Abstract
BACKGROUND
Multiple lines of evidence suggest that the adrenergic system can modulate sensitivity to anesthetic-induced immobility and anesthetic-induced hypnosis as well. However, several considerations prevent the conclusion that the endogenous adrenergic ligands norepinephrine and epinephrine alter anesthetic sensitivity.
METHODS
Using dopamine β-hydroxylase (Dbh−/−) mice genetically engineered to lack the adrenergic ligands and their siblings with normal adrenergic levels, we test the contribution of the adrenergic ligands upon volatile anesthetic induction and emergence. Moreover, we investigate the effects of intravenous dexmedetomidine in adrenergic-deficient mice and their siblings using both righting reflex and processed electroencephalographic measures of anesthetic hypnosis.
RESULTS
We demonstrate that the loss of norepinephrine and epinephrine and not other neuromodulators copackaged in adrenergic neurons is sufficient to cause hypersensitivity to induction of volatile anesthesia. However, the most profound effect of adrenergic deficiency is retarding emergence from anesthesia, which takes two to three times as long in Dbh−/− mice for sevoflurane, isoflurane, and halothane. Having shown that Dbh−/− mice are hypersensitive to volatile anesthetics, we further demonstrate that their hypnotic hypersensitivity persists at multiple doses of dexmedetomidine. Dbh−/− mice exhibit up to 67% shorter latencies to loss of righting reflex and up to 545% longer durations of dexmedetomidine-induced general anesthesia. Central rescue of adrenergic signaling restores control-like dexmedetomidine sensitivity. A novel continuous electroencephalographic analysis illustrates that the longer duration of dexmedetomidine-induced hypnosis is not due to a motor confound, but occurs because of impaired anesthetic emergence.
CONCLUSIONS
Adrenergic signaling is essential for normal emergence from general anesthesia. Dexmedetomidine-induced general anesthesia does not depend upon inhibition of adrenergic neurotransmission.
INTRODUCTION
Adrenergic neurons that utilize norepinephrine and epinephrine play an important role in the regulation of numerous physiological events, including thermoregulation, stress responses, attention, learning, and the sleep-wake cycle.1 While previous studies have shown that manipulations of adrenergic neurons affect the onset and dissipation of the anesthetic state, it remains unproven whether norepinephrine and epinephrine themselves are directly sufficient to alter anesthetic hypnosis. Acute administration of reserpine and alpha-methyldopa, agents known to reduce norepinephrine release, significantly decreases the amount of halothane required to prevent movement to noxious stimuli in animals.2,3 Conversely, acute administration of agents such as cocaine, amphetamine, and the monoamine oxidase inhibitor, iproniazid, which generate increased norepinephrine release or prevent norepinephrine reuptake at synapses, increases the dose of halothane required to prevent movement.2–4 Systemic delivery of dexmedetomidine, a highly selective alpha2 adrenoceptor agonist, is an anesthetic on its own5 but also decreases halothane anesthetic requirements,6 providing support for anesthetic-induced suppression of adrenergic signaling in producing or altering an anesthetic state. Although these results consistently suggest a role for adrenergic signaling in modulating anesthetic sensitivity, inherent problems with pharmacologic specificity remain as a potential confounder.
A second class of studies supporting a role for adrenergic signaling in altering anesthetic sensitivity has been conducted using lesions. Systemic injections of DSP-4 or 6-hydroxydopamine combined with a tyrosine hydroxylase inhibitor severely deplete central nervous system norepinephrine levels and are associated with decreased halothane minimum alveolar concentration and prolonged duration of barbiturate-induced hypnosis.6–9 Nevertheless, pharmacologic lesions cannot cleanly eliminate the entire adrenergic neuron population. Bilateral electrolytic lesions of the locus coeruleus (LC), implicated in mediating hypnosis and predominantly composed of noradrenergic neurons, significantly decrease halothane and cyclopropane anesthetic requirements.10 However, in destroying LC neurons, the electrolytic lesions cause a loss of other cotransmitters in addition to norepinephrine, as well as a loss of non-adrenergic cells and fibers.
We previously reported that inactivation of LC neurons was not required for anesthetic action by halothane, questioning whether the removal of adrenergic signaling from the LC was the sole source behind hypnosis.11 This finding heightens the importance of the question of whether the release of norepinephrine itself or that of copackaged neurotransmitters, including neuropeptide Y, adenosine, and galanin, may be held responsible for modifying anesthetic sensitivity.
Using dopamine β-hydroxylase knockout (Dbh−/−) mice that lack the adrenergic ligands norepinephrine and epinephrine, we previously demonstrated that loss of norepinephrine and epinephrine produced increased sensitivity to isoflurane.12 Herein, we investigated the effects of sevoflurane and halothane on this genetically engineered model to extend our previous finding. We hypothesize that adrenergic deficiency in Dbh−/− mice accounts for hypersensitivity to induction of and delayed emergence from volatile anesthesia. Under the supposition that adrenergic signaling suffices to alter anesthetic hypnosis, we test the possibility that dexmedetomidine acts solely on adrenergic neurons.13,14 If inhibition of adrenergic signaling were responsible for dexmedetomidine-induced hypnosis, we hypothesize that the intravenous anesthetic would produce no hypnotic effect in the norepinephrine-deficient Dbh−/− mice. As a control, we re-examined anesthetic effects upon restoring norepinephrine and epinephrine to the central nervous system using L-threo-3,4-dihydroxyphenylserine and benserazide, a peripheral L-amino acid decarboxylase inhibitor.15,16
MATERIALS AND METHODS
Animals
All efforts were made to minimize animal suffering and reduce the number of animals used. Dbh heterozygous and knockout mice were maintained on a hybrid C57BL/6J x 129/SvCPJ genetic background and included equal numbers of males and females. Dbh+/− females were mated to Dbh−/− males and treated with 100 µg/ml each of phenylephrine and isoproterenol (Sigma, St. Louis, MO) from embryonic days 8.5 to 16.5 followed by 2 mg/ml L-threo-3,4-dihydroxyphenylserine (Sumitomo Pharmaceuticals, Osaka, Japan) until birth in the drinking water to increase likelihood of fetal survival. Given that neither norepinephrine nor epinephrine is essential for postnatal survival, litters were not provided treatment after birth.17 Heterozygous Dbh+/− siblings were used as controls as tissue samples revealed levels of norepinephrine and epinephrine indistinguishable from wildtypes.16 The animals were housed under controlled conditions (12 h of light starting at 19:00, 22–24°C) in an isolated ventilated room and given food and water ad libidum. As Dbh−/− mice exhibit ptosis, a condition absent in their Dbh+/− siblings,16 investigators could not be blinded to genotype. All studies were performed with approval from the Institutional Animal Care and Use Committee at the University of Pennsylvania (Philadelphia, Pennsylvania) and in accordance with National Institutes of Health guidelines.
Loss and Return of Righting Reflex
Anesthetic sensitivity was assessed behaviorally using loss of righting reflex (LORR). A mouse unable to turn itself prone onto all four feet was considered to have lost its righting reflex and entered a hypnotic state. All mice were observed until they had regained the righting reflex (RORR) at which point the mouse was able to right itself two consecutive times within one minute of each other. In single-step anesthetic wash-in and washout studies, the times to induction and emergence were recorded.
Dbh+/− (n=30) and Dbh−/− (n=36) mice ranged from 4–6 months in volatile anesthetic experiments. To acclimate animals to the testing environment, mice were placed in 200 ml cylindrical open circuit chambers and exposed to 200 ml/min of fresh oxygen flow for two hours daily in the four days prior to anesthetic testing. Mice were exposed to an average initial concentration of 0.63% isoflurane, 0.73% halothane, or 0.96% sevoflurane in 100% oxygen. After every 15-minute period, chambers were rotated 180° to assess LORR. Anesthetic gas concentrations were determined in triplicate during the last two minutes of each 15-minute interval using a Riken FI-21 refractometer (AM Bickford, Wales Center, NY). The concentration of volatile anesthetic was increased incrementally by 0.05%, 0.06%, or 0.10% to peak values of 1.10%, 1.32%, and 1.92% for isoflurane, halothane, and sevoflurane, respectively. After the last mouse had lost its righting reflex, volatile anesthetic was discontinued and time until emergence as defined by the return of the righting reflex was determined. Body temperature was maintained at 36.6 ± 0.2 °C by submerging the chambers in a heated water bath.
