Abstract
Background
The purpose of this study was to investigate the changes in the Power Spectrum Density (PSD) of the electroencephalography (EEG) in common sensorimotor balance training tasks of varying difficulty. Sensorimotor balance exercises including alteration of vision, base of support or surface compliance are used to improve postural control. These exercises are presumed to induce supraspinal adaptation, however, there were no studies that investigated the power changes of the cortical activity in these static balance tasks. Our objective was to provide evidence in the cortical involvement with the static balance tasks frequently used in sensorimotor training.
Material/Methods
Postural sway and EEG changes of alpha, beta and sigma wave bands were measured in seventeen participants during eight balance tasks of varying difficulty with eyes open and closed, feet in tandem or apart and on foam or a firm surface.
Results
The power of beta and sigma bands increased significantly at the parietal and central area of the brain in tasks with eyes open together with one sensory factor (base of support or surface compliance) or two sensory factors (base of support and surface compliance) altered, and in task with three sensory factors (vision, base of support and surface compliance) altered from the control task.
Conclusions
This study demonstrated the cortical involvement in the sensorimotor balance tasks, suggesting that these exercises may induce cortical adaptation for postural control. The results support subcortical control with increased task difficulty and the increase in cortical processing when task became extremely challenging.
Keywords: EEG, posture, sensorimotor, training, balance
Background
Maintaining an upright posture is a complex motor skill based on the integration of dynamic sensorimotor information [1]. The central nervous system plays an integral role in integrating the afferent information from the visual, somatosensory and vestibular systems [2,3]. It was assumed that postural regulation is under the control of subcortical structures of the cerebrum and the spinal cord [4], but more studies have emerged to suggest cortical involvement in the postural response. Cerebellum vermis and prefrontal cortex were shown to be significantly involved in the postural control [5]. Prefrontal cortex was shown to be activated after external perturbation with or without auditory warning [6]. Jacobs and colleagues also reported cortical involvement in the control of postural tasks and [7]; Goble and colleagues demonstrated central processing of proprioceptive signals from the foot during balance control [8]. Movement-related cortical potentials were reported preceding self-paced initiation of postural sway and after voluntary limb movement [9,10]. An increase in cortical negative potential was exhibited following an application of nudges during gait or surface translation [11]. Same phenomenon was reported during perturbation with cues suggesting that cerebral cortex contributed to the modification of upcoming postural responses to external perturbation when provided with pre-warning cues [12]. Mochizuki and colleagues also reported that cortical activity was observed prior to and following predictable and un-predictable perturbation of balance [13].
Contribution of various cortical structures in postural control has been examined. Posterior-parietal cortex has been an area of interest in the studies of sensorimotor integration as it is heavily interconnected with the motor and premotor areas [2,14]. Studies have suggested that posterior-parietal cortex is crucial in the sensorimotor processing [15–17]. Cortical activities in the fronto-central and parietal area have also shown to be enhanced during visual recognition of postural instability [18] and in postural perturbation with warning cue [12]. Other studies reported the activation of the fronto-central region of the brain during self-initiated postural movement [10,13,19] and unpredicted postural perturbations [11], suggesting the supplementary motor area and the foot area of sensori-motor cortex were the possible sources in the initiating of postural movement.
It has been well documented that balance training improves postural control [20,21]. Studies have shown that balance training program was effective in improving functional status [22] and, reducing the risk of falling [23]. Group and home-based exercise programs consisting of balance and strengthening exercises were shown to be effective in reducing falls [24]. Group balance training has also exhibited long and short term effect on gait speed, balance and fear of falling [25]. However, balance-training programs are very diverse, even spinal stabilization exercises were shown to be effective in reducing postural sway [26]. In general, the guideline for balance exercises is to include static and dynamic exercises on stable or unstable surfaces with eyes open or closed while standing in a bipedal or mono-pedal position [27,28]. Specific sensorimotor balance exercises are used commonly by clinicians to address the deficit in the integration of sensory inputs in the postural control. Sensory inputs such as vision and somatosensory inputs are routinely altered individually or simultaneously to challenge the remaining sensory systems for postural control in balance training. With visual input altered such as eyes closed, one has to rely more on the somatosensory and vestibular inputs for balance. With somatosensory input reduced such as standing on a foam surface, one has to rely on the visual and vestibular inputs for balance. With the alteration of combined visual and somatosensory inputs such as standing with eyes closed on a foam surface, one has to rely heavily on the vestibular information for balance. Additionally, narrowing base of support such as standing with one foot in front of the other (tandem standing) can further challenge the sensorimotor system [29,30]. While these balance exercises are presumed to induce adaptation in the central nervous system, there is no scientific evidence to show any cortical involvement in these exercises.
Balance training has been shown to induce supraspinal adaptation [31]. Studies have shown that short-term motor skill training was associated with cortical adaptation [32,33]. Taube and colleagues have reported a decrease in corticospinal and cortical excitability with four weeks of balance training and suggested that the balance improvement relied mostly on the supraspinal adaptation [31]. In addition, other studies have demonstrated an association between reduced supraspinal excitability and improvement in balance performance with balance training and suggested an enhancement of subcortical control of muscles [34,35].
Most of the studies that investigated the neural response associated with balance training used electrophysiological and imaging techniques. Positron Emission Tomography (PET), a nuclear medicine imaging technique used to study the functioning of the brain by detecting the signal from a delivered radioactive material in the body, has been used to study the brain activation during maintenance of standing postures [5]. Functional near-infrared spectroscopy (fNIRS), a non-invasive and affordable neuroimaging technique used to monitor the blood hemoglobin concentrations and tissue oxygenation associated with neural activity by measuring the changes in near-infrared light in the brain, has been selected to study the role of prefrontal cortex in balance control [6]. Functional Magnetic Resonance imaging (fMRI), a neuroimaging technique used to detect the changes in blood flow and oxygenation in response to neural activity, has been selected to investigate the cortical involvement in shaping the postural responses [7]. While these imaging techniques have excellent spatial resolution and provide great access to subcortical areas, they only measure the cerebral blood flow or metabolic activity during the performance of the tasks. Electroencephalogram (EEG) is the only electrophysiological recording technique that provides a more accurate temporal resolution of the brain activity in a millisecond time frame. EEG is traditionally used in the evaluation of neurological conditions, but it has often been used to quantify cortical response in relation to event-related changes.
Previous studies have investigated the EEG changes associated with postural control by examining the event-related potential (ERP). Components of the ERP, such as amplitude of the responses N1 and Contingent Negative Variation (CNV) have been used as measures of the brain activity in response to perturbation to postural stability [11–13]. Some studies have examined the Movement-Related Cortical Potentials (MRCP) preceding the onset of postural adjustment [9,10]. A type of ERP with low frequency negative potential, MRCP, is recorded in the motor cortex preceding voluntary movement [9]. It was interpreted as a reflection of the cortical processes in planning and preparation of voluntary movement [36].
