Abstract
In the epidermis, retinoids regulate the expression of keratins, the intermediate filament proteins of epithelial cells. We have cloned the 5' regulatory regions of four human epidermal keratin genes, K#5, K#6, K#10, and K#14, and engineered constructs in which these regions drive the expression of the CAT reporter gene. By co-transfecting the constructs into epithelial cells along with the vectors expressing nuclear receptors for retinoic acid (RA) and thyroid hormone, we have demonstrated that the receptors can suppress the promoters of keratin genes. The suppression is ligand dependent; it is evident both in established cell lines and in primary cultures of epithelial cells. The three RA receptors have similar effects on keratin gene transcription. Our data indicate that the nuclear receptors for RA and thyroid hormone regulate keratin synthesis by binding to negative recognition elements in the upstream DNA sequences of the keratin genes. RA thus has a twofold effect on epidermal keratin expression: qualitatively, it regulates the regulators that effect the switch from basal cell-specific keratins to differentiation-specific ones; and quantitatively, it determines the level of keratin synthesis within the cell by direct interaction of its receptors with the keratin gene promoters.
Full text
PDF
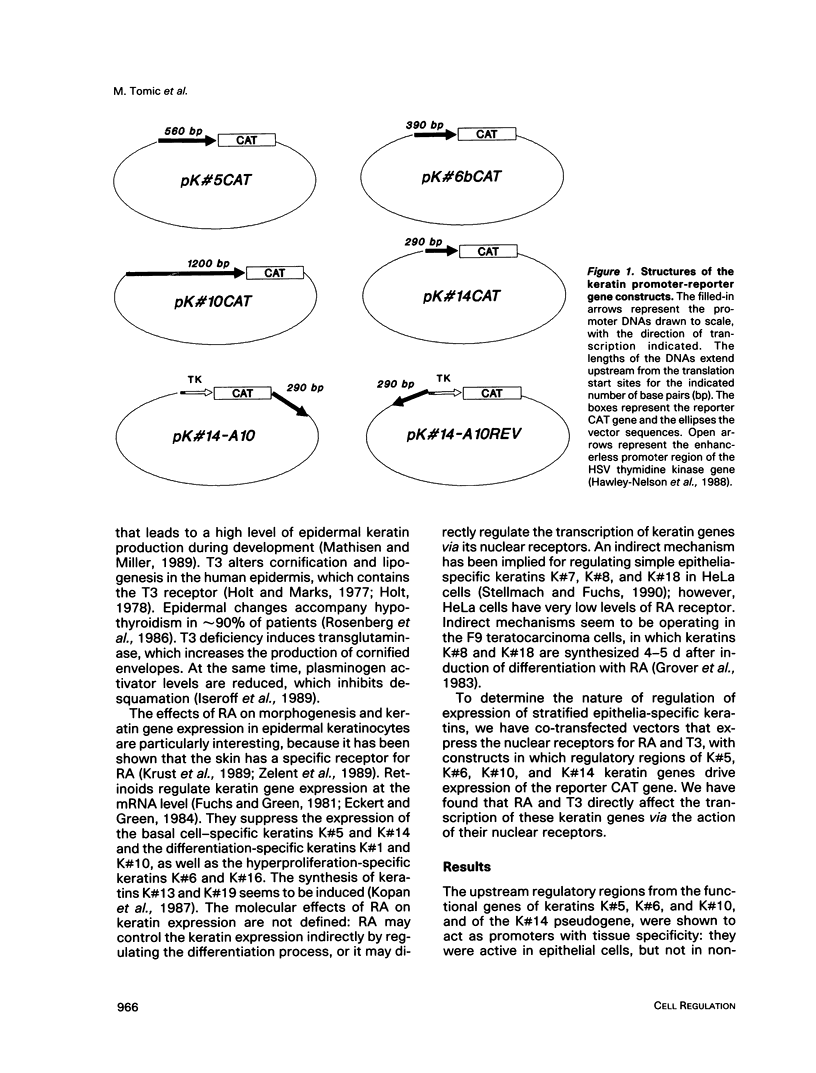
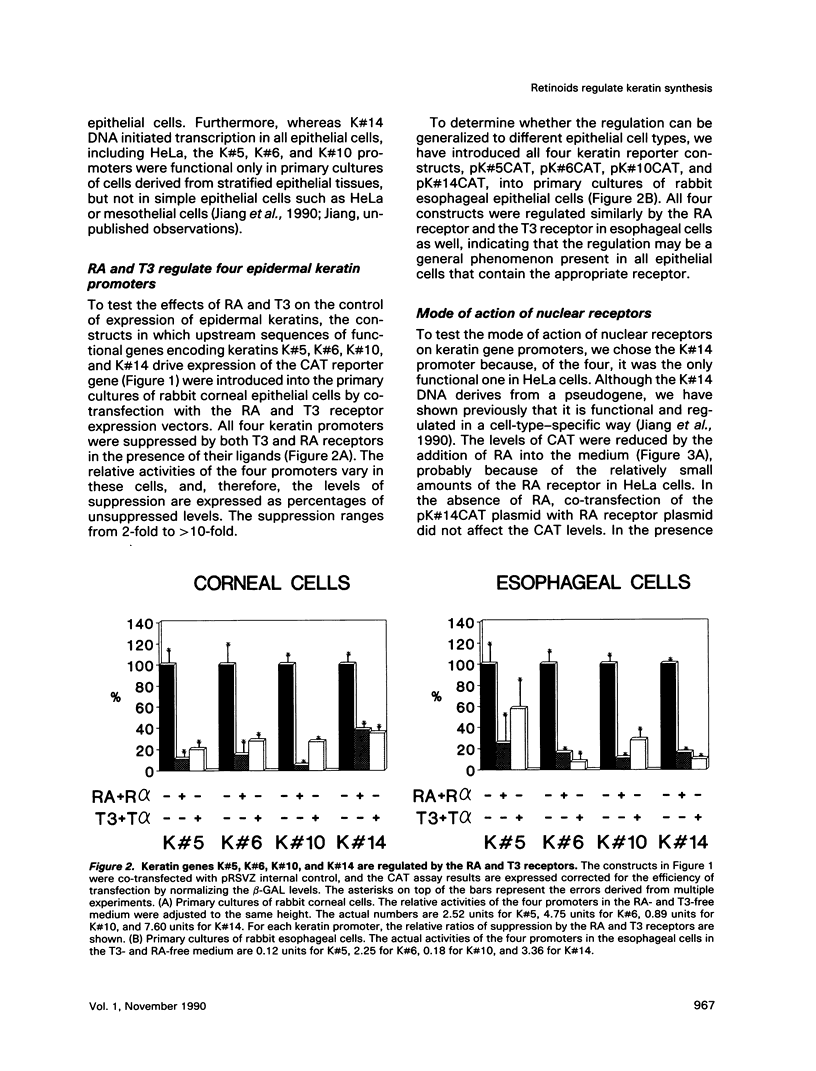
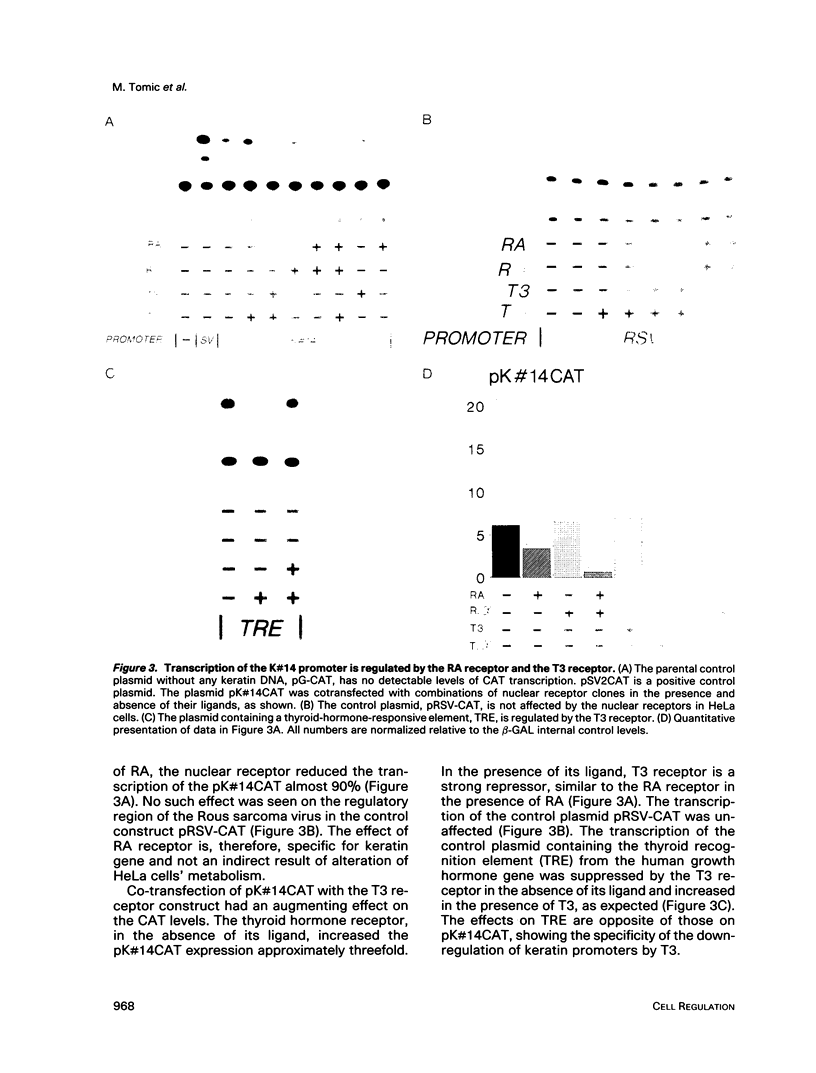
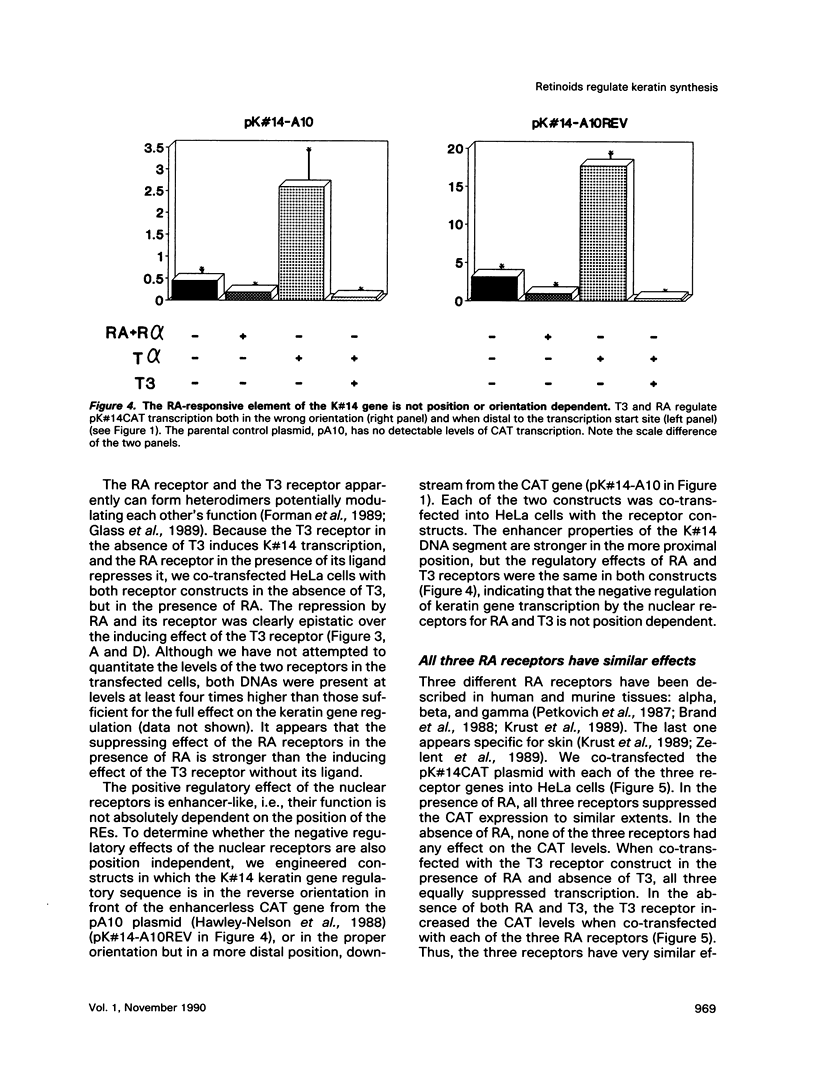



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselineau D., Bernard B. A., Bailly C., Darmon M. Retinoic acid improves epidermal morphogenesis. Dev Biol. 1989 Jun;133(2):322–335. doi: 10.1016/0012-1606(89)90037-7. [DOI] [PubMed] [Google Scholar]