After fitting Dbh+/− and Dbh−/− LORR data with separate sigmoidal dose-response curves in Prism 4.0c (GraphPad Software, Inc., La Jolla, CA) to characterize induction in each genotype, volatile anesthetic concentrations required to elicit LORR in 95% of mice (ED95) were extrapolated for both Dbh+/− and Dbh−/− mice. To control for genotype differences in volatile anesthetic induction, a second round of single-step anesthetic wash-in and washout experiments was conducted with all mice exposed to their corresponding ED95 induction dose in oxygen for two hours, after which the anesthetic gas was discontinued (n=36/genotype). Times to LORR and RORR were recorded.
In dexmedetomidine experiments, Dbh+/− (n=20) and Dbh−/− (n=23) mice ranged from 8–14 weeks. Animals received an intravenous injection in one of four possible doses: 50, 100, 200, or 400 mg/kg, administered in identical volumes of 5 ml/kg. Immediately following injection, mice were held in 200 ml cylindrical open circuit chambers under a heating lamp and above a heating pad in order to prevent hypothermia. Chambers were rotated 180° every 30 seconds until a mouse failed to right itself. A total of 4 of 43 mice had failed intravenous injections and were consequently excluded from the study. Rectal temperatures, both pre-injection and post-anesthesia were measured to ensure that the mice remained euthermic during the experiment.
Pharmacologic Rescue of Adrenergic Signaling
Rescue of adrenergic signaling in Dbh−/− mice was performed according to published protocols.16,18 Five hours in advance of anesthetic sensitivity testing, four Dbh−/− mice received a subcutaneous injection of 20 mg/ml pH neutralized L-threo-3,4-dihydroxyphenylserine, 2 mg/ml vitamin C (Sigma), and 1 mg/ml benserazide (Sigma), a peripheral aromatic L-amino acid decarboxylase inhibitor, in a total volume of 50 ml/g. This treatment restores norepinephrine to near-normal levels for 12 hours with peaks around 5 hours post-injection. As a control for injection-induced stress, four additional Dbh−/− mice received a subcutaneous injection of 50 ml/g vehicle. Dbh+/− mice were not treated with L-threo-3,4-dihydroxyphenylserine and benserazide as such therapy can cause supranormal levels of norepinephrine in these control mice.16
Surgical Procedure
Dbh+/− (n=12) and Dbh−/− (n=8) mice ranged in age from 5–9 months. Animals were deeply anesthetized with 1.5%–2.0% isoflurane and warmed during surgery. Mice were treated aseptically, given cefazolin 40mg/kg intraperitoneally (Sagent Pharmaceuticals, Schamburg, IL), placed in a Model 940 stereotaxic frame (David Kopf Instruments, Tujunga, CA) and given 0.3 ml 0.25% bupivicaine (Hospira, Inc., Lake Forest, IL) subcutaneously prior to opening a 3 cm vertical scalp incision. After exposing the periosteum, four burr holes were drilled into the skull over primary motor (relative to bregma, 1.5 mm anterior and ±1.6 mm lateral) and visual cortices (3.5 mm posterior, ± 1.7 mm lateral) bilaterally.19 Four miniature stainless steel screws (Small Parts, Miami Lakes, FL) were inserted into each hole to form epidural electroencephalographic leads and secured with dental acrylic (Co-Oral-Ite Dental Manufacturing Company, Diamond Springs, CA). A fifth burr hole was drilled (1.0 mm posterior and 1.7 mm lateral) to permit placement of a 600 µm wide, calibrated 10-kΩ microthermistor (AB6E3-GC16KA103L, GE Infrastructure Sensing, St. Marys, PA) inserted to acquire continuous cortical brain temperatures20 (Tbr) and secured with dental acrylic. Two Teflon coated silver wires (A-M Systems, Inc., Sequim, WA) were inserted along the neck muscles to form electromyographic leads. The four screw electrodes, two electromyographic leads, and thermistor had previously been electrically connected to an insulated 2×4 pin row connector (Digi-Key, Thief River Falls, MN) to form an ultra-light weight 0.6 g socket headpiece.20 Headpieces were secured with dental acrylic. The surgical wound was treated with a triple antibiotic ointment (Neomycin and Polymyxin B sulfates and Bacitracin Zinc, E. Fougera & Co., Mellville, NY). Skin edges were reapproximated with suture. Immediately following surgery, mice were injected with 1.0 ml 0.9% sterile saline and given a single injection of buprenorphine 0.3 mg/kg intraperitoneally.
Physiological Recordings
Mice were allowed at least seven days to recover from surgery before experimentation. All experiments were performed at room temperature (22–24°C). Mice were connected to the recording apparatus via a light weight flexible shielded tether and then back loaded into a custom modified plexiglass tail vein injector with free access to the mouse’s head. Electroencephalographic and electromyographic analog signals were amplified (factor of 5000) and filtered (high-pass 1.0Hz, low-pass 100Hz, 60 Hz Notch filter for electroencephalogram; high-pass 10Hz, low-pass 5000 Hz, 60 Hz Notch filter for electromyogram). The thermistor signal was amplified by means of a direct-current powered, custom-made bridge (Intec Associates Ltd., Surrey, United Kingdom), and had been calibrated prior to implantation using a water bath and precision thermometer. All signals were converted from analog to digital with Biopac’s MP150 System (BIOPAC Systems, Inc., Goleta, CA) and visualized using Acknowledge 3.9.2 software for Mac (BIOPAC Systems, Inc). All biopotential signals were digitally sampled at 200 Hz except for Tbr, which was sampled at 1.5 Hz. Recordings began a minimum of 30 minutes before and continued for 4–6 hours after a single intravenous tail injection of 50 µg/kg dexmedetomidine (Hospira, Inc.) given in a volume of 5 µl/g. Dexmedetomidine dosing was based upon postoperative weight less the weight of the headpiece. Tbr was continuously monitored throughout the recording, while rectal temperatures were measured both pre-injection and post-anesthesia. During these physiological recording sessions, mice were actively warmed with a servo-controlled heat lamp set to shut off at 37°C.
Analysis of Electroencephalographic and Electromyographic Signals
For each mouse, three channels of electroencephalographic and one channel of electromyographic data were imported into the Somnologica 3.2 rodent sleep scoring software module (Embla Systems, Broomfield, CO). Each 10-second epoch was automatically scored for the presence of wakefulness. Automatically scored epochs were visually inspected to confirm correct assignments. Any epoch containing artifact was excluded from subsequent analyses. During the baseline period, prior to dexmedetomidine, sample entropy was only determined during epochs of wakefulness. Sample entropy values were calculated for all channels using a freely available module21 written for Matlab Student 7.4 (The Mathworks, Inc., Natic, MA) with an N=1000, m=2, r=0.2. Integrated electromyography values were computed directly using AcqKnowledge 3.9.2 and were normalized so that the highest value obtained during a baseline five-second epoch of wakefulness was defined as 100%.
Statistical Analysis
Investigators were blinded to genotype during data processing and statistical analysis and simply identified mice by ear tag number. For volatile anesthetic experiments, dose responses for induction were fit with a sigmoidal curve (Prism 4.0c) to obtain EC50 and hill slopes along with their corresponding 95% confidence limits.22,23 For analysis of times to LORR and RORR after stepwise or single-step ED95 volatile anesthetic experiments, a two-way ANOVA with Bonferroni post-tests was used with main factors of genotype and volatile anesthetic. Using JMP (SAS Institute, Inc., Cary, NC), a log transformation was used to normalize the unequal variances in emergence times. Two-way ANOVA with Bonferroni post-tests was also used for comparison of latency to and duration of dexmedetomidine-induced LORR values with main factors of genotype and dexmedetomidine dose using Prism 4.0c. An independent two-sample student’s t-test was used to compare the duration of and latency to LORR values between Dbh−/− mice involving rescue or sham-rescue of adrenergic signaling. LORR values are reported as mean ± standard error. Continuous temperature data were imported into Prism 4.0c and analyzed as change from average baseline wakefulness temperature. The Wilcoxon test was used for comparison of continuous temperature data between genotypes while the independent two-sample student’s t-test was used for comparison of rectal temperatures. Integrated electromyographic and sample entropy raw data were independently fit with five linear segments to approximate baseline, induction, duration at nadir values, slope of recovery, and post-dexmedetomidine recovery states using least-squares regression to the mean. A Wilcoxon test was used to assess genotypic differences both for integrated electromyographic and sample entropy values. Due to technical problems with screw implantation, one to two electroencephalographic leads in three of twelve Dbh+/− and two of eight Dbh−/− had to be excluded from data analysis. The exact number of mice represented for each electroencephalographic lead is shown in the corresponding boxplot. All comparisons were unpaired and performed using two-tailed hypothesis testing with the exception of one-tailed testing for sample entropy values. A p-value of <0.05 was considered statistically significant.