An alternative EEG analysis is Power spectral density analysis (PSD). It is one of the conventional methods for the analysis of EEG signals. Power spectral density reflects the distribution of signal power over frequency. It has been used widely to assess changes in the cortical activity during cognitive and motor tasks [37–39]. To the best of our knowledge, there are no studies examining the change of power spectrum density of the cortical activity during static standing balance tasks. Therefore, the objective of this study was to provide evidence in the cortical involvement by investigating the changes in the PSD of EEG in eight balance-training tasks that are frequently used in sensorimotor training. We predicted that the PSD of all wavebands would alter when the number of sensory inputs in the balance tasks altered from the control task. Previous study has shown that the balance tasks difficulty increased as the number of altered sensory inputs increased [40]. We hypothesized that there would be measurable changes in the power of the cortical response with changes in the difficulty of the balance tasks.
Material and Methods
Overall design
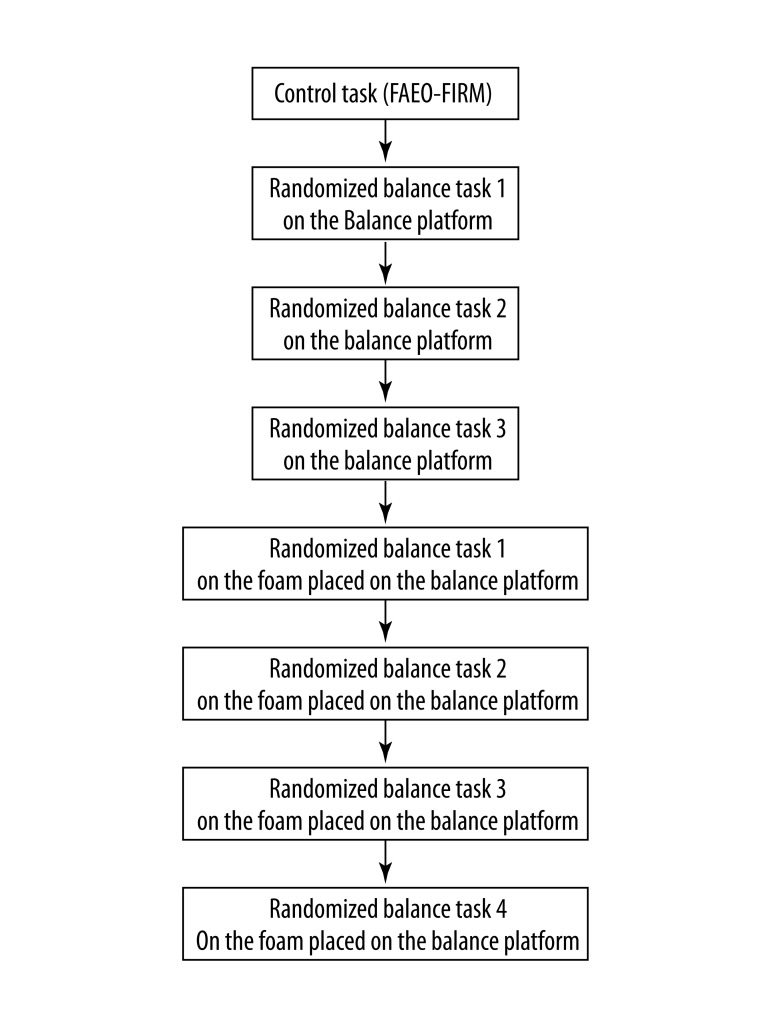
This study was a 2×2×2 repeated measures design with three independent variables. There were two levels for each repeated factor: vision (eyes open or closed), surface compliance (foam or firm surface) and base of support (feet apart or tandem stand). Each participant was exposed to eight test conditions with four tasks on the firm surface and 4 tasks on foam (Table 1). All participants were first tested in the control task, FAEO-FIRM, by standing with feet apart, eyes open on the firm balance platform. To control the order effects, each participant was then randomized to the three remaining balance tasks on the firm surface by drawing, and then randomized to the four balance tasks on the foam (Figure 1). We recorded the postural sway and the PSD of the alpha, beta and sigma wavebands at the EEG electrode sites Fz, Cz and POz in the eight balance tasks. The response of each variable in each task was compared to that in the control task for each participant to generate the relative response. The average relative response of each variable from the three trials of each task of all the participants was then used for statistical analysis.
Table 1.
2×2×2 factorial design of the eight balance tasks in this study.
| Feet position | Firm Surface | Foam | ||
|---|---|---|---|---|
| Eyes open | Eyes closed | Eyes open | Eyes closed | |
| Feet apart | FAEO-FIRM (Control task) | FAEC-FIRM | FAEO-FOAM | FAEC-FOAM |
| Tandem | TEO-FIRM | TEC-FIRM | TEO-FOAM | TEC-FOAM |
FA – Feet apart; T – Tandem; EO – Eyes open; EC – Eyes closed; Firm – firm surface; Foam – foam.
Figure 1.
Sequence of the balance tasks tested in each participant.
Subjects
Twenty young healthy volunteers were recruited from the Inland Empire in Southern California. To ensure the external validity of the study, equal numbers (n=10) of male and female were recruited. Data collected from three participants were incomplete; therefore, only data from seventeen participants were used for analysis. The seventeen participants (9 males, 8 females) with ages of 24–32 were free of headaches, diabetes mellitus, and orthopedic or neurological conditions. To control the extraneous variables, a homogeneous group of sedentary individuals who did not participate in any regular balance exercises was recruited. Participants were instructed not to take any medication or central nervous stimulants that might affect their balance the day before the study. The general characteristics of the participants are shown in Table 2. The experimental protocol, approved by the Institutional Review Board of Loma Linda University, was explained to each participant and they gave their written informed consent for the study.
Table 2.
Mean ±SD of the general characteristics by gender.
| Age* (years) | Height* (cm) | Weight** (kg) | |
|---|---|---|---|
| Female (n=8) | 26.4±2.4 | 165.4±9.3 | 62.8±14.2 |
| Male (n=9) | 27.8±3.4 | 173.9±6.1 | 78.9±15.1 |
| Test statistic | t=−1.0 | t=−2.3 | Z=−2.0 |
| p-value | 0.37 | 0.06 | 0.04*** |
Independent t-test;
Mann-Whitney U-test;
p<0.05.
