Abstract
Members of the seven-transmembrane receptor (7TMR), or G protein-coupled receptor (GPCR), superfamily represent some of the most successful targets of modern drug therapy, with proven efficacy in the treatment of a broad range of human conditions and disease processes. It is now appreciated that β-arrestins, once viewed simply as negative regulators of traditional 7TMR-stimulated G protein signaling, act as multifunctional adapter proteins that regulate 7TMR desensitization and trafficking and promote distinct intracellular signals in their own right. Moreover, several 7TMR biased agonists, which selectively activate these divergent signaling pathways, have been identified. Here we highlight the diversity of G protein- and β-arrestin-mediated functions and the therapeutic potential of selective targeting of these in disease states.
Signaling by seven transmembrane receptors (7TMRs)
7TMRs represent the largest single family of cell surface receptors [1]. Members of this receptor superfamily are uniquely expressed throughout the body and are activated by a diverse host of ligands, including biogenic amines, hormones, peptides, proteins, growth factors, lipids, nucleic acids, odorants, tastants, protons (H+), ions (Ca2+) and light (photons) [1]. There are also several 7TMRs for which no clear ligand has yet been identified, the so-called orphan receptors [2]. The combination of ligand diversity and unique tissue expression makes 7TMRs attractive drug targets, with approximately 40% of modern drug therapy targeting 7TMRs, either directly or indirectly [3]. These include blockbusters such as opiates, antihistamines, α- and β-blockers, β-agonists, dopamine receptor blockers, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors and selective serotonin reuptake inhibitors.
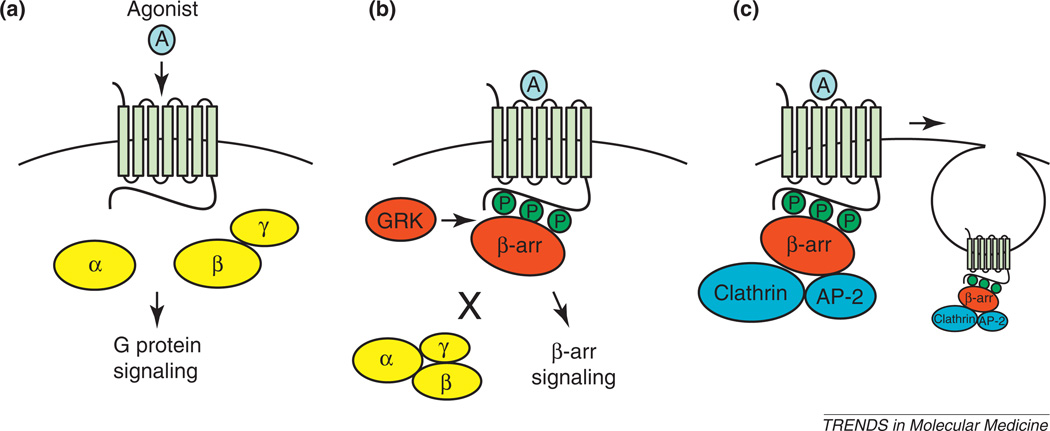
Drugs that directly target a 7TMR have been classically described as either agonists or antagonists for G protein signaling. The binding of an agonist to a 7TMR promotes a conformational change that results in the activation of receptor-associated heterotrimeric G proteins and consequent downstream signaling (Figure 1a). In addition to G protein activation, agonist binding promotes a process known as desensitization, which involves rapid phosphorylation of the receptor at the C terminus and intracellular loops, in large part by the G protein-coupled receptor kinases (GRKs). This combination of conformational change and phosphorylation dramatically increases the affinity of the receptor for a small family of multifunctional 7TMR regulatory or adaptor proteins known as the β-arrestins (β-arrestin1 and β-arrestin2). This association blocks subsequent G protein activation and plays an almost universal role in facilitating traditional 7TMR desensitization (Figure 1b). This process is complemented by the association of β-arrestins with second-messenger-metabolizing or -converting enzymes and components of the internalization machinery, which target the degradation of G protein-mediated signals and facilitate the removal of receptors from the cell surface (Figure 1c).
Figure 1.
7TMR signaling and regulation by the GRKs and β-arrestins. (a) Agonist-stimulated, 7TMR-mediated G protein activation. (b) Subsequent desensitization of 7TMR-mediated G protein signaling and activation of β-arrestin-mediated signals. (c) β-Arrestin-mediated 7TMR receptor internalization involving clathrin and AP-2.
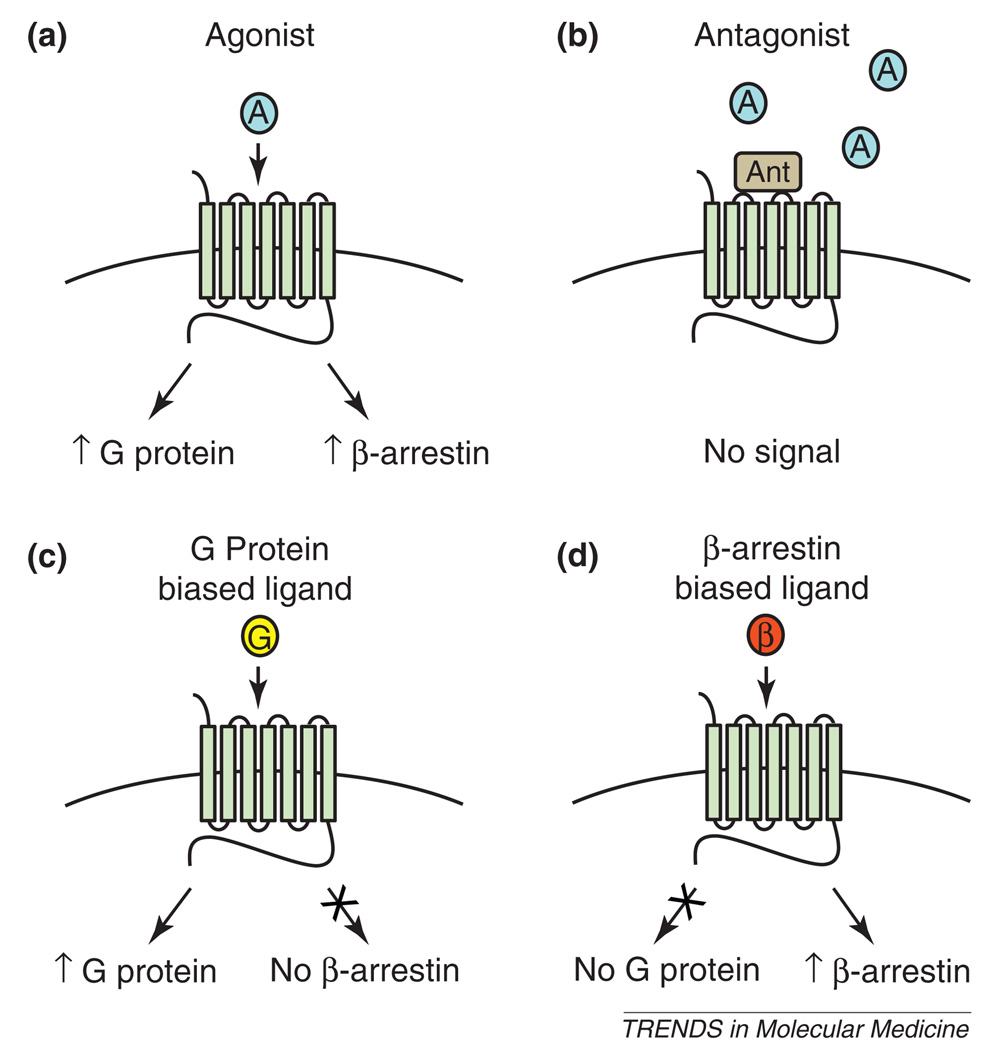
Classically, agonists have been described as having linear efficacy [4], in which activation of the multiple signaling pathways downstream of a receptor (e.g. G protein signaling, receptor phosphorylation, β-arrestin recruitment and internalization) correlates with the degree of agonist-mediated receptor activation from partial to full (Figure 2a). In this scheme, antagonists have been described almost exclusively by their ability to block agonist-stimulated G protein activation. This view, in which agonists and antagonists can be defined purely in the context of a single form of signaling (most commonly G protein-mediated signaling), has guided the vast majority of modern 7TMR-based drug discovery (Figure 2a,b).
Figure 2.
Differential 7TMR-stimulated G protein- and β-arrestin-mediated signaling. (a) Traditional agonist with linear efficacy. (b) Traditional antagonist, blocking all aspects of 7TMR signaling. (c) G protein-biased ligand, promoting 7TMR G protein signaling in the absence of β-arrestin-mediated desensitization, internalization and signaling. (d) β-Arrestin-biased ligand, promoting β-arrestin-mediated signaling and internalization in the absence of G protein activation.
However, it has recently been appreciated that in addition to regulating receptor-stimulated G protein signaling, the β-arrestins are also capable of initiating distinct signals in their own right (Figure 1b). These signals are often both spatially and temporally distinct, and result in unique cellular and physiological or pathophysiological consequences (see [5–7] for a detailed review of β-arrestin signaling). With the discovery of β-arrestin-mediated signaling has come a new appreciation of biased agonism [4,8–10]. Biased agonism is best understood in a model in which different 7TMR active conformations are either competent for the full range of receptor activities or only for a restricted subset of them. Whereas balanced ligands stabilize the conformations that are competent for signaling to all downstream pathways, biased ligands stabilize only those conformations that are capable of promoting a subset of signaling effects [4,8–10]. For example, ligands can show bias for either G protein- (G protein-biased) or β-arrestin-mediated (β-arrestin-biased) signaling (Figure 2c,d). Such bias clearly adds a layer of texture [11] to the definition of ligand action. Using this framework, we could hypothesize that such ligands could more selectively target beneficial signaling and even block or negate detrimental or unwanted actions of receptor activation (e.g. side effects, toxicity or tolerance). Indeed, over the last decade a diversity of biased ligands for 7TMRs have been identified that selectively activate G proteins or β-arrestins (Table 1), and several of these seem to have distinct functional consequences when compared to traditional ligands with more linear efficacy [9]. Realization of the complexity of 7TMR signaling impacts both the way we describe 7TMR functions and how we apply these definitions to future 7TMR-based discovery and drug therapy.
Table 1.
