Abstract
G protein-coupled receptor (GPCR) kinase 2 (GRK2) regulates cellular signaling via desensitization of GPCRs and by direct interaction with intracellular signaling molecules. We recently described that ischemic brain injury decreases cerebral GRK2 levels. Here we studied the effect of astrocyte GRK2-deficiency on neonatal brain damage in vivo. As astrocytes protect neurons by taking up glutamate via plasma-membrane transporters, we also studied the effect of GRK2 on the localization of the GLutamate ASpartate Transporter (GLAST).
Brain damage induced by hypoxia-ischemia was significantly reduced in GFAP-GRK2+/− mice, which have a 60% reduction in astrocyte GRK2 compared to GFAP-WT littermates. In addition, GRK2-deficient astrocytes have higher plasma-membrane levels of GLAST and an increased capacity to take up glutamate in vitro.
In search for the mechanism by which GRK2 regulates GLAST expression, we observed increased GFAP levels in GRK2-deficient astrocytes. GFAP and the cytoskeletal protein ezrin are known regulators of GLAST localization. In line with this evidence, GRK2-deficiency reduced phosphorylation of the GRK2 substrate ezrin and enforced plasma-membrane GLAST association after stimulation with the group I mGluR-agonist DHPG. When ezrin was silenced, the enhanced plasma-membrane GLAST association in DHPG-exposed GRK2-deficient astrocytes was prevented.
In conclusion, we identified a novel role of astrocyte GRK2 in regulating plasma-membrane GLAST localization via an ezrindependent route. We demonstrate that the 60% reduction in astrocyte GRK2 protein level that is observed in GFAP-GRK2+/− mice is sufficient to significantly reduce neonatal ischemic brain damage. These findings underline the critical role of GRK2 regulation in astrocytes for dampening the extent of brain damage after ischemia.
Keywords: ischemic brain injury, neuroprotection, astrocytes, G protein-coupled receptor kinase 2 (GRK2), excitotoxicity, glutamate transporter GLAST, GFAP, cytoskeleton, ezrin
Introduction
Astrocytes form the largest glial cell population in the brain; they outnumber neurons by 5 times and comprise ~50% of human brain volume (Rossi et al., 2007; Sofroniew and Vinters, 2010). Astrocytes provide metabolic support for neurons and regulate the extracellular ion balance (Rossi et al., 2007; Sofroniew and Vinters, 2010; Takano et al., 2009). Under pathological conditions like cerebral ischemia, astrocytes are thought to contribute to preventing neuronal cell death by reducing extracellular glutamate to non-excitotoxic levels (Swanson et al., 2004; Takano et al., 2009). They do so by enveloping neuronal synapses with processes that express the glutamate transporters GLAST and GLT-1, which remove glutamate from the synaptic cleft (Rothstein et al., 1994). Genetic ablation, pharmacological inhibition or antisense oligonucleotide-mediated downregulation of GLAST or GLT-1 exacerbate excitotoxic neurodegeneration or ischemic brain injury in rodents (Rao et al., 2001; Rothstein et al., 1996; Watase et al., 1998). Conversely, increasing the expression of glutamate transporters has been shown to be neuroprotective in in vitro models of ischemic damage and an in vivo model of amyotrophic lateral sclerosis (Fontana et al., 2007; Rothstein et al., 2005). Collectively, these data indicate a neuroprotective role of astrocytes by glutamate uptake via their glutamate transporters.
G protein-coupled receptor kinase 2 (GRK2) is a widely expressed kinase that regulates phosphorylation and subsequent desensitization of multiple G protein-coupled receptors (GPCRs), including metabotropic glutamate receptors (mGluRs). Desensitization of GPCRs is a crucial regulatory process to ensure attenuation of signaling and internalization of receptors to prevent cellular overstimulation (Gainetdinov et al., 2004; Reiter and Lefkowitz, 2006). GRK2 also regulates signaling via direct interaction with intracellular signaling molecules like p38 MAP kinase, MEK1/2, PI3 kinase and the cAMP sensor Epac (Eijkelkamp et al., 2010b; Kavelaars et al., 2011; Kleibeuker et al., 2008; Peregrin et al., 2006; Reiter and Lefkowitz, 2006; Ribas et al., 2007). Moreover, cytoskeletal proteins like tubulin, radixin and ezrin have also been shown to be substrates for GRK2 (Cant and Pitcher, 2005; Kahsai et al., 2010; Pitcher et al., 1998).
