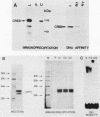
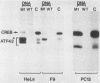
Abstract
Promoter elements containing the sequence motif CGTCA are important for a variety of inducible responses at the transcriptional level. Multiple cellular factors specifically bind to these elements and are encoded by a multigene family. Among these factors, polypeptides termed activating transcription factor 43 (ATF-43) and ATF-47 have been purified from HeLa cells and a factor referred to as cyclic AMP response element-binding protein (CREB) has been isolated from PC12 cells and rat brain. We demonstrated that CREB and ATF-47 are identical and that CREB and ATF-43 form protein-protein complexes. We also found that the cis requirements for stable DNA binding by ATF-43 and CREB are different. Using antibodies to ATF-43 we have identified a group of polypeptides (ATF-43) in the size range from 40 to 43 kDa. ATF-43 polypeptides are related by their reactivity with anti-ATF-43, DNA-binding specificity, complex formation with CREB, heat stability, and phosphorylation by protein kinase A. Certain cell types vary in their ATF-43 complement, suggesting that CREB activity is modulated in a cell-type-specific manner through interaction with ATF-43. ATF-43 polypeptides do not appear simply to correspond to the gene products of the ATF multigene family, suggesting that the size of the ATF family at the protein level is even larger than predicted from cDNA-cloning studies.
Full text
PDF


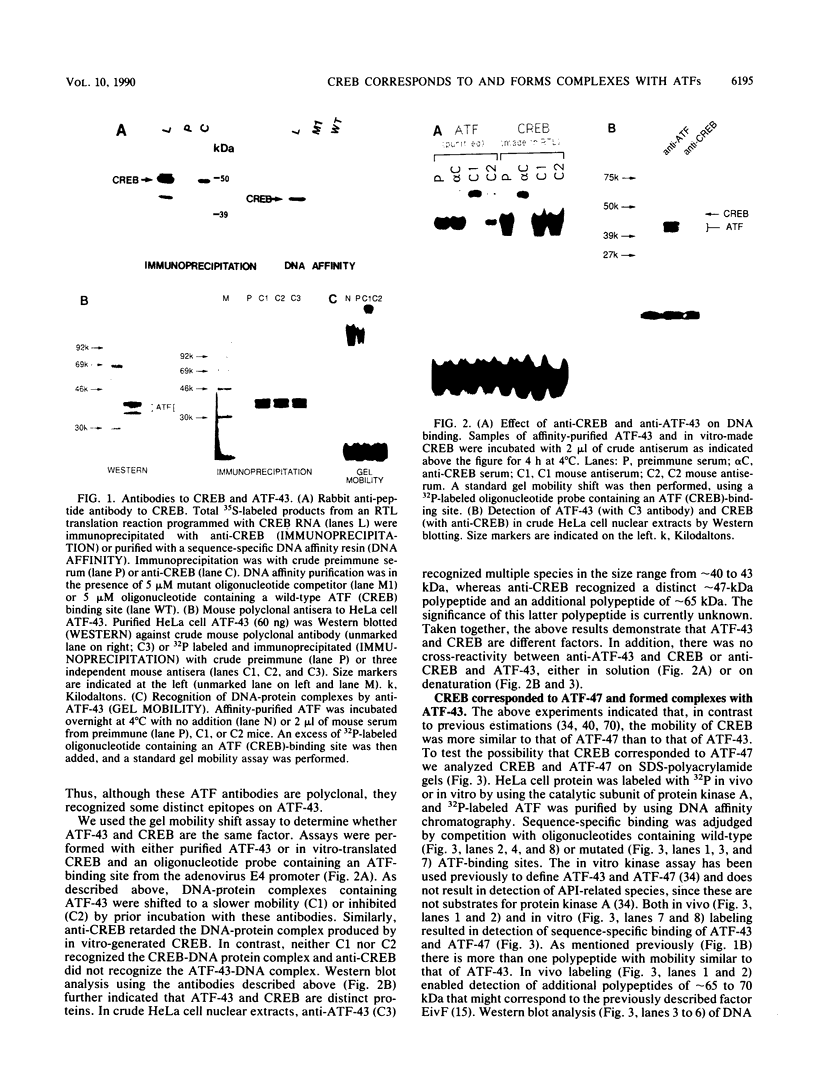


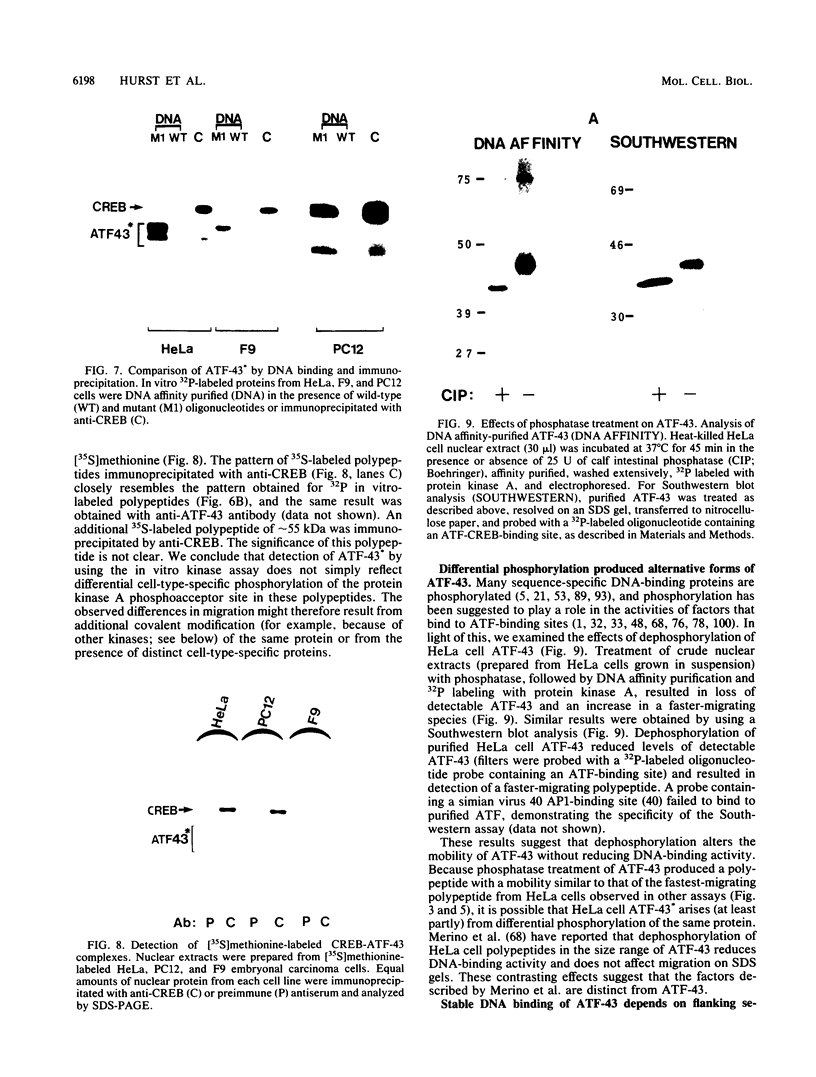





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrisani O. M., Hayes T. E., Roos B., Dixon J. E. Identification of the promoter sequences involved in the cell specific expression of the rat somatostatin gene. Nucleic Acids Res. 1987 Jul 24;15(14):5715–5728. doi: 10.1093/nar/15.14.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrisani O. M., Pot D. A., Zhu Z., Dixon J. E. Three sequence-specific DNA-protein complexes are formed with the same promoter element essential for expression of the rat somatostatin gene. Mol Cell Biol. 1988 May;8(5):1947–1956. doi: 10.1128/mcb.8.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrisani O. M., Zhu Z. N., Pot D. A., Dixon J. E. In vitro transcription directed from the somatostatin promoter is dependent upon a purified 43-kDa DNA-binding protein. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2181–2185. doi: 10.1073/pnas.86.7.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrisani O., Dixon J. E. Identification and purification of a novel 120-kDa protein that recognizes the cAMP-responsive element. J Biol Chem. 1990 Feb 25;265(6):3212–3218. [PubMed] [Google Scholar]
- Bagchi S., Raychaudhuri P., Nevins J. R. Phosphorylation-dependent activation of the adenovirus-inducible E2F transcription factor in a cell-free system. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4352–4356. doi: 10.1073/pnas.86.12.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D. M., Jones N. C. Heterodimer formation between CREB and JUN proteins. Oncogene. 1990 Mar;5(3):295–302. [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Boeuf H., Zajchowski D. A., Tamura T., Hauss C., Kédinger C. Specific cellular proteins bind to critical promoter sequences of the adenovirus early EIIa promoter. Nucleic Acids Res. 1987 Jan 26;15(2):509–527. doi: 10.1093/nar/15.