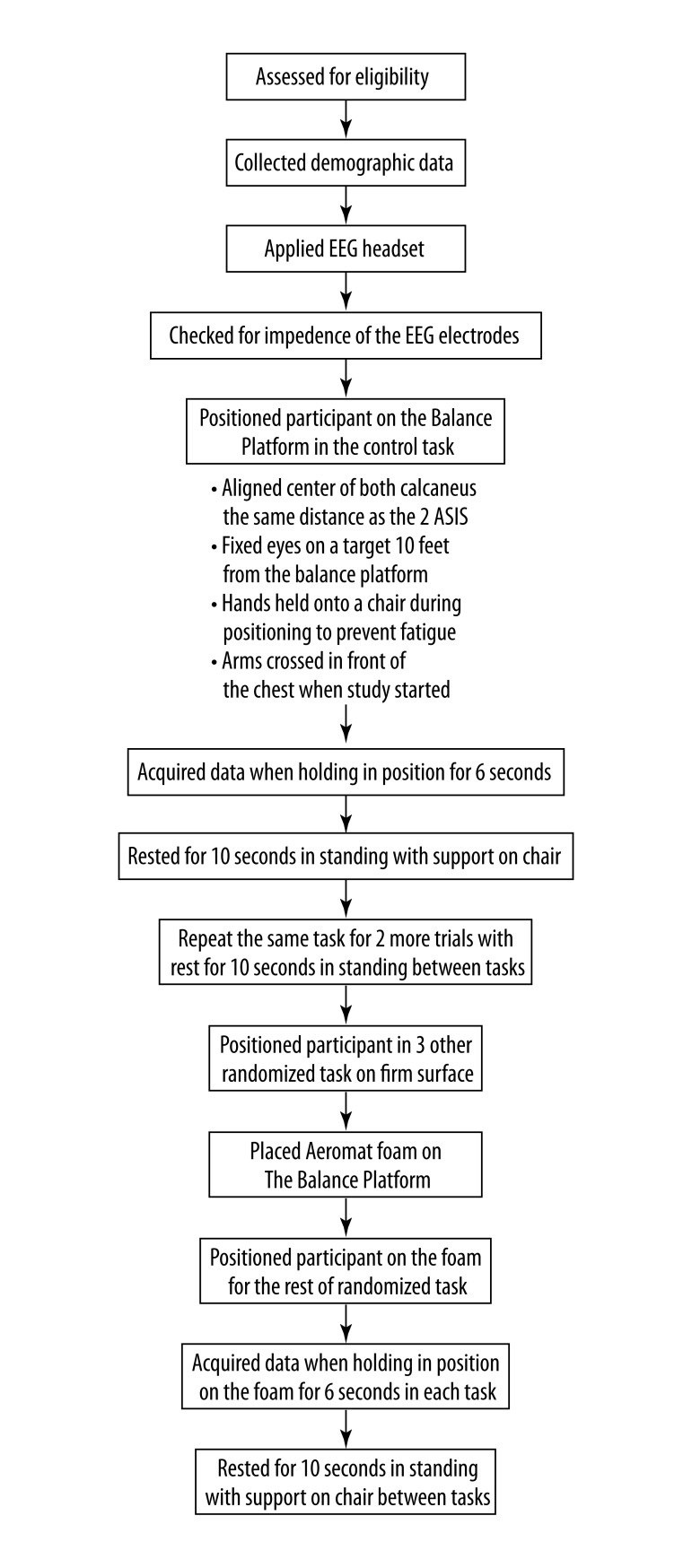
Experimental procedures (Figure 2)
Figure 2.
Experimental procedure of this study (ASIS – Anterior Superior Iliac Spine).
Baseline demographic data including age, height, weight and side of dominance were collected from each subject at the beginning of the study. The B-Alert X10 wireless EEG 9 channels headset was placed on the skull. Bilateral mastoids were linked as reference. Electrode impedance was then checked. All the participants started with the control task, in which they stood with feet apart on the balance platform for 6 seconds. Their feet were aligned with the centers of the calcaneus the same distance as that of the two Anterior Superior Iliac Spine. They were instructed to fix their eyes on a target on the wall 10 feet away from the balance platform with arms crossed in front of their chests to minimize the artifacts generated by any eye movement or excessive trunk and arm movements. The task was repeated 3 times. To minimize fatigue, participants were instructed to hold onto a chair to rest in standing for 10 seconds between the tasks. Thereafter, the subject was randomized to the rest of the balance tasks on the firm surface. Then an Aeromat balance block was placed on top of the balance platform and data was collected during the randomized balance tasks on the foam.
Balance tasks
Eight quiet standing balance tasks each lasting for 6 seconds were included in this study [40]. Sensory variables such as the vision, base of support and surface compliance were altered individually or simultaneously in the balance tasks. To alter the visual input, 2 levels of vision (eyes open & closed) were used in the balance tasks. To alter the somatosensory input, 2 different surface compliances (firm surface & foam) were used. The Aeromat balance block, a PVC/NBR foam with size 16×19×2.5 inches and density around 0.04–0.06 g/cm3 (AGM Group, Aeromat Fitness Product, Fremont, CA), was placed on top of the balance platform as the foam surface in this study. Participants were asked to stand in two different stance positions with feet apart (centers of the calcaneus in the same distance as the two Anterior Superior Iliac Spine) or in tandem (feet in a heel-toe position with non-dominant foot in front).
In a previous study, Tse et. al. caterogized the difficulty of the eight balance tasks based on the postural sway and it was reported that the tasks difficulty was affected by the number of sensory variables altered [40]. The eight balance tasks in the order of tasks difficulty are listed in Table 3. Participants were randomized to the balance tasks. Detail of randomization was explained in the overall design.
Table 3.
Eight balance tasks in the increasing order of tasks difficulty [40].
| Balance tasks in the increasing order of difficulty | Position in standing | The sensory factors altered from the control task | The number of sensory factors altered |
|---|---|---|---|
| FAEO-FIRM | Feet apart, eyes open on firm surface | Control task | 0 |
| TEO-FIRM | Tandem stand, eyes open on firm surface | Base of support | 1 |
| FAEO-FOAM | Feet apart, eyes open on foam | Surface compliance | 1 |
| FAEC-FIRM | Feet apart, eyes closed on firm surface | Vision | 1 |
| TEC-FIRM | Tandem stand, eyes closed on firm surface | Base of support & vision | 2 |
| TEO-FOAM | Tandem stand, eyes open on foam | Base of support & surface compliance | 2 |
| FAEC-FOAM | Feet apart, eyes closed on foam | Vision & surface compliance | 2 |
| TEC-FOAM | Tandem stand, eyes closed on foam | Base of support, vision & surface compliance | 3 |
Measurement of postural sway
The displacement of the subject’s center of pressure was measured using a valid and reliable balance platform of 1 m by 1 m in size and 0.1 m in height (Figure 3) [41]. Four stainless steel bars, each with four strain gauges, were mounted at the four corners under the platform (TML Strain Gauge FLA-6, 350-17, Tokyo, Japan). The output of the 4 Wheatstone strain gauge bridges was amplified with BioPac 100C low-level bio-potential amplifiers and recorded on a BioPac MP-150 system through a 24-bit A/D converter (Figure 4). The sampling rate was 2000 samples per second [41].
Figure 3.

Balance platform used in the study.
Figure 4.

BioPac MP-150 system.
To calculate the load and the center of the pressure of the force on the platform, the output of the four sensors was used to measure the X and Y coordinates of the center of gravity of the subject. This data was converted to a movement vector giving a magnitude and angular displacement. By averaging the vector magnitude over 6 seconds, mean and standard deviation (SD) were obtained for this measure. From this, the Coefficient of Variation (CV) was calculated (SD ÷ Mean ×100%) as a measure of the postural sway [41]. The average CV of each task was then determined by averaging the CVs of the 3 trials for each participant.