7TMR ligands showing β-arrestin or G protein bias. For effects that are merely consistent with bias, ligands are in italics
| Receptor | Biased ligands | Bias | Reference(s) |
|---|---|---|---|
| A3A adenosine | CCPA, DPMA, MRS542, MRS1760 | β-Arrestin | [105] |
| DBXRM | G protein | [105] | |
| APJ (Apelin) | Apelin 13, Apelin 36 | Differential receptor trafficking | [106] |
| AT1 | Sar1,Ile4,Ile8 Ang II (SII), Sar1,Lys5,Ala8 Ang II (TRV120023) | β-arrestin | [61–64,66,67,69,107–114] |
| Sar1,Tyr5,Pro7-NH2 Ang II (TRV120026) | |||
| Sar1,D-Ala8 Ang II (TRV120027), troglitazone | |||
| α2A-Adrenergic | Clonidine, guanfacine | Differential signaling, regulation and trafficking | [115] |
| Multiple ligands | Differential G protein signaling | [116] | |
| β1-Adrenergic | Alprenolol, carvedilol | β-Arrestin | [57] |
| β2-Adrenergic | Carvedilol, ICI118551, propranolol cyclopenylbutanephrine | β-Arrestin | [58,59,117] |
| Norepinephrin | G protein | [118] | |
| Bombesin | [D-Arg1,D-Phe5,D-Trp7,9,Leu11] substance P | Differential signaling | [119] |
| CB1 | Δ9-Tetrahydrocannabinol | Differential tolerance (WT vs β-arrestin2 KO mice) | [120] |
| Δ9-Tetrahydrocannabinol, anandamide, WIN 55,212-2, CP 55940, 2-arachidonoyl glycerol | Differential receptor trafficking | [121] | |
| CCKA | D-Tyr-Gly-([Nle28,31,D-Trp30]CCK-26–32)-phenethyl ester | Internalizing antagonist | [122] |
| CCR2 | GMME1 | G protein | [123] |
| CCR5 | AOP–RANTES, CAP-RANTES, MET-RANTES, NNY-RANTES, PSC-RANTES | Differential internalization and trafficking | [42–44,124,125] |
| Monoclonal antibody MC-1 | Internalizing antagonist | [126] | |
| Monoclonal antibody MC-4 | Allosteric blocker of internalization | [126] | |
| CCR7 | CCL19, CCL21 | G protein, differential GRK | [127–129] |
| CTR2 | Multiple ligands | Reversal of potency | [130] |
| CXCR2 | IL-8, GROα | Reversal of potency | [131] |
| CXCR4 | ASLW | G protein (allosteric) | [132] |
| CRTH2 | 1-(4-Ethoxyphenyl)-5-methoxy-2-methylindole-3-carboxylic acid, Nα-tosyltryptophan | G protein (allosteric) | [133] |
| D1 dopamine | Multiple ligands | G protein, desensitization and internalization | [134–136] |
| D2 dopamine | Multiple ligands | Block D2 stimulated β-arrestin recruitment | [137,138] |
| Dihydrexidine (DHX), N-n-propyl-DHX | Differential signaling | [139,140] | |
| EDG1 | FTY720 (fingolimod) | β-Arrestin | [141,142] |
| EDG3 | FTY720-P (in vivo activated FTY720) | G protein | [142,143] |
| EP4 | Multiple ligands | β-Arrestin and G protein | [144] |
| ETAR | BQ123 | Internalizing antagonist | [145] |
| FSH | Multiple ligands | β-Arrestin and G protein | [146,147] |
| GLP-1 | Oxyntomodulin, glucagon | G protein | [148] |
| Novo Nordisk compound 2 (6,7-dichloro2-methylsulfonyl-3-tert-butylaminoquinoxaline), quercetin | Allosteric ligands promote differential signaling | [149] | |
| GnRH | Multiple ligands | Internalizing antagonists, altered trafficking and differential signaling | [150–152] |
| GPR109a | Multiple ligands | Reduced internalization | [74] |
| M1 muscarinic | AC260584, TBPB | Allosteric G protein | [153] |
| AC-42, AC-260584, N-desmethylclozapine, xanomeline, BQCA | Allosteric ligands promote differential signaling and receptor trafficking | [154,155] | |
| AF102B, AF150, AF151, pilocarpine | Differential signaling | [156,157] | |
| M2 muscarinic | Multiple ligands | Allosteric ligands promote differential signaling | [158] |
| Motilin (GPR38) | ABT-229 | Differential receptor trafficking | [159] |
| δ-OR | TAN-67, morphine | G protein | [158,160–162] |
| κ-OR | Etorphine, levorphanol | G protein | [163] |
| norBNI, JDTic | Differential signaling | [164] | |
| µ-OR | Morphine, heroin | G protein | [20,161,162,165–167] |
| OX1 | Atosiban | Differential signaling | [168] |
| P2Y2 | ATP, UTP | Differential β-arrestin trafficking | [169,170] |
| PAF | WEB2086 | Internalizing antagonist | [171] |
| PAR-2 | rPAR2-Ala37–38, rPAR2-Leu37Ser38 | G protein | [172] |
| (receptor-tethered mutant ligands) | |||
| PTH1 | (D-Trp12,Tyr34)-PTH(7–34) | β-Arrestin | [90,91] |
| [Trp1]PTHrp-(1–36) | G protein | [89,90] | |
| 5-HT1A | Ipsaperone, F14,679, flesinoxan | Differential signaling | [173,174] |
| 5-HT1B | Naratriptan | Differential signaling | [175] |
| 5-HT2A | Multiple ligands | Internalizing antagonist and differential signaling | [176–184] |
| 5-HT2C | Multiple ligands | Differential signaling | [184–187] |
| SST2 | Pasireotide (SOM230) | G protein | [188] |
| SST5 | KE108, BIM-23244, L-817,818 | Reduced internalization | [189] |
| V2 vasopressin | MCF14, MCF18, MCF57 | G protein and chaperones that modulate trafficking | [37] |
| SR49059 | Chaperone that modulates trafficking | [36] | |
| VRQ397 (CRAVKY) | Allosteric ligand promoting differential signaling | [190] |
As described above, the β-arrestins are involved in multiple aspects of 7TMR-mediated signaling and regulation, and ligand bias has been described for numerous receptor subtypes (Table 1). Moreover, the β-arrestins have been implicated in numerous aspects of physiology and the pathophysiology of disease [6,7]. In the following sections, we highlight several examples for which targeting of selected β-arrestin-mediated activities might have or actually has therapeutic benefit (Table 2). Although these different activities might be linked functionally, for the purposes of discussion we broadly split the different roles of β-arrestin into desensitization of G protein signaling, receptor internalization or trafficking, and β-arrestin-mediated signaling.
Table 2.
Physiology and pathophysiology of β-arrestin-mediated processes
| Therapeutic area | β-Arrestin-mediated process (isoform) | Function | References |
|---|---|---|---|
| Cardiovascular | AT1R-stimulated increase in cardiac performance and preserved stroke volume | Signaling | [64] |
| AT1R-stimulated cardiomyocyte growth or hypertrophy, proliferation and contractility | Signaling | [60–63,114] | |
| AT1R-stimulated vascular smooth-muscle-cell protein synthesis and antiapoptosis | Signaling | [65,66] | |
| AT1R-stimulated salt appetite | Signaling | [70] | |
| A G protein-uncoupled AT1R mutant stimulated cardiac hypertrophy | Signaling | [60] | |
| Mechanical stress induces AT1R-stimulated β-arrestin recruitment and signaling | Signaling | [191,192] | |
| Differential effects on experimental vascular injury (1 and 2) | Signaling | [193] | |
| Cardiac βAR desensitization (1) | Desensitization | [12] | |
| β1AR-stimulated EGFR transactivation and cardioprotection (1 and 2) | Signaling | [52] | |
| β-Arrestin1 regulates vascular smooth muscle P2Y-purinoreceptor signaling | Desensitization | [194] | |
| Renal | AT1R-stimulated adrenocortical aldosterone secretion (1) | Signaling | [67] |
| cNDI and the nephrogenic syndrome of inappropriate antidiuresis are associated with V2R mutations that alter receptor and β-arrestin dynamics, signaling, desensitization and trafficking | Desensitization and trafficking | [34–38] | |
| Pulmonary | β-Arrestin2 KO mice are resistant to airway inflammation and airway smooth-muscle hyperactivity associated with experimental allergic asthma | Signaling | [195,196] |
| Airway smooth-muscle cell and bronchial β2AR desensitization (1 and 2) | Desensitization | [18,19] | |
| β-Arrestin2 involved in B1-bradykinin-stimulated iNOS activation in human lung microvascular endothelial cells | Signaling | [197] | |
| Significant β-arrestin2 upregulation in a cellular model of cystic fibrosis (2) | Unknown | [198] | |
| Rheumatology | Chemokine-stimulated leukocyte chemotaxis involves β-arrestins | Signaling | [199] |
| and immunology | Toll-like receptors involved in innate immunity signaling through β-arrestins | Signaling | [200–202] |
| Knockdown of β-arrestin1 enhances IFN-γ-induced antiviral response (1) | Signaling | [203] | |
| β-Arrestin2 KO mice show decreased susceptibility to mouse CMV infection | Signaling | [204] | |
| β-Arrestins play important roles in the regulation of neutrophils, natural killer cells and lymphocytes | Signaling and desensitization | [204–206] | |
| β-Arrestin2 KO mice show greater inflammatory responses and are more susceptible to endotoxin-mediated shock (2) | Desensitization | [207] | |
| β-Arrestin2 negatively regulates the inflammatory response to microbial sepsis | Unknown | [208] | |
| β-Arrestin KO mice are protected from LPS stimulated TLR4-mediated endotoxic shock and lethality (1 and 2) | Signaling | [209] | |
| CCR2-stimulated β-arrestin-mediated signaling is a mediator of inflammation in a mouse model of arthritis | Signaling | [123] | |
| β-Arrestin1 expression of is increased in an animal model of multiple sclerosis (MS) and MS patients | Unknown | [210] | |
| A C-terminal truncation mutation of CXCR4 affects receptor signaling and β-arrestin-mediated regulation and is associated with WHIM syndrome | Desensitization and trafficking | [39] | |
| Defective chemotaxis observed in lymphocytes from β-arrestin2 KO mice | Signaling | [199,211] | |
| Significant increase in neutrophil chemotaxis and wound re-epithelialization in β-arrestin2 KO mice, which is thought to involve CXCR2 | Desensitization | [212] | |
| Differential regulation of adenovirus-vector-induced innate immune responses | Unknown | [213] | |
| Endocrinology Metabolic | The niacin-induced flushing response is dramatically reduced in β-arrestin1 KO mice whereas the beneficial lipolytic effects remain intact | Signaling | [73] |
| β-Arrestin2 is down-regulated in both db/db and high-fat diet mouse models of type 2 diabetes | Unknown | [214] | |
| β-Arrestin2 KO mice exhibit significant insulin resistance, whereas mice overexpressing | Signaling | [214] | |
| β-arrestin2 show improved glucose metabolism and insulin sensitivity | |||
| GLP-1 receptor-stimulated pancreatic β-cell insulin secretion, as well as ERK activation and associated antiapoptotic signals, involve β-arrestin1 | Signaling | [215,216] | |
| Endocrinology Bone mineral homeostasis | β-Arrestin2 plays a role in regulating PTH-receptor-mediated signaling, with effects on bone formation and resorption, as well as related gene expression | Signaling | [79–90] |
| The β-arrestin-biased ligand (D-Trp12,Tyr34)-PTH(7–34) promotes anabolic bone formation in the absence of bone resorption, and this activity is absent in β-arrestin2 KO mice | Signaling | [91] | |
| Endocrinology Reproductive | The use of oxytocin to induce or augment labor is often limited by tachyphylaxis, and oxytocin receptor regulation is thought to involve β-arrestin | Desensitization | [217–221] |
| β-Arrestin1 involved in prostaglandin E2-stimulated human mesenchymal stem cell growth | Signaling | [222] | |
| GnRH receptor β-arrestin-biased ligands have potent antiproliferative activities | Signaling | [152] | |
| Gastrointestinal | PAR2-receptor-mediated β-arrestin-signaling provides a mechanistic link between inflammation and stress-induced alterations in colonic permeability | Signaling | [223] |
| β-Arrestin2 KO mice are resistant to the effects of morphine and loperamide (Imodium®) on fecal bolus accumulation | Signaling | [92,93] | |
| Oncology | Elevated β-arrestin1 mRNA levels have been observed in multiple tumorigenic cancer cell lines, and elevated β-arrestin2 mRNA has been observed in advanced breast cancer tumors | Unknown | [94] |
| β-Arrestin1 plays a role in nicotine induced proliferation of non-small-cell lung cancer cells | Signaling | [95] | |
