs. Ran, a small GTPase of the Ras superfamily controls transport due to its asymmetric distribution across the nuclear envelope, with Ran-GTP being concentrated in the nucleus and Ran-GDP being concentrated in the cytoplasm. RanGTP binds the “Ran-binding domain” of karyopherin β superfamily members, thereby regulating their conformation – which governs their affinity for cargo. For nuclear import, RanGTP binding causes karyopherin βs to release their cargoes in the nucleus. For nuclear export, RanGTP stabilizes the interaction of exportins with cargo. These complexes are then translocated through the NPC and the absence of Ran-GTP in the cytoplasm leads to dissociation of the cargo-exportin complexes.
Machinery of nucleocytoplasmic transport
There are 14 members of karyopherin β superfamily in S. cerevisiae and 20 in man [10, 11]. Although their sequence similarities are quite low, their molecular weights range from 95-115 kD and they share the following structural features: an N-terminal Ran-binding domain, NPC-binding sites, and 18~21 HEAT repeats. Karyopherin βs can import cargoes bearing both cNLS and ncNLSs. When cargoes bear a cNLS, the cNLS is recognized by adaptor importin αs. Importin β1 then interacts with the IBB domain of importin α and carries the importin α/β-cargo complex through the NPC. Several karyopherin βs import cargoes by directly recognizing their ncNLSs.
Leucine-rich NESs are recognized by the exportin, Crm1, which is also a member of karyopherin β superfamily [1]. A recent crystal structure shows that the leucine-rich NES occupies a hydrophobic groove between the outer helices of Crm1 HEAT repeats 11 and 12 [12]. Crm1, exportin 5 and exportin T (Xpo-t) export microRNAs, tRNA and rRNPs [13-17]. Although most karyopherin βs appear to function in either import or export, interestingly, three karyopherin βs (Msn5p [18, 19], importin 13 [20-22] and exportin 4 [23]) can transport cargoes both into and out of the nucleus, suggesting that they have a flexible structure, and may - in fact - participate in yet-uncharacterized cyclic transport events.
Importin α (karyopherin α) adaptors contain an N-terminal IBB that binds karyopherin β and a structure comprising ten tandem armadillo repeats [8]. These repeats include cNLS-binding sites. There are six importin α’s in man and five α’s in mice [24, 25]. Each importin α is highly conserved among species [26]. Based on sequence comparisons, importin α’s can be subdivided into three subtypes, one including importin α1, one including importin α3 and α4, and one including importin α5, α6 and α7 [27, 28].
The nuclear pore complex consists of about 30 different nucleoporins (Nups). Structurally and functionally, there are three classes of Nups: “structural Nups” which contribute to overall NPC architecture; “pore membrane proteins“ (Poms), which include a transmembrane domain and could contribute to anchoring the NPC in the nuclear envelope; and “FG-Nups”, which include multiple phenylalanine-glycine (FG), GLFG or FxFG repeat motifs which are interspersed among sequences of varying polarity. The FG-rich domains of Nups are unstructured and are essential for maintaining the NPC permeability barrier [29]. During translocation, the surfaces of karyopherin βs are thought to engage in multiple low-affinity interactions with FG-repeats [30, 31].
Homeoproteins
Homeodomain proteins (homeoproteins) are master control transcription factors that are important for diverse functions in development [32]. Homeoproteins regulate axial patterning, segment or cell identity and proliferation by modulating expression patterns of target genes in a temporal, spatial, and tissue-specific manner. Their name derives from the original identification of proteins that bind Drosophila homeotic loci [33]. The sequence similarity of homeodomains is 50-80% for a given sub-type and 20% among all homeodomain sequences [34]. The homeodomain itself has a 60 residue-long conserved DNA-binding motif (Figure 1). Activities of homeoproteins are regulated by post-translational phosphorylation [35-37] and sumoylation [38]. The subcellular distribution of homeoproteins is critical for their functions. In Drosophila, for example, Extradenticle (Exd) is necessary for proximal leg development, but is not required for distal leg development [39-41]. Accordingly, Exd concentrates in the nucleus in cells that will give rise to proximal leg segments, while it is cytoplasmic in cells with distal leg fates [39-43]. The behavior of Oct6 also exemplifies the same theme. Oct6 is mainly cytoplasmic in undifferentiated ES cells, but localizes increasingly to the nucleus during retinoic acid-induced differentiation, and becomes predominantly nuclear in differentiated neurons, where it is required for function [44].
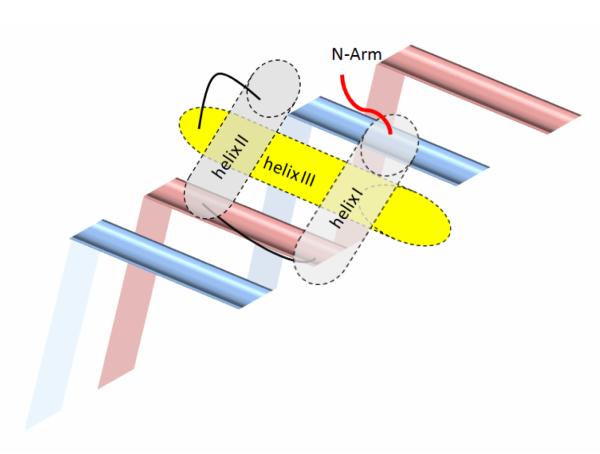
Figure 1. Cartoon of the DNA binding architecture of homeodomains.
Helix III of the homeodomain binds the major groove of DNA, with helix I and II lying outside the double helix. Helix III, as a recognition helix, contains the C-terminal basic cluster that contacts both the phosphate backbone and specific bases. The N-terminal arm containing the N-terminal basic cluster lies in the minor groove and makes additional contacts.
The homeodomain
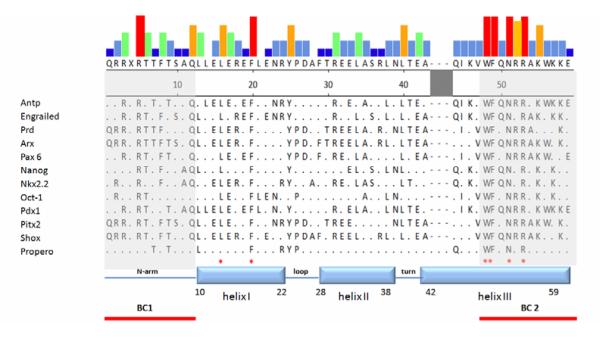
Each homeodomain has an N-terminal flexible arm, a short helix I (a.a.10-22), helix II (a.a. 28-38) linked to helix I by a short loop, and helix III (a.a. 42-59), forming a classical helix-turn-helix motif along with helix II (Figure 2) [32, 34, 45]. Homeodomain-DNA interactions have been identified by NMR and by X-ray crystallography [46-48]. The basic residues preceding the N-terminal arm support contact with the DNA minor groove, and the helix-turn-helix motif binds to the major groove of DNA. Helix III serves as the major helix for interaction with DNA and harbors several highly conserved amino acid residues [32, 49]. In helix III, interactions between the conserved Arg52, Arg53 and DNA have been found in all homeodomains studied to date [32, 48-53]. Interestingly, Arg52 and Arg53 are also mutational hot spots in homeoproteins [49, 54]. Most homeoproteins contain not only a conserved homeodomain but also other functionally-important domains. These domains are either found alone as a DNA-binding motif or in tandem with another module. For example, members of the Pax family have both a “paired domain” and a homeodomain. “Paired domains” contain two DNA binding subdomains named PAI and RED. Both sub-domains include a helix-turn-helix structure [55-57]. The “POU-domain” is derived from three mammalian genes, PIT-l, OCT-l, and OCT-2 and the C. elegans gene Unc-86, which share a region of homology. The POU domain is a bipartite DNA-binding domain, consisting of two highly conserved regions tethered by a variable linker. The 75-amino acid N-terminal region is called the POU-specific domain and the carboxy-terminal 60-amino acid region is called the POU homeodomain [58]. The “Cut domain” contains three highly homologous regions of −70 amino acids, the Cut repeats. Cut repeats are specific DNA binding domains, and Cut repeat lll cooperates with the Cut homeodomain to bind DNA with high affinity [59]. The “LIM domain” is composed of two contiguous zinc finger domains, separated by a two residue-long hydrophobic linker. It functions as a modular protein-binding interface and is named after its initial characterization in Lin11, Isl-1 & Mec-3 [60].
Figure 2. Sequences of homeodomains.