- Bedo G., Santisteban P., Aranda A. Retinoic acid regulates growth hormone gene expression. Nature. 1989 May 18;339(6221):231–234. doi: 10.1038/339231a0. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Casanova J., Copp R. P., Janocko L., Samuels H. H. 5'-Flanking DNA of the rat growth hormone gene mediates regulated expression by thyroid hormone. J Biol Chem. 1985 Sep 25;260(21):11744–11748. [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Robertson K. A., Mueller L. Retinoic acid-induced granulocytic differentiation of HL-60 myeloid leukemia cells is mediated directly through the retinoic acid receptor (RAR-alpha). Mol Cell Biol. 1990 May;10(5):2154–2163. doi: 10.1128/mcb.10.5.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Green H. Cloning of cDNAs specifying vitamin A-responsive human keratins. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4321–4325. doi: 10.1073/pnas.81.14.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P. M. Epidermal effects of retinoids: supramolecular observations and clinical implications. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):797–809. doi: 10.1016/s0190-9622(86)70236-3. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B. M., Yang C. R., Au M., Casanova J., Ghysdael J., Samuels H. H. A domain containing leucine-zipper-like motifs mediate novel in vivo interactions between the thyroid hormone and retinoic acid receptors. Mol Endocrinol. 1989 Oct;3(10):1610–1626. doi: 10.1210/mend-3-10-1610. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Yang C. R., Stanley F., Casanova J., Samuels H. H. c-erbA protooncogenes mediate thyroid hormone-dependent and independent regulation of the rat growth hormone and prolactin genes. Mol Endocrinol. 1988 Oct;2(10):902–911. doi: 10.1210/mend-2-10-902. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Gilfix B. M., Eckert R. L. Coordinate control by vitamin A of keratin gene expression in human keratinocytes. J Biol Chem. 1985 Nov 15;260(26):14026–14029. [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupner G., Wills K. N., Tzukerman M., Zhang X. K., Pfahl M. Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature. 1989 Aug 24;340(6235):653–656. doi: 10.1038/340653a0. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Grover A., Oshima R. G., Adamson E. D. Epithelial layer formation in differentiating aggregates of F9 embryonal carcinoma cells. J Cell Biol. 1983 Jun;96(6):1690–1696. doi: 10.1083/jcb.96.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P., Androphy E. J., Lowy D. R., Schiller J. T. The specific DNA recognition sequence of the bovine papillomavirus E2 protein is an E2-dependent enhancer. EMBO J. 1988 Feb;7(2):525–531. doi: 10.1002/j.1460-2075.1988.tb02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. J. In vitro responses of the epidermis to triiodothyronine. J Invest Dermatol. 1978 Sep;71(3):202–204. doi: 10.1111/1523-1747.ep12547158. [DOI] [PubMed] [Google Scholar]
- Holt P. J., Marks R. The epidermal response to change in thyroid status. J Invest Dermatol. 1977 May;68(5):299–301. doi: 10.1111/1523-1747.ep12494564. [DOI] [PubMed] [Google Scholar]
- Isseroff R. R., Chun K. T., Rosenberg R. M. Triiodothyronine alters the cornification of cultured human keratinocytes. Br J Dermatol. 1989 Apr;120(4):503–510. doi: 10.1111/j.1365-2133.1989.tb01323.x. [DOI] [PubMed] [Google Scholar]
- Jiang C. K., Epstein H. S., Tomic M., Freedberg I. M., Blumenberg M. Epithelial-specific keratin gene expression: identification of a 300 base-pair controlling segment. Nucleic Acids Res. 1990 Jan 25;18(2):247–253. doi: 10.1093/nar/18.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Schwartz F., Fuchs E. Differences in keratin synthesis between normal epithelial cells and squamous cell carcinomas are mediated by vitamin A. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4280–4284. doi: 10.1073/pnas.81.