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Jeang K. T., Duvall J., Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987 Jul;61(7):2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch S. J., Sassone-Corsi P. Dimers, leucine zippers and DNA-binding domains. Trends Genet. 1990 Feb;6(2):36–40. doi: 10.1016/0168-9525(90)90071-d. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Ferreira P. C., Gentz R., Franza B. R., Jr, Curran T. The product of a fos-related gene, fra-1, binds cooperatively to the AP-1 site with Jun: transcription factor AP-1 is comprised of multiple protein complexes. Genes Dev. 1989 Feb;3(2):173–184. doi: 10.1101/gad.3.2.173. [DOI] [PubMed] [Google Scholar]
- Cortes P., Buckbinder L., Leza M. A., Rak N., Hearing P., Merino A., Reinberg D. EivF, a factor required for transcription of the adenovirus EIV promoter, binds to an element involved in EIa-dependent activation and cAMP induction. Genes Dev. 1988 Aug;2(8):975–990. doi: 10.1101/gad.2.8.975. [DOI] [PubMed] [Google Scholar]
- Dean D. C., Blakeley M. S., Newby R. F., Ghazal P., Hennighausen L., Bourgeois S. Forskolin inducibility and tissue-specific expression of the fibronectin promoter. Mol Cell Biol. 1989 Apr;9(4):1498–1506. doi: 10.1128/mcb.9.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delegeane A. M., Ferland L. H., Mellon P. L. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987 Nov;7(11):3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Habener J. F. Cyclic AMP and phorbol ester-stimulated transcription mediated by similar DNA elements that bind distinct proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7922–7926. doi: 10.1073/pnas.85.21.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Lin J. C., Habener J. F. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem. 1988 Dec 5;263(34):18466–18472. [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989 Jan 12;337(6203):138–143. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S. Modularity in promoters and enhancers. Cell. 1989 Jul 14;58(1):1–4. doi: 10.1016/0092-8674(89)90393-0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. Interaction of nuclear factors with multiple sites in the somatic cytochrome c promoter. Characterization of upstream NRF-1, ATF, and intron Sp1 recognition sequences. J Biol Chem. 1989 Aug 25;264(24):14361–14368. [PubMed] [Google Scholar]
- Fink J. S., Verhave M., Kasper S., Tsukada T., Mandel G., Goodman R. H. The CGTCA sequence motif is essential for biological activity of the vasoactive intestinal peptide gene cAMP-regulated enhancer. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6662–6666. doi: 10.1073/pnas.85.18.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher C., Heintz N., Roeder R. G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987 Dec 4;51(5):773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Fujisawa J., Toita M., Yoshida M. A unique enhancer element for the trans activator (p40tax) of human T-cell leukemia virus type I that is distinct from cyclic AMP- and 12-O-tetradecanoylphorbol-13-acetate-responsive elements. J Virol. 1989 Aug;63(8):3234–3239. doi: 10.1128/jvi.63.8.3234-3239.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz R., Rauscher F. J., 3rd, Abate C., Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989 Mar 31;243(4899):1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Giam C. Z., Xu Y. L. HTLV-I tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J Biol Chem. 1989 Sep 15;264(26):15236–15241. [PubMed] [Google Scholar]