Measurement of cortical response
A 10–20 system, B-Alert X10 wireless EEG 9 channels headset (Advanced Brain Monitoring Inc., Carlsbad, CA, USA), integrated with the AcqKnowledge MP-150 acquisition software (BioPac systems, Inc., Goleta, CA) was used to acquire the EEG data from 9 channels in a monopolar configuration referenced to the linked mastoids (Figure 5). Data from three channels (Fz, Cz and POz) was used for analysis. The impedance of each electrode was kept below 40 kΩ. Bandpass filters 0.1 Hz and 65 Hz at 3 dB attenuation were used to remove environmental artifact. The data was sampled at a frequency of 256 samples per second and analyzed using signal processing techniques to identify and decontaminate biological and environmental artifacts including eye blinks, EMG, excursions, saturations and spikes [42]. Detailed artifact decontamination process was described in a previous study [38].
Figure 5.
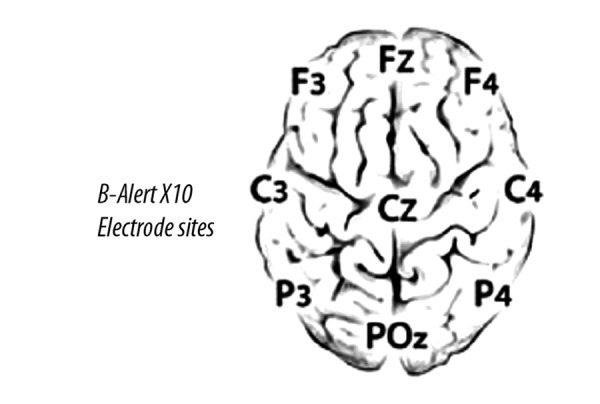
EEG electrode positions.
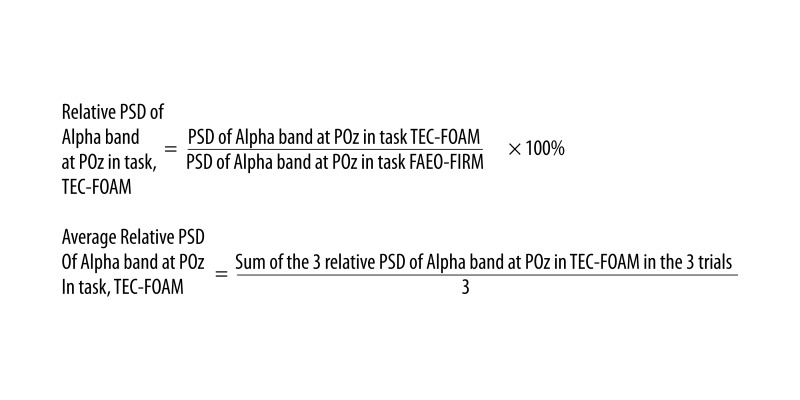
All uncontaminated EEG data for each task was epoched into 1-second blocks with the B-Alert Software version 2.90 (Advanced Brain Monitoring Inc., Carlsbad, CA, USA). The power spectral densities (PSD) of alpha (8–12 Hz), beta (13–19 Hz) and sigma (30–40 Hz) frequency bands were computed for each task in each electrode site using a Fast-Fourier transform with a 50% overlapping window. Three of the one-second overlays were used to obtain the average PSD for an epoch. The PSD of a specific frequency band in each of the balance tasks was then divided by the PSD of the corresponding frequency band in the control task at the same electrode site for each participant. This provides the percentage of the PSD of each frequency band relative to the control task in each individual task at a specific electrode site (Figure 6). The average relative PSD was then computed using the three relative PSD from the three trials. The same process was done on all the wave bands at all the electrode sites in the seven balance tasks for each participant. All the average relative PSD calculated was then analyzed using SPSS.
Figure 6.
Formulation of Relative PSD and Average Relative PSD of Alpha band at POz in one of the tasks, TEC-FOAM.
Data analysis
Statistical analysis was performed using SPSS for Windows version 20.0 [43]. The significance level was set at 0.05. The Kolmogorov-Smirnov test was used to assess for normality of the continuous variables. The distribution of EEG and weight were not approximately normal, therefore, non-parametric tests were used in the analyses of these variables. Mann-Whitney U-test was used to compare the mean weight by gender. Friedman test was used to examine if significant differences existed in the average relative PSD of a specific wave band at each EEG electrode site among the eight balance tasks. Wilcoxon signed ranks test was then used to assess whether differences in the average relative PSD were significantly different between balance tasks.
Mean age and height by gender were compared using independent t-test. Repeated measures analysis of variance (ANOVA), was used to examine if significant differences existed in the postural sway (average Coefficient of Variation) among the eight balance tasks and Bonferroni test was then used as post hoc pairwise comparison to assess for significant differences in postural sway within the balance tasks.
Results
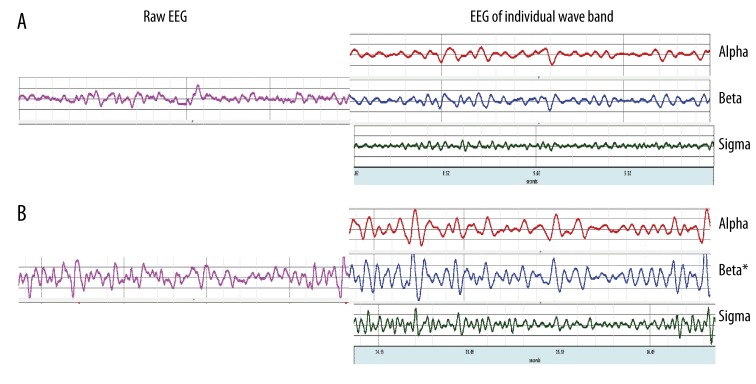
Figure 7 showed the raw EEG data and EEG of individual wave bands at POz during the least difficult task and the most difficult task.
Figure 7.
EEG activity at POz during the least difficult task, FAEO-FIRM (A) and the most difficult balance task, TEC-FOAM (B). Raw EEG is shown on the left side of the Figure and EEG of individual wave bands (alpha, beta and sigma bands) are shown on the right side of the Figure. * indicates significant difference relative to the control task, p<0.05.
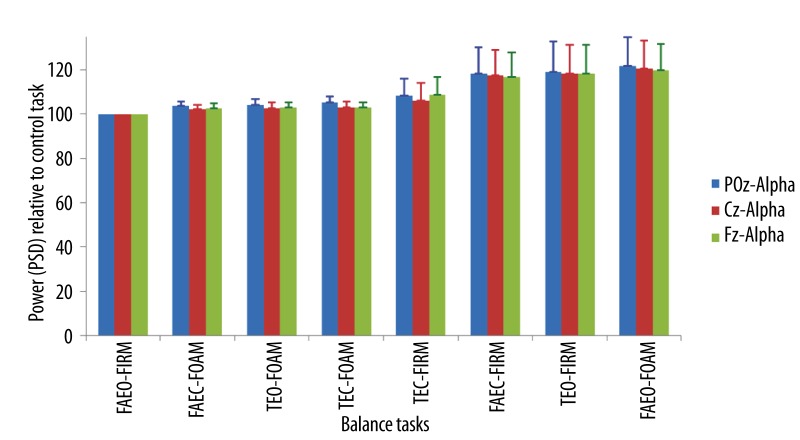
The average increase of alpha band power ranged from 4–22% at POz, 2–18% at Cz and, 3–20% at Fz when compared to the control task. These changes were not significant relative to the control task (Figure 8).