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R., Traska G., Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987 Jul;105(1):427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lersch R., Stellmach V., Stocks C., Giudice G., Fuchs E. Isolation, sequence, and expression of a human keratin K5 gene: transcriptional regulation of keratins and insights into pairwise control. Mol Cell Biol. 1989 Sep;9(9):3685–3697. doi: 10.1128/mcb.9.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Mitchell P. J., Williams T., Tjian R. Regulation of transcription factor AP-2 by the morphogen retinoic acid and by second messengers. Genes Dev. 1989 Oct;3(10):1507–1517. doi: 10.1101/gad.3.10.1507. [DOI] [PubMed] [Google Scholar]
- Mathisen P. M., Miller L. Thyroid hormone induces constitutive keratin gene expression during Xenopus laevis development. Mol Cell Biol. 1989 May;9(5):1823–1831. doi: 10.1128/mcb.9.5.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Nagae S., Lichti U., De Luca L. M., Yuspa S. H. Effect of retinoic acid on cornified envelope formation: difference between spontaneous envelope formation in vivo or in vitro and expression of envelope competence. J Invest Dermatol. 1987 Jul;89(1):51–58. doi: 10.1111/1523-1747.ep12580383. [DOI] [PubMed] [Google Scholar]
- Nunez E. A. The erb-A family receptors for thyroid hormones, steroids, vitamin D and retinoic acid: characteristics and modulation. Curr Opin Cell Biol. 1989 Apr;1(2):177–185. doi: 10.1016/0955-0674(89)90083-5. [DOI] [PubMed] [Google Scholar]
- O'Guin W. M., Galvin S., Schermer A., Sun T. T. Patterns of keratin expression define distinct pathways of epithelial development and differentiation. Curr Top Dev Biol. 1987;22:97–125. doi: 10.1016/s0070-2153(08)60100-3. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Rieger M., Franke W. W. Identification of an orthologous mammalian cytokeratin gene. High degree of intron sequence conservation during evolution of human cytokeratin 10. J Mol Biol. 1988 Dec 20;204(4):841–856. doi: 10.1016/0022-2836(88)90045-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. M., Isseroff R. R., Ziboh V. A., Huntley A. C. Abnormal lipogenesis in thyroid hormone-deficient epidermis. J Invest Dermatol. 