Selected homeoproteins were aligned by the Clustal W program [137] embedded in MegAlign (DNASTAR, Inc). The colors of the top panel represent the frequency of given residues among sequences shown. The red column represents perfectly conserved residues for which there are no exceptions. The yellow and green columns indicate conserved residues with exceptions (frequency around 70%-90%). The grey and blue columns indicate residues that are much less well conserved among the homeoproteins (from high to low: red>yellow>green>grey>blue). Four amino acid residues in helix III and two amino acid residues in helix I indicated by ‘*’ are conserved among all homeoproteins [32, 34, 49]. Note that two basic clusters are located at both ends of the homeodomain. The shaded region between helix II and helix III can form an extended loop for specific types of homeodomains.
Nucleocytoplasmic transport of homeoproteins
Homeodomains include functional NLSs and NESs
Interestingly, many mutations in the homeodomain not only reduce DNA binding but also impair the nuclear localization of homeoproteins, suggesting that the homeodomain overlaps with functional transport signals [49]. Moreover, the basic amino-acid clusters (BCs) at both ends of the homeodomain (Figure 2) structurally resemble classical NLSs and can function as NLSs when coupled to an irrelevant cytoplasmic protein [61, 62]. For the homeoproteins Chx10, Nkx2.5, Oct6, and Otx1, the N-terminal basic clusters (BC1) have been shown to function as a NLS [63-66]. For Pdx1, Pitx2 and Shox2, the C-terminal basic clusters (BC2) function as a NLS [62, 67, 68]. In Arx, Cart1, HB9, Nanog, Nkx2.2, and Pax6, both clusters (BC1/BC2) are required for NLS function [22, 61, 69-72]. As shown in Figure 3A, all the basic amino-acid residues in BC1 can vary among homeoproteins, while several basic amino-acid residues in BC2 are highly conserved (Arg52 and Arg53) (Figure 3B). Indeed these arginine residues are required for both DNA binding and NLS function. Thus, homeoprotein Pitx2 localizes to the cytoplasm when Arg 53 is mutated to Pro [67]; Arx localizes to the cytoplasm when Arg 52 or Arg 53 is mutated [22, 73]; and Nkx2.2 localizes to the cytoplasm upon mutation of Arg 53 when BC1 is absent (unpublished observation) [69]. Studies of Pdx1 [68, 74], Pax6 [70] and Nanog [71] show that helix III of the homeodomain is also important for NLS function.
Figure 3A. Sequences of the N-terminal basic clusters.
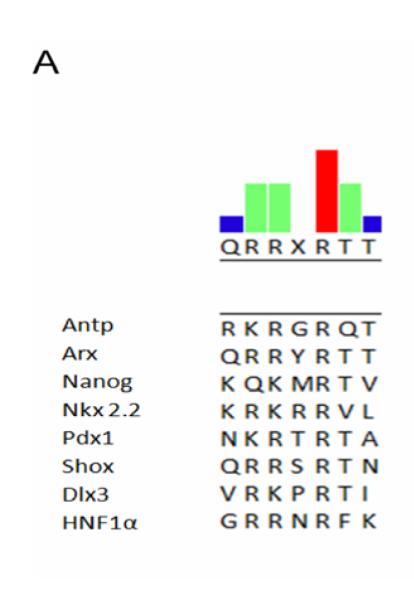
Selected homeoproteins were aligned by the Clustal W program. Note that amino acid R5 in this cluster is highly conserved. Mutation of this amino acid in HNF1α causes it to be sequestered in the cytoplasm [49].
Figure 3B. Sequences of the C-terminal basic cluster.
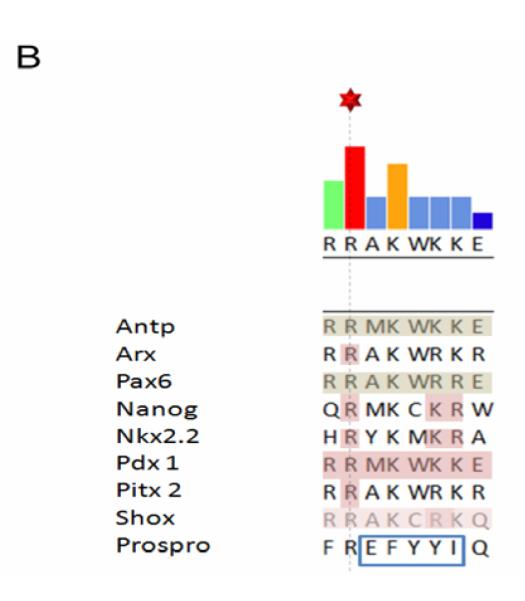
Selected homeoproteins were aligned by the Clustal W program. Note that the shaded region represents identified core residues for NLS function. The boxed region indicates a hydrophobic group of amino acids in the Prospro homeodomain which functions as a NES.
In addition to these basic motifs, some homeoproteins contain at least one additional NLS [22]. For two homeodomain proteins belonging to the Pax family, Pax5 and Pax8, the functional NLSs are located outside their homeodomains [75, 76]. In a rare case, import of homeoprotein Vsx1, a member of Vsx family, was reported to be mediated by its binding partner (Ubc9) and the Vsx1 homeodomain is dispensable for its nuclear localization [77]. By contrast, a functional NLS is found in the homeodomain of the PLC-HD protein, another member of the Vsx family [64].
Nuclear export of homeoproteins has been proposed to regulate their functions [78, 79]. Vax2 is a homeoprotein that ventralizes the vertebrate eye field by repressing transcription of PAX6. Thus, constitutively nuclear Vax2 in the chick optic vesicle results in constitutive repression of PAX6, resulting in the formation of an eyeless embryo [80]. Cytoplasmic retention of Exd, a homeoprotein of the PBC family which includes products of vertebrate PBX1, PBX2, PBX3, Drosophila extradenticle (exd) and C.elegans ceh-20 [81], is critical for patterning the proximal–distal axes of appendages, and for the development of both the eye and antennae in D. melanogaster [82, 83].
NESs have been characterized in several homeoproteins. NESs can overlap with their homeodomains and are either leucine-rich NESs, e.g. in Engrailed and Oct6 [84, 85], or “divergent” leucine-rich NESs, e.g. in Prospero [86, 87] (Figure 3B). NESs are also found outside the homeodomain, e.g. in the “octapeptide” domain of CVC paired-like homeoproteins [64], in the tryptophan-rich region in Nanog [78] and in the PBC-B region of Exd [43, 88]. As expected, export of most of these homeoproteins is sensitive to leptomycin B (LMB), the inhibitor of Crm1-mediated export (Table).
Table 1.
Summary of importin αs and karyopherin βs known to be responsible for nuclear transport of homeoproteins
| Homeoprotein | Homeoprotein functions | Karyopherins/importins with a transport role | Sensitivity to LMB |
|---|---|---|---|
| Arx | early development of multiple tissues [94, 138, 139] | importin β1, 9, 13 [22, 73, 95] importin α3, α5 (unpublished observation) |
|
| Brn2 | differentiation of Schwann cells [140] | importin α5 [90] | |
| Caudal | anteroposterior body axis of Drosophila [97, 98] | Dim7 (importin 7) [99] | |
| Cdx2 | pattern formation in the developing embryo [141] | Yes [130] | |
| Exd (Pbx) | embryogenesis [41, 43, 82, 83] | Yes [134] | |
| Hex | embryonic patterning [115] | importin 7 [117] | |
| Nkx 2.2 | early development of multiple tissues [100, 101] | importin β1, 4, 7, 9, 13 (unpublished observation) | |
| Oct 3/4 | differentiation of neuronal cells [142] | importin α1, α3, α5 [90] | |
| Oct6 | differentiation of neuronal cells [143] | importin α3, α5 [90] | Yes [85, 90] |
| Pax6 | development of CNS system and pancreas [104-109] | importin 13 [70] | |
| Pdx1 | pancreatic cell-type maintaining [110-112] | importin β1 [114] | |
| PLC-HDP | ocular development [144, 145] | Yes [64] | |
| prospero | regulation of cell fate [146] | Yes [86, 87] | |
| Yox1p | regulating G1/S transition [91, 92] | importin α [92] |
Nuclear import of homeoproteins via their homeodomains is mediated by diverse karyopherin βs
Being transcription factors, homeoproteins must be in the nucleus at the correct time. Mislocalization can be catastrophic. For example, inhibition of Bcd (bicoid) import can result from mutation of the Drosophila semushi (semi) gene, which encodes an E2 enzyme that modifies the NLS of Bcd. Inhibition of Bcd import results in multiple defects in anterior segmentation of embryos [89].