| Prostaglandin E2-stimulated EP2- and EP4-receptor-mediated transactivation of EGFR uses β-arrestin1, and is involved in mouse skin papilloma development and colorectal carcinoma cell migration and metastasis | Signaling | [96,97] | |
| β-Arrestins mediate ETA–receptor-stimulated EGFR transactivation in ovarian cancer cells, which is thought to be involved in both cell invasion and metastasis | Signaling | [98,99] | |
| Knockdown of either β-arrestin1 or -2 reduces LPA-stimulated cancer cell migration and invasion, and transgenic overexpression of β-arrestin1 promotes tumor angiogenesis and progression | Signaling | [100,102] | |
| TGF-β inhibits epithelial and cancer cell migration via β-arrestin2-mediated activation of CDC42 | Signaling | [101] | |
| β-Arrestin2 is involved in thromboxane-receptor-β-stimulated bladder cancer cell migration | Signaling | [224] | |
| β-Arrestin2 KO mice show increased tumor growth and metastasis in an experimental model of lung cancer | Unknown | [102] | |
| In androgen-dependent prostate-cancer cells, β-arrestin2 acts as an androgen receptor corepressor | Desensitization | [103] | |
| Neurology and behavior Opioids | β-Arrestin2 KO mice experience enhanced and prolonged morphine-induced analgesia and are resistant to the development of chronic morphine-induced tolerance | Desensitization | [20–22] |
| β-Arrestin2 KO mice are resistant to morphine-induced respiratory suppression and constipation | Signaling | [7,92,93] | |
| β-Arrestin2 knockdown in vivo with either antisense or siRNA results in reduced tolerance, whereas overexpression in the brain attenuates morphine analgesia | Desensitization | [23–25] | |
| Chronic morphine treatment affects β-arrestin and GRK expression in the brain | Unknown | [225–227] | |
| β-Arrestin2 is involved in limiting opioid-induced reward | Unknown | [228] | |
| Neurology and behavior Dopamine | D2 dopamine receptor signals via a β-arrestin2-scaffolded complex of protein phosphatase 2A and Akt, acting as a positive mediator of dopamine-associated signals and behavior | Signaling | [229] |
| A common feature of clinically effective antipsychotics, acting at D2 dopamine receptors, is their ability to block D2-receptor-stimulated β-arrestin recruitment | Desensitization | [137,138] | |
| The antidepressant effects of lithium involve disruption of a β-arrestin2 signaling complex | Signaling | [230] | |
| β-Arrestin2 KO mice are resistant to both direct or indirect dopamine-receptor-stimulated locomotor sensitization | Signaling | [229,231] | |
| Changes in central β-arrestin and GRK expression are observed in several neural and behavioral disorders | Unknown | [232–236] | |
| Data suggest that D1 dopamine-receptor-stimulated increases in striatal neuron apoptosis involve β-arrestin | Signaling | [237] | |
| Neurology and behavior | Studies suggest that κ-OR dysphoria involves GRK3 and β-arrestin, which can be differentiated from κ-OR-stimulated analgesic effects | Signaling | [238,239] |
| Other effects | β-Arrestin2 KO mice show enhanced Δ9-tetrahydrocannabinol-stimulated CB1 cannabinoid-receptor-mediated analgesic and hypothermic responses | Desensitization | [120] |
| β-Arrestin2 plays a role in alcohol exposure, consumption and reward | Unknown | [240,241] | |
| β-Arrestin2 plays a positive role in nicotine sensitization and dependence | Unknown | [242,243] | |
| β-Arrestin2 plays a role in 5-HT2A-receptor-stimulated hallucinations | Signaling | [176] | |
| β-Arrestin2 KO mice are resistant to α2-AR-stimulated sedation | Signaling | [244] |
G protein signaling and desensitization
Treatment with 7TMR agonists can be limited by the development of tachyphylaxis, a decrease in responsiveness to a drug with repeated dosing, and tolerance, whereby a higher drug dose is required with repeated doses to obtain the same effect. Both processes, which limit the utility of therapeutics, are largely thought to be regulated by β-arrestin-dependent receptor desensitization and downregulation. The design of G protein-biased ligands, which avoid β-arrestin activation and the associated negative consequences, represents a novel strategy for the development of improved agonist therapies with more sustained efficacy.
The β1 adrenergic receptor in heart failure
The β-arrestins are involved in a variety of cardiovascular processes, with a significant body of work focused on the prototypic β-adrenergic receptors (β-ARs). Some of the earliest work in this area involved β-arrestin1 knockout (KO) mice, which were more sensitive to the cardiac stimulatory effects of β-agonist when compared to wild-type (WT) mice, which strongly suggests the involvement of β-arrestin1 in cardiac β-AR desensitization [12]. Clinically, the β-agonist dobutamine is often used to provide inotropic support in patients with severe heart failure, but is associated with the development of tachyphylaxis. A G protein-biased ligand that limits β-arrestin-mediated desensitization and downregulation could provide a more consistent and efficacious therapeutic option.
The β2 adrenergic receptor in obstructive lung disease
In the pulmonary system, the β2-AR plays a major role in regulating airway smooth-muscle-cell contraction and bronchial tone. One major problem in the treatment of asthma is tachyphylaxis to β-agonist-stimulated bronchodilation, whereby repeated doses of either short- or long-acting agonists result in decreased bronchodilation [13]. This decreased responsiveness can lead patients to increase their use of β-agonists, thus increasing the health risks associated with elevated sympathetic activation and decreasing the effectiveness of rescue inhaler use during an asthma attack, and increasing the risk of death from such attacks [14]. β2-AR tachyphylaxis is also thought to result from increased receptor desensitization and down-regulation after agonist stimulation, an effect thought to be mediated by β-arrestins. β-Arrestin2 regulates receptor desensitization by sterically preventing the interaction between the receptor and G protein [15], by internalizing receptor [16], and by actively recruiting phosphodiesterases to degrade the cAMP generated by G protein signaling [17]. Studies have shown that the airway relaxation stimulated by the β-agonist albuterol is augmented in β-arrestin2 KO mice compared to WT controls [18]. Moreover, comparable results were obtained in transgenic mice expressing a mutant β2-AR deficient in GRK phosphorylation sites; thus, this mutant β2-AR is probably resistant to β-arrestin-mediated regulation [19]. These data therefore predict that a G protein-biased ligand that does not promote β-arrestin recruitment would result in less tachyphylaxis and would be a more effective therapeutic agent.
The µ opioid receptor (OR) and analgesia
The influence of β-arrestins on opioid analgesia and tolerance has been the subject of several studies [20–22]. β-Arrestin2 KO mice experienced enhanced and prolonged morphine-induced analgesia [22] (effects not observed in β-arrestin1 KO mice [20]), as well as increased hypothermic responses compared to WT mice [22]. The β-arrestin2 KO mice also showed greater and more efficient central OR G protein coupling [22]. Interestingly, in addition to morphine, heroin also produced augmented analgesic efficacy in the β-arrestin2 KO mice; however, other µ-OR agonists, including etorphine, fentanyl and methadone, did not [20]. Together, these studies show that the analgesic efficacy of µ-OR agonists can be differentially regulated by β-arrestin2 in vivo. Consistent with these findings, β-arrestin2 KO mice were also resistant to the development of chronic morphine-induced tolerance, which correlated with preserved central OR G protein coupling [21]. Subsequent studies showed comparable results when β-arrestin2 was knocked down in vivo with either antisense [23] or small interfering (si)-RNA [24]. Conversely, adenoviral overexpression of β-arrestin2 in the brain attenuated morphine analgesia as evaluated by measuring response latencies in the hot-plate test [25]. These findings clearly show that β-arrestin2 is involved in regulating both morphine-stimulated OR desensitization and tolerance in vivo, and strongly suggest that a G protein-biased ligand produces enhanced and prolonged morphine-induced analgesia with reduced tolerance.
β-Arrestins and 7TMR internalization and trafficking
It has long been known that β-arrestins regulate the internalization and trafficking of 7TMRs through a variety of mechanisms. β-Arrestins interact with AP-2, an adapter protein for clathrin, thereby regulating receptor endocytosis into clathrin-coated pits [26,27]. Ubiquitination of β-arrestins regulates 7TMR trafficking: transient β-arrestin ubiquitination results in rapid recycling of the receptor, whereas stably ubiquinated β-arrestin targets the receptor to endosomes [28,29]. More broadly, β-arrestins act as adapters for several E3 ligases that catalyze ubiquitination, such as MDM2 [30], which ubiquitinates β-arrestin2; and other ligases such as NEDD4 [31] and AIP4 [32], which ubiquitinate 7TMRs and regulate their downregulation. The β-arrestins also interact with deubiquitinating enzymes such as the ubiquitin specific protease USP33 [29,33], thus providing a mechanism for the regulation of the β-arrestin–7TMR interaction [33]. Not surprisingly, there are several examples of disease states associated with alterations in receptor trafficking that might benefit from therapies that modulate β-arrestin-mediated functions.
The V2 vasopressin receptor in kidney function
Over 150 mutations of the V2 vasopressin receptor (V2R) have been identified that result in congenital nephrogenic diabetes insipidus (cNDI), a condition in which the kidney is unable to properly reabsorb water because the V2R does not respond appropriately to vasopressin [34]. Most of these mutations result in intracellular retention of a misfolded V2R, which limits its expression on the cell membrane and therefore the cell response to vasopressin. Mutation of one of the critical residues in the highly conserved DRY motif, R137H, results in a receptor that constitutively associates with β-arrestins in intracellular vesicles even in the absence of agonist [34], and is associated with constitutive receptor desensitization [35]. Thismutant receptor can be rescued by a cell-permeable V2R antagonist that acts as a pharmacological chaperone and restores the ability of the receptor to fold [36]. However, the constitutive β-arrestin association of R137H is not affected by this chaperone, even though patients with this mutation showed a clinical response to this therapy. Surprisingly, chaperones that are capable of rescuing other V2R mutants restore cAMP signaling in the absence of β-arrestin recruitment or receptor internalization, which suggests a role for G protein-biased agonists in the treatment of cNDI [37].
Conversely, the nephrogenic syndrome of inappropriate antidiuresis, a disorder associated with inappropriate retention of water, results from constitutive activity of the V2R [38]. It is caused by specific mutations of the V2R at R137 (R137C and R137L), the same residue that is mutated in cNDI [38]. Both R137C and R137L V2R mutants interact with β-arrestins in an agonist-independent manner but traffic considerably more efficiently to the plasma membrane than R137H [38], which suggests that receptor expression at the membrane is required for receptor function. As with cNDI, these findings suggest that ligands that restore V2R trafficking might have therapeutic utility in the treatment of the nephrogenic syndrome of inappropriate antidiuresis.