The cytoskeleton is an organized network of protein fibers, consisting of microtubules, microfilaments and intermediate filaments, which provide shape, strength, movement and intracellular transport of cell organelles. The intermediate filament glial fibrillary acidic protein (GFAP) is an astrocyte-specific cytoskeletal protein and it is widely acknowledged that GFAP levels are upregulated in response to astrocyte activation. A recent study by Sullivan et al. (2007) suggested an essential role for GFAP in regulating glutamate transporter trafficking and function. These authors suggested that GFAP plays a role in retaining GLAST in the plasma-membrane via a mechanism depending on the adaptor proteins ezrin and NHERF1 (Na+-H+ exchanger regulatory factor 1).
The aim of this study was to determine whether a reduction in astrocyte GRK2 affects the capacity of astrocytes to regulate glutamate uptake as well as hypoxic-ischemic brain damage in vivo. In search for the mechanism by which GRK2 might regulate glutamate homeostasis, we studied the effects of GRK2-deficiency on GLAST expression and on GFAP and ezrin, two possible regulators of GLAST localization.
Materials and Methods
Animal experiments: breeding, HI brain damage and histology
Experiments were performed in accordance with national guidelines and were approved by the UMC Utrecht-experimental animal care committee.
We used heterozygous GRK2+/− and wildtype (WT) C57BL/6J littermates (Jaber et al., 1996). Mice with cell-specific reduction of GRK2 in astrocytes were obtained using Cre-Lox technology using GFAP-Cre+/− (FVB-Tg(GFAP-cre)25Mes/J (Jackson Laboratory) and GRK2f/fmice on a mixed 129/C57BL/6J background (Matkovich et al., 2006). Pups had either normal GRK2 levels (GFAPWT) or reduced GRK2 levels in astrocytes (GFAP-GRK2+/−) (Eijkelkamp et al., 2010a).
Hypoxic-ischemic (HI) brain damage was induced on postnatal day 9 (P9) in mouse pups of both sexes by permanent occlusion of the right carotid artery followed by 45 min 10% O2. This model induces neuronal damage in primarly the hippocampus and also in the cortical and thalamic regions of the brain as we described before (van der Kooij et al., 2010; Nijboer et al., 2008). Neuronal damage was quantified as loss of staining for microtubule-associated protein 2 (MAP2) as described earlier (Nijboer et al., 2008).
Paraffin-embedded sections cut at hippocampal level (−1.8 mm from bregma) were stained with mouse-anti-GFAP (Acris, Herford, Germany) followed by biotinylated horse-anti-mouse antibody (Vector laboratories, Burlingame, CA). Visualization was performed using Vectastain ABC kit (Vector Laboratories) and diaminobenzamidine (DAB).
For immunofluorescent detection of GRK2 levels in astrocytes and neurons, brain sections were incubated with mouse-anti-GFAP (Acris), mouse-anti-MAP2 (Sigma-Aldrich, Steinheim, Germany) and rabbit-anti-GRK2 (Santa Cruz Biotechnology, Santa Cruz, CA) followed by Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary antibodies (Invitrogen, Paisley, UK). Photographs were obtained using a Zeiss Apotome fluorescence microscope (Zeiss, Oberkochen, Germany). GRK2 levels in GFAP-positive cells were quantified using Image J software (http://rsb.info.nih.gov/ij/, 1997–2006).