NLSs within homeodomains can be recognized either by importin αs with importin β1 or directly by karyopherin βs. Import of Brn2, Oct3/4 and Oct6 in mouse ES cells provides examples of import via the classical import pathway [90]. Moreover, in fission yeast, homeoprotein Yox1p, which regulates transcription during G1/S [91], relocates to the cytoplasm when importin α/Srp1p is mutated [92], suggesting that the classical importin α/β pathway is involved. Additionally, several homeoproteins (e.g. Arx, Pax6) are recognized directly by karyopherin βs and cannot be imported by any importin α. Given the approximate constancy of structure of the homeodomain itself, the diversity of import pathways suggests that the structures of NLSs of homeodomains could be modulated by flanking sequences or post-translational modifications. The following is a summary of nuclear import of several key homeoproteins and their transport receptors (Table 1):
Arx
The aristaless-related homeobox protein (Arx) is a paired-like homeoprotein that is predominantly expressed in the brain [93]. It is important for the development of the forebrain, testis and pancreas [94]. Both basic amino-acid clusters (BC1, BC2) cooperate to form a functional NLS which is targeted by importin β1, importin 9 and importin 13, but not by importin αs [22, 73, 95]. Interestingly, importin β1 mainly interacts with BC2 but importin 13 prefers binding to BC1. Using in vitro nuclear import analysis, GST-pull downs and interfering small RNAs, importin β1 was found to play a major role in import of Arx. Arg53 (R382) in its homeodomain is a core amino acid for recognition by importin β1 [22]. Curiously, our unpublished observations show that in vitro expressed homeodomains from Arx can interact with importin αs. Thus, import of β-galactosidase-EGFP tagged with either BC1 or BC2 of the Arx homeodomain is inhibited by Bimax, a specific inhibitor of the importin α/β pathway [96]. Nevertheless, the subcellular distribution of wild type Arx is not affected by Bimax, showing that the classical import pathway is not the principal pathway for Arx import.
Caudal
Caudal regulates the anteroposterior body axis of Drosophila [97, 98]. The moleskin protein DIM7 (Drosophila homologue of importin-7) binds BC2. Moreover, RNA interference of moleskin inhibits Caudal nuclear localization, suggesting that moleskin mediates its import [99].
Nkx2.2
Nkx2.2 regulates development of multiple tissues [100, 101]. Both basic clusters of its homeodomain are functional NLSs, but each is inefficient for nuclear localization of Nkx2.2 [69]. We observe that, although intact Nkx2.2 binds importin α1, α3 and α5 in GST-pull down assays, nuclear import of wildtype Nkx2.2 is not affected by Bimax, suggesting that its import is normally mediated by nonclassical pathways. Multiple karyopherin βs such as importin β1, importin 4 and importin 13 interact with the homeodomain of Nkx2.2 and can import Nkx2.2 in in vitro nuclear import assays (unpublished observation).
Pax6
Pax6, encoded by PAIRED BOX gene 6, was the first paired-type homeoprotein to be identified [102, 103]. This highly-conserved vertebrate transcription factor is important for development of multiple tissues including the CNS, eyes and pancreas [104-109]. The NLS of the Pax6 homeodomain includes both BC1 and BC2 and is recognized by importin 13 [70]. Deletion of either cluster dramatically reduces the interaction between Pax6 and importin 13 as well as nuclear localization of Pax6 in in vitro nuclear import assays. Interestingly, neither importin α/β nor importin β1 imports Pax6 efficiently. Two other members of the Pax family, Pax3 and Crx, are also imported by importin 13 [70]. In in vitro assays, importin αs can directly interact with basic amino-acid clusters of exogenously expressed homeodomains of Pax proteins [75]. There is, however, no direct evidence that nuclear import of Pax proteins is mediated by importin α/β [75].
Pdx1
Pdx1 (Pancreatic duodenal homeobox-1) is essential for pancreatic development [110-112]. It rapidly accumulates in the nucleoplasm when cells are stimulated with glucose, insulin or sodium arsenite [113]. Importin β1 itself binds strongly to the Pdx1 homeodomain and microinjection of MIN6 cells with an antibody to importin β1 maintains Pdx1 in the cytoplasm [114], suggesting that nuclear import of Pdx1 is mediated mainly by importin β1.
PRH/Hex
PRH/Hex (proline-rich homeobox/hematopoietically expressed homeobox) plays an important role in early embryonic patterning and hematopoiesis and has been reported to act as both a tumor suppressor and as an oncoprotein [115]. Aberrant exclusion of PRH/Hex from the nucleus has been associated with thyroid and breast cancers and a subset of myeloid leukemia. Interestingly, nuclear localization of PRH is necessary for the inhibition of eIF4E-dependent transformation [116]. Importin 7 (imp7) is a direct binding partner for PRH/Hex and the imp7-PRH complex dissociates in the presence of RanGTP, as expected for a nuclear import complex. Imp7 mediates the import of PRH/Hex in digitonin-permeabilized cells and in vivo depletion of imp7 dramatically reduces the accumulation of PRH/Hex in the nucleus [117].
Regulation of nuclear transport of homeoproteins by multiple mechanisms
Nucleocytoplasmic transport of homeoproteins such as Exd, Otx1 and Pdx1 is regulated in development and this regulation is essential for their functions [82, 113, 118, 119]. The subcellular localization of homeoproteins depends on expression of specific karyopherins, on post-translational modifications of homeoproteins, and on interactions of homeoproteins with additional proteins (and perhaps DNA-binding sites).
Expression of importin αs/karyopherin βs [7, 10, 120-123]
Metazoans express multiple karyopherin αs that control cell differentiation [5] and development [5, 26, 90, 124-126]. Good examples are found during mammalian spermatogenesis [127] and in mouse ES cell differentiation into neurons, which is controlled by Brn2, Oct3/4, Oct6 and Sox2 [90]. Brn2, Oct6 and Sox2 are imported only by importin α3 and/or α5, not by importin α1. By contrast, import of Oct3/4 is mediated by importin α1. A switch in expression of importin α subtype in ES cells thus determines neuronal differentiation [90]: only importin α1 is expressed in undifferentiated ES cells, so Brn2, Oct6 and Sox2 remain in the cytoplasm, while Oct3/4 is in the nucleus. When cells start to differentiate, importin α1 is down-regulated and importin α3 and α5 are upregulated. Therefore, nuclear import of Oct3/4 is blocked but Brn2, Oct6, and Sox2 are imported. Nuclear Oct3/4 prevents differentiation, while nuclear Brn2, Oct6 and Sox2 promote neuronal differentiation.
Expression of karyopherin βs is also regulated [10, 122, 128] and is crucial for regulation of nucleocytoplasmic transport [7, 10, 121]. Although there is no direct evidence that they regulate the subcellular distribution of Arx, we observe that importin β1 and importin 13 import Arx through different mechanisms, suggesting that expression of different karyopherin βs could regulate Arx import and its functions in different contexts [22].
Phosphorylation
Phosphorylation regulates the subcellular localization of homeoproteins [80, 113, 129, 130]. One example is that of Vax2, a homeoprotein that as mentioned, ventralizes the vertebrate eye field by repressing transcription of PAX6. The subcellular localization of Vax2 is controlled by phosphorylation of serine 170. Wildtype Vax2 is in the nucleus but phosphorylation of S170 results in the exclusion of Vax2 from the nucleus. Exclusion likely reflects inactivation of its NLS – since expression of a nonphosphorylatable, constitutively nuclear Vax2 protein in the chick optic vesicle results in constitutive repression of PAX6, and leads to the formation of an eyeless embryo [80].
Exposure/concealment of transport motifs
The indirect masking of NLS/NES sequences of homeoproteins by association with other proteins can regulate their nucleocytoplasmic distribution [123, 131]. A good example is provided by Drosophila Exd, that is critical for embryogenesis [82, 83]. Exd localization correlates perfectly with the expression of a second homeoprotein, Homothorax (Hth) [42, 132, 133]. Both Hth and Exd are necessary for proximal leg development but are not required for distal leg development [39-41]. In the absence of Hth, Exd is cytoplasmic, but the co-expression of Hth causes it to localize to the nucleus. Exd has two NLSs in its homeodomain and one NES in its PBC-B domain, a region of ~90 amino acids located between the PBC-A domain and the homeodomain. Hth binds Exd through its PBC-A domain. When Hth is present, the NES of Exd is masked and Exd is nuclear. When Hth is absent, the NES is bound by Crm1 and Exd is cytoplasmic [43, 79, 88, 133, 134]. Although Hth regulates the nuclear localization of Exd, the nuclear export of its mammalian homolog, Pbx, appears to be regulated by a fragment of murine nonmuscle myosin II heavy chain B, which conceals its NLSs [135]. Moreover, the N-terminal fragment of Pbx appears to interact with its homeodomain and mask its NLS. Thus, when Hth binds Pbx to release this interaction, the NLS of Pbx is exposed and Pbx is imported [136].