CXCR4 in autoimmune disease
A cytoplasmic tail truncation mutation in the chemokine receptor CXCR4 is found in the warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome, and leukocytes from patients with this disease display defective CXCR4 desensitization and enhanced chemotaxis [39]. The agonist-induced receptor internalization of CXCR4 is regulated specifically by GRK3 and β-arrestin2 and is defective in WHIM syndrome, whereas the chemotaxis of WHIM mutant leukocytes requires β-arrestin2 signaling, which suggests that the truncated receptor is capable of regulating β-arrestin-mediated signaling but not β-arrestin-mediated internalization or endocytosis [39].
CCR5 in HIV
HIV requires cell-surface coreceptors, either the CCR5 or CXCR4 chemokine receptor, to attach and gain entry to target cells. CCR5-tropic viruses are the predominant species in the early stages of infection, and patients with a homozygous truncation mutation of CCR5 (CCR5-Δ32) are resistant to HIV [40]. Accordingly there has been significant interest in targeting this receptor in the treatment of HIV infection [41]. A modified CCR5 ligand, aminooxypentane-RANTES (AOP-RANTES), promotes receptor internalization similar to endogenous RANTES, but unlike RANTES, AOP-RANTES does not allow the internalized CCR5 to recycle [42,43]. More highly potent derivatives of AOP-RANTES seem to be effective in preventing HIV infection of peripheral blood mononuclear cells [44]. The β-arrestins regulate this CCR5 trafficking, recycling and degradation [45], and these ligands probably regulate CCR5 trafficking via changes in β-arrestin activity. Thus, use of a ligand that modifies β-arrestin-regulated CCR5 trafficking might represent an attractive therapy in the treatment of HIV.
β-Arrestin-mediated signaling
It has now been over a decade since the first reports of β-arrestin-mediated signaling were published [46–48]. β-Arrestin-mediated signaling encompasses a diverse range of pathways [5,49–51], including kinase activation, transcriptional regulation and receptor transactivation. The best-characterized of these responses is the regulation of protein kinases, such as members of the mitogen-activated protein (MAP) and Src kinase families [5]. The list of physiologic responses that are regulated by β-arrestins continues to grow (Table 2), and drugs that selectively target β-arrestin-mediated signaling are currently being developed as therapeutic agents.
The β1-AR in heart failure
Recent studies have shown that cardiac β1-ARs can stimulate β-arrestin1- and -2-dependent signaling in the heart that results in transactivation of the epidermal growth factor receptor (EGFR), which is cardioprotective [52]. In addition, it is thought that chronic β-AR activation is cardiotoxic [53–55] and that this in large part involves Gs signaling [55,56]. These combined observations suggest that a β-arrestin-biased ligand acting as a classical antagonist of cardiotoxic G protein signaling, while engaging cardioprotective β-arrestin signaling, could be therapeutically beneficial. Moreover, β-arrestin-biased ligands for both the β1-AR [57] and β2-AR [58,59] have recently been identified, with the β-blocker carvedilol a β-arrestin-biased ligand of both receptor subtypes [57,58]. Whether β-arrestin-mediated signaling plays a role in the cardioprotection associated with such compounds remains to be determined.
The angiotensin II type 1A receptor (AT1R) in cardiovascular disease
In addition to the β-ARs, AT1R-stimulated β-arrestin signaling has been the subject of several cardiovascular studies. In the heart and in isolated cardiomyocytes, AT1R-stimulated β-arrestin-dependent andGprotein-independent signaling promotes growth and hypertrophy [60], myocyte proliferation [61,62] and increased myocyte contractility [63,64]. Moreover, the newly identified β-arrestin-biased ligand Sar1,D-Ala8 angiotensin II (TRV120027) reduces mean arterial pressure, increases cardiac performance and preserves stroke volume in the anesthetized rat, whereas unbiased antagonists reduce cardiac performance [64]. In vascular smooth muscle cells, β-arrestin signaling increases protein synthesis [65] and is antiapoptotic [66]. Furthermore, cardiovascular function is in large part influenced by changes in body fluid homeostasis and salt balance, and AT1R-stimulated β-arrestin-associated signaling is involved in several related processes. For example, AT1R-stimulated β-arrestin1 signaling promotes adrenocortical aldosterone secretion both in vitro (cultured human adrenocortical carcinomacells, H295) and in vivo (adrenal-targeted adenoviral βarr1 overexpression in rats) [67]. Furthermore, central AT1R activation is associated with increased thirst and salt appetite [68]. Interestingly, the β-arrestin-biased AT1R ligand Sar1,Ile4,Ile8 angiotensin II (SII) [69] when injected directly into the brain stimulates central MAP kinase activation and salt intake, while blocking G protein-associated increases in central inositol triphosphate production and water intake [70]. Thus, AT1R-stimulated salt appetite would seem to be a β-arrestin-dependent process, at least in part. Overall, it seems that AT1R can stimulate a variety of β-arrestin-mediated cellular signals and associated processes involved in the maintenance of cardiovascular homeostasis.
GPR109A and the regulation of lipid homeostasis
A unique role for β-arrestin1-mediated signaling has recently been identified that involves the niacin receptor, GPR109A. Niacin is one of the most effective therapies for increasing HDL-cholesterol and decreasing triglycerides in the treatment of dyslipidemia [71,72]. However, the therapeutic utility of niacin is limited by the rather unpleasant side effect of cutaneous flushing, which significantly reduces patient compliance [71,72]. Recent studies have shown that the niacin-induced flushing response is dramatically reduced in β-arrestin1 KO mice, whereas the beneficial lipolytic effects remain intact [73]. This supports the hypothesis that a G protein-biased ligand would maintain beneficial effects on plasma lipids in the absence of β-arrestin-mediated flushing. Interestingly, GPR109A agonists have been identified that exhibit antilipolytic actions with significantly reduced cutaneous flushing [74–78]. Some of these compounds also show reduced receptor internalization and extracellular signal-regulated kinase (ERK) activation [74], which could result from non-engagement of β-arrestin.
The parathyroid hormone receptor and bone mineral homeostasis
Bone mineral homeostasis requires a complex process of renewal involving a continuous cycle of bone formation and resorption. The parathyroid hormone (PTH) receptor plays an important role in regulating these processes, with recombinant human PTH 1–34 (Forteo®) currently approved for the treatment of osteoporosis. Previous studies have shown that β-arrestin2 plays a role in regulating PTH-receptor-mediated signaling [79–84], with effects on bone formation and resorption [85,86], as well as related gene expression [87,88]. Furthermore, studies have shown that the PTH receptor can stimulate both G protein- and β-arrestin-mediated signals, and that these signals can be selectively engaged by both G protein- and β-arrestin-biased ligands [89,90]. Most recently, it has been shown that the β-arrestin-biased ligand (D-Trp12,Tyr34)-PTH(7–34), which simultaneously stimulates receptor-mediated β-arrestin signaling and blocks G protein signaling, promotes anabolic bone formation in the absence of bone resorption, and that this activity is abolished in β-arrestin2 KO mice [91]. These studies have thus identified a novel β-arrestin-mediated pathway and a unique β-arrestin-biased ligand that positively affects anabolic bone formation. Targeting of this newly identified mechanism of action could represent a novel therapeutic strategy for the treatment of osteoporosis.
Opioid side effects
Opioid therapy is associated with several adverse side effects, including respiratory suppression, constipation, and the development of tolerance and physical dependence. β-Arrestin2 is responsible for desensitization of the receptor after chronic morphine treatment, and β-arrestin2 KO mice are protected from the development of tolerance, the requirement for increasing doses of opioids to maintain the same antinociceptive effect [21]. Studies have shown that β-arrestin2 KO mice are also resistant to morphine-induced respiratory suppression when compared to WT mice [92]. In addition, β-arrestin2 KO mice were less sensitive to some of the adverse gastrointestinal effects of µ-OR agonists. Specifically, β-arrestin2 KO mice were resistant to the effects of morphine on fecal boli accumulation and to a lesser extent colonic propulsion when compared to WT mice [92,93]. The β-arrestin2 KO mice were not, however, resistant to the inhibitory effects of morphine on small intestinal transit. Interestingly, the peripherally restricted µ-OR agonist and antidiarrheal agent loperamide significantly reduced colonic propulsion in WT mice, and these effects were completely abolished in β-arrestin2 KO mice [92]. This suggests that peripherally restricted β-arrestin-biased agonists might be useful in the treatment of diarrhea and other hypermotility disorders.
Cancer cell metastasis and cell motility
The β-arrestins are involved in several cancer-related signals and processes via a range of receptor subtypes. Moreover, elevated β-arrestin mRNA levels have been observed in cancerous tumors and tumorigenic cancer cell lines [94]. Of note, β-arrestin1 plays a role in nicotine-induced proliferation of non-small-cell lung cancer cells via a process involving a β-arrestin1-scaffolded complex of the nicotinic acetylcholine receptor and Src, as well as downstream signaling via the MAP kinase and Rb-Raf-1 pathways [95]. Prostaglandin-E2-stimulated EP2- and EP4-receptor-mediated transactivation of EGFR also involves a complex of receptor, β-arrestin1 and Src, which is involved in mouse skin papilloma development [96] and colorectal carcinoma cell migration and metastasis [97], respectively. Similarly, in ovarian cancer cells the β-arrestins mediate endothelin type A (ETA)-receptor-stimulated EGFR transactivation via Src, as well as effects on β-catenin, which are thought to be involved in both cell invasion and metastasis [98,99]. Lysophosphatidic acid (LPA) receptor activation is associated with enhanced breast cancer cell metastasis [94], and siRNA-mediated knockdown of either β-arrestin 1 or 2 in breast cancer cells reduces LPA-stimulated transwell migration and 3D Matrigel invasion [94]. Conversely, transgenic overexpression of β-arrestin1 in mice promotes tumor angiogenesis and progression [100].
In addition to activating proliferative signaling pathways, β-arrestins are also involved in several pathways that suppress tumor growth and metastasis. For example, transforming growth factor (TGF)-β inhibits epithelial and cancer cell migration via β-arrestin2-mediated activation of cell division cycle (CDC)-42 [101]. β-Arrestin2 KO mice also have increased tumor growth and metastasis mediated by host changes in inflammation and angiogenesis in a heterotopic model of lung cancer in which both WT and β-arrestin2 KO mice were injected with cells derived from a spontaneously occurring lung cancer tumor in C57BL/6 mice [102]. In androgen-dependent prostate cancer cells, β-arrestin2 acts as an androgen receptor corepressor and promotes the association of MDM2 with the receptor and its consequent ubiquitylation and degradation [103]. The above studies clearly demonstrate that the β-arrestins play roles in a variety of pro- and anticancer-related signals and processes in both neoplastic cells and the surrounding host environment via several different receptor subtypes. The diversity of this influence might provide insight into potential targets for future chemotherapeutic strategies.
Emerging areas of interest
In addition to the specific examples highlighting β-arrestin-mediated functions (Table 2), several reports describe processes that are probably β-arrestin-mediated. One recent example, which might have broad therapeutic implications in the treatment of cognitive disorders, involves the M3 muscarinic receptor. Specifically, M3-receptor-dependent learning and memory require processes that depend on receptor phosphorylation, consistent with β-arrestin recruitment and apparently independent of G protein signaling [104]. These data suggest that a β-arrestin-biased ligand at the M3 receptor might promote both learning and memory, and might thus be beneficial in the treatment of cognitive disorders such as Alzheimer’s disease. The exact role of β-arrestin in such processes and the therapeutic potential of targeting β-arrestin-mediated signaling will require further study.