Figure 8.
Mean ±SEM of the power (PSD) of alpha band in the balance tasks relative to the control task at different EEG sites.
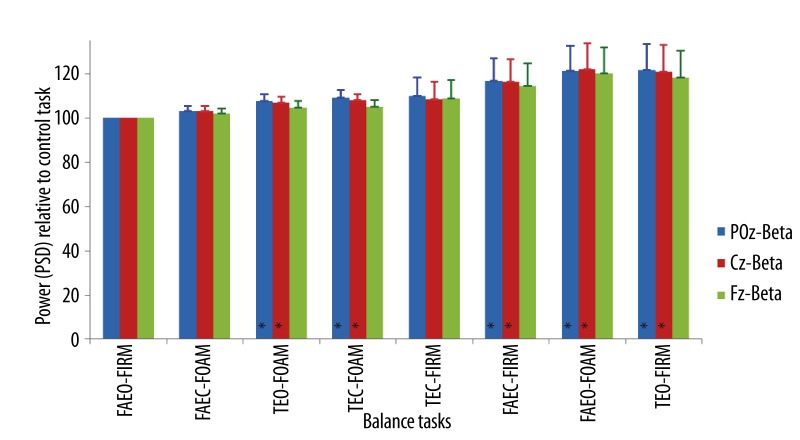
The average increase of beta band power ranged from 3–22% at POz and Cz and, 2–18% at Fz when compared to the control task. There were significant increases in beta band power at POz (Z=18.96, p<0.01) and Cz (Z=16.67, p=0.02) relative to the control task (Figure 9). In task, TEO-FOAM, eyes were open with two sensory factors (base of support and surface compliance) altered relative to the control task, beta power increased by 8% and 9% at POz (Z=−2.34, p=0.02) and Cz (Z=−2.20, p=0.03) respectively. When all three factors (vision, base of support and surface compliance) were altered from the control tasks in TEC-FOAM, beta power increased by 9% at POz (Z=−2.30, p=0.02) and 8% at Cz (Z=−2.49, p=0.01). When eyes were closed in FAEC-FIRM, beta power increased by 17% and 16% at POz (Z=−2.15, p=0.03) and Cz (Z=−2.01, p=0.04) respectively. When eyes were open with one sensory factor changed, such as the surface compliance was altered to foam in FAEO-FOAM, beta power increased by 21% at POz (Z=−2.44, p=0.02) and 22% at Cz (Z=−2.53, p=0.01). When eyes were open with another sensory factor, base of support, altered to tandem standing in TEO-FIRM, beta power increased by 22% at POz (Z=−2.63, p<0.01) and 21% at Cz (Z=−2.34, p=0.02).
Figure 9.
Mean ±SEM of the power (PSD) of beta band in the balance tasks relative to the control task at different EEG sites. * p<0.05.
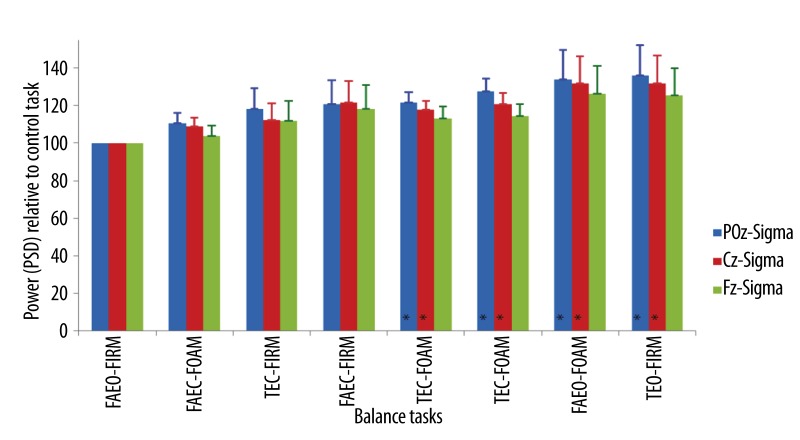
The average increase of the sigma band power ranged from 11–36% at POz, 9–32% at Cz and 4–26% at Fz when compared to the control task (Figure 10). There were significant increases in sigma band power at POz (Z=28.96, p<0.001) and Cz (Z=18.12, p=0.01) relative to the control task. When eyes were open with both sensory factors (base of support and surface compliance) were altered relative to the control task in TEO-FOAM, sigma power increased by 21% at POz (Z=−2.96, p<0.01) and 18% at Cz (Z=−2.68, p<0.01). When all the sensory factors (vision, base of support and surface compliance) were altered from the control tasks in TEC-FOAM, sigma power increased by 27% at POz (Z=−3.01, p<0.01) and 21% at Cz (Z=−2.68, p<0.01). When eyes were open with one sensory factor, surface compliance, changed to foam in FAEO-FOAM, the sigma power increased by 34% and 32% at POz (Z=−2.86, p<0.01) and Cz (Z=−2.63, p<0.01) respectively. When eyes were open with another sensory factor, base of support, altered to tandem standing in TEO-FIRM, the sigma power increased by 36% at POz (Z=−2.82, p<0.01) and 32% at Cz (Z=−2.63, p<0.01).
Figure 10.
Mean ±SEM of the power (PSD) of sigma band in the balance tasks relative to the control task at different EEG sites. * p<0.05.
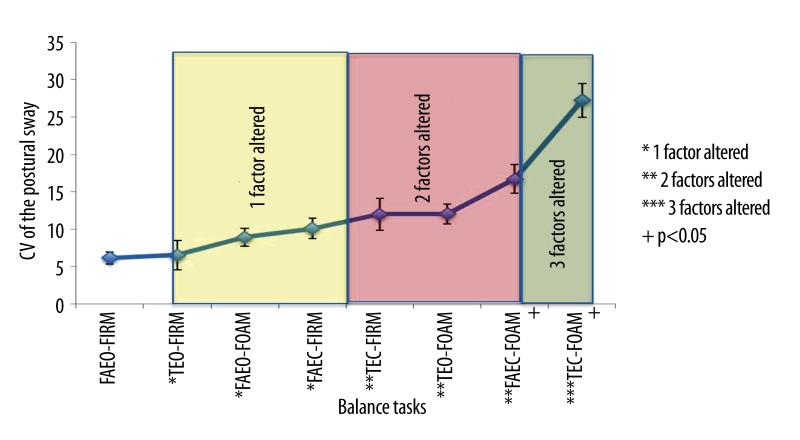
The result of the postural sway ranked in the order of task difficulty has been reported in a previous study [40]. Figure 11 showed that there was more postural sway when the number of sensory factors altered in the balance tasks increased. The powers (PSD) of all the wave bands at POz, Cz and Fz in response to the increasing order of task difficulty are shown in Figures 12–14.