RESULTS
Adrenergic-deficient mice are hypersensitive to volatile anesthetic induction and exhibit delayed emergence from volatile anesthetics
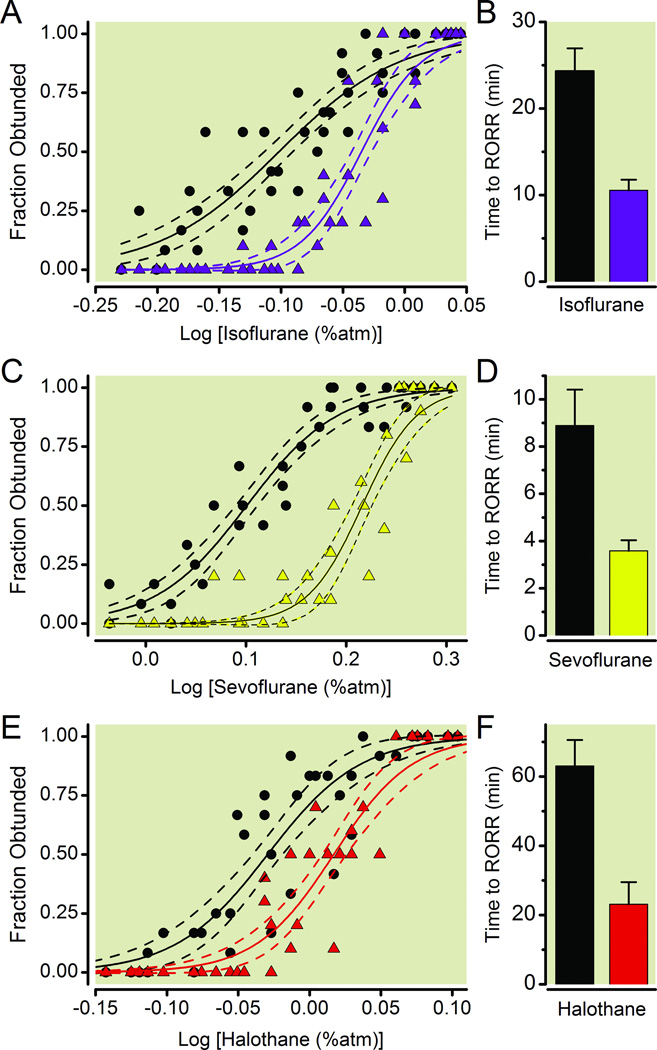
Using the LORR assay, Dbh−/− mice, which completely lack epinephrine and norepinephrine, exhibit hypersensitivity to the induction of isoflurane, sevoflurane, and halothane anesthesia, compared to Dbh+/− sibling controls (Fig. 1) shown previously to have normal catecholamine levels.16 EC50 values (95% confidence interval) for induction of isoflurane, sevoflurane, and halothane anesthesia are 0.79% (0.77% to 0.81%), 1.26% (1.23% to 1.29%), 0.93% (0.91% to 0.96%) in Dbh−/− mice and 0.92% (0.90% to 0.94%), 1.64% (1.61% to 1.68%), 1.04% (1.02% to 1.06%) in Dbh+/− mice respectively. Moreover, the effects of genotype upon emergence from volatile anesthetics were significant (F1,236=46.7, p < 0.0001). When exposed to identical concentrations of a volatile anesthetic, emergence takes two to three times as long in Dbh−/− mice relative to their sibling controls (Fig. 1B, 1D, 1F). Not surprisingly, time to emerge also depended significantly upon the anesthetic drug (F2,236=41.6, p < 0.0001). There was no significant interaction between genotype and volatile anesthetic (F2,236=5.8, p = 0.06).
Fig.1.
Adrenergic-deficient mice exhibit volatile anesthetic hypersensitivity relative to sibling controls with normal levels of norepinephrine/epinephrine. Symbols respectively depict the fraction of Dbh+/− (n=10, triangles) and Dbh−/− (n=12, circles) mice that exhibit a loss of righting reflex (LORR) at each specified anesthetic dose for (A) isoflurane, (C) sevoflurane, and (E) halothane. Solid lines denote the best-fit curves with dashed lines showing 95% confidence interval bracketing the best-fit curves. Bars represent mean ± SEM time lapsed from the termination of anesthetic exposure (shown in A, C, E) until the return of righting reflex (RORR) for Dbh−/− (black) and Dbh+/− (colored) mice for (B) isoflurane, (D) sevoflurane, and (F) halothane. Effects of genotype are significant F1,236=46.7, p < 0.0001. Atm: atmosphere; Dbh: dopamine β-hydroxylase.
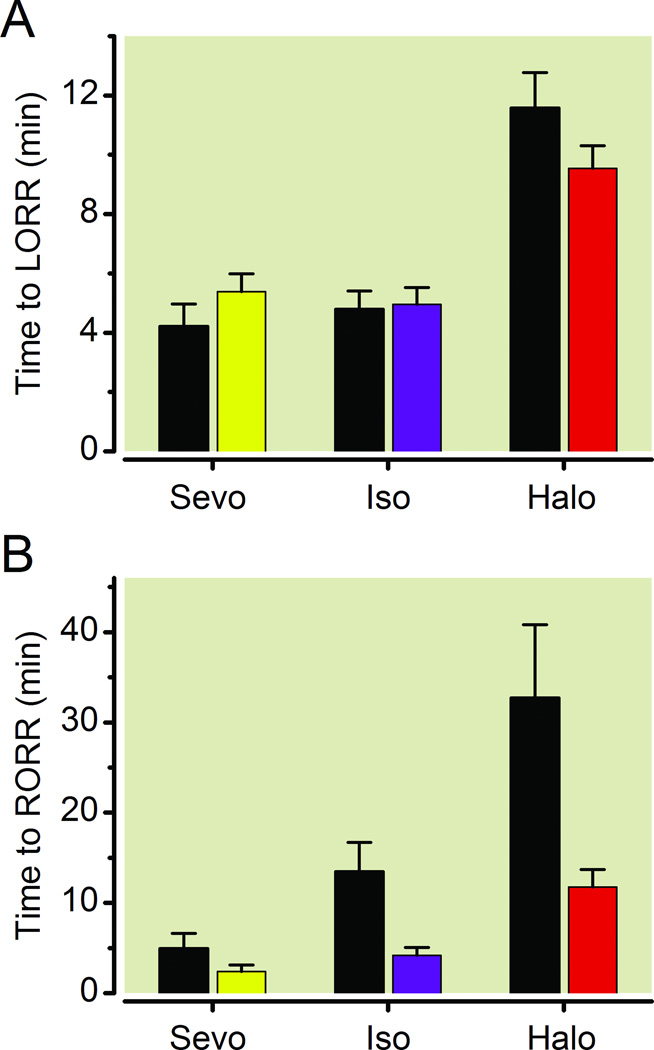
Since the induction sensitivity of Dbh−/− mice is left-shifted compared to their sibling controls (Fig. 1A, 1C, 1E), exposing both Dbh−/− mice and Dbh+/− siblings to identical concentrations of volatile anesthetics leads to a relative overdosing of the Dbh−/− mice that could affect their subsequent emergence. To avoid this confound, Dbh+/− and Dbh−/− mice were each exposed to their respective ED95 for LORR based upon the best-fit curves generated in Fig. 1. Times to induction and emergence were assessed by LORR and RORR. Upon exposure to their ED95 dose for volatile anesthetics, Dbh+/− and Dbh−/− exhibited a significant difference in latency to LORR with respect to anesthetic (F2,66=35.67, p < 0.0001), which may be explained by differences in volatile anesthetic solubility. However, no significant difference was observed with respect to genotype (F1,66=0.14, p=0.7132), and no significant interaction (F2,66=2.20, p=0.1193) occurred between anesthetic and genotype, suggesting an identical rate of volatile anesthetic induction (Fig. 2A). Upon discontinuation of anesthetic gases after two hours, Dbh+/− and Dbh−/− mice similarly exhibited a significant difference in time to emergence with a main effect of the anesthetic (F2,66=27.04, p < 0.0001). Crucially, a significant difference was observed with respect to genotype (F1,66=12.33, p < 0.0003) while no significant interaction (F2,66=0.51, p=0.6046) occurred between anesthetic and genotype (Fig. 2B). These results emphasize that despite correcting for intrinsic changes in sensitivity to anesthetic induction, the Dbh−/− mice continue to exhibit significant impairments in their ability to emerge following volatile anesthetic exposures.