- Gilardi P., Perricaudet M. The E4 promoter of adenovirus type 2 contains an E1A dependent cis-acting element. Nucleic Acids Res. 1986 Nov 25;14(22):9035–9049. doi: 10.1093/nar/14.22.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Allegretto E. A., Karin M., Green M. R. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev. 1988 Oct;2(10):1216–1226. doi: 10.1101/gad.2.10.1216. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Halazonetis T. D., Georgopoulos K., Greenberg M. E., Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988 Dec 2;55(5):917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- Hardy S., Shenk T. Adenoviral control regions activated by E1A and the cAMP response element bind to the same factor. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4171–4175. doi: 10.1073/pnas.85.12.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington C. A., Lewis E. J., Krzemien D., Chikaraishi D. M. Identification and cell type specificity of the tyrosine hydroxylase gene promoter. Nucleic Acids Res. 1987 Mar 11;15(5):2363–2384. doi: 10.1093/nar/15.5.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Yun Y., Jameson J. L., Habener J. F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Hurst H. C., Jones N. C. Identification of factors that interact with the E1A-inducible adenovirus E3 promoter. Genes Dev. 1987 Dec;1(10):1132–1146. doi: 10.1101/gad.1.10.1132. [DOI] [PubMed] [Google Scholar]
- Hyman S. E., Comb M., Lin Y. S., Pearlberg J., Green M. R., Goodman H. M. A common trans-acting factor is involved in transcriptional regulation of neurotransmitter genes by cyclic AMP. Mol Cell Biol. 1988 Oct;8(10):4225–4233. doi: 10.1128/mcb.8.10.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv L. B., Liou H. C., Kara C. J., Lamph W. W., Verma I. M., Glimcher L. H. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol Cell Biol. 1990 Apr;10(4):1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalinot P., Devaux B., Kédinger C. The abundance and in vitro DNA binding of three cellular proteins interacting with the adenovirus EIIa early promoter are not modified by the EIa gene products. Mol Cell Biol. 1987 Oct;7(10):3806–3817. doi: 10.1128/mcb.7.10.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalinot P., Wintzerith M., Gaire M., Hauss C., Egly J. M., Kédinger C. Purification and functional characterization of a cellular transcription factor that binds to an enhancer element within the adenovirus early EIIa promoter. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2484–2488. doi: 10.1073/pnas.85.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Boros I., Brady J., Radonovich M., Khoury G. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J Virol. 1988 Dec;62(12):4499–4509. doi: 10.1128/jvi.62.12.4499-4509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Widen S. G., Semmes O. J., 4th, Wilson S. H. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990 Mar 2;247(4946):1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Jones R. H., Jones N. C. Mammalian cAMP-responsive element can activate transcription in yeast and binds a yeast factor(s) that resembles the mammalian transcription factor ANF. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2176–2180. doi: 10.1073/pnas.86.7.