Conclusion
Given the diversity of structure and DNA-binding specificity of homeoproteins, it is not surprising that their nucleocytoplasmic transport is crucial in many biological contexts including cell differentiation, proliferation and tissue development. It is especially striking that their nuclear import is primarily mediated by NLSs that overlap the two basic clusters at the margins of homeodomains that bind DNA. Presumably, when they do bind DNA, these homeoproteins – like most import cargoes – will already have been efficiently released from their corresponding karyopherins. This functional overlap nevertheless could restrict sequence options at these sites. These options in turn could dictate the identity of the karyopherins that can be used.
A further possible spatial restriction that may limit interaction with transport factors is the tendency of homeoproteins to form homo- or heterodimers, as well as their interactions with additional proteins that modulate their transcriptional potency. An area for continued cell biological interest will be clarification of whether such protein-protein interactions occur prior to import. Especially when the cargo as well as the transport factors can be subject to post-translational modifications, the interplay between DNA-binding and nucleocytoplasmic transport – in light of the importance of proper nucleocytoplasmic distribution of homeoproteins for development – poses an intriguing challenge for coordinated evolution.
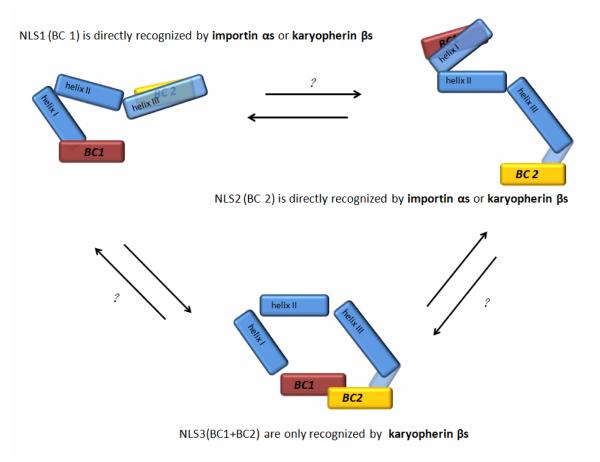
According to the homeoproteins under consideration, functional NLSs can be either in the N-terminal basic cluster (BC1/NLS1), in the C-terminal basic cluster (BC2/NLS2), or require both BC1 and BC2 (NLS3). Both BC1 and BC2 can be targeted by either importin αs or karyopherin βs, and both the classical and nonclassical pathways can mediate nuclear import of homeoproteins. When import of homeoproteins requires both BC1 and BC2, direct interaction with karyopherin βs is involved (Figure 4). Although it is too soon for conclusive comment, it is already seems surprising that one of the few “bidirectional” karyopherins, importin 13, imports several homeoproteins. At present, there is no evidence that importin 13 also participates in their export. For those homeoproteins that have been studied, export is mediated by leucine-rich NESs (that are recognized by Crm1), some of which also overlap with homeodomains.
Figure 4. Model of nuclear import of homeoproteins mediated by the homeodomain.
There are two basic amino-acid clusters (BC1 and BC2) at the ends of the homeodomain of homeoproteins. There are three forms of NLS found in the homeodomains: both BC1 and BC2 can function as an NLS independently (NLS1 or NLS2). BC1 can function as an NLS (NLS3) in conjunction with BC2. NLS1 and NLS2 can be recognized by either importin αs or karyopherin βs directly but NLS3 is only targeted by karyopherin βs. Therefore, nuclear import of homeoproteins can be mediated by both the classical and the nonclassical pathways. The homeodomain could use only a single NLS in certain conditions. As shown in the top left panel, if BC2 is structurally concealed, BC1/NLS1 could be functional. Alternatively as shown in the top right panel, if BC1 is structurally concealed, its BC2/NLS2 could function. When the homeodomain shows the NLS3 conformation (lower panel), only importin βs interact with NLS3 and nuclear import is mediated by a nonclassical pathway. The causes of possible interconversions among the three different NLS conformations are largely unknown (indicated by question marks).
The entire issue of whether homeoproteins can and do shuttle rapidly in and out of the nucleus is largely untouched. Closely linked to this uncertainty is the question of their stability. Some transcription factors turn over quickly within the nucleus; however, this possibility has not been addressed systematically for homeoproteins.
Given initial indications that the subcellular localization of homeoproteins can be regulated by phosphorylation, it will be of great interest to identify signals that adjust expression of karyopherins, covalently modify homeoproteins and coordinate the interactions of homeoproteins with additional proteins during development.
Acknowledgements
Research in the laboratory of Tao Tao is currently supported by grants from the National Foundation of Science of China (#30971669, #81071670), the Ministry of Science and Technology, China (#2006AA02A310), the Ministry of Education, China (#20090121110014) and the Department of Science & Technology, Fujian Province, China (#2009I0026). Research in the laboratory of Alan Tartakoff is supported by the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tartakoff AM, Tao T. Comparative and evolutionary aspects of macromolecular translocation across membranes. Int J Biochem Cell Biol. 2010;42:214–229. doi: 10.1016/j.biocel.2009.07.013. [DOI] [PubMed] [Google Scholar]
- [2].Adam SA. The nuclear transport machinery in Caenorhabditis elegans: A central role in morphogenesis. Semin Cell Dev Biol. 2009;20:576–581. doi: 10.1016/j.semcdb.2009.03.013. [DOI] [PubMed] [Google Scholar]
- [3].Kelly SM, Corbett AH. Messenger RNA export from the nucleus: a series of molecular wardrobe changes. Traffic. 2009;10:1199–1208. doi: 10.1111/j.1600-0854.2009.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jordan BA, Kreutz MR. Nucleocytoplasmic protein shuttling: the direct route in synapse-to-nucleus signaling. Trends Neurosci. 2009;32:392–401. doi: 10.1016/j.tins.2009.04.001. [DOI] [PubMed] [Google Scholar]
- [5].Yasuhara N, Oka M, Yoneda Y. The role of the nuclear transport system in cell differentiation. Semin Cell Dev Biol. 2009;20:590–599. doi: 10.1016/j.semcdb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- [6].Itman C, Miyamoto Y, Young J, Jans DA, Loveland KL. Nucleocytoplasmic transport as a driver of mammalian gametogenesis. Semin Cell Dev Biol. 2009;20:607–619. doi: 10.1016/j.semcdb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [7].Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- [8].Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH. Classical nuclear localization signals: definition, function, and interaction with importin alpha. J Biol Chem. 2007;282:5101–5105. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McLane LM, Corbett AH. Nuclear localization signals and human disease. IUBMB Life. 2009;61:697–706. doi: 10.1002/iub.194. [DOI] [PubMed] [Google Scholar]
- [10].Quan Y, Ji ZL, Wang X, Tartakoff AM, Tao T. Evolutionary and transcriptional analysis of karyopherin beta superfamily proteins. Mol Cell Proteomics. 2008;7:1254–1269. doi: 10.1074/mcp.M700511-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- [12].Dong X, Biswas A, Suel KE, Jackson LK, Martinez R, Gu H, Chook YM. Structural basis for leucine-rich nuclear export signal recognition by CRM1. Nature. 2009;458:1136–1141. doi: 10.1038/nature07975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Castanotto D, Lingeman R, Riggs AD, Rossi JJ. CRM1 mediates nuclear-cytoplasmic shuttling of mature microRNAs. Proc Natl Acad Sci U S A. 2009;106:21655–21659. doi: 10.1073/pnas.0912384106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bussing I, Yang JS, Lai EC, Grosshans H. The nuclear export receptor XPO-1 supports primary miRNA processing in C. elegans and Drosophila. EMBO J. 2010 doi: 10.1038/emboj.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Okada C, Yamashita E, Lee SJ, Shibata S, Katahira J, Nakagawa A, Yoneda Y, Tsukihara T. A high-resolution structure of the pre-microRNA nuclear export machinery. Science. 2009;326:1275–1279. doi: 10.1126/science.1178705. [DOI] [PubMed] [Google Scholar]