Concluding remarks
The β-arrestins are intimately involved in numerous aspects of 7TMR signaling and regulation, and accordingly influence manifold physiological and pathophysiological processes. β-Arrestin-mediated signaling is a relatively new area of 7TMR research, especially when compared to the study of more traditional G protein signals, and continued efforts in this area will undoubtedly lead to the discovery of additional roles for β-arrestins in 7TMR biology. Of particular interest, the ability of biased ligands to differentiate between β-arrestin and G protein functions at the receptor level should facilitate selective engagement of a subset of signals from a particular 7TMR. In most instances, identification of biased ligands for a specific 7TMR target is rather straightforward. The major bottleneck in determining the therapeutic potential of G protein- and β-arrestin-biased agonists is the lack of knowledge regarding the roles of these distinct signaling pathways in both health and disease. Therefore, studies should focus on the development and use of biased agonists as tool compounds in cellular and animal models of disease to more clearly delineate the physiologic consequences of these two signaling mechanisms. We expect that clearer definitions of the role of β-arrestins and G proteins in 7TMR signaling will facilitate the development of improved therapeutic agents that target 7TMRs (and potentially β-arrestin- and G protein-mediated signals directly) with improved efficacy and fewer side effects.
Glossary
- Antinociceptive effect
effect that leads to a decreased sensation of pain
- Congenital nephrogenic diabetes insipidus (cNDI)
condition in which the kidney is unable to properly reabsorb water because it does not respond appropriately to vasopressin
- Inotropic support
therapy aimed at improving the contractile function of the heart
- Nephrogenic syndrome of inappropriate antidiuresis
disorder in which the body inappropriately retains water due to a defect in signaling in the kidney
- Tachyphylaxis
decrease in responsiveness to a drug with repeated dosing
- Warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome
congenital immunodeficiency due to mutation of CXCR4 that results in neutropenia
References
- 1.Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 2.Chung S, et al. Orphan GPCR research. Br. J. Pharmacol. 2008;153(Suppl 1):S339–S346. doi: 10.1038/sj.bjp.0707606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma P, Zemmel R. Value of novelty? Nat. Rev. Drug Discov. 2002;1:571–572. doi: 10.1038/nrd884. [DOI] [PubMed] [Google Scholar]
- 4.Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat. Rev. Drug Discov. 2005;4:919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- 5.DeWire SM, et al. beta-Arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 6.Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol. Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmid CL, Bohn LM. Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol. Ther. 2009;121:285–293. doi: 10.1016/j.pharmthera.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajagopal S, et al. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Violin JD, Lefkowitz RJ. beta-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenakin T. Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- 12.Conner DA, et al. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ. Res. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- 13.Haney S, Hancox RJ. Recovery from bronchoconstriction and bronchodilator tolerance. Clin. Rev. Allergy Immunol. 2006;31:181–196. doi: 10.1385/CRIAI:31:2:181. [DOI] [PubMed] [Google Scholar]
- 14.Abramson MJ, et al. Adverse effects of beta-agonists: are they clinically relevant? Am. J. Respir. Med. 2003;2:287–297. doi: 10.1007/BF03256657. [DOI] [PubMed] [Google Scholar]
- 15.Lohse MJ, et al. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 16.Ahn S, et al. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry SJ, et al. Targeting of cyclic AMP degradation to beta 2- adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande DA, et al. beta-Arrestins specifically constrain beta2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 2008;22:2134–2141. doi: 10.1096/fj.07-102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang WC, et al. Targeted transgenesis reveals discrete attenuator functions of GRK and PKA in airway beta2-adrenergic receptor physiologic signaling. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15007–15012. doi: 10.1073/pnas.0906034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohn LM, et al. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol. Pharmacol. 2004;66:106–112. doi: 10.1124/mol.66.1.106. [DOI] [PubMed] [Google Scholar]
- 21.Bohn LM, et al. mu-Opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 22.Bohn LM, et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 23.Przewlocka B, et al. Knockdown of spinal opioid receptors by antisense targeting beta-arrestin reduces morphine tolerance and allodynia in rat. Neurosci. Lett. 2002;325:107–110. doi: 10.1016/s0304-3940(02)00246-x. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, et al. Improvement of morphine-mediated analgesia by inhibition of beta-arrestin 2 expression in mice periaqueductal gray matter. Int. J. Mol. Sci. 2009;10:954–963. doi: 10.3390/ijms10030954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang B, et al. Decreased morphine analgesia in rat overexpressing beta-arrestin 2 at periaqueductal gray. Neurosci. Lett. 2006;400:150–153. doi: 10.1016/j.neulet.2006.02.071. [DOI] [PubMed] [Google Scholar]
- 26.Goodman OB, Jr, et al. beta-Arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 27.Laporte SA, et al. The beta2-adrenergic receptor/beta-arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shenoy SK, et al. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J. Biol. Chem. 2007;282:29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shenoy SK, et al. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenoy SK, et al. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 31.Shenoy SK, et al. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. J. Biol. Chem. 2008;283:22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhandari D, et al. Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J. Biol. Chem. 2007;282:36971–36979. doi: 10.1074/jbc.M705085200. [DOI] [PubMed] [Google Scholar]
- 33.Berthouze M, et al. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. EMBO J. 2009;28:1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barak LS, et al. Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc. Natl. Acad. Sci. U.S.A. 2001;98:93–98. doi: 10.1073/pnas.011303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilbanks AM, et al. Apparent loss-of-function mutant GPCRs revealed as constitutively desensitized receptors. Biochemistry. 2002;41:11981–11989. doi: 10.1021/bi020275m. [DOI] [PubMed] [Google Scholar]
- 36.Bernier V, et al. Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Mol. Endocrinol. 2004;18:2074–2084. doi: 10.1210/me.2004-0080. [DOI] [PubMed] [Google Scholar]
- 37.Jean-Alphonse F, et al. Biased agonist pharmacochaperones of the AVP V2 receptor may treat congenital nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 2009;20:2190–2203. doi: 10.1681/ASN.2008121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kocan M, et al. Agonist-independent interactions between beta-arrestins and mutant vasopressin type II receptors associated with nephrogenic syndrome of inappropriate antidiuresis. Mol. Endocrinol. 2009;23:559–571. doi: 10.1210/me.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balabanian K, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 40.Samson M, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 41.Simmons G, et al. Co-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligands. Immunol. Rev. 2000;177:112–126. doi: 10.1034/j.1600-065x.2000.17719.x. [DOI] [PubMed] [Google Scholar]
- 42.Mack M, et al. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J. Exp. Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Signoret N, et al. Endocytosis and recycling of the HIV coreceptor CCR5. J. Cell Biol. 2000;151:1281–1294. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartley O, et al. Medicinal chemistry applied to a synthetic protein: development of highly potent HIV entry inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16460–16465. doi: 10.1073/pnas.0404802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oppermann M. Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal. 2004;16:1201–1210. doi: 10.1016/j.cellsig.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 46.DeFea KA, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin-dependent scaffolding complex. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeFea KA, et al. beta-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luttrell LM, et al. beta-Arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 49.Christensen GL, et al. Quantitative phosphoproteomics dissection of seven-transmembrane receptor signaling using full and biased agonists. Mol. Cell Proteomics. 2010;9:1540–1553. doi: 10.1074/mcp.M900550-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao K, et al. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao K, et al. Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc. Natl. Acad. Sci. U.S.A. 2010;107:15299–15304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noma T, et al. beta-Arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J. Clin. Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bristow MR. beta-Adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 54.Cohn JN, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 55.Lohse MJ, et al. What is the role of beta-adrenergic signaling in heart failure? Circ. Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 56.Xiao RP. beta-Adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to Gs and Gi proteins. Sci. STKE. 2001:re15. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- 57.Kim IM, et al. beta-Blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc. Natl. Acad. Sci. U.S.A. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wisler JW, et al. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drake MT, et al. beta-Arrestin-biased agonism at the beta2-adrenergic receptor. J. Biol. Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 60.Zhai P, et al. Cardiac-specific overexpression of AT1 receptor mutant lacking G alpha q/G alpha i coupling causes hypertrophy and bradycardia in transgenic mice. J. Clin. Invest. 2005;115:3045–3056. doi: 10.1172/JCI25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aplin M, et al. Differential extracellular signal-regulated kinases 1 and 2 activation by the angiotensin type 1 receptor supports distinct phenotypes of cardiac myocytes. Basic Clin. Pharmacol. Toxicol. 2007;100:296–301. doi: 10.1111/j.1742-7843.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- 62.Hansen JL, et al. The human angiotensin AT1 receptor supports G protein-independent extracellular signal-regulated kinase 1/2 activation and cellular proliferation. Eur. J. Pharmacol. 2008;590:255–263. doi: 10.1016/j.ejphar.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 63.Rajagopal K, et al. beta-Arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Violin JD, et al. Selectively engaging beta-arrestins at the AT1R reduces blood pressure and increases cardiac performance. J. Pharmacol. Exp. Ther. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 65.DeWire SM, et al. beta-Arrestin-mediated signaling regulates protein synthesis. J. Biol. Chem. 2008;283:10611–10620. doi: 10.1074/jbc.M710515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahn S, et al. beta-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J. Biol. Chem. 2009;284:8855–8865. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lymperopoulos A, et al. An adrenal beta-arrestin 1-mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5825–5830. doi: 10.1073/pnas.0811706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- 69.We H, et al. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daniels D, et al. Divergent behavioral roles of angiotensin receptor intracellular signaling cascades. Endocrinology. 2005;146:5552–5560. doi: 10.1210/en.2005-0774. [DOI] [PubMed] [Google Scholar]