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanei-Ishii C., Ishii S. Dual enhancer activities of the cyclic-AMP responsive element with cell type and promoter specificity. Nucleic Acids Res. 1989 Feb 25;17(4):1521–1536. doi: 10.1093/nar/17.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara C. J., Liou H. C., Ivashkiv L. B., Glimcher L. H. A cDNA for a human cyclic AMP response element-binding protein which is distinct from CREB and expressed preferentially in brain. Mol Cell Biol. 1990 Apr;10(4):1347–1357. doi: 10.1128/mcb.10.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Ikawa Y. Bovine leukemia virus trans-activator p38tax activates heterologous promoters with a common sequence known as a cAMP-responsive element or the binding site of a cellular transcription factor ATF. EMBO J. 1989 Feb;8(2):497–503. doi: 10.1002/j.1460-2075.1989.tb03403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Krause H. M., Klemenz R., Gehring W. J. Expression, modification, and localization of the fushi tarazu protein in Drosophila embryos. Genes Dev. 1988 Aug;2(8):1021–1036. doi: 10.1101/gad.2.8.1021. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Fink J. S., Goodman R. H., Green M. R. Distinguishable promoter elements are involved in transcriptional activation by E1a and cyclic AMP. Mol Cell Biol. 1989 Oct;9(10):4390–4397. doi: 10.1128/mcb.9.10.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Green M. R. A cellular transcription factor E4F1 interacts with an E1a-inducible enhancer and mediates constitutive enhancer function in vitro. EMBO J. 1987 May;6(5):1345–1353. doi: 10.1002/j.1460-2075.1987.tb02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Hai T. Y., SivaRaman L., Thimmappaya B., Hurst H. C., Jones N. C., Green M. R. A cellular protein, activating transcription factor, activates transcription of multiple E1A-inducible adenovirus early promoters. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8355–8359. doi: 10.1073/pnas.84.23.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Lewis E. J., Harrington C. A., Chikaraishi D. M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leza M. A., Hearing P. Cellular transcription factor binds to adenovirus early region promoters and to a cyclic AMP response element. J Virol. 1988 Aug;62(8):3003–3013. doi: 10.1128/jvi.62.8.3003-3013.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Identification and purification of a Saccharomyces cerevisiae protein with the DNA binding specificity of mammalian activating transcription factor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):109–113. doi: 10.1073/pnas.86.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Interaction of a common cellular transcription factor, ATF, with regulatory elements in both E1a- and cyclic AMP-inducible promoters. Proc Natl Acad Sci U S A. 1988 May;85(10):3396–3400. doi: 10.1073/pnas.85.10.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor P. F., Abate C., Curran T. Direct cloning of leucine zipper proteins: Jun binds cooperatively to the CRE with CRE-BP1. Oncogene. 1990 Apr;5(4):451–458. [PubMed] [Google Scholar]