- [16].Okamura K, Hagen JW, Duan H, Tyler DM, Lai EC. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007;130:89–100. doi: 10.1016/j.cell.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bohnsack MT, Regener K, Schwappach B, Saffrich R, Paraskeva E, Hartmann E, Gorlich D. Exp5 exports eEF1A via tRNA from nuclei and synergizes with other transport pathways to confine translation to the cytoplasm. EMBO J. 2002;21:6205–6215. doi: 10.1093/emboj/cdf613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kaffman A, Rank NM, O’Neill EM, Huang LS, O’Shea EK. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature. 1998;396:482–486. doi: 10.1038/24898. [DOI] [PubMed] [Google Scholar]
- [19].Yoshida K, Blobel G. The karyopherin Kap142p/Msn5p mediates nuclear import and nuclear export of different cargo proteins. J Cell Biol. 2001;152:729–740. doi: 10.1083/jcb.152.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mingot JM, Kostka S, Kraft R, Hartmann E, Gorlich D. Importin 13: a novel mediator of nuclear import and export. EMBO J. 2001;20:3685–3694. doi: 10.1093/emboj/20.14.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tao T, Lan J, Lukacs GL, Hache RJ, Kaplan F. Importin 13 regulates nuclear import of the glucocorticoid receptor in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;35:668–680. doi: 10.1165/rcmb.2006-0073OC. [DOI] [PubMed] [Google Scholar]
- [22].Lin W, Ye W, Cai L, Meng X, Ke G, Huang C, Peng Z, Yu Y, Golden JA, Tartakoff AM, Tao T. The roles of multiple importins for nuclear import of murine aristaless-related homeobox protein. J Biol Chem. 2009;284:20428–20439. doi: 10.1074/jbc.M109.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gontan C, Guttler T, Engelen E, Demmers J, Fornerod M, Grosveld FG, Tibboel D, Gorlich D, Poot RA, Rottier RJ. Exportin 4 mediates a novel nuclear import pathway for Sox family transcription factors. J Cell Biol. 2009;185:27–34. doi: 10.1083/jcb.200810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kohler M, Speck C, Christiansen M, Bischoff FR, Prehn S, Haller H, Gorlich D, Hartmann E. Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import. Mol Cell Biol. 1999;19:7782–7791. doi: 10.1128/mcb.19.11.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kohler M, Ansieau S, Prehn S, Leutz A, Haller H, Hartmann E. Cloning of two novel human importin-alpha subunits and analysis of the expression pattern of the importin-alpha protein family. FEBS Lett. 1997;417:104–108. doi: 10.1016/s0014-5793(97)01265-9. [DOI] [PubMed] [Google Scholar]
- [26].Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol. 2004;14:505–514. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- [27].Fagerlund R, Kinnunen L, Kohler M, Julkunen I, Melen K. NF-{kappa}B is transported into the nucleus by importin {alpha}3 and importin {alpha}4. J Biol Chem. 2005;280:15942–15951. doi: 10.1074/jbc.M500814200. [DOI] [PubMed] [Google Scholar]
- [28].Mason DA, Stage DE, Goldfarb DS. Evolution of the metazoan-specific importin alpha gene family. J Mol Evol. 2009;68:351–365. doi: 10.1007/s00239-009-9215-8. [DOI] [PubMed] [Google Scholar]
- [29].Denning DP, Patel SS, Uversky V, Fink AL, Rexach M. Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded. Proc Natl Acad Sci U S A. 2003;100:2450–2455. doi: 10.1073/pnas.0437902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Frey S, Gorlich D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell. 2007;130:512–523. doi: 10.1016/j.cell.2007.06.024. [DOI] [PubMed] [Google Scholar]
- [31].Ribbeck K, Gorlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 2002;21:2664–2671. doi: 10.1093/emboj/21.11.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gehring WJ, Affolter M, Burglin T. Homeodomain proteins. Annu Rev Biochem. 1994;63:487–526. doi: 10.1146/annurev.bi.63.070194.002415. [DOI] [PubMed] [Google Scholar]
- [33].McGinnis W, Levine MS, Hafen E, Kuroiwa A, Gehring WJ. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. Nature. 1984;308:428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- [34].Banerjee-Basu S, Baxevanis AD. Molecular evolution of the homeodomain family of transcription factors. Nucleic Acids Res. 2001;29:3258–3269. doi: 10.1093/nar/29.15.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Seo JH, Jin YH, Jeong HM, Kim YJ, Jeong HG, Yeo CY, Lee KY. Calmodulin-dependent kinase II regulates Dlx5 during osteoblast differentiation. Biochem Biophys Res Commun. 2009;384:100–104. doi: 10.1016/j.bbrc.2009.04.082. [DOI] [PubMed] [Google Scholar]
- [36].Soufi A, Noy P, Buckle M, Sawasdichai A, Gaston K, Jayaraman PS. CK2 phosphorylation of the PRH/Hex homeodomain functions as a reversible switch for DNA binding. Nucleic Acids Res. 2009;37:3288–3300. doi: 10.1093/nar/gkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Janody F, Sturny R, Catala F, Desplan C, Dostatni N. Phosphorylation of bicoid on MAP-kinase sites: contribution to its interaction with the torso pathway. Development. 2000;127:279–289. doi: 10.1242/dev.127.2.279. [DOI] [PubMed] [Google Scholar]
- [38].Shan SF, Wang LF, Zhai JW, Qin Y, Ouyang HF, Kong YY, Liu J, Wang Y, Xie YH. Modulation of transcriptional corepressor activity of prospero-related homeobox protein (Prox1) by SUMO modification. FEBS Lett. 2008;582:3723–3728. doi: 10.1016/j.febslet.2008.09.057. [DOI] [PubMed] [Google Scholar]
- [39].Gonzalez-Crespo S, Morata G. Genetic evidence for the subdivision of the arthropod limb into coxopodite and telopodite. Development. 1996;122:3921–3928. doi: 10.1242/dev.122.12.3921. [DOI] [PubMed] [Google Scholar]
- [40].Casares F, Mann RS. Control of antennal versus leg development in Drosophila. Nature. 1998;392:723–726. doi: 10.1038/33706. [DOI] [PubMed] [Google Scholar]
- [41].Gonzalez-Crespo S, Abu-Shaar M, Torres M, Martinez AC, Mann RS, Morata G. Antagonism between extradenticle function and Hedgehog signalling in the developing limb. Nature. 1998;394:196–200. doi: 10.1038/28197. [DOI] [PubMed] [Google Scholar]
- [42].Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS. Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell. 1997;91:171–183. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- [43].Abu-Shaar M, Ryoo HD, Mann RS. Control of the nuclear localization of Extradenticle by competing nuclear import and export signals. Genes Dev. 1999;13:935–945. doi: 10.1101/gad.13.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ilia M, Bazigou E, Price J. Expression of the POU domain transcription factor, Oct-6, is attenuated in the adult mouse telencephalon, but increased by neurotoxic damage. Exp Neurol. 2003;181:159–169. doi: 10.1016/s0014-4886(03)00047-5. [DOI] [PubMed] [Google Scholar]
- [45].Qian YQ, Billeter M, Otting G, Muller M, Gehring WJ, Wuthrich K. The structure of the Antennapedia homeodomain determined by NMR spectroscopy in solution: comparison with prokaryotic repressors. Cell. 1989;59:573–580. doi: 10.1016/0092-8674(89)90040-8. [DOI] [PubMed] [Google Scholar]
- [46].Genis C, Scone P, Kasahara H, Nam HJ. Crystallization and preliminary X-ray analysis of the NKX2.5 homeodomain in complex with DNA. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64:1079–1082. doi: 10.1107/S1744309108033447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wilson DS, Sheng G, Jun S, Desplan C. Conservation and diversification in homeodomain-DNA interactions: a comparative genetic analysis. Proc Natl Acad Sci U S A. 1996;93:6886–6891. doi: 10.1073/pnas.93.14.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gehring WJ, Qian YQ, Billeter M, Furukubo-Tokunaga K, Schier AF, Resendez-Perez D, Affolter M, Otting G, Wuthrich K. Homeodomain-DNA recognition. Cell. 1994;78:211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- [49].Chi YI. Homeodomain revisited: a lesson from disease-causing mutations. Hum Genet. 2005;116:433–444. doi: 10.1007/s00439-004-1252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu B, Kissinger CR, Pabo CO. Crystallization and preliminary X-ray diffraction studies of the engrailed homeodomain and of an engrailed homeodomain/DNA complex. Biochem Biophys Res Commun. 1990;171:257–259. doi: 10.1016/0006-291x(90)91385-6. [DOI] [PubMed] [Google Scholar]