Fig. 2.
Delayed emergence in Dbh−/− mice is not due to a relative volatile anesthetic overdose. (A) Time to LORR in Dbh−/− and Dbh+/− mice when each group is exposed to their respective ED95 dose for eliciting LORR. (B) Time until RORR in Dbh−/− (black) and Dbh+/− (colored) mice following a 2 hour exposure to each group’s respective volatile anesthetic ED95 (n=12/group). There is a significant effect of genotype on emergence F1,66=12.33, p < 0.0003, but not upon induction F1,66=0.14, p=0.7132. Bars show mean ± SEM; LORR: loss of righting reflex; RORR: return of righting reflex; Sevo: sevoflurane; Iso: isoflurane; Halo: halothane; Dbh: dopamine β-hydroxylase.
Cumulatively, these results suggest that loss of norepinephrine and epinephrine in Dbh−/− mice is sufficient to cause hypersensitivity to volatile anesthetic induction as well as delayed emergence following volatile anesthetic exposure. Consequently we proceeded to test the hypothesis that Dbh−/− mice would be insensitive to dexmedetomidine, an intravenous anesthetic that inhibits noradrenergic neurons in the LC.
Adrenergic-deficient mice are hypersensitive to dexmedetomidine
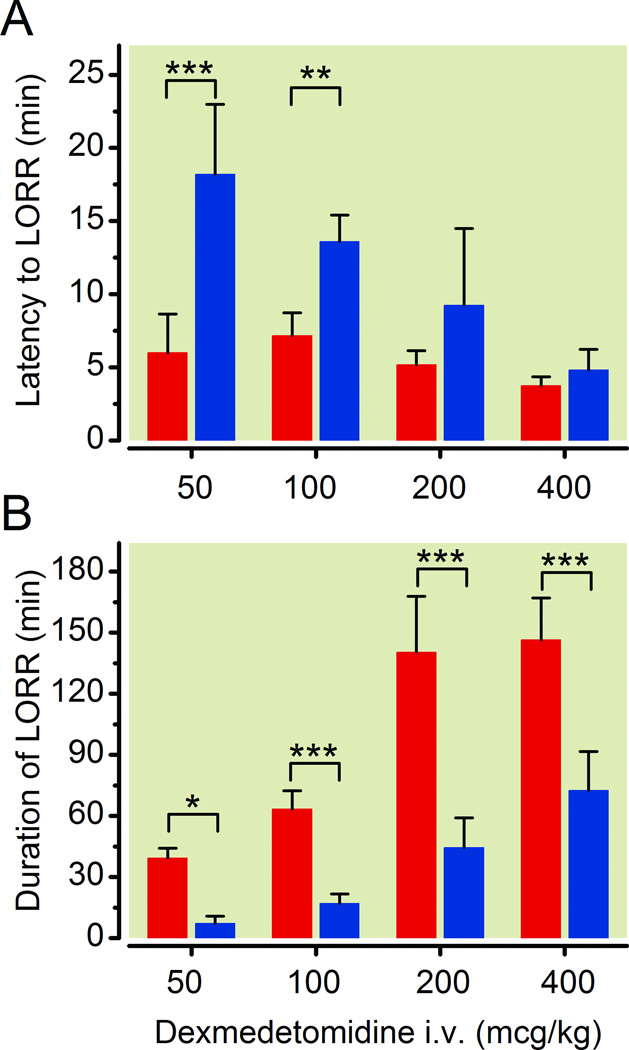
Surprisingly, as with the volatile anesthetics, the Dbh−/− mice illustrated hypersensitivity to dexmedetomidine-induced hypnosis at all tested doses. With intravenous dexmedetomidine, there were significant differences in latency to LORR with respect to both genotype (F1,35=53.40, p < 0.0001) and dose (F3,35=18.07, p < 0.0001), with a significant interaction between genotype and dose (F3,35=8.37, p=0.0003) (Fig. 3A). Moreover, two-way ANOVA revealed significant differences in the duration of LORR with respect to both genotype (F1,35=158.77, p < 0.0001) and dose (F3,35=69.87, p < 0.0001), and the interaction between the two factors is significant (F3,35=8.32, p=0.0003) (Fig. 3B). Larger doses of dexmedetomidine were associated with decreased latency to LORR and longer duration of LORR. At the highest tested intravenous dose of dexmedetomidine, 400 µg/kg, Dbh−/− mice showed significant mortality with four of six mice dying. All deaths occurred within 120 minutes of dexmedetomidine administration, and these data were excluded. Meanwhile, all sibling control mice survived.
Fig. 3.
Loss of adrenergic ligands is sufficient to cause hypersensitivity to dexmedetomidine as assessed by LORR. Dbh−/− mice illustrate hypersensitivity relative to Dbh+/− mice in response to varying intravenous doses of dexmedetomidine. (A) Latency to loss of righting reflex and (B) Duration of LORR in Dbh−/− (red) and Dbh+/− (blue) mice (n=5–6/group). Significant genotypic effects were found for both latency to F1,35=53.40, p < 0.0001 and duration of F1,35=158.77, p < 0.0001 dexmedetomidine-induced hypnosis. * p < 0.05, ** p < 0.01, and *** p < 0.001 relative to Dbh+/− mouse latency to LORR or duration of LORR times. Bars show mean ± SEM; LORR: loss of righting reflex; Dbh: dopamine β-hydroxylase.