Primary astrocyte cultures and subcellular protein fractionation
P1 pups were used to obtain mixed primary cultures of cortical astrocytes and microglia as described earlier (Nijboer et al., 2010). In short, both cortices of one animal were isolated, dissected and cultured separately resulting in a mixed primary culture of astrocytes and microglia. After 10–12 DIV, these mixed cultures were shaken for 3 hours to detach the microglia that were discarded thereafter. The purity of the astrocyte culture was > 95% GFAP-positive cells. For further experiments, astrocyte cultures from 10–14 animals per genotype were pooled per genotype and were cultured for 48 h in poly-L-ornithine coated 24-well plates at a density of 0.4×106 cells/ml to reach 90% confluency before the experimental procedures. Astrocytes were stimulated for 10 min with 50 µM 3,5-dihydroxyphenylglycine (DHPG; Sigma-Aldrich, Steinheim, Germany) at 37°C.
Cytosol and plasma-membrane fractions were prepared as described earlier (Lombardi et al., 1999). In short, cells were homogenized in ice-cold buffer (20 mM Tris (pH 7.5), 2 mM EDTA and protease inhibitors) followed by centrifugation at 800 g for 5 min at 4°C. The supernatant was centrifuged at 48.000 g for 20 min at 4°C to obtain crude cytosolic fractions containing intracellular vesicle membranes. Pellets containing plasma-membrane fractions were homogenized in ice-cold buffer containing 20 mM Tris (pH 7.5), 2 mM EDTA, 1% Triton X-100 and protease inhibitors.
Total cell lysates of astrocytes or microglia were prepared as described earlier (Nijboer et al., 2010).
Glutamate uptake assay
Glutamate uptake was measured as described by Tavares et al. (2002). Astrocytes were incubated in HBSS supplemented with 5 mM Hepes for 15 min at 37°C. Uptake was started by adding 0.33 µCi/ml of L-[3H]glutamate (GE Healthcare; Eindhoven, The Netherlands) mixed with unlabeled L-glutamate to a final concentration of 100 µM. After incubation of 0-10 minutes at 37°C, uptake was terminated by two washes with ice-cold HBSS, immediately followed by cell lysis with 0.1N NaOH/0.01% SDS for 15 minutes on ice. Aliquots were taken for protein assay prior to liquid scintillation counting.
Immunoprecipitation and Western Blotting
WT astrocytes were homogenized in ice-cold buffer containing 20 mM Tris-HCl (pH 8.0), 2 mM EDTA (pH 8), 137 mM NaCl, 10% glycerol, 1% Triton X-100 and protease inhibitors. Homogenates were centrifuged at 14.000g for 15 min at 4°C and supernatants were used for immunoprecipitation. Brains were homogenized in ice-cold buffer containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40 and protease inhibitors using Ultra-Turrax T25 and were incubated for 4 hours at 4°C under gentle rotation. Homogenates were centrifuged for 60 min at 30.000 g at 4°C and supernatants were used for immunoprecipitation. Immunoprecipitation was performed as described earlier using 5 µg mouse-anti-GRK2 antibody (Millipore, Watford, UK) or control anti-mouse IgG (IgG2a kappa) (Abcam, Cambridge, UK) (Eijkelkamp et al., 2010a).
Total brain homogenates of adult or P9 mice were made as described earlier (Nijboer et al., 2007).
Proteins samples were separated by SDS-PAGE and transferred to nitrocellulose membranes (Hybond-C; Amersham Biosciences, Roosendaal, the Netherlands). Membranes were stained with rabbit-anti-GRK2 (dilution 1:1000), mouse-anti-ezrin (dilution 1:500), goat-anti-β-actin (dilution 1:2000) (all Santa Cruz Biotechnology), rabbit-anti-calreticulin (dilution 1:1000) (Enzo Life Sciences, Antwerp, Belgium), rabbit-anti-Na+/K+ ATPase (dilution 1:1000) (kind gift of Dr. J. Koenderink), mouse-anti-GFAP (dilution 1:500) (Acris), rabbit-anti-GLAST (dilution 1:5000) (kind gift of Dr. D. Pow), rabbit-anti-phospho ezrin-radixin-moesin (P-ERM) (dilution 1:1000) (Millipore), rabbit-anti-NHERF1 (dilution 1:3000) (kind gift of Dr. C. Yun) or rabbit-anti-GLT-1 (dilution 1:500) (Abcam) followed by incubation with HRP-labeled secondary antibodies. Specific bands were visualised by chemiluminescence (ECL; Amersham Biosciences) with X-ray film exposure, scanned with a GS-700 Imaging Densitometer and analyzed with Quantity One Software (Bio-Rad, Hercules, CA).