Figure 11.
Mean ±SEM of postural sway (Coefficient of Variation of the postural sway) in 8 balance tasks.
Figure 12.
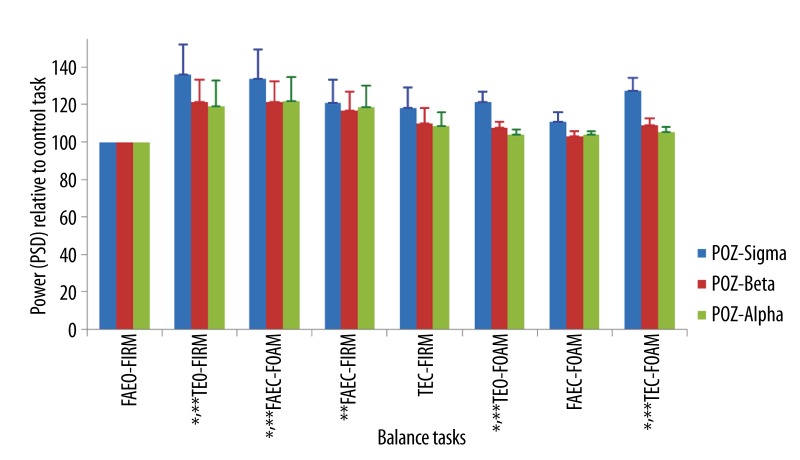
Mean ±SEM of the power of all wave bands in the order of the balance task difficulty at POz. * p<0.05 for sigma wave; ** p<0.05 for beta wave.
Figure 14.
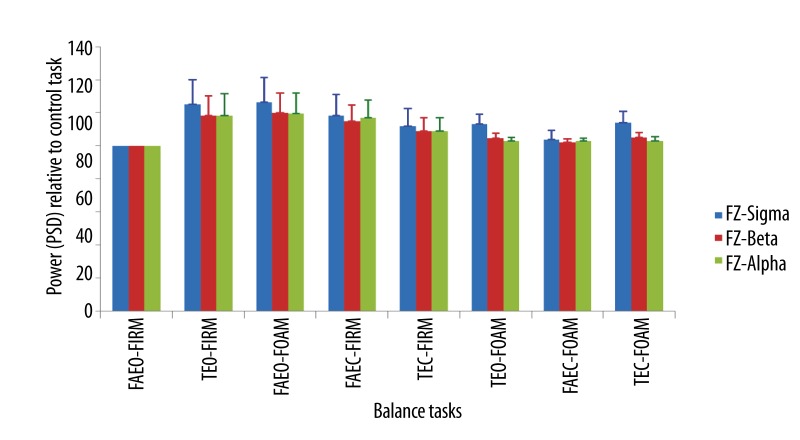
Mean ±SEM of the power of all wave bands in the order of the balance task difficulty at Fz.
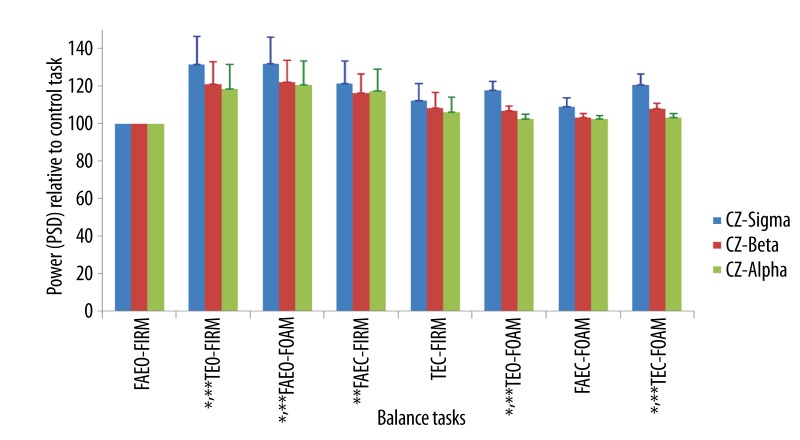
The power of all the wave bands at the three electrode sites increased in all the balance tasks when compared to the control tasks. The relative power of beta and sigma wave bands increased significantly at the parietal (Figure 12) and central area (Figure 13) of the brain in tasks, TEO-FIRM, FAEO-FOAM, TEO-FOAM, in which eyes were open together with either one sensory factor (base of support or surface compliance) or two sensory factors (base of support and surface compliance) altered, and in task, TEC-FOAM, with three sensory factors (vision, base of support and surface compliance) altered from the control task. The relative power of beta and sigma bands decreased when eyes were closed together with one sensory factor altered in TEC-FIRM and FAEC-FOAM. When only one sensory factor, vision, was altered from the control task in FAEC-FIRM, the power of beta wave bands increased significantly. No significant increase in the relative PSD of all the wave bands was found at Fz electrode site (Figure 14).
Figure 13.
Mean ±SEM of the power of all wave bands in the order of the balance task difficulty at Cz. * p<0.05 for sigma wave; ** p<0.05 for beta wave.
Discussion
Effective balance training challenges sensorimotor integration and induces adaptation in the central nervous system. However, little is known about the cortical response to static balance exercises routinely used in sensorimotor training. The purpose of this study was to investigate the changes in the PSD of the EEG in eight balance tasks with different levels of task difficulty.
The power of beta and sigma bands increased significantly at the parietal and central area of the brain in tasks with eyes open together with one sensory factor (base of support or surface compliance) or two sensory factors (base of support and surface compliance) altered, and in task with three sensory factors (vision, base of support and surface compliance) altered from the control task.
Our results provide evidence that there was an increase in cortical activity during the commonly used static balance tasks. Our finding is consistent with previous studies that reported the presence of cortical excitability during normal unperturbed quiet standing [44], and an increase of the corticospinal excitability during unstable stance [45,46]. Although Slobounov and colleagues used a different EEG analysis technique, they also reported cortical activity preceding and accompanying the postural movements [10].
Our results showed that the power of beta and sigma bands was higher when eyes were opened in combination with one or two sensory factors (base of support or surface compliance) altered in the balance tasks, but with no significant changes in the postural sway. This may be due to the processing of visual information available since the eyes were open. This finding concurs with a previous study showing that there is increased activation in the parietal area with visual demand [47]. Barry and colleagues also provided evidence for the cortical processing with visual input [48]. One study has shown that the central nervous system is able to re-weight the sensory information based on the sensory context [2]. It is possible that our normal participants with no impairment in their sensory systems, were able to re-weight the dependence from the somatosensory system to the visual input for balance and consequently postural sway was not significantly affected.