1986 Mar;86(3):244–248. doi: 10.1111/1523-1747.ep12285364. [DOI] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Forman B. M., Horowitz Z. D., Ye Z. S. Regulation of gene expression by thyroid hormone. J Clin Invest. 1988 Apr;81(4):957–967. doi: 10.1172/JCI113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer A., Galvin S., Sun T. T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986 Jul;103(1):49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits H. L., Floyd E. E., Jetten A. M. Molecular cloning of gene sequences regulated during squamous differentiation of tracheal epithelial cells and controlled by retinoic acid. Mol Cell Biol. 1987 Nov;7(11):4017–4023. doi: 10.1128/mcb.7.11.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellmach V. M., Fuchs E. Exploring the mechanisms underlying cell type-specific and retinoid-mediated expression of keratins. New Biol. 1989 Dec;1(3):305–317. [PubMed] [Google Scholar]
- Tseng S. C., Hatchell D., Tierney N., Huang A. J., Sun T. T. Expression of specific keratin markers by rabbit corneal, conjunctival, and esophageal epithelia during vitamin A deficiency. J Cell Biol. 1984 Dec;99(6):2279–2286. doi: 10.1083/jcb.99.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner A. L., Eichman M. J., Fuchs E. The sequence of a type II keratin gene expressed in human skin: conservation of structure among all intermediate filament genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4683–4687. doi: 10.1073/pnas.82.14.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Weiss J. S., Ellis C. N., Headington J. T., Tincoff T., Hamilton T. A., Voorhees J. J. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. JAMA. 1988 Jan 22;259(4):527–532. [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]