Small Interfering RNA (siRNA)
To knockdown ezrin in astrocytes we used 21-base pairs, annealed siRNA duplexes with 2 UU overhangs (5'-CAAGAAGGCACCUGACUUU-3' or scrambled control; Dharmacon RNA Technologies, Lafayette, CO) (Rasmussen et al., 2008) and Astrocytes Transfection Reagent (Altogen Biosystems, Las Vegas, NV) according to the manufacturer’s protocol. After 48 h, silencing of ezrin was confirmed by Western Blotting and astrocytes were used for DHPG stimulation (see above).
Statistical Analysis
All data are presented as mean and SEM. T-test, or two-way ANOVA with Bonferroni post-tests was used to analyze the effect of genotype or genotype and time/treatment.
Results
Effect of low GRK2 in astrocytes on ischemic brain damage
To determine the contribution of astrocyte GRK2 to brain damage, we used GFAP-WT and GFAP-GRK2+/− mice in this study. We have previously shown that GFAP-GRK2+/− mice have a cell-specific 60% reduction in GRK2 protein in astrocytes, whereas GRK2 levels in microglia are not affected compared to GFAP-WT littermates (Eijkelkamp et al., 2010a). In addition we show here that neuronal levels of GRK2 are similar in GFAP-WT and GFAP-GRK2+/− brains (Fig. 1A).
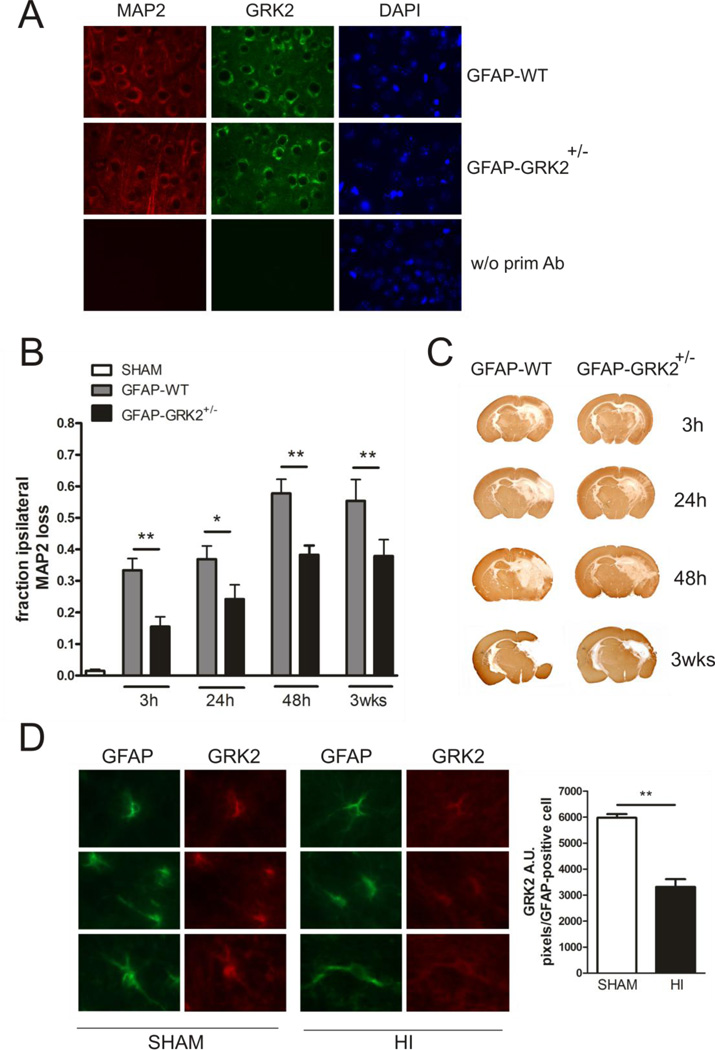
Figure 1. Functional consequences of astrocyte GRK2-deficiency for brain damage in vivo.