When the tasks became more difficult with vision and somatosensory information altered, the postural sway increased but the EEG band power decreased relative to the less difficult tasks. Studies have shown that H-reflexes diminished when eyes were closed suggesting that there was an increase in the supraspinal excitability in the postural control when vision was compromised [49,50]. In our study, the reduced power in the EEG with eyes closed may due to a shift of the postural control from the cortex to the subcortical structures. These findings are consistent with previous studies suggesting the importance of subcortical structure in the postural control [51,52], and an increase in the subcortical activity when postural demand increases [5].
During the most difficult task with vision, base of support and surface compliance altered, postural sway became the highest among all the tasks, but the band power of beta and sigma increased significantly at the central and parietal area of the brain relative to the control task. Although there may have been an increase in the subcortical activity as the tasks became more difficult, the increase in the EEG power in the most difficult task suggests that increased cortical activity was required in the more challenging tasks. Previous studies have suggested that cerebral cortex contributes to the postural control by sensorimotor processing of postural instability [10,11] or modification of postural responses through cortical response loops [7,53]. In addition, Teasdale and colleagues have also reported that more cognitive processing was required when the postural task became more difficult [54]. It is possible when balance task becomes extremely challenging with both visual and somatosensory information altered, the demand for cortical processing increases.
In our study, young healthy participants were recruited; the result should not be extrapolated to other populations. Studies on older adults or patient populations are recommended. Also, this may be of special interest in individuals with diabetes when neuropathies are known. Furthermore, our sample size is small; a larger sample size is suggested in future studies. Future research is also recommended to examine the EEG power change of each wave band with balance training.
Conclusions
The results of this study provide evidence that there were cortical involvement in the static balance tasks routinely used in the sensorimotor training for postural control. Our findings concur with previous studies that showed that cortical activation increased when eyes were open in the balance tasks [47,48]. Our study also supports previous findings that there was increased in the subcortical activity when tasks became more difficult [5]. When task became extremely challenging with all the sensory factors altered, the demand on the cortical processing increased as reported in other study [55]. The results of this study indicated that these balance tasks may induce cortical adaptation for postural control when used in balance training.
Acknowledgements
We thank Ms. Diamond Nguyen, Sophia Rodrigues, Riya D. Lodha and Pooja A. Potnis for their help in the data collection and computing process. Special thanks also goes to Advanced Brain Monitoring for the software support.
Footnotes
Source of support: Departmental sources
References
- 1.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 2.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 3.Horak FB, Shupert C. Vestibular rehabilitation. Second ed. F. A. Davis; 2000. Role of the vestibular system in postural control. [Google Scholar]
- 4.Keck ME, Pijnappels M, Schubert M, et al. Stumbling reactions in man: influence of corticospinal input. Electroencephalogr Clin Neurophysiol. 1998;109:215–23. doi: 10.1016/s0924-980x(98)00009-5. [DOI] [PubMed] [Google Scholar]
- 5.Ouchi Y, Okada H, Yoshikawa E, et al. Brain activation during maintenance of standing postures in humans. J Neurol. 1999;122( Pt 2):329–38. doi: 10.1093/brain/122.2.329. [DOI] [PubMed] [Google Scholar]
- 6.Mihara M, Miyai I, Hatakenaka M, et al. Role of the prefrontal cortex in human balance control. NeuroImage. 2008;43:329–36. doi: 10.1016/j.neuroimage.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114:1339–48. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goble DJ, Coxon JP, Van Impe A, et al. Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. J Neurosci. 2011;31:16344–52. doi: 10.1523/JNEUROSCI.4159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saitou K, Washimi Y, Koike Y, et al. Slow negative cortical potential preceding the onset of postural adjustment. Electroencephalogr Clin Neurophysiol. 1996;98:449–55. doi: 10.1016/0013-4694(96)95004-x. [DOI] [PubMed] [Google Scholar]
- 10.Slobounov S, Hallett M, Stanhope S, Shibasaki H. Role of cerebral cortex in human postural control: an EEG study. Clin Neurophysiol. 2005;116:315–23. doi: 10.1016/j.clinph.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Adkin AL, Quant S, Maki BE, McIlroy WE. Cortical responses associated with predictable and unpredictable compensatory balance reactions. Exp Brain Res. 2006;172:85–93. doi: 10.1007/s00221-005-0310-9. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs JV, Fujiwara K, Tomita H, et al. Changes in the activity of the cerebral cortex relate to postural response modification when warned of a perturbation. Clin Neurophysiol. 2008;119:1431–42. doi: 10.1016/j.clinph.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mochizuki G, Sibley KM, Esposito JG, et al. Cortical responses associated with the preparation and reaction to full-body perturbations to upright stability. Clin Neurophysiol. 2008;119:1626–37. doi: 10.1016/j.clinph.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Iwai K, Mori N, Oie M, et al. Human T-cell leukemia virus type 1 tax protein activates transcription through AP-1 site by inducing DNA binding activity in T cells. Virology. 2001;279:38–46. doi: 10.1006/viro.2000.0669. [DOI] [PubMed] [Google Scholar]
- 15.Anand V, Buckley JG, Scally A, Elliott DB. Postural stability changes in the elderly with cataract simulation and refractive blur. Invest Ophthalmol Vis Sci. 2003;44:4670–75. doi: 10.1167/iovs.03-0455. [DOI] [PubMed] [Google Scholar]
- 16.Lyon IN, Day BL. Control of frontal plane body motion in human stepping. Exp Brain Res. 1997;115:345–56. doi: 10.1007/pl00005703. [DOI] [PubMed] [Google Scholar]
- 17.Jeka JJ, Schoner G, Dijkstra T, et al. Coupling of fingertip somatosensory information to head and body sway. Exp Brain Res. 1997;113:475–83. doi: 10.1007/pl00005600. [DOI] [PubMed] [Google Scholar]
- 18.Eiken HG, Oie E, Damas JK, et al. Myocardial gene expression of leukaemia inhibitory factor, interleukin-6 and glycoprotein 130 in end-stage human heart failure. Eur J Clin Invest. 2001;31:389–97. doi: 10.1046/j.1365-2362.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- 19.Liaw MY, Chen CL, Pei YC, et al. Comparison of the static and dynamic balance performance in young, middle-aged, and elderly healthy people. Chang Gung Med J. 2009;32:297–304. [PubMed] [Google Scholar]
- 20.Granacher U, Gollhofer A, Kriemler S. Effects of balance training on postural sway, leg extensor strength, and jumping height in adolescents. Res Q Exerc Sport. 2010;81:245–51. doi: 10.1080/02701367.2010.10599672. [DOI] [PubMed] [Google Scholar]