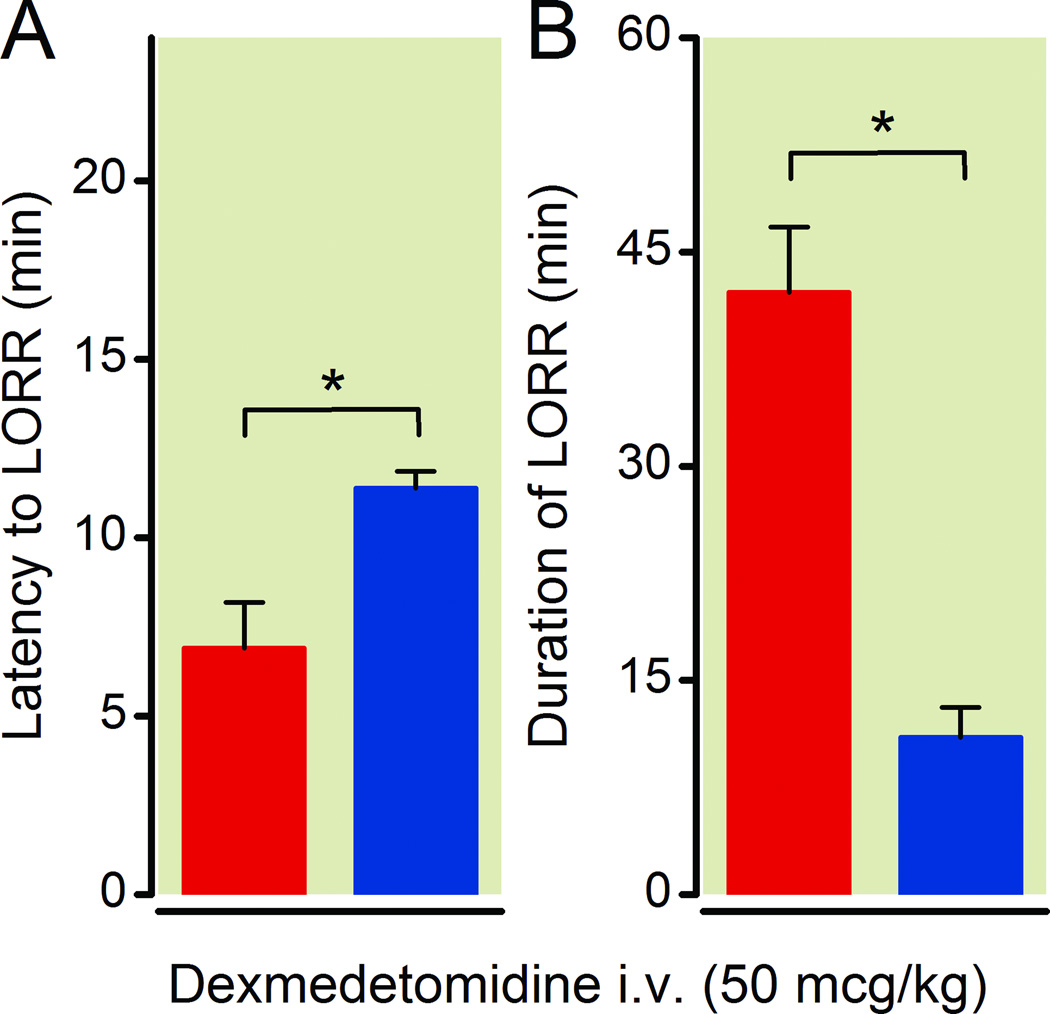
To address whether this apparent hypersensitivity to dexmedetomidine in Dbh−/− mice could be the result of a developmental compensation, we rescued adrenergic signaling specifically in the central nervous system of adult Dbh−/− mice. Five hours following treatment with vehicle or L-threo-3,4-dihydroxyphenylserine plus benserazide, Dbh−/− mice received 50 µg/kg dexmedetomidine intravenously. Restoration of adrenergic signaling partially, but significantly restored the latency to dexmedetomidine-induced LORR (p=0.021) (Fig. 4A). However, central rescue of adrenergic signaling in Dbh−/− mice fully rescued the duration of dexmedetomidine anesthesia (p=0.016) (Fig. 4B). Rescued Dbh−/− averaged 11.0 ± 2.1 minutes for duration of LORR compared to 42.2 ± 4.6 minutes in sham rescued Dbh−/− mice. These values are respectively identical to untreated Dbh−/− animals, that averaged 39.3 ± 2.1 minutes and to Dbh+/− mice, which averaged 7.2 ± 1.6 minutes following 50 µg/kg intravenous dosing (Fig. 3).
Fig. 4.
CNS-specific rescue of norepinephrine and ephinephrine in adrenergic-deficient mice restores duration of dexmedetomidine-induced hypnosis to control levels. Both the latency to LORR following 50 µg/kg of intravenous dexmedetomidine (A) and the duration of LORR (B) are rescued in Dbh−/− mice receiving L-threo-3.4-dihydroxyphenylserine and benserazide rescue treatment (blue) compared to Dbh−/− mice that received vehicle treatment (red) (n=4/group). * p < 0.05. Bars show mean ± SEM; CNS: central nervous system; LORR: loss of righting reflex; Dbh: dopamine β-hydroxylase.
Electroencephalographic evidence of dexmedetomidine hypersensitivity in adrenergic-deficient mice is not accompanied by corresponding motor atonic hypersensitivity
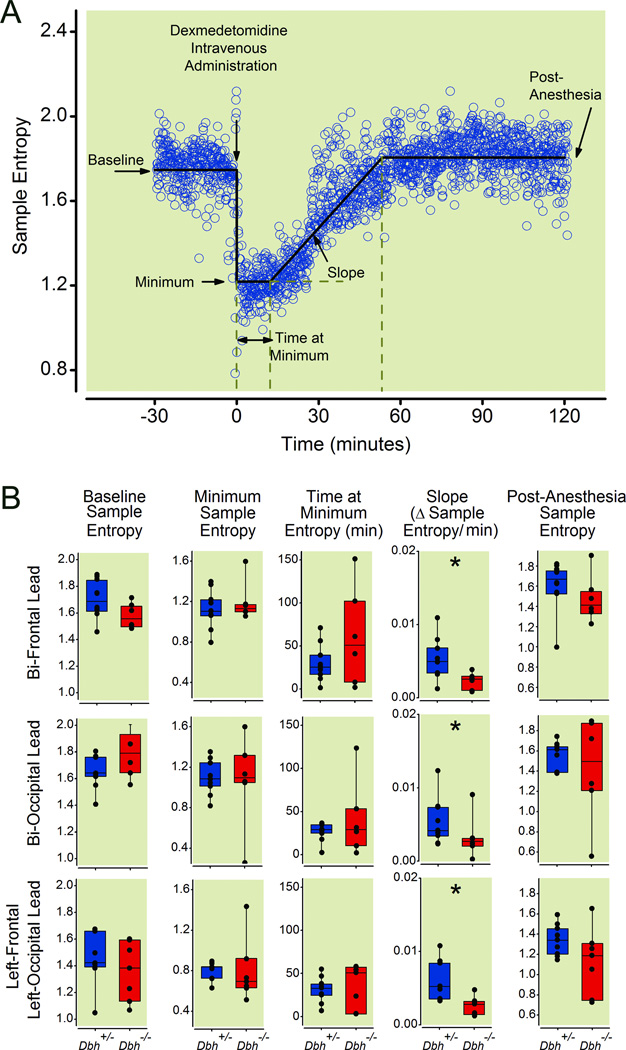
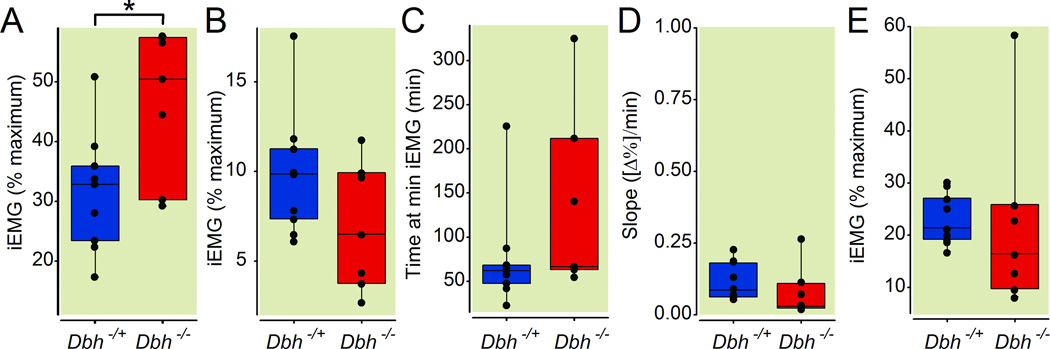
To avoid a potential motor confound and to isolate the hypnotic properties of anesthetics, we measured sample entropy of the electroencephalogram in multiple leads acquired simultaneously from in Dbh−/− mice and their sibling Dbh+/− controls. A typical example depicting how sample entropy changes following administration of dexmedetomidine is shown (Fig. 5A). In practice, no significant allelic effects at the Dbh locus were found in the bi-frontal, bi-occipital, or left-occipital to left-frontal leads for 1) baseline entropy level prior to dexmedetomidine injection (p= 0.06, p=0.10, p=0.18), 2) minimum entropy level immediately following intravenous dexmedetomidine (p=0.60, p=0.70, p=0.20), 3) the duration of time spent at the minimum entropy level (p=0.84, p=0.57, p=0.70), or 4) post-dexmedetomidine emergence entropy levels (p=0.09, p=0.48, p=0.08). However, Dbh−/− mice showed a significantly reduced rate of emergence based upon the entropy recovery slope compared with Dbh+/− mice in the bi-frontal (p=0.008), bi-occipital (p=0.044), and left frontal to left occipital (p=0.028) leads (Fig. 5B). Dbh−/− mice demonstrated no significant differences from Dbh+/− mice with regard to slope from minimum integrated electromyogram to post-anesthesia integrated electromyogram (p=1.00) or in any other measure of motor activity save for baseline muscle tone (p=0.042) where integrated electromyographic tone during wakefulness in the knockouts exceeded that of their siblings (Fig. 6). Finally, no significant effects of genotype were found for changes in temperature following dexmedetomidine administration either for continuously recorded Tbr (p=0.1905) or for baseline rectal temperature minus that recorded immediately following emergence (p=0.07) (not shown).