- [51].Clarke ND, Kissinger CR, Desjarlais J, Gilliland GL, Pabo CO. Structural studies of the engrailed homeodomain. Protein Sci. 1994;3:1779–1787. doi: 10.1002/pro.5560031018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wolberger C. Homeodomain interactions. Curr Opin Struct Biol. 1996;6:62–68. doi: 10.1016/s0959-440x(96)80096-0. [DOI] [PubMed] [Google Scholar]
- [53].Billeter M. Homeodomain-type DNA recognition. Prog Biophys Mol Biol. 1996;66:211–225. doi: 10.1016/s0079-6107(97)00006-0. [DOI] [PubMed] [Google Scholar]
- [54].D’Elia AV, Tell G, Paron I, Pellizzari L, Lonigro R, Damante G. Missense mutations of human homeoboxes: A review. Hum Mutat. 2001;18:361–374. doi: 10.1002/humu.1207. [DOI] [PubMed] [Google Scholar]
- [55].Czerny T, Schaffner G, Busslinger M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993;7:2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- [56].Noll M. Evolution and role of Pax genes. Curr Opin Genet Dev. 1993;3:595–605. doi: 10.1016/0959-437x(93)90095-7. [DOI] [PubMed] [Google Scholar]
- [57].Miskiewicz P, Morrissey D, Lan Y, Raj L, Kessler S, Fujioka M, Goto T, Weir M. Both the paired domain and homeodomain are required for in vivo function of Drosophila Paired. Development. 1996;122:2709–2718. doi: 10.1242/dev.122.9.2709. [DOI] [PubMed] [Google Scholar]
- [58].Ryan AK, Rosenfeld MG. POU domain family values: flexibility, partnerships, and developmental codes. Genes Dev. 1997;11:1207–1225. doi: 10.1101/gad.11.10.1207. [DOI] [PubMed] [Google Scholar]
- [59].Coqueret O, Berube G, Nepveu A. The mammalian Cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO J. 1998;17:4680–4694. doi: 10.1093/emboj/17.16.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kadrmas JL, Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol. 2004;5:920–931. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- [61].Furukawa K, Iioka T, Morishita M, Yamaguchi A, Shindo H, Namba H, Yamashita S, Tsukazaki T. Functional domains of paired-like homeoprotein Cart1 and the relationship between dimerization and transcription activity. Genes Cells. 2002;7:1135–1147. doi: 10.1046/j.1365-2443.2002.00587.x. [DOI] [PubMed] [Google Scholar]
- [62].Sabherwal N, Schneider KU, Blaschke RJ, Marchini A, Rappold G. Impairment of SHOX nuclear localization as a cause for Leri-Weill syndrome. J Cell Sci. 2004;117:3041–3048. doi: 10.1242/jcs.01152. [DOI] [PubMed] [Google Scholar]
- [63].Kasahara H, Izumo S. Identification of the in vivo casein kinase II phosphorylation site within the homeodomain of the cardiac tisue-specifying homeobox gene product Csx/Nkx2.5. Mol Cell Biol. 1999;19:526–536. doi: 10.1128/mcb.19.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Knauer SK, Carra G, Stauber RH. Nuclear export is evolutionarily conserved in CVC paired-like homeobox proteins and influences protein stability, transcriptional activation, and extracellular secretion. Mol Cell Biol. 2005;25:2573–2582. doi: 10.1128/MCB.25.7.2573-2582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sock E, Enderich J, Rosenfeld MG, Wegner M. Identification of the nuclear localization signal of the POU domain protein Tst-1/Oct6. J Biol Chem. 1996;271:17512–17518. doi: 10.1074/jbc.271.29.17512. [DOI] [PubMed] [Google Scholar]
- [66].Zhang YA, Okada A, Lew CH, McConnell SK. Regulated nuclear trafficking of the homeodomain protein otx1 in cortical neurons. Mol Cell Neurosci. 2002;19:430–446. doi: 10.1006/mcne.2001.1076. [DOI] [PubMed] [Google Scholar]
- [67].Kozlowski K, Walter MA. Variation in residual PITX2 activity underlies the phenotypic spectrum of anterior segment developmental disorders. Hum Mol Genet. 2000;9:2131–2139. doi: 10.1093/hmg/9.14.2131. [DOI] [PubMed] [Google Scholar]
- [68].Hessabi B, Ziegler P, Schmidt I, Hessabi C, Walther R. The nuclear localization signal (NLS) of PDX-1 is part of the homeodomain and represents a novel type of NLS. Eur J Biochem. 1999;263:170–177. doi: 10.1046/j.1432-1327.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- [69].Hessabi B, Schmidt I, Walther R. The homeodomain of Nkx2.2 carries two cooperatively acting nuclear localization signals. Biochem Biophys Res Commun. 2000;270:695–700. doi: 10.1006/bbrc.2000.2491. [DOI] [PubMed] [Google Scholar]
- [70].Ploski JE, Shamsher MK, Radu A. Paired-type homeodomain transcription factors are imported into the nucleus by karyopherin 13. Mol Cell Biol. 2004;24:4824–4834. doi: 10.1128/MCB.24.11.4824-4834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Do HJ, Lim HY, Kim JH, Song H, Chung HM. An intact homeobox domain is required for complete nuclear localization of human Nanog. Biochem Biophys Res Commun. 2007;353:770–775. doi: 10.1016/j.bbrc.2006.12.100. [DOI] [PubMed] [Google Scholar]
- [72].Kosaka Y, Akimoto Y, Yokozawa K, Obinata A, Hirano H. Localization of HB9 homeodomain protein and characterization of its nuclear localization signal during chick embryonic skin development. Histochem Cell Biol. 2004;122:237–247. doi: 10.1007/s00418-004-0698-5. [DOI] [PubMed] [Google Scholar]
- [73].Shoubridge C, Tan MH, Fullston T, Cloosterman D, Coman D, McGillivray G, Mancini GM, Kleefstra T, Gecz J. Mutations in the nuclear localization sequence of the Aristaless related homeobox; sequestration of mutant ARX with IPO13 disrupts normal subcellular distribution of the transcription factor and retards cell division. Pathogenetics. 2010;3:1. doi: 10.1186/1755-8417-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Moede T, Leibiger B, Pour HG, Berggren P, Leibiger IB. Identification of a nuclear localization signal, RRMKWKK, in the homeodomain transcription factor PDX-1. FEBS Lett. 1999;461:229–234. doi: 10.1016/s0014-5793(99)01446-5. [DOI] [PubMed] [Google Scholar]
- [75].Kovac CR, Emelyanov A, Singh M, Ashouian N, Birshtein BK. BSAP (Pax5)-importin alpha 1 (Rch1) interaction identifies a nuclear localization sequence. J Biol Chem. 2000;275:16752–16757. doi: 10.1074/jbc.M001551200. [DOI] [PubMed] [Google Scholar]
- [76].Poleev A, Okladnova O, Musti AM, Schneider S, Royer-Pokora B, Plachov D. Determination of functional domains of the human transcription factor PAX8 responsible for its nuclear localization and transactivating potential. Eur J Biochem. 1997;247:860–869. doi: 10.1111/j.1432-1033.1997.00860.x. [DOI] [PubMed] [Google Scholar]
- [77].Kurtzman AL, Schechter N. Ubc9 interacts with a nuclear localization signal and mediates nuclear localization of the paired-like homeobox protein Vsx-1 independent of SUMO-1 modification. Proc Natl Acad Sci U S A. 2001;98:5602–5607. doi: 10.1073/pnas.101129698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chang DF, Tsai SC, Wang XC, Xia P, Senadheera D, Lutzko C. Molecular characterization of the human NANOG protein. Stem Cells. 2009;27:812–821. doi: 10.1634/stemcells.2008-0657. [DOI] [PubMed] [Google Scholar]
- [79].Affolter M, Marty T, Vigano MA. Balancing import and export in development. Genes Dev. 1999;13:913–915. doi: 10.1101/gad.13.8.913. [DOI] [PubMed] [Google Scholar]
- [80].Kim JW, Lemke G. Hedgehog-regulated localization of Vax2 controls eye development. Genes Dev. 2006;20:2833–2847. doi: 10.1101/gad.1462706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Burglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25:4173–4180. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mann RS, Abu-Shaar M. Nuclear import of the homeodomain protein extradenticle in response to Wg and Dpp signalling. Nature. 1996;383:630–633. doi: 10.1038/383630a0. [DOI] [PubMed] [Google Scholar]