- 21.Granacher U, Wick C, Rueck N, et al. Promoting balance and strength in the middle-aged workforce. Int J Sports Med. 2011;32:35–44. doi: 10.1055/s-0030-1267214. [DOI] [PubMed] [Google Scholar]
- 22.Madureira MM, Takayama L, Gallinaro AL, et al. Balance training program is highly effective in improving functional status and reducing the risk of falls in elderly women with osteoporosis: a randomized controlled trial. Osteoporos Int. 2007;18:419–25. doi: 10.1007/s00198-006-0252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009:CD007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halvarsson A, Franzen E, Faren E, et al. Long-term effects of new progressive group balance training for elderly people with increased risk of falling – a randomized controlled trial. Clin Rehabil. :2012. doi: 10.1177/0269215512462908. [DOI] [PubMed] [Google Scholar]
- 26.Rhee HS, Kim YH, Sung PS. A randomized controlled trial to determine the effect of spinal stabilization exercise intervention based on pain level and standing balance differences in patients with low back pain. Med Sci Monit. 2012;18(3):CR174–81. doi: 10.12659/MSM.882522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granacher U, Muehlbauer T, Zahner L, et al. Comparison of traditional and recent approaches in the promotion of balance and strength in older adults. Sports Med. 2011;41:377–400. doi: 10.2165/11539920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.DiStefano LJ, Clark MA, Padua DA. Evidence supporting balance training in healthy individuals: a systemic review. J Strength Cond Res. 2009;23:2718–31. doi: 10.1519/JSC.0b013e3181c1f7c5. [DOI] [PubMed] [Google Scholar]
- 29.Amiridis IG, Hatzitaki V, Arabatzi F. Age-induced modifications of static postural control in humans. Neurosci Lett. 2003;350:137–40. doi: 10.1016/s0304-3940(03)00878-4. [DOI] [PubMed] [Google Scholar]
- 30.Era P, Sainio P, Koskinen S, et al. Postural balance in a random sample of 7,979 subjects aged 30 years and over. Gerontology. 2006;52:204–13. doi: 10.1159/000093652. [DOI] [PubMed] [Google Scholar]
- 31.Taube W, Gruber M, Beck S, et al. Cortical and spinal adaptations induced by balance training: correlation between stance stability and corticospinal activation. Acta Physiol (Oxf) 2007;189:347–58. doi: 10.1111/j.1748-1716.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 32.Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- 33.Liepert J, Terborg C, Weiller C. Motor plasticity induced by synchronized thumb and foot movements. Exp Brain Res. 1999;125:435–39. doi: 10.1007/s002210050700. [DOI] [PubMed] [Google Scholar]
- 34.Beck S, Taube W, Gruber M, et al. Task-specific changes in motor evoked potentials of lower limb muscles after different training interventions. Brain Res. 2007;1179:51–60. doi: 10.1016/j.brainres.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 35.Schubert M, Beck S, Taube W, et al. Balance training and ballistic strength training are associated with task-specific corticospinal adaptations. Eur J Neurosci. 2008;27:2007–18. doi: 10.1111/j.1460-9568.2008.06186.x. [DOI] [PubMed] [Google Scholar]
- 36.Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–56. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Dorneich MC, Whitlow SD, Mathan S, et al. Supporting real-time cognitive state classification one mobile individual. Journal of Cognitive Engineering and Decision Making. 2007;1:2240–70. [Google Scholar]
- 38.Berka C, Levendowski DJ, Lumicao MN, et al. EEG correlates of task engagement and mental workload in vigilance, learning, and memory tasks. Aviat Space, Environ Med. 2007;78:B231–44. [PubMed] [Google Scholar]
- 39.Stevens RH, Galloway TL, Wang P, Berka C. Cognitive neurophysiologic synchronies: what can they contribute to the study of teamwork? Human factors. 2012;54:489–502. doi: 10.1177/0018720811427296. [DOI] [PubMed] [Google Scholar]
- 40.Tse YYF, Petrofsky JS, Lee B, et al. Postural sway and EMG analysis of hip and ankle muscles during eight balance training tasks. Int J Ther Rehabil. 2012 (submitted) [Google Scholar]
- 41.Petrofsky JS, Lohman E, Lohman T. A device to evaluate motor and autonomic impairment. Med Eng Phys. 2009;31:705–12. doi: 10.1016/j.medengphy.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Kimura T, Kouzaki M, Masani K, Moritani T. Unperceivable noise to active light touch effects on fast postural sway. Neurosci Lett. 2012;506:100–3. doi: 10.1016/j.neulet.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 43.Kouzaki M, Masani K. Postural sway during quiet standing is related to physiological tremor and muscle volume in young and elderly adults. Gait Posture. 2012;35:11–17. doi: 10.1016/j.gaitpost.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 44.Qiu F, Cole MH, Davids KW, et al. Enhanced somatosensory information decreases postural sway in older people. Gait Posture. 2012;35:630–35. doi: 10.1016/j.gaitpost.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Solopova IA, Kazennikov OV, Deniskina NB, et al. Postural instability enhances motor responses to transcranial magnetic stimulation in humans. Neurosci Lett. 2003;337:25–2. doi: 10.1016/s0304-3940(02)01297-1. [DOI] [PubMed] [Google Scholar]
- 46.Lavoie BA, Cody FW, Capaday C. Cortical control of human soleus muscle during volitional and postural activities studied using focal magnetic stimulation. Exp Brain Res. 1995;103:97–107. doi: 10.1007/BF00241968. [DOI] [PubMed] [Google Scholar]
- 47.Mizelle JC, Forrester L, Hallett M, Wheaton LA. Electroencephalographic reactivity to unimodal and bimodal visual and proprioceptive demands in sensorimotor integration. Exp Brain Res. 2010;203:659–70. doi: 10.1007/s00221-010-2273-8. [DOI] [PubMed] [Google Scholar]
- 48.Jeka J, Kiemel T, Creath R, et al. Controlling human upright posture: velocity information is more accurate than position or acceleration. J Neurophysiol. 2004;92:2368–79. doi: 10.1152/jn.00983.2003. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman MA, Koceja DM. The effects of vision and task complexity on Hoffmann reflex gain. Brain Res. 1995;700:303–7. doi: 10.1016/0006-8993(95)01082-7. [DOI] [PubMed] [Google Scholar]
- 50.Earles DR, Koceja DM, Shively CW. Environmental changes in soleus H-reflex excitability in young and elderly subjects. Int J Neurosci. 2000;105:1–13. doi: 10.3109/00207450009003261. [DOI] [PubMed] [Google Scholar]
- 51.Doyon J, Gaudreau D, Laforce R, Jr, et al. Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain Cogn. 1997;34:218–45. doi: 10.1006/brcg.1997.0899. [DOI] [PubMed] [Google Scholar]
- 52.Prentice SD, Drew T. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol. 2001;85:679–98. doi: 10.1152/jn.2001.85.2.679. [DOI] [PubMed] [Google Scholar]
- 53.Maki BE, McIlroy WE. Cognitive demands and cortical control of human balance-recovery reactions. J Neural Transm. 2007;114:1279–96. doi: 10.1007/s00702-007-0764-y. [DOI] [PubMed] [Google Scholar]
- 54.Teasdale N, Simoneau M. Attentional demands for postural control: the effects of aging and sensory reintegration. Gait Posture. 2001;14:203–10. doi: 10.1016/s0966-6362(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 55.Teasdale N, Stelmach GE, Breunig A. Postural sway characteristics of the elderly under normal and altered visual and support surface conditions. J Gerontol. 1991;46:B238–44. doi: 10.1093/geronj/46.6.b238. [DOI] [PubMed] [Google Scholar]