- Maekawa T., Sakura H., Kanei-Ishii C., Sudo T., Yoshimura T., Fujisawa J., Yoshida M., Ishii S. Leucine zipper structure of the protein CRE-BP1 binding to the cyclic AMP response element in brain. EMBO J. 1989 Jul;8(7):2023–2028. doi: 10.1002/j.1460-2075.1989.tb03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Marriott S. J., Boros I., Duvall J. F., Brady J. N. Indirect binding of human T-cell leukemia virus type I tax1 to a responsive element in the viral long terminal repeat. Mol Cell Biol. 1989 Oct;9(10):4152–4160. doi: 10.1128/mcb.9.10.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino A., Buckbinder L., Mermelstein F. H., Reinberg D. Phosphorylation of cellular proteins regulates their binding to the cAMP response element. J Biol Chem. 1989 Dec 15;264(35):21266–21276. [PubMed] [Google Scholar]
- Montagne J., Béraud C., Crenon I., Lombard-Platet G., Gazzolo L., Sergeant A., Jalinot P. Tax1 induction of the HTLV-I 21 bp enhancer requires cooperation between two cellular DNA-binding proteins. EMBO J. 1990 Mar;9(3):957–964. doi: 10.1002/j.1460-2075.1990.tb08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Niki M., Ohtani K., Sugamura K. Differential activation of the 21-base-pair enhancer element of human T-cell leukemia virus type I by its own trans-activator and cyclic AMP. Nucleic Acids Res. 1989 Jul 11;17(13):5207–5221. doi: 10.1093/nar/17.13.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborne T. F., Gil G., Brown M. S., Kowal R. C., Goldstein J. L. Identification of promoter elements required for in vitro transcription of hamster 3-hydroxy-3-methylglutaryl coenzyme A reductase gene. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3614–3618. doi: 10.1073/pnas.84.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J., Missotten M. Functional characterization of a cAMP-responsive element of the rat insulin I gene. J Biol Chem. 1990 Jan 25;265(3):1465–1469. [PubMed] [Google Scholar]
- Poteat H. T., Chen F. Y., Kadison P., Sodroski J. G., Haseltine W. A. Protein kinase A-dependent binding of a nuclear factor to the 21-base-pair repeat of the human T-cell leukemia virus type I long terminal repeat. J Virol. 1990 Mar;64(3):1264–1270. doi: 10.1128/jvi.64.3.1264-1270.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri P., Bagchi S., Nevins J. R. DNA-binding activity of the adenovirus-induced E4F transcription factor is regulated by phosphorylation. Genes Dev. 1989 May;3(5):620–627. doi: 10.1101/gad.3.5.620. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri P., Rooney R., Nevins J. R. Identification of an E1A-inducible cellular factor that interacts with regulatory sequences within the adenovirus E4 promoter. EMBO J. 1987 Dec 20;6(13):4073–4081. doi: 10.1002/j.1460-2075.1987.tb02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D. A., Aitken L. D., Vandenbark G. R., Mouw A. R., Franklin A., Schimmer B. P., Parker K. L. A cAMP-responsive element regulates expression of the mouse steroid 11 beta-hydroxylase gene. J Biol Chem. 1989 Aug 25;264(24):14011–14015. [PubMed] [Google Scholar]
- Rickles R. J., Darrow A. L., Strickland S. Differentiation-responsive elements in the 5' region of the mouse tissue plasminogen activator gene confer two-stage regulation by retinoic acid and cyclic AMP in teratocarcinoma cells. Mol Cell Biol. 1989 Apr;9(4):1691–1704. doi: 10.1128/mcb.9.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P. Cyclic AMP induction of early adenovirus promoters involves sequences required for E1A trans-activation. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7192–7196. doi: 10.1073/pnas.85.19.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Ransone L. J., Lamph W. W., Verma I. M. Direct interaction between fos and jun nuclear oncoproteins: role of the 'leucine zipper' domain. Nature. 