- 71.Guyton JR. Extended-release niacin for modifying the lipoprotein profile. Expert Opin. Pharmacother. 2004;5:1385–1398. doi: 10.1517/14656566.5.6.1385. [DOI] [PubMed] [Google Scholar]
- 72.Guyton JR. Niacin in cardiovascular prevention: mechanisms, efficacy, and safety. Curr. Opin. Lipidol. 2007;18:415–420. doi: 10.1097/MOL.0b013e3282364add. [DOI] [PubMed] [Google Scholar]
- 73.Walters RW, et al. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J. Clin. Invest. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richman JG, et al. Nicotinic acid receptor agonists differentially activate downstream effectors. J. Biol. Chem. 2007;282:18028–18036. doi: 10.1074/jbc.M701866200. [DOI] [PubMed] [Google Scholar]
- 75.Semple G, et al. 3-(1H-Tetrazol-5-yl)-1,4,5,6-tetrahydro-cyclopentapyrazole (MK-0354): a partial agonist of the nicotinic acid receptor, G-protein coupled receptor 109a, with antilipolytic but no vasodilatory activity in mice. J. Med. Chem. 2008;51:5101–5108. doi: 10.1021/jm800258p. [DOI] [PubMed] [Google Scholar]
- 76.Shen HC, et al. Discovery of novel tricyclic full agonists for the G-protein-coupled niacin receptor 109A with minimized flushing in rats. J. Med. Chem. 2009;52:2587–2602. doi: 10.1021/jm900151e. [DOI] [PubMed] [Google Scholar]
- 77.Shen HC, et al. Discovery of biaryl anthranilides as full agonists for the high affinity niacin receptor. J. Med. Chem. 2007;50:6303–6306. doi: 10.1021/jm700942d. [DOI] [PubMed] [Google Scholar]
- 78.Shen HC, et al. Discovery of a biaryl cyclohexene carboxylic acid (MK-6892): a potent and selective high affinity niacin receptor full agonist with reduced flushing profiles in animals as a preclinical candidate. J. Med. Chem. 2010;53:2666–2670. doi: 10.1021/jm100022r. [DOI] [PubMed] [Google Scholar]
- 79.Castro M, et al. Dual regulation of the parathyroid hormone (PTH)/PTH-related peptide receptor signaling by protein kinase C and beta-arrestins. Endocrinology. 2002;143:3854–3865. doi: 10.1210/en.2002-220232. [DOI] [PubMed] [Google Scholar]
- 80.Ferrari SL, et al. Endocytosis of ligand–human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves beta-arrestin2. Real-time monitoring by fluorescence microscopy. J. Biol. Chem. 1999;274:29968–29975. doi: 10.1074/jbc.274.42.29968. [DOI] [PubMed] [Google Scholar]
- 81.Ferrari SL, Bisello A. Cellular distribution of constitutively active mutant parathyroid hormone (PTH)/PTH-related protein receptors and regulation of cyclic adenosine 3′,5′-monophosphate signaling by beta-arrestin2. Mol. Endocrinol. 2001;15:149–163. doi: 10.1210/mend.15.1.0587. [DOI] [PubMed] [Google Scholar]
- 82.Sneddon WB, Friedman PA. beta-Arrestin-dependent parathyroid hormone-stimulated extracellular signal-regulated kinase activation and parathyroid hormone type 1 receptor internalization. Endocrinology. 2007;148:4073–4079. doi: 10.1210/en.2007-0343. [DOI] [PubMed] [Google Scholar]
- 83.Vilardaga JP, et al. Differential conformational requirements for activation of G proteins and the regulatory proteins arrestin and G protein-coupled receptor kinase in the G protein-coupled receptor for parathyroid hormone (PTH)/PTH-related protein. J. Biol. Chem. 2001;276:33435–33443. doi: 10.1074/jbc.M011495200. [DOI] [PubMed] [Google Scholar]
- 84.Vilardaga JP, et al. Internalization determinants of the parathyroid hormone receptor differentially regulate beta-arrestin/receptor association. J. Biol. Chem. 2002;277:8121–8129. doi: 10.1074/jbc.M110433200. [DOI] [PubMed] [Google Scholar]
- 85.Bouxsein ML, et al. beta-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J. Bone Miner Res. 2005;20:635–643. doi: 10.1359/JBMR.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferrari SL, et al. Bone response to intermittent parathyroid hormone is altered in mice null for beta-arrestin2. Endocrinology. 2005;146:1854–1862. doi: 10.1210/en.2004-1282. [DOI] [PubMed] [Google Scholar]
- 87.Bianchi EN, Ferrari SL. Beta-arrestin2 regulates parathyroid hormone effects on a p38 MAPK and NFkappaB gene expression network in osteoblasts. Bone. 2009;45:716–725. doi: 10.1016/j.bone.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pierroz DD, et al. beta-Arrestin2 regulates RANKL and ephrins gene expression in response to bone remodeling in mice. J. Bone Miner Res. 2009;24:775–784. doi: 10.1359/JBMR.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bisello A, et al. Selective ligand-induced stabilization of active and desensitized parathyroid hormone type 1 receptor conformations. J. Biol. Chem. 2002;277:38524–38530. doi: 10.1074/jbc.M202544200. [DOI] [PubMed] [Google Scholar]
- 90.Gesty-Palmer D, et al. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J. Biol. Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 91.Gesty-Palmer D, et al. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci. Transl. Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raehal KM, et al. Morphine side effects in beta-arrestin 2 knockout mice. J. Pharmacol. Exp. Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 93.Bohn LM, Raehal KM. Opioid receptor signaling: relevance for gastrointestinal therapy. Curr. Opin. Pharmacol. 2006;6:559–563. doi: 10.1016/j.coph.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 94.Li TT, et al. beta-Arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol. Cancer Res. 2009;7:1064–1077. doi: 10.1158/1541-7786.MCR-08-0578. [DOI] [PubMed] [Google Scholar]
- 95.Dasgupta P, et al. Nicotine induces cell proliferation by beta-arrestin- mediated activation of Src and Rb-Raf-1 pathways. J. Clin. Invest. 2006;116:2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chun KS, et al. The prostaglandin receptor EP2 activates multiple signaling pathways and beta-arrestin1 complex formation during mouse skin papilloma development. Carcinogenesis. 2009;30:1620–1627. doi: 10.1093/carcin/bgp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buchanan FG, et al. Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1492–1497. doi: 10.1073/pnas.0510562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosano L, Bagnato A. Convergent pathways link the endothelin A receptor to the beta-catenin: the beta-arrestin connection. Cell Cycle. 2009;8:1462–1463. [PubMed] [Google Scholar]
- 99.Rosano L, et al. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zou L, et al. Rapid xenograft tumor progression in beta-arrestin1 transgenic mice due to enhanced tumor angiogenesis. FASEB J. 2008;22:355–364. doi: 10.1096/fj.07-9046com. [DOI] [PubMed] [Google Scholar]
- 101.Mythreye K, Blobe GC. The type III TGF-beta receptor regulates epithelial and cancer cell migration through beta-arrestin2-mediated activation of Cdc42. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8221–8226. doi: 10.1073/pnas.0812879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raghuwanshi SK, et al. Depletion of beta-arrestin-2 promotes tumor growth and angiogenesis in a murine model of lung cancer. J. Immunol. 2008;180:5699–5706. doi: 10.4049/jimmunol.180.8.5699. [DOI] [PubMed] [Google Scholar]
- 103.Lakshmikanthan V, et al. Identification of βarrestin2 as a corepressor of androgen receptor signaling in prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9379–9384. doi: 10.1073/pnas.0900258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poulin B, et al. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 2010;107:9440–9445. doi: 10.1073/pnas.0914801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao ZG, Jacobson KA. Translocation of arrestin induced by human A3 adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways. Pharmacol. Res. 2008;57:303–311. doi: 10.1016/j.phrs.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee DK, et al. The fate of the internalized apelin receptor is determined by different isoforms of apelin mediating differential interaction with beta-arrestin. Biochem. Biophys. Res. Commun. 2010;395:185–189. doi: 10.1016/j.bbrc.2010.03.151. [DOI] [PubMed] [Google Scholar]
- 107.Ahn S, et al. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J. Biol. Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 108.Ahn S, et al. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J. Biol. Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- 109.Aplin M, et al. The angiotensin type 1 receptor activates extracellular signal-regulated kinases 1 and 2 by G protein-dependent and -independent pathways in cardiac myocytes and Langendorff-perfused hearts. Basic Clin. Pharmacol. Toxicol. 2007;100:289–295. doi: 10.1111/j.1742-7843.2007.00063.x. [DOI] [PubMed] [Google Scholar]
- 110.Barnes WG, et al. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- 111.Hunton DL, et al. beta-Arrestin 2-dependent angiotensin II type 1A receptor-mediated pathway of chemotaxis. Mol. Pharmacol. 2005;67:1229–1236. doi: 10.1124/mol.104.006270. [DOI] [PubMed] [Google Scholar]
- 112.Kim J, et al. Independent beta-arrestin2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J. Biol. Chem. 2009;284:11953–11962. doi: 10.1074/jbc.M808176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morinelli TA, et al. Angiotensin II-induced cyclooxygenase 2 expression in rat aorta vascular smooth muscle cells does not require heterotrimeric G protein activation. J. Pharmacol. Exp. Ther. 2009;330:118–124. doi: 10.1124/jpet.109.151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tilley DG, et al. Troglitazone stimulates beta-arrestin-dependent cardiomyocyte contractility via the angiotensin II type 1A receptor. Biochem. Biophys. Res. Commun. 2010;396:921–926. doi: 10.1016/j.bbrc.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu R, et al. Epitope-tagged receptor knock-in mice reveal that differential desensitization of alpha2-adrenergic responses is because of ligand-selective internalization. J. Biol. Chem. 2009;284:13233–13243. doi: 10.1074/jbc.M807535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kukkonen JP, et al. Agonist trafficking of Gi/o-mediated alpha2A-adrenoceptor responses in HEL 92.1.7 cells. Br. J. Pharmacol. 2001;132:1477–1484. doi: 10.1038/sj.bjp.0703964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Azzi M, et al. beta-Arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reiner S, et al. Differential signaling of the endogenous agonists at the beta2-adrenergic receptor. J. Biol. Chem. 2010;285:31688–31698. doi: 10.1074/jbc.M110.175604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.MacKinnon AC, et al. Bombesin and substance P analogues differentially regulate G-protein coupling to the bombesin receptor. Direct evidence for biased agonism. J. Biol. Chem. 2001;276:28083–28091. doi: 10.1074/jbc.M009772200. [DOI] [PubMed] [Google Scholar]
- 120.Breivogel CS, et al. Sensitivity to delta9-tetrahydrocannabinol is selectively enhanced in beta-arrestin2 –/– mice. Behav. Pharmacol. 2008;19:298–307. doi: 10.1097/FBP.0b013e328308f1e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu DF, et al. Role of receptor internalization in the agonist-induced desensitization of cannabinoid type 1 receptors. J. Neurochem. 2008;104:1132–1143. doi: 10.1111/j.1471-4159.2007.05063.x. [DOI] [PubMed] [Google Scholar]
- 122.Roettger BF, et al. Antagonist-stimulated internalization of the G protein-coupled cholecystokinin receptor. Mol. Pharmacol. 1997;51:357–362. [PubMed] [Google Scholar]
- 123.Rafei M, et al. An engineered GM–CSF–CCL2 fusokine is a potent inhibitor of CCR2-driven inflammation as demonstrated in a murine model of inflammatory arthritis. J. Immunol. 2009;183:1759–1766. doi: 10.4049/jimmunol.0900523. [DOI] [PubMed] [Google Scholar]
- 124.Blanpain C, et al. CCR5 and HIV infection. Receptors Channels. 2002;8:19–31. [PubMed] [Google Scholar]