Fig. 5.
Dbh−/− mice show delayed emergence from dexmedetomidine relative to Dbh+/− mice using a motor-independent electroencephalographic measure. (A) Panel shows a characteristic segmental best-fit (solid line) analysis of sample entropy values calculated from raw electroencephalogram in a control mouse before, during, and after intravenous dexmedetomidine. Raw sample entropy values (circles) were calculated from the electroencephalogram, which were fit with five linear segments to approximate five variables as labeled. Dexmedetomidine was administered intravenously at time=0 as denoted by the arrow. (B) Box plots illustrate sample entropy values along with lower quartile, upper quartile, group minimum and group maximum for pre-drug baseline wakefulness, minimum entropy level, the duration of time at the minimum entropy level, the slope of sample entropy recovery that defines emergence, and sample entropy post-dexmedetomidine emergence for Dbh+/− (n=12) and Dbh−/− (n=8) mice as computed from right frontal-left frontal, right occipital-left occipital, and left frontal-left occipital leads. * denotes p < 0.05 in Dbh−/− mice relative to Dbh+/− mice. Dbh: dopamine β-hydroxylase.
Fig 6.
Adrenergic-deficient mice show no significant differences from sibling controls with respect to motor tone following intravenous dexmedetomidine administration. Box plots depict integrated electromyographic intensity (normalized to percentage of maximum during wakefulness) along with lower quartile, upper quartile, group minimum, and group maximum for (A) pre-drug baseline wakefulness, (B) minimum integrated electromyogram values, (C) the duration of time at the minimum integrated electromyogram values, (D) the slope of integrated electromyogram recovery, and (E) stabilized integrated electromyogram values post-dexmedetomidine emergence in Dbh+/− (n=12) and Dbh−/− (n=8) mice. * denotes p < 0.05 in Dbh−/− mice relative to Dbh+/− mice. iEMG: integrated electromyogram; Dbh: dopamine β-hydroxylase.
DISCUSSION
Loss of the adrenergic ligands norepinephrine and epinephrine and not of neurotransmitters copackaged in adrenergic neurons is sufficient to cause hypersensitivity to induction of anesthesia. Impairing central adrenergic signaling left-shifts induction dose-response curves for many anesthetics,2,3,7,12,24,25 including isoflurane, but our work extends this association to other volatile anesthetics and dexmedetomidine as well. Moreover, our results address a lingering question about the role of dopamine in modulating anesthetic induction. While pharmacological treatments that deplete norepinephrine may also deplete other monoamines such as dopamine,2 in the Dbh−/− model dopamine levels increase;17 yet, we demonstrate induction hypersensitivity indicating that modulation of dopamine may not predominate as has been implied.26
Adrenergic deficits also affect anesthetic emergence, causing significant temporal delays in RORR, our surrogate marker for recovery of consciousness in mice. By comparing the magnitude of this temporal effect with our previously published study on isoflurane,12 we assert that the most profound effect in the Dbh−/− mice occurs during emergence. In support of this conclusion, when adrenergic-deficient mice and their sibling controls are exposed to their respective equipotent induction doses of isoflurane, sevoflurane, or halothane, we find no differences in the time to LORR. While much is known about the cardiorespiratory physiology in adrenergic-deficient mice,27–29 formal evaluation of their cardiac output and minute ventilation remains lacking. As anesthetic uptake and distribution depend upon cardiac output and minute ventilation, it is reasonable to assume that such values may differ between Dbh−/− and sibling control mice. However, within the limits of sensitivity for righting reflex assay, and by using each genotype’s respective ED95 as a driving dose, we are unable to detect differences between Dbh−/− and sibling controls in the times to induction. This places a small upper limit on the degree to which anesthetic uptake and distribution might differ between Dbh−/− and their siblings. Nonetheless, under identical methodological constraints, we find significant differences for emergence in Dbh−/− mice (Fig. 1). Even when corrected for intrinsic differences in volatile anesthetic sensitivity, adrenergic-deficient mice still take two to three times longer to emerge from volatile anesthetics than sibling controls (Fig. 2). Considering that metabolism of isoflurane, sevoflurane, and halothane varies over one hundred-fold, and that elimination also depends upon cardiac output and minute ventilation, these results point to large and important differences in the pharmacodynamic effects of volatile anesthetics in adrenergic-deficient Dbh−/− mice.
Adrenergic Signaling and Emergence from Volatile Anesthesia
The loss of norepinephrine and epinephrine is associated with a protracted RORR, signifying delayed emergence. These studies reveal a prominent role for norepinephrine and epinephrine and more broadly, a necessary reactivation of adrenergic signaling, in facilitating anesthetic emergence. During emergence from halothane, LC neurons exhibit burst-like firing patterns,30 causing endogenous release of norepinephrine. During emergence from sevoflurane and isoflurane, microdialysis studies confirm surges in norepinephrine in the preoptic area of the hypothalamus.31 Within basal forebrain, norepinephrine can directly promote transient arousals.32 If released upon sleep-active ventrolateral preoptic hypothalamus (VLPO) and median preoptic hypothalamic neurons, norepinephrine’s hyperpolarizing actions should inhibit activity in these groups, which would release inhibition of other wake-active systems, thus enhancing arousal.33,34 Studies conducted in humans demonstrate an arousal-promoting effect of ephedrine upon processed electroencephalographic measures of anesthetic hypnosis.35,36 As a mixed indirect adrenergic agonist, ephedrine could affect dopamine levels in addition to norepinephrine. There is accumulating evidence to suggest that facilitation of dopaminergic signaling itself may enhance emergence from anesthesia via multiple mechanisms.37,38 However, the relative contributions of noradrenergic versus dopaminergic mechanisms merit additional clarification.37,39
Mechanism of Action of Dexmedetomidine
Despite the studies supporting the locus coeruleus as the key site of action underlying the hypnotic actions of dexmedetomidine,13,14 we find that inhibition of adrenergic neurons within LC or elsewhere is unlikely to mediate the hypnotic properties of dexmedetomidine. Having established that loss of adrenergic ligands (and not a copackaged neuromodulator) is sufficient for hypnotic hypersensitivity to and delayed emergence from volatile anesthetics, the causal association of dexmedetomidine-induced hypnosis proceeding via inhibition of the LC and ensuing disinhibition of the VLPO must be challenged.13 We demonstrate significantly reduced latencies and prolonged duration of dexmedetomidine action in Dbh−/− mice, compared to sibling controls (Fig. 3). These changes are unlikely to arise from potential developmental compensation because postnatal central nervous system-specific rescue of adrenergic signaling reverted the hypnotic duration in Dbh−/− mice to levels indistinguishable from controls. Rather, the rescue of norepinephrine and epinephrine suggests that adrenergic signaling restoration is sufficient for normal emergence.12 While dexmedetomidine still should inhibit LC neuronal activity via alpha2A adrenoceptors in Dbh−/− mice,40 these mice have no norepinephrine to release at the synapse. Consequently LC targets normally inhibited by norepinephrine, such as the VLPO,41–43 hypothetically should not be affected unless dopamine or another copackaged neurotransmitter could substitute for norepinephrine. If inhibiting release of copackaged neuromodulators from the LC and other adrenergic neurons such as galanin, neuropeptide Y, or adenosine were sufficient to cause anesthesia, then Dbh−/− mice should not have had altered responses to volatile anesthetics. This leaves two viable alternative explanations that might still rescue the Nelson model13 for dexmedetomidine or the possibility that the model requires revision. Dopamine accumulates in adrenergic neurons of Dbh−/− mice.17 While the exact adrenoceptors mediating the hyperpolarizing effects of norepinephrine upon VLPO remain unknown, presynaptic alpha2A adrenoceptors abutting putative sleep-promoting VLPO neurons are involved.44 Dopamine is more than 1000-fold less potent than norepinephrine at alpha2A adrenoceptors and 10- to 10000-fold less potent than norepinephrine at other adrenoceptors.45 While this does not formally exclude dopamine released from adrenergic neurons in Dbh−/− mice, two studies demonstrate that adrenergic neurons themselves are not required for alpha2 agonist-induced hypnosis. Following pharmacologic depletion of catecholamines, dexmedetomidine retains an additional profound volatile anesthetic-sparing effect.6 Moreover, medetomidine retains its hypnotic properties in mice that lack alpha2A adrenoceptors on adrenergic neurons,46 proving that inhibition of adrenergic neurons, including those in the LC, is not necessary. Finally, it could be possible that the apparent interpretation of hypnosis in Dbh−/− mice given either medetomidine46 or dexmedetomidine (Fig. 3) could simply be loss of postural muscle tone leading to LORR with preserved consciousness. It is known that exhaustion of norepinephrine from its terminals is sufficient to mimic cataplexy, inhibiting movement.47 Here our studies of electroencephalographic effects of dexmedetomidine in Dbh−/− mice and sibling controls are particularly informative. We demonstrate no difference in the onset, depth, duration, or recovery from motor inhibition in both groups of mice given intravenous dexmedetomidine (Fig. 6). Although we detect an increased baseline resting motor tone in Dbh−/−, the presence of significantly higher delta power during wakefulness in these animals18 may have confounded our scoring of wakefulness; with a greater fraction of active, high-motor tone wakeful epochs selected for Dbh−/− than in control siblings. Nevertheless, even if Dbh−/− have heightened resting motor tone, this finding would not confound our righting reflex results since only muscle weakness might cloud the interpretation of LORR. By applying a novel segmental best-fit algorithm to continuous sample entropy measurements, we confirm a processed electroencephalographic parameter that conclusively demonstrates delayed rate of emergence in Dbh−/− mice given dexmedetomidine (Fig. 5). Together with the LORR studies we demonstrate that Dbh−/− are hypersensitive to anesthetics using methodology that permits a dissociation between the myorelaxant and hypnotic effects of anesthetic drugs. It is possible that we were underpowered to detect differences in awake baseline sample entropy between Dbh−/− and Dbh+/− siblings. Increased sleepiness in Dbh−/− mice18 would be predicted to result in lower sample entropy values.