1988 Dec 15;336(6200):692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Visvader J., Ferland L., Mellon P. L., Verma I. M. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 1988 Dec;2(12A):1529–1538. doi: 10.1101/gad.2.12a.1529. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Heguy A., Roeder R. G. Identification and purification of a human lymphoid-specific octamer-binding protein (OTF-2) that activates transcription of an immunoglobulin promoter in vitro. Cell. 1987 Dec 4;51(5):783–793. doi: 10.1016/0092-8674(87)90101-2. [DOI] [PubMed] [Google Scholar]
- Silver B. J., Bokar J. A., Virgin J. B., Vallen E. A., Milsted A., Nilson J. H. Cyclic AMP regulation of the human glycoprotein hormone alpha-subunit gene is mediated by an 18-base-pair element. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2198–2202. doi: 10.1073/pnas.84.8.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SivaRaman L., Subramanian S., Thimmappaya B. Identification of a factor in HeLa cells specific for an upstream transcriptional control sequence of an EIA-inducible adenovirus promoter and its relative abundance in infected and uninfected cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5914–5918. doi: 10.1073/pnas.83.16.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Stamminger T., Fickenscher H., Fleckenstein B. Cell type-specific induction of the major immediate early enhancer of human cytomegalovirus by cyclic AMP. J Gen Virol. 1990 Jan;71(Pt 1):105–113. doi: 10.1099/0022-1317-71-1-105. [DOI] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Tan T. H., Horikoshi M., Roeder R. G. Purification and characterization of multiple nuclear factors that bind to the TAX-inducible enhancer within the human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1989 Apr;9(4):1733–1745. doi: 10.1128/mcb.9.4.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Fink J. S., Mandel G., Goodman R. H. Identification of a region in the human vasoactive intestinal polypeptide gene responsible for regulation by cyclic AMP. J Biol Chem. 1987 Jun 25;262(18):8743–8747. [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Wada T., Handa H. Transcription factor E4TF1 contains two subunits with different functions. EMBO J. 1990 Mar;9(3):841–847. doi: 10.1002/j.1460-2075.1990.tb08181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widen S. G., Kedar P., Wilson S. H. Human beta-polymerase gene. Structure of the 5'-flanking region and active promoter. J Biol Chem. 1988 Nov 15;263(32):16992–16998. [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Menzel P., Rivier J., Montminy M. R. Characterization of a bipartite activator domain in transcription factor CREB. Cell. 1990 Feb 23;60(4):611–617. doi: 10.1016/0092-8674(90)90664-z. [DOI] [PubMed] [Google Scholar]
- Zajchowski D. A., Boeuf H., Kédinger C. E1a inducibility of the adenoviral early E2a promoter is determined by specific combinations of sequence elements. Gene. 1987;58(2-3):243–256. doi: 10.1016/0378-1119(87)90379-9. [DOI] [PubMed] [Google Scholar]
- von der Ahe D., Janich S., Scheidereit C., Renkawitz R., Schütz G., Beato M. Glucocorticoid and progesterone receptors bind to the same sites in two hormonally regulated promoters. Nature. 1985 Feb 21;313(6004):706–709. doi: 10.1038/313706a0. [DOI] [PubMed] [Google Scholar]