- 125.Vila-Coro AJ, et al. Characterization of RANTES- and aminooxypentane-RANTES-triggered desensitization signals reveals differences in recruitment of the G protein-coupled receptor complex. J. Immunol. 1999;163:3037–3044. [PubMed] [Google Scholar]
- 126.Blanpain C, et al. Multiple active states and oligomerization of CCR5 revealed by functional properties of monoclonal antibodies. Mol. Biol. Cell. 2002;13:723–737. doi: 10.1091/mbc.01-03-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Byers MA, et al. Arrestin 3 mediates endocytosis of CCR7 following ligation of CCL19 but not CCL21. J. Immunol. 2008;181:4723–4732. doi: 10.4049/jimmunol.181.7.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kohout TA, et al. Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J. Biol. Chem. 2004;279:23214–23222. doi: 10.1074/jbc.M402125200. [DOI] [PubMed] [Google Scholar]
- 129.Zidar DA, et al. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Watson C, et al. The use of stimulus-biased assay systems to detect agonist-specific receptor active states: implications for the trafficking of receptor stimulus by agonists. Mol. Pharmacol. 2000;58:1230–1238. doi: 10.1124/mol.58.6.1230. [DOI] [PubMed] [Google Scholar]
- 131.Hall DA, et al. Signalling by CXC-chemokine receptors 1 and 2 expressed in CHO cells: a comparison of calcium mobilization, inhibition of adenylyl cyclase and stimulation of GTPgammaS binding induced by IL-8 and GROalpha. Br. J. Pharmacol. 1999;126:810–818. doi: 10.1038/sj.bjp.0702329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sachpatzidis A, et al. Identification of allosteric peptide agonists of CXCR4. J. Biol. Chem. 2003;278:896–907. doi: 10.1074/jbc.M204667200. [DOI] [PubMed] [Google Scholar]
- 133.Mathiesen JM, et al. Identification of indole derivatives exclusively interfering with a G protein-independent signaling pathway of the prostaglandin D2 receptor CRTH2. Mol. Pharmacol. 2005;68:393–402. doi: 10.1124/mol.104.010520. [DOI] [PubMed] [Google Scholar]
- 134.Lewis MM, et al. Homologous desensitization of the D1A dopamine receptor: efficacy in causing desensitization dissociates from both receptor occupancy and functional potency. J. Pharmacol. Exp. Ther. 1998;286:345–353. [PubMed] [Google Scholar]
- 135.Ryman-Rasmussen JP, et al. Functional selectivity of dopamine D1 receptor agonists in regulating the fate of internalized receptors. Neuropharmacology. 2007;52:562–575. doi: 10.1016/j.neuropharm.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ryman-Rasmussen JP, et al. Differential activation of adenylate cyclase and receptor internalization by novel dopamine D1 receptor agonists. Mol. Pharmacol. 2005;68:1039–1048. doi: 10.1124/mol.105.012153. [DOI] [PubMed] [Google Scholar]
- 137.Klewe IV, et al. Recruitment of beta-arrestin2 to the dopamine D2 receptor: insights into anti-psychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology. 2008;54:1215–1222. doi: 10.1016/j.neuropharm.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Masri B, et al. Antagonism of dopamine D2 receptor/beta-arrestin-2 interaction is a common property of clinically effective antipsychotics. Proc. Natl. Acad. Sci. U.S.A. 2008;105:13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mottola DM, et al. Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J. Pharmacol. Exp. Ther. 2002;301:1166–1178. doi: 10.1124/jpet.301.3.1166. [DOI] [PubMed] [Google Scholar]
- 140.Kilts JD, et al. Functional selectivity of dopamine receptor agonists. II. Actions of dihydrexidine in D2L receptor-transfected MN9D cells and pituitary lactotrophs. J. Pharmacol. Exp. Ther. 2002;301:1179–1189. doi: 10.1124/jpet.301.3.1179. [DOI] [PubMed] [Google Scholar]
- 141.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 142.Wetter JA, et al. Utilization of the Tango beta-arrestin recruitment technology for cell-based EDG receptor assay development and interrogation. J. Biomol. Screen. 2009;14:1134–1141. doi: 10.1177/1087057109343809. [DOI] [PubMed] [Google Scholar]
- 143.Sensken SC, et al. Selective activation of G alpha i mediated signalling of S1P3 by FTY720-phosphate. Cell Signal. 2008;20:1125–1133. doi: 10.1016/j.cellsig.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 144.Leduc M, et al. Functional selectivity of natural and synthetic prostaglandin EP4 receptor ligands. J. Pharmacol. Exp. Ther. 2009;331:297–307. doi: 10.1124/jpet.109.156398. [DOI] [PubMed] [Google Scholar]
- 145.Bhowmick N, et al. The endothelin subtype A receptor undergoes agonist- and antagonist-mediated internalization in the absence of signaling. Endocrinology. 1998;139:3185–3192. doi: 10.1210/endo.139.7.6105. [DOI] [PubMed] [Google Scholar]
- 146.Wehbi V, et al. Selective modulation of follicle-stimulating hormone signaling pathways with enhancing equine chorionic gonadotropin/antibody immune complexes. Endocrinology. 2010;151:2788–2799. doi: 10.1210/en.2009-0892. [DOI] [PubMed] [Google Scholar]
- 147.Wehbi V, et al. Partially deglycosylated equine LH preferentially activates {beta}-arrestin-dependent signaling at the follicle-stimulating hormone receptor. Mol. Endocrinol. 2010;24:561–573. doi: 10.1210/me.2009-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jorgensen R, et al. Oxyntomodulin differentially affects glucagon-like peptide-1 receptor beta-arrestin recruitment and signaling through Galpha. J. Pharmacol. Exp. Ther. 2007;322:148–154. doi: 10.1124/jpet.107.120006. [DOI] [PubMed] [Google Scholar]
- 149.Koole C, et al. Allosteric ligands of the glucagon-like peptide 1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: implications for drug screening. Mol. Pharmacol. 2010;78:456–465. doi: 10.1124/mol.110.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jennes L, et al. Receptor-mediated binding and uptake of GnRH agonist and antagonist by pituitary cells. Peptides. 1984;5(Suppl 1):215–220. doi: 10.1016/0196-9781(84)90279-1. [DOI] [PubMed] [Google Scholar]
- 151.Jennes L, et al. Receptor-mediated uptake of GnRH agonist and antagonists by cultured gonadotropes: evidence for differential intracellular routing. Peptides. 1986;7:459–463. doi: 10.1016/0196-9781(86)90015-x. [DOI] [PubMed] [Google Scholar]
- 152.Millar RP, et al. Diversity of actions of GnRHs mediated by ligand-induced selective signaling. Front Neuroendocrinol. 2008;29:17–35. doi: 10.1016/j.yfrne.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Davis AA, et al. Differential effects of allosteric M1 muscarinic acetylcholine receptor agonists on receptor activation, arrestin 3 recruitment, and receptor down-regulation. ACS Chem. Neurosci. 2010;1:542–551. doi: 10.1021/cn100011e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Davis CN, et al. Differential regulation of muscarinic M1 receptors by orthosteric and allosteric ligands. BMC Pharmacol. 2009;9:14. doi: 10.1186/1471-2210-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ma L, et al. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15950–15955. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fisher A, et al. Selective signaling via unique M1 muscarinic agonists. Ann. N. Y. Acad. Sci. 1993;695:300–303. doi: 10.1111/j.1749-6632.1993.tb23070.x. [DOI] [PubMed] [Google Scholar]
- 157.Gurwitz D, et al. Discrete activation of transduction pathways associated with acetylcholine M1 receptor by several muscarinic ligands. Eur. J. Pharmacol. 1994;267:21–31. doi: 10.1016/0922-4106(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 158.Gregory KJ, et al. Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias. J. Biol. Chem. 2010;285:7459–7474. doi: 10.1074/jbc.M109.094011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mitselos A, et al. Delineation of the motilin domain involved in desensitization and internalization of the motilin receptor by using full and partial antagonists. Biochem. Pharmacol. 2007;73:115–124. doi: 10.1016/j.bcp.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 160.Bradbury FA, et al. G protein independent phosphorylation and internalization of the delta-opioid receptor. J. Neurochem. 2009;109:1526–1535. doi: 10.1111/j.1471-4159.2009.06082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang J, et al. Agonist-specific regulation of delta-opioid receptor trafficking by G protein-coupled receptor kinase and beta-arrestin. J. Recept. Signal. Transduct. Res. 1999;19:301–313. doi: 10.3109/10799899909036653. [DOI] [PubMed] [Google Scholar]
- 162.Molinari P, et al. Morphine-like opiates selectively antagonize receptor–arrestin interactions. J. Biol. Chem. 2010;285:12522–12535. doi: 10.1074/jbc.M109.059410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li JG, et al. Differential regulation of the human kappa opioid receptor by agonists: etorphine and levorphanol reduced dynorphin A-and U50,488H-induced internalization and phosphorylation. J. Pharmacol. Exp. Ther. 2003;305:531–540. doi: 10.1124/jpet.102.045559. [DOI] [PubMed] [Google Scholar]
- 164.Bruchas MR, et al. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J. Biol. Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu Y, et al. mu Opioid receptor phosphorylation, desensitization, and ligand efficacy. J. Biol. Chem. 1997;272:28869–28874. doi: 10.1074/jbc.272.46.28869. [DOI] [PubMed] [Google Scholar]
- 166.Zhang J, et al. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reversi A, et al. The oxytocin receptor antagonist atosiban inhibits cell growth via a “biased agonist” mechanism. J. Biol. Chem. 2005;280:16311–16318. doi: 10.1074/jbc.M409945200. [DOI] [PubMed] [Google Scholar]
- 169.Hoffmann C, et al. Agonist-selective, receptor-specific interaction of human P2Y receptors with beta-arrestin-1 and -2. J. Biol. Chem. 2008;283:30933–30941. doi: 10.1074/jbc.M801472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Velazquez B, et al. Differential agonist-induced desensitization of P2Y2 nucleotide receptors by ATP and UTP. Mol. Cell Biochem. 2000;206:75–89. doi: 10.1023/a:1007091127392. [DOI] [PubMed] [Google Scholar]
- 171.Dupre DJ, et al. Inverse agonist-induced signaling and down-regulation of the platelet-activating factor receptor. Cell Signal. 2007;19:2068–2079. doi: 10.1016/j.cellsig.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 172.Ramachandran R, et al. Agonist-biased signaling via proteinase activated receptor-2: differential activation of calcium and mitogen-activated protein kinase pathways. Mol. Pharmacol. 2009;76:791–801. doi: 10.1124/mol.109.055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pauwels PJ, Colpaert FC. Ca2+ responses in Chinese hamster ovary-K1 cells demonstrate an atypical pattern of ligand-induced 5-HT1A receptor activation. J. Pharmacol. Exp. Ther. 2003;307:608–614. doi: 10.1124/jpet.103.055871. [DOI] [PubMed] [Google Scholar]
- 174.Gettys TW, et al. Selective activation of inhibitory G-protein alpha-subunits by partial agonists of the human 5-HT1A receptor. Biochemistry. 1994;33:4283–4290. doi: 10.1021/bi00180a024. [DOI] [PubMed] [Google Scholar]
- 175.Akin D, et al. Agonist-directed trafficking explaining the difference between response pattern of naratriptan and sumatriptan in rabbit common carotid artery. Br. J. Pharmacol. 2002;136:171–176. doi: 10.1038/sj.bjp.0704710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schmid CL, et al. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kurrasch-Orbaugh DM, et al. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J. Pharmacol. Exp. Ther. 2003;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- 178.Berry SA, et al. Rapid agonist-induced internalization of the 5-hydroxytryptamine2A receptor occurs via the endosome pathway in vitro. Mol. Pharmacol. 1996;50:306–313. [PubMed] [Google Scholar]
- 179.Bhatnagar A, et al. The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J. Biol. Chem. 2001;276:8269–8277. doi: 10.1074/jbc.M006968200. [DOI] [PubMed] [Google Scholar]