One issue that remains is the question of whether modulation of adrenergic signaling mechanistically underlies anesthetic hypnosis. Proving a mechanistic link would require demonstrating that all anesthetics inhibit adrenergic signaling as a requirement for induction and that direct inhibition (enhancement) of adrenergic signaling is sufficient to facilitate (retard) induction. In the case of volatile anesthetic induction, this study and others demonstrates the latter. However, while many anesthetics do inhibit adrenergic output or downstream adrenoceptor coupled signal transduction, this is not uniformly true.2,3,6,7,9,11–13,24,48,49,50,51 When considering anesthetic emergence, a parallel analysis of necessity and sufficiency would require the demonstration both of surges in adrenergic signaling preceding emergence and the association that increased (decreased) adrenergic signaling is sufficient to facilitate (retard) emergence.12,30–32,37 Formally meeting such strict requirements would require detailed understanding of anesthetic effects on every step from adrenergic neuronal activity, synaptic release of adrenergic ligands, receptor-ligand binding, post-receptor signal transduction, and modulation of ligand metabolism or reuptake. Complete characterization for each and every step in the presence of distinct anesthetics is lacking. Hence, we cannot formally exclude the possibility that modulation of adrenergic signaling independently affects arousal parallel to but independent of anesthetic drug effects.
Concluding remarks
Our results with the alpha2 selective agonist, dexmedetomidine, are consistent with those obtained with volatile anesthetics in which adrenergic-deficient mice also exhibit delayed emergence. Cumulatively, our results suggest that current understanding of the mechanisms through which dexmedetomidine works are incomplete. While dexmedetomidine may indeed inhibit the LC and subsequently disinhibit the VLPO, the initial step of LC inhibition is not required11 nor sufficient46 to explain anesthetic hypnosis. Dexmedetomidine’s hypnotic effects must arise via actions on post-synpatic alpha2A adrenoceptors located on non-adrenergic neurons. Determining the identity of the non-adrenergic neurons should become a priority for subsequent studies, as targeted inhibition of this neural substrate may prove important for existing as well as novel therapeutics.
Summary Statement.
Adrenergic ligand deficiency causes hypersensitivity to volatile anesthetic induction and more profoundly impedes volatile anesthetic emergence. Surprisingly, such results also hold for dexmedetomidine, indicating that inhibition of adrenergic neurons cannot account for dexmedetomidine’s hypnotic properties.
Final Box Summary.
What we already know about this topic
-
*
Modulation of adrenergic signaling inversely affects volatile anesthetic potency in vivo, suggesting a critical role anesthetic mechanisms
-
*
Loss of norepinephrine and epinephrine in mutant mice deficient in dopamine beta-hydroxylase increases sensitivity to isoflurane
What this article tells us that is new
-
*
Loss of norepinephrine and epinephrine causes hypersensitivity to induction of and delayed emergence from volatile anesthetics, as well as hypersensitivity to the highly selective alpha2 adrenoceptor agonist dexmedetomidine
-
*
These findings indicate that adrenergic signaling is essential for normal emergence from volatile anesthesia, but is not required for dexmedetomidine's hypnotic effects
Acknowledgments
The authors wish to thank Mark Opp, Ph.D. (Professor, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA) for his advice on redesigning ultra lightweight socket headpieces with thermistor capability and Matthew Young (Ph.D. Graduate Student, Department of Neuroscience, University of Pennsylvania, Philadelphia, PA) for his assistance with central adrenergic rescue treatments in mice.
Support: This work was supported by research grant numbers GM077357, GM088156, and the American Recovery and Reinvestment Act (ARRA) funds through grant GM077357 from the National Institutes of Health, Bethesda, Maryland, from the Mary Elizabeth Groff Foundation, Radnor PA, the Foundation for Anesthesia Education and Research, Rochester, MN, and by the Department of Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Attribution: Department of Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA
REFERENCES
- 1.Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller RD, Way WL, Eger EI., 2nd The effects of alpha-methyldopa, reserpine, guanethidine, and iproniazid on minimum alveolar anesthetic requirement (MAC) Anesthesiology. 1968;29:1153–1158. doi: 10.1097/00000542-196811000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Johnston RR, White PF, Eger EI., 2nd Comparative effects of dextroamphetamine and reserpine on halothane and cyclopropane anesthetic requirements. Anesth Analg. 1975;54:655–659. doi: 10.1213/00000539-197509000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Johnston RR, Way WL, Miller RD. Alteration of anesthetic requirement by amphetamine. Anesthesiology. 1972;36:357–363. doi: 10.1097/00000542-197204000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Doze VA, Chen BX, Maze M. Dexmedetomidine produces a hypnotic-anesthetic action in rats via activation of central alpha-2 adrenoceptors. Anesthesiology. 1989;71:75–79. doi: 10.1097/00000542-198907000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Segal IS, Vickery RG, Walton JK, Doze VA, Maze M. Dexmedetomidine diminishes halothane anesthetic requirements in rats through a postsynaptic alpha 2 adrenergic receptor. Anesthesiology. 1988;69:818–823. doi: 10.1097/00000542-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Mueller RA, Smith RD, Spruill WA, Breese GR. Central monaminergic neuronal effects on minimum alveolar concentrations (MAC) of halothane and cyclopropane in rats. Anesthesiology. 1975;42:143–152. doi: 10.1097/00000542-197502000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Kushikata T, Yoshida H, Kudo M, Kudo T, Hirota K. Role of coerulean noradrenergic neurones in general anaesthesia in rats. Br J Anaesth. 2011;107:924–929. doi: 10.1093/bja/aer303. [DOI] [PubMed] [Google Scholar]
- 9.Mason ST, King RA, Banks P, Angel A. Brain noradrenaline and anaesthesia: Behavioural and electrophysiological evidence. Neuroscience. 1983;10:177–185. doi: 10.1016/0306-4522(83)90091-x. [DOI] [PubMed] [Google Scholar]
- 10.Roizen MF, White PF, Eger EI, 2nd, Brownstein M. Effects of ablation of serotonin or norepinephrine brain-stem areas on halothane and cyclopropane MACs in rats. Anesthesiology. 1978;49:252–255. doi: 10.1097/00000542-197810000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Gompf HS, Chen J, Sun Y, Yanagisawa M, Aston-Jones G, Kelz MB. Halothane-induced Hypnosis is not Accompanied by Inactivation of Orexinergic Output in Rodents. Anesthesiology. 2009;111:1001–1009. doi: 10.1097/ALN.0b013e3181b764b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman EB, Sun Y, Moore JT, Hung HT, Meng QC, Perera P, Joiner WJ, Thomas SA, Eckenhoff RG, Sehgal A, Kelz MB. A Conserved Behavioral State Barrier Impedes Transitions between Anesthetic-Induced Unconsciousness and Wakefulness: Evidence for Neural Inertia. PLoS One. 2010;5:e11903. doi: 10.1371/journal.pone.0011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- 16.Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- 17.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 18.Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–2082. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- 19.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd Edition. Vol. 18. New York: Elsevier Academic Press; 2008. p. 60. [Google Scholar]