A: Representative photographs of immunofluorescent staining of MAP2 (red), GRK2 (green) and DAPI (blue) in the cortex of GFAP-WT and GFAP-GRK2+/− P9 mice, illustrating neuronal GRK2 levels. As a control the primary antibodies were ommitted (w/o prim Ab).
B: Quantification of HI-induced MAP2 loss in the ipsilateral hemisphere at hippocampal level at 3 h, 24 h, 48 h and 3 wks post- HI in GFAP-WT and GFAP-GRK2+/− animals and in sham-operated controls. MAP2 loss was calculated as 1- {MAP2-positive staining in ipsilateral/contralateral hemisphere}. Sham-operated controls of both genotypes did not show significant MAP2 loss and are represented as one group. n=10-12 per genotype per time point.
C: Representative photographs of immunohistochemical staining of MAP2 as an indicator of neuronal integrity in GFAP-WT and GFAP-GRK2+/− mice at the different time points indicated.
D: Left: Representative photographs of immunofluorescent staining of GFAP (green) and GRK2 (red) in the white matter tract of sham-operated (SHAM) or HI-treated (HI) P9 mice at 3 h post-HI. Right: quantification of GRK2 level in GFAP-positive cells.
*p<0.05; **p<0.01 GFAP-WT vs GFAP-GRK2+/−; **p<0.01 SHAM vs HI.
We induced hypoxic-ischemic (HI) brain damage in P9 GFAP-WT and GFAP-GRK2+/− mice by permanent unilateral carotid artery occlusion and hypoxia and neuronal damage was analyzed as loss of ipsilateral microtubule-associated protein 2 (MAP2) staining. Neuronal damage is significantly reduced in GFAP-GRK2+/− mice as compared to GFAP-WT littermate controls at all time points tested (Figs. 1B and C). Figure 1C shows that at 3h after the insult MAP2 loss is apparent in the hippocampal area. At 48 h after the insult, MAP2 loss is also visible in the cortical areas and the thalamic region. At 3 weeks after HI, a cystic infarct has formed with loss of hippocampal and thalamic area in the GFAP-GRK2+/− animals and loss of hippocampal, thalamic and large cortical area loss in GFAP-WT animals. MAP2 loss did not further increase between 48 h and 3 weeks after induction of HI in both genotypes (Figs. 1B and C), indicating that maximal loss of MAP2 was obtained already at 48 h after the insult.
Brain sections stained for GRK2 and GFAP show reduced (44%) levels of GRK2 in GFAP-positive cells in HI-brains compared to sham-control brains at 3 h post-HI, indicating that the level of GRK2 is actually reduced in astrocytes after HI in vivo (Fig. 1D).
Functional consequences of low astrocyte GRK2 for plasma-membrane GLAST expression and glutamate uptake
Next, we investigated whether GRK2-deficiency affected levels of glutamate transporters at the astrocyte plasma-membrane. We observed that primary cultures of astrocytes from P1 mice express GLAST. The GLT-1 glutamate transporter was not detectable in these cultures (Fig. 2A, left panel) which is in line with other studies (Beaulé et al., 2009; Guillet et al., 2002; Suzuki et al., 2001). GLAST and GLT-1 were both highly expressed in the adult mouse brain with a higher expression of GLT-1 as was earlier described by Lehre and Danbolt (1998) (Fig. 2A, left panel). In the immature mouse brain at P9 however, GLAST is the predominant glutamate transporter, with lower levels of GLT-1 expression (Fig. 2A, right panels). No differences in total GLAST levels were observed between WT and GRK2+/− and GFAP-WT and GFAP-GRK2+/− mouse brains at P9.