- 180.Cussac D, et al. Agonist-directed trafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur. J. Pharmacol. 2008;594:32–38. doi: 10.1016/j.ejphar.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 181.Hanley NR, Hensler JG. Mechanisms of ligand-induced desensitization of the 5-hydroxytryptamine2A receptor. J. Pharmacol. Exp. Ther. 2002;300:468–477. doi: 10.1124/jpet.300.2.468. [DOI] [PubMed] [Google Scholar]
- 182.Newton RA, Elliott JM. Mianserin-induced down-regulation of human 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptors stably expressed in the human neuroblastoma cell line SH-SY5Y. J. Neurochem. 1997;69:1031–1038. doi: 10.1046/j.1471-4159.1997.69031031.x. [DOI] [PubMed] [Google Scholar]
- 183.Willins DL, et al. Clozapine and other 5-hydroxytryptamine-2A receptor antagonists alter the subcellular distribution of 5- hydroxytryptamine-2A receptors in vitro and in vivo. Neuroscience. 1999;91:599–606. doi: 10.1016/s0306-4522(98)00653-8. [DOI] [PubMed] [Google Scholar]
- 184.Berg KA, et al. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- 185.Stout BD, et al. Rapid desensitization of the serotonin2C receptor system: effector pathway and agonist dependence. J. Pharmacol. Exp. Ther. 2002;302:957–962. doi: 10.1124/jpet.302.3.957. [DOI] [PubMed] [Google Scholar]
- 186.Werry TD, et al. Characterization of serotonin 5-HT2C receptor signaling to extracellular signal-regulated kinases 1 and 2. J. Neurochem. 2005;93:1603–1615. doi: 10.1111/j.1471-4159.2005.03161.x. [DOI] [PubMed] [Google Scholar]
- 187.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 188.Lesche S, et al. Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. J. Clin. Endocrinol. Metab. 2009;94:654–661. doi: 10.1210/jc.2008-1919. [DOI] [PubMed] [Google Scholar]
- 189.Cescato R, et al. Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. J. Nucl. Med. 2006;47:502–511. [PubMed] [Google Scholar]
- 190.Rihakova L, et al. VRQ397 (CRAVKY): a novel noncompetitive V2 receptor antagonist. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R1009–R1018. doi: 10.1152/ajpregu.90766.2008. [DOI] [PubMed] [Google Scholar]
- 191.Rakesh K, et al. beta-Arrestin-biased agonismof the angiotensin receptor induced by mechanical stress. Sci. Signal. 2010;3:ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mederos y Schnitzler M, et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim J, et al. Beta-arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ. Res. 2008;103:70–79. doi: 10.1161/CIRCRESAHA.108.172338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Morris GE, et al. G protein-coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y-purinoceptor signalling. Cardiovasc Res. 2010 doi: 10.1093/cvr/cvq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hollingsworth JW, et al. Both hematopoietic- and non-hematopoietic- derived {beta}-arrestin-2 regulates murine allergic airway disease. AmJRespir. Cell Mol. Biol. 2009;43:269–275. doi: 10.1165/rcmb.2009-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Walker JK, et al. beta-Arrestin-2 regulates the development of allergic asthma. J. Clin. Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kuhr FK, et al. beta-Arrestin 2 is required for B1 receptor-dependent post-translational activation of inducible nitric oxide synthase. FASEB J. 2010;24:2475–2483. doi: 10.1096/fj.09-148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Manson ME, et al. cAMP-mediated regulation of cholesterol accumulation in cystic fibrosis and Niemann-Pick type C cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L809–L819. doi: 10.1152/ajplung.90402.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fong AM, et al. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kizaki T, et al. beta2-Adrenergic receptor regulates Toll-like receptor-4-induced nuclear factor-kappaB activation through beta-arrestin-2. Immunology. 2008;124:348–356. doi: 10.1111/j.1365-2567.2007.02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li Y, et al. Morphine promotes apoptosis via TLR2, and this is negatively regulated by beta-arrestin 2. Biochem. Biophys. Res. Commun. 2009;378:857–861. doi: 10.1016/j.bbrc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 202.Loniewski K, et al. Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages. Mol. Immunol. 2008;45:2312–2322. doi: 10.1016/j.molimm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 203.Mo W, et al. Nuclear beta-arrestin1 functions as a scaffold for the dephosphorylation of STAT1 and moderates the antiviral activity of IFN-gamma. Mol. Cell. 2008;31:695–707. doi: 10.1016/j.molcel.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 204.Yu MC, et al. An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat. Immunol. 2008;9:898–907. doi: 10.1038/ni.1635. [DOI] [PubMed] [Google Scholar]
- 205.Basher F, et al. beta-Arrestin 2: a negative regulator of inflammatory responses in polymorphonuclear leukocytes. IntJClin. Exp. Med. 2008;1:32–41. [PMC free article] [PubMed] [Google Scholar]
- 206.Shi Y, et al. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat. Immunol. 2007;8:817–824. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- 207.Wang Y, et al. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 208.Fan H, et al. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–351. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Porter KJ, et al. Regulation of lipopolysaccharide-induced inflammatory response and endotoxemia by beta-arrestins. J. Cell Physiol. 2010;225:406–416. doi: 10.1002/jcp.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tsutsui S, et al. Glucocorticoids regulate innate immunity in a model of multiple sclerosis: reciprocal interactions between the A1 adenosine receptor and beta-arrestin-1 in monocytoid cells. FASEB J. 2008;22:786–796. doi: 10.1096/fj.07-9002com. [DOI] [PubMed] [Google Scholar]
- 211.DeFea KA. Stop that cell! beta-Arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu. Rev. Physiol. 2007;69:535–560. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- 212.Su Y, et al. Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. J. Immunol. 2005;175:5396–5402. doi: 10.4049/jimmunol.175.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Seregin SS, et al. beta-Arrestins modulate adenovirus-vector-induced innate immune responses: differential regulation by beta-arrestin-1 and beta-arrestin-2. Virus Res. 2010;147:123–134. doi: 10.1016/j.virusres.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Luan B, et al. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457:1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- 215.Quoyer J, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J. Biol. Chem. 2010;285:1989–2002. doi: 10.1074/jbc.M109.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sonoda N, et al. beta-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6614–6619. doi: 10.1073/pnas.0710402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Oakley RH, et al. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor–beta-arrestin complexes after receptor endocytosis*. J. Biol. Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 218.Phaneuf S, et al. Effect of oxytocin antagonists on the activation of human myometrium in vitro: atosiban prevents oxytocin-induced desensitization. Am. J. Obstet. Gynecol. 1994;171:1627–1634. doi: 10.1016/0002-9378(94)90414-6. [DOI] [PubMed] [Google Scholar]
- 219.Phaneuf S, et al. Oxytocin signalling in human myometrium. Adv. Exp. Med. Biol. 1995;395:453–467. [PubMed] [Google Scholar]
- 220.Phaneuf S, et al. Loss of myometrial oxytocin receptors during oxytocin-induced and oxytocin-augmented labour. J. Reprod. Fertil. 2000;120:91–97. doi: 10.1530/jrf.0.1200091. [DOI] [PubMed] [Google Scholar]
- 221.Robinson C, et al. Oxytocin-induced desensitization of the oxytocin receptor. Am. J. Obstet. Gynecol. 2003;188:497–502. doi: 10.1067/mob.2003.22. [DOI] [PubMed] [Google Scholar]
- 222.Yun SP, et al. Interaction of profiling-1 and F-actin via a beta-arrestin-1/JNK signaling pathway involved in prostaglandin E2- induced human mesenchymal stem cell migration and proliferation. J. Cell Physiol. 2010 doi: 10.1002/jcp.22366. [DOI] [PubMed] [Google Scholar]
- 223.Jacob C, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J. Biol. Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 224.Moussa O, et al. Novel role of thromboxane receptors beta isoform in bladder cancer pathogenesis. Cancer Res. 2008;68:4097–4104. doi: 10.1158/0008-5472.CAN-07-6560. [DOI] [PubMed] [Google Scholar]
- 225.Fan X, et al. Acute and chronic morphine treatments and morphine withdrawal differentially regulate GRK2 and GRK5 gene expression in rat brain. Neuropharmacology. 2002;43:809–816. doi: 10.1016/s0028-3908(02)00147-8. [DOI] [PubMed] [Google Scholar]
- 226.Fan XL, et al. Differential regulation of beta-arrestin 1 and beta-arrestin 2 gene expression in rat brain by morphine. Neuroscience. 2003;117:383–389. doi: 10.1016/s0306-4522(02)00930-2. [DOI] [PubMed] [Google Scholar]
- 227.Terwilliger RZ, et al. Chronic morphine administration increases beta-adrenergic receptor kinase (beta ARK) levels in the rat locus coeruleus. J. Neurochem. 1994;63:1983–1986. doi: 10.1046/j.1471-4159.1994.63051983.x. [DOI] [PubMed] [Google Scholar]
- 228.Bohn LM, et al. Enhanced rewarding properties of morphine, but not cocaine, in βarrestin-2 knock-out mice. J. Neurosci. 2003;23:10265–10273. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 230.Beaulieu JM, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 231.Gainetdinov RR, et al. Desensitization of G protein-coupled receptors and neuronal functions. Annu. Rev. Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 232.Ahmed MR, et al. Haloperidol and clozapine differentially affect the expression of arrestins, receptor kinases, and extracellular signal-regulated kinase activation. J. Pharmacol. Exp. Ther. 2008;325:276–283. doi: 10.1124/jpet.107.131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Amar S, et al. Possible involvement of post-dopamine D2 receptor signalling components in the pathophysiology of schizophrenia. Int. J. Neuropsychopharmacol. 2008;11:197–205. doi: 10.1017/S1461145707007948. [DOI] [PubMed] [Google Scholar]
- 234.Bezard E, et al. L-DOPA reverses the MPTP-induced elevation of the arrestin2 and GRK6 expression and enhanced ERK activation in monkey brain. Neurobiol. Dis. 2005;18:323–335. doi: 10.1016/j.nbd.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 235.Bychkov ER, et al. Arrestins and two receptor kinases are upregulated in Parkinson’s disease with dementia. Neurobiol. Aging. 2008;29:379–396. doi: 10.1016/j.neurobiolaging.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Guigoni C, et al. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism Relat. Disord. 2005;11(Suppl 1):S25–S29. doi: 10.1016/j.parkreldis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 237.Chen J, et al. Dopamine promotes striatal neuronal apoptotic death via ERK signaling cascades. Eur. J. Neurosci. 2009;29:287–306. doi: 10.1111/j.1460-9568.2008.06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bruchas MR, et al. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J. Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bruchas MR, et al. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J. Biol. Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bjork K, et al. Modulation of voluntary ethanol consumption by beta-arrestin 2. FASEB J. 2008;22:2552–2560. doi: 10.1096/fj.07-102442. [DOI] [PubMed] [Google Scholar]
- 241.Rimondini R, et al. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- 242.Correll JA, et al. Nicotine sensitization and analysis of brain-derived neurotrophic factor in adolescent beta-arrestin-2 knockout mice. Synapse. 2009;63:510–519. doi: 10.1002/syn.20625. [DOI] [PubMed] [Google Scholar]
- 243.Sun D, et al. Beta-arrestins 1 and 2 are associated with nicotine dependence in European American smokers. Mol. Psychiatry. 2008;13:398–406. doi: 10.1038/sj.mp.4002036. [DOI] [PubMed] [Google Scholar]
- 244.Wang Q, et al. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]