- 20.Baracchi F, Opp MR. Sleep-wake behavior and responses to sleep deprivation of mice lacking both interleukin-1 beta receptor 1 and tumor necrosis factor-alpha receptor 1. Brain Behav Immun. 2008;22:982–993. doi: 10.1016/j.bbi.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation. 2000;101:E215–E220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 22.Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, Yanagisawa M. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Chen J, Pruckmayr G, Baumgardner JE, Eckmann DM, Eckenhoff RG, Kelz MB. High throughput modular chambers for rapid evaluation of anesthetic sensitivity. BMC Anesthesiology. 2006;6:13. doi: 10.1186/1471-2253-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason ST, Angel A. Behavioural evidence that chronic treatment with the antidepressant desipramine causes reduced functioning of brain noradrenaline systems. Psychopharmacology (Berl) 1983;81:73–77. doi: 10.1007/BF00439277. [DOI] [PubMed] [Google Scholar]
- 25.Mason ST, Angel A. Anaesthesia: The role of adrenergic mechanisms. Eur J Pharmacol. 1983;91:29–39. doi: 10.1016/0014-2999(83)90358-8. [DOI] [PubMed] [Google Scholar]
- 26.Nakao H, Ono J, Nogaya J, Yokono S, Yube K. The relationship of brain catecholamine levels to enflurane requirements among three strains of mice with different anesthetic sensitivities. J Anesth. 2001;15:88–92. doi: 10.1007/s005400170033. [DOI] [PubMed] [Google Scholar]
- 27.Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Dbh(−/−) mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. Am J Physiol Regul Integr Comp Physiol. 2004;286:R108–R113. doi: 10.1152/ajpregu.00405.2003. [DOI] [PubMed] [Google Scholar]
- 28.Cho MC, Rao M, Koch WJ, Thomas SA, Palmiter RD, Rockman HA. Enhanced contractility and decreased beta-adrenergic receptor kinase-1 in mice lacking endogenous norepinephrine and epinephrine. Circulation. 1999;99:2702–2707. doi: 10.1161/01.cir.99.20.2702. [DOI] [PubMed] [Google Scholar]
- 29.Hilaire G, Viemari JC, Coulon P, Simonneau M, Bevengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Saunier CF, Akaoka H, de La Chapelle B, Charlety PJ, Chergui K, Chouvet G, Buda M, Quintin L. Activation of brain noradrenergic neurons during recovery from halothane anesthesia: Persistence of phasic activation after clonidine. Anesthesiology. 1993;79:1072–1082. doi: 10.1097/00000542-199311000-00026. [DOI] [PubMed] [Google Scholar]
- 31.Anzawa N, Kushikata T, Ohkawa H, Yoshida H, Kubota T, Matsuki A. Increased noradrenaline release from rat preoptic area during and after sevoflurane and isoflurane anesthesia. Can J Anaesth. 2001;48:462–465. doi: 10.1007/BF03028309. [DOI] [PubMed] [Google Scholar]
- 32.Pillay S, Vizuete JA, McCallum JB, Hudetz AG. Norepinephrine infusion into nucleus basalis elicits microarousal in desflurane-anesthetized rats. Anesthesiology. 2011;115:733–742. doi: 10.1097/ALN.0b013e31822c5ee1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 34.Zecharia AY, Nelson LE, Gent TC, Schumacher M, Jurd R, Rudolph U, Brickley SG, Maze M, Franks NP. The involvement of hypothalamic sleep pathways in general anesthesia: Testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse. J Neurosci. 2009;29:2177–2187. doi: 10.1523/JNEUROSCI.4997-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takizawa D, Takizawa E, Miyoshi S, Kawahara F, Ito N, Ishizeki J, Koizuka S, Hiraoka H. The effect of ephedrine and phenylephrine on BIS values during propofol anaesthesia. Eur J Anaesthesiol. 2006;23:654–657. doi: 10.1017/S0265021506000433. [DOI] [PubMed] [Google Scholar]
- 36.Ishiyama T, Oguchi T, Iijima T, Matsukawa T, Kashimoto S, Kumazawa T. Ephedrine, but not phenylephrine, increases bispectral index values during combined general and epidural anesthesia. Anesth Analg. 2003;97:780–784. doi: 10.1213/01.ANE.0000073355.63287.E4. [DOI] [PubMed] [Google Scholar]
- 37.Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115:791–803. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active Emergence from Propofol General Anesthesia Is Induced by Methylphenidate. Anesthesiology. 2012;116:998–1005. doi: 10.1097/ALN.0b013e3182518bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segal IS, Walton JK, Irwin I, DeLanney LE, Ricaurte GA, Langston JW, Maze M. Modulating role of dopamine on anesthetic requirements. Eur J Pharmacol. 1990;186:9–15. doi: 10.1016/0014-2999(90)94055-3. [DOI] [PubMed] [Google Scholar]
- 40.Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. Substitution of a mutant alpha2a-adrenergic receptor via "hit and run" gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci U S A. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, Audinat E, Muhlethaler M, Serafin M. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 42.Gallopin T, Luppi PH, Cauli B, Urade Y, Rossier J, Hayaishi O, Lambolez B, Fort P. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience. 2005;134:1377–1390. doi: 10.1016/j.neuroscience.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 43.Gallopin T, Luppi PH, Rambert FA, Frydman A, Fort P. Effect of the wake-promoting agent modafinil on sleep-promoting neurons from the ventrolateral preoptic nucleus: An in vitro pharmacologic study. Sleep. 2004;27:19–25. [PubMed] [Google Scholar]
- 44.Matsuo S, Jang IS, Nabekura J, Akaike N. Alpha 2-adrenoceptor-mediated presynaptic modulation of GABAergic transmission in mechanically dissociated rat ventrolateral preoptic neurons. J Neurophysiol. 2003;89:1640–1648. doi: 10.1152/jn.00491.2002. [DOI] [PubMed] [Google Scholar]
- 45.Zhang WP, Ouyang M, Thomas SA. Potency of catecholamines and other L-tyrosine derivatives at the cloned mouse adrenergic receptors. Neuropharmacology. 2004;47:438–449. doi: 10.1016/j.neuropharm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Gilsbach R, Roser C, Beetz N, Brede M, Hadamek K, Haubold M, Leemhuis J, Philipp M, Schneider J, Urbanski M, Szabo B, Weinshenker D, Hein L. Genetic dissection of alpha2-adrenoceptor functions in adrenergic versus nonadrenergic cells. Mol Pharmacol. 2009;75:1160–1170. doi: 10.1124/mol.109.054544. [DOI] [PubMed] [Google Scholar]
- 47.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–393. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- 49.Pentyala S, Moller D, Chowdhury A, Sung KY, Rebecchi M. Effects of inhalational anesthetics on alpha2-adrenergic signaling in isolated platelets. Toxicol Lett. 1998;100–101:115–120. doi: 10.1016/s0378-4274(98)00174-x. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka S, Tsuchida H. Effects of halothane and isoflurane on beta-adrenoceptor-mediated responses in the vascular smooth muscle of rat aorta. Anesthesiology. 1998;89:1209–1217. doi: 10.1097/00000542-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 51.Sato K, Seki S, Murray PA. Effects of halothane and enflurane anesthesia on sympathetic beta-adrenoreceptor-mediated pulmonary vasodilation in chronically instrumented dogs. Anesthesiology. 2002;97:478–487. doi: 10.1097/00000542-200208000-00027. [DOI] [PubMed] [Google Scholar]