- [83].Aspland SE, White RA. Nucleocytoplasmic localisation of extradenticle protein is spatially regulated throughout development in Drosophila. Development. 1997;124:741–747. doi: 10.1242/dev.124.3.741. [DOI] [PubMed] [Google Scholar]
- [84].Maizel A, Bensaude O, Prochiantz A, Joliot A. A short region of its homeodomain is necessary for engrailed nuclear export and secretion. Development. 1999;126:3183–3190. doi: 10.1242/dev.126.14.3183. [DOI] [PubMed] [Google Scholar]
- [85].Baranek C, Sock E, Wegner M. The POU protein Oct-6 is a nucleocytoplasmic shuttling protein. Nucleic Acids Res. 2005;33:6277–6286. doi: 10.1093/nar/gki947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bi X, Kajava AV, Jones T, Demidenko ZN, Mortin MA. The carboxy terminus of Prospero regulates its subcellular localization. Mol Cell Biol. 2003;23:1014–1024. doi: 10.1128/MCB.23.3.1014-1024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Demidenko Z, Badenhorst P, Jones T, Bi X, Mortin MA. Regulated nuclear export of the homeodomain transcription factor Prospero. Development. 2001;128:1359–1367. doi: 10.1242/dev.128.8.1359. [DOI] [PubMed] [Google Scholar]
- [88].Stevens KE, Mann RS. A balance between two nuclear localization sequences and a nuclear export sequence governs extradenticle subcellular localization. Genetics. 2007;175:1625–1636. doi: 10.1534/genetics.106.066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Epps JL, Tanda S. The Drosophila semushi mutation blocks nuclear import of bicoid during embryogenesis. Curr Biol. 1998;8:1277–1280. doi: 10.1016/s0960-9822(07)00538-6. [DOI] [PubMed] [Google Scholar]
- [90].Yasuhara N, Shibazaki N, Tanaka S, Nagai M, Kamikawa Y, Oe S, Asally M, Kamachi Y, Kondoh H, Yoneda Y. Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat Cell Biol. 2007;9:72–79. doi: 10.1038/ncb1521. [DOI] [PubMed] [Google Scholar]
- [91].Aligianni S, Lackner DH, Klier S, Rustici G, Wilhelm BT, Marguerat S, Codlin S, Brazma A, de Bruin RA, Bahler J. The fission yeast homeodomain protein Yox1p binds to MBF and confines MBF-dependent cell-cycle transcription to G1-S via negative feedback. PLoS Genet. 2009;5:e1000626. doi: 10.1371/journal.pgen.1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Pulliam KF, Fasken MB, McLane LM, Pulliam JV, Corbett AH. The classical nuclear localization signal receptor, importin-alpha, is required for efficient transition through the G1/S stage of the cell cycle in Saccharomyces cerevisiae. Genetics. 2009;181:105–118. doi: 10.1534/genetics.108.097303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bienvenu T, Poirier K, Friocourt G, Bahi N, Beaumont D, Fauchereau F, Jeema L. Ben, Zemni R, Vinet MC, Francis F, Couvert P, Gomot M, Moraine C, van Bokhoven H, Kalscheuer V, Frints S, Gecz J, Ohzaki K, Chaabouni H, Fryns JP, Desportes V, Beldjord C, Chelly J. ARX, a novel Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in X-linked mental retardation. Hum Mol Genet. 2002;11:981–991. doi: 10.1093/hmg/11.8.981. [DOI] [PubMed] [Google Scholar]
- [94].Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, Matsuo M, Kamijo S, Kasahara M, Yoshioka H, Ogata T, Fukuda T, Kondo I, Kato M, Dobyns WB, Yokoyama M, Morohashi K. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–369. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- [95].Shoubridge C, Cloosterman D, Parkinson-Lawerence E, Brooks D, Gecz J. Molecular pathology of expanded polyalanine tract mutations in the Aristaless-related homeobox gene. Genomics. 2007;90:59–71. doi: 10.1016/j.ygeno.2007.03.005. [DOI] [PubMed] [Google Scholar]
- [96].Kosugi S, Hasebe M, Entani T, Takayama S, Tomita M, Yanagawa H. Design of peptide inhibitors for the importin alpha/beta nuclear import pathway by activity-based profiling. Chem Biol. 2008;15:940–949. doi: 10.1016/j.chembiol.2008.07.019. [DOI] [PubMed] [Google Scholar]
- [97].Mlodzik M, Gehring WJ. Expression of the caudal gene in the germ line of Drosophila: formation of an RNA and protein gradient during early embryogenesis. Cell. 1987;48:465–478. doi: 10.1016/0092-8674(87)90197-8. [DOI] [PubMed] [Google Scholar]
- [98].Moreno E, Morata G. Caudal is the Hox gene that specifies the most posterior Drosophile segment. Nature. 1999;400:873–877. doi: 10.1038/23709. [DOI] [PubMed] [Google Scholar]
- [99].Han SH, Ryu JH, Oh CT, Nam KB, Nam HJ, Jang IH, Brey PT, Lee WJ. The moleskin gene product is essential for Caudal-mediated constitutive antifungal Drosomycin gene expression in Drosophila epithelia. Insect Mol Biol. 2004;13:323–327. doi: 10.1111/j.0962-1075.2004.00491.x. [DOI] [PubMed] [Google Scholar]
- [100].Doyle MJ, Loomis ZL, Sussel L. Nkx2.2-repressor activity is sufficient to specify alpha-cells and a small number of beta-cells in the pancreatic islet. Development. 2007;134:515–523. doi: 10.1242/dev.02763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Price M, Lazzaro D, Pohl T, Mattei MG, Ruther U, Olivo JC, Duboule D, Di Lauro R. Regional expression of the homeobox gene Nkx-2.2 in the developing mammalian forebrain. Neuron. 1992;8:241–255. doi: 10.1016/0896-6273(92)90291-k. [DOI] [PubMed] [Google Scholar]
- [102].Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- [103].Ton CC, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, van Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- [104].Heller RS, Stoffers DA, Liu A, Schedl A, Crenshaw EB, 3rd, Madsen OD, Serup P. The role of Brn4/Pou3f4 and Pax6 in forming the pancreatic glucagon cell identity. Dev Biol. 2004;268:123–134. doi: 10.1016/j.ydbio.2003.12.008. [DOI] [PubMed] [Google Scholar]
- [105].Ashery-Padan R, Zhou X, Marquardt T, Herrera P, Toube L, Berry A, Gruss P. Conditional inactivation of Pax6 in the pancreas causes early onset of diabetes. Dev Biol. 2004;269:479–488. doi: 10.1016/j.ydbio.2004.01.040. [DOI] [PubMed] [Google Scholar]
- [106].Hever AM, Williamson KA, van Heyningen V. Developmental malformations of the eye: the role of PAX6, SOX2 and OTX2. Clin Genet. 2006;69:459–470. doi: 10.1111/j.1399-0004.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- [107].Osumi N, Shinohara H, Numayama-Tsuruta K, Maekawa M. Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells. 2008;26:1663–1672. doi: 10.1634/stemcells.2007-0884. [DOI] [PubMed] [Google Scholar]
- [108].Wen J, Hu Q, Li M, Wang S, Zhang L, Chen Y, Li L. Pax6 directly modulate Sox2 expression in the neural progenitor cells. Neuroreport. 2008;19:413–417. doi: 10.1097/WNR.0b013e3282f64377. [DOI] [PubMed] [Google Scholar]
- [109].Hanson I, Churchill A, Love J, Axton R, Moore T, Clarke M, Meire F, van Heyningen V. Missense mutations in the most ancient residues of the PAX6 paired domain underlie a spectrum of human congenital eye malformations. Hum Mol Genet. 1999;8:165–172. doi: 10.1093/hmg/8.2.165. [DOI] [PubMed] [Google Scholar]
- [110].Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Leonard J, Peers B, Johnson T, Ferreri K, Lee S, Montminy MR. Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cells. Mol Endocrinol. 1993;7:1275–1283. doi: 10.1210/mend.7.10.7505393. [DOI] [PubMed] [Google Scholar]
- [112].Watada H, Kajimoto Y, Kaneto H, Matsuoka T, Fujitani Y, Miyazaki J, Yamasaki Y. Involvement of the homeodomain-containing transcription factor PDX-1 in islet amyloid polypeptide gene transcription. Biochem Biophys Res Commun. 1996;229:746–751. doi: 10.1006/bbrc.1996.1875. [DOI] [PubMed] [Google Scholar]
- [113].Elrick LJ, Docherty K. Phosphorylation-dependent nucleocytoplasmic shuttling of pancreatic duodenal homeobox-1. Diabetes. 2001;50:2244–2252. doi: 10.2337/diabetes.50.10.2244. [DOI] [PubMed] [Google Scholar]
- [114].Guillemain G, Da Silva Xavier G, Rafiq I, Leturque A, Rutter GA. Importin beta1 mediates the glucose-stimulated nuclear import of pancreatic and duodenal homeobox-1 in pancreatic islet beta-cells (MIN6) Biochem J. 2004;378:219–227. doi: 10.1042/BJ20031549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Soufi A, Jayaraman PS. PRH/Hex: an oligomeric transcription factor and multifunctional regulator of cell fate. Biochem J. 2008;412:399–413. doi: 10.1042/BJ20080035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Topisirovic I, Culjkovic B, Cohen N, Perez JM, Skrabanek L, Borden KL. The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. EMBO J. 2003;22:689–703. doi: 10.1093/emboj/cdg069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ploski JE, Topisirovic I, Park KW, Borden KL, Radu A. A mechanism of nucleocytoplasmic trafficking for the homeodomain protein PRH. Mol Cell Biochem. 2009;332:173–181. doi: 10.1007/s11010-009-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ryoo HD, Mann RS. The control of trunk Hox specificity and activity by Extradenticle. Genes Dev. 1999;13:1704–1716. doi: 10.1101/gad.13.13.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Weimann JM, Zhang YA, Levin ME, Devine WP, Brulet P, McConnell SK. Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron. 1999;24:819–831. doi: 10.1016/s0896-6273(00)81030-2. [DOI] [PubMed] [Google Scholar]
- [120].Wang H, Tao T, Tang J, Mao YH, Li W, Peng J, Tan G, Zhou YP, Zhong JX, Tseng SC, Kawakita T, Zhao YX, Liu ZG. Importin 13 serves as a potential marker for corneal epithelial progenitor cells. Stem Cells. 2009;27:2516–2526. doi: 10.1002/stem.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Jans DA. Nuclear transport in development and disease - The importance of importins. Semin Cell Dev Biol. 2009;20:575. doi: 10.1016/j.semcdb.2009.05.007. [DOI] [PubMed] [Google Scholar]
- [122].Loveland KL, Hogarth C, Szczepny A, Prabhu SM, Jans DA. Expression of nuclear transport importins beta 1 and beta 3 is regulated during rodent spermatogenesis. Biol Reprod. 2006;74:67–74. doi: 10.1095/biolreprod.105.042341. [DOI] [PubMed] [Google Scholar]
- [123].Poon IK, Jans DA. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–186. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- [124].Geles KG, Johnson JJ, Jong S, Adam SA. A role for Caenorhabditis elegans importin IMA-2 in germ line and embryonic mitosis. Mol Biol Cell. 2002;13:3138–3147. doi: 10.1091/mbc.E02-02-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Geles KG, Adam SA. Germline and developmental roles of the nuclear transport factor importin alpha3 in C. elegans. Development. 2001;128:1817–1830. doi: 10.1242/dev.128.10.1817. [DOI] [PubMed] [Google Scholar]
- [126].Fang X, Chen T, Tran K, Parker CS. Developmental regulation of the heat shock response by nuclear transport factor karyopherin-alpha3. Development. 2001;128:3349–3358. doi: 10.1242/dev.128.17.3349. [DOI] [PubMed] [Google Scholar]
- [127].Hogarth CA, Calanni S, Jans DA, Loveland KL. Importin alpha mRNAs have distinct expression profiles during spermatogenesis. Dev Dyn. 2006;235:253–262. doi: 10.1002/dvdy.20569. [DOI] [PubMed] [Google Scholar]
- [128].Hogarth C, Itman C, Jans DA, Loveland KL. Regulated nucleocytoplasmic transport in spermatogenesis: a driver of cellular differentiation? Bioessays. 2005;27:1011–1025. doi: 10.1002/bies.20289. [DOI] [PubMed] [Google Scholar]
- [129].Kilstrup-Nielsen C, Alessio M, Zappavigna V. PBX1 nuclear export is regulated independently of PBX-MEINOX interaction by PKA phosphorylation of the PBC-B domain. EMBO J. 2003;22:89–99. doi: 10.1093/emboj/cdg010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Boulanger J, Vezina A, Mongrain S, Boudreau F, Perreault N, Auclair BA, Laine J, Asselin C, Rivard N. Cdk2-dependent phosphorylation of homeobox transcription factor CDX2 regulates its nuclear translocation and proteasome-mediated degradation in human intestinal epithelial cells. J Biol Chem. 2005;280:18095–18107. doi: 10.1074/jbc.M502184200. [DOI] [PubMed] [Google Scholar]
- [131].Tartakoff AM, Lichtenstein M, Nanduri J, Tsao HM. Review: dynamic stability of the interphase nucleus in health and disease. J Struct Biol. 2000;129:144–158. doi: 10.1006/jsbi.2000.4225. [DOI] [PubMed] [Google Scholar]
- [132].Kurant E, Pai CY, Sharf R, Halachmi N, Sun YH, Salzberg A. Dorsotonals/homothorax, the Drosophila homologue of meis1, interacts with extradenticle in patterning of the embryonic PNS. Development. 1998;125:1037–1048. doi: 10.1242/dev.125.6.1037. [DOI] [PubMed] [Google Scholar]
- [133].Pai CY, Kuo TS, Jaw TJ, Kurant E, Chen CT, Bessarab DA, Salzberg A, Sun YH. The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, extradenticle, and suppresses eye development in Drosophila. Genes Dev. 1998;12:435–446. doi: 10.1101/gad.12.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Berthelsen J, Kilstrup-Nielsen C, Blasi F, Mavilio F, Zappavigna V. The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH. Genes Dev. 1999;13:946–953. doi: 10.1101/gad.13.8.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Huang H, Paliouras M, Rambaldi I, Lasko P, Featherstone M. Nonmuscle myosin promotes cytoplasmic localization of PBX. Mol Cell Biol. 2003;23:3636–3645. doi: 10.1128/MCB.23.10.3636-3645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Saleh M, Huang H, Green NC, Featherstone MS. A conformational change in PBX1A is necessary for its nuclear localization. Exp Cell Res. 2000;260:105–115. doi: 10.1006/excr.2000.5010. [DOI] [PubMed] [Google Scholar]
- [137].Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gecz J, Cloosterman D, Partington M. ARX: a gene for all seasons. Curr Opin Genet Dev. 2006;16:308–316. doi: 10.1016/j.gde.2006.04.003. [DOI] [PubMed] [Google Scholar]
- [139].Friocourt G, Poirier K, Rakic S, Parnavelas JG, Chelly J. The role of ARX in cortical development. Eur J Neurosci. 2006;23:869–876. doi: 10.1111/j.1460-9568.2006.04629.x. [DOI] [PubMed] [Google Scholar]
- [140].Jaegle M, Ghazvini M, Mandemakers W, Piirsoo M, Driegen S, Levavasseur F, Raghoenath S, Grosveld F, Meijer D. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 2003;17:1380–1391. doi: 10.1101/gad.258203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].James R, Erler T, Kazenwadel J. Structure of the murine homeobox gene cdx-2. Expression in embryonic and adult intestinal epithelium. J Biol Chem. 1994;269:15229–15237. [PubMed] [Google Scholar]
- [142].Shimozaki K, Nakashima K, Niwa H, Taga T. Involvement of Oct3/4 in the enhancement of neuronal differentiation of ES cells in neurogenesis-inducing cultures. Development. 2003;130:2505–2512. doi: 10.1242/dev.00476. [DOI] [PubMed] [Google Scholar]
- [143].Jaegle M, Meijer D. Role of Oct-6 in Schwann cell differentiation. Microsc Res Tech. 1998;41:372–378. doi: 10.1002/(SICI)1097-0029(19980601)41:5<372::AID-JEMT4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- [144].Chow RL, Volgyi B, Szilard RK, Ng D, McKerlie C, Bloomfield SA, Birch DG, McInnes RR. Control of late off-center cone bipolar cell differentiation and visual signaling by the homeobox gene Vsx1. Proc Natl Acad Sci U S A. 2004;101:1754–1759. doi: 10.1073/pnas.0306520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ohtoshi A, Wang SW, Maeda H, Saszik SM, Frishman LJ, Klein WH, Behringer RR. Regulation of retinal cone bipolar cell differentiation and photopic vision by the CVC homeobox gene Vsx1. Curr Biol. 2004;14:530–536. doi: 10.1016/j.cub.2004.02.027. [DOI] [PubMed] [Google Scholar]
- [146].Doe CQ, Chu-LaGraff Q, Wright DM, Scott MP. The prospero gene specifies cell fates in the Drosophila central nervous system. Cell. 1991;65:451–464. doi: 10.1016/0092-8674(91)90463-9. [DOI] [PubMed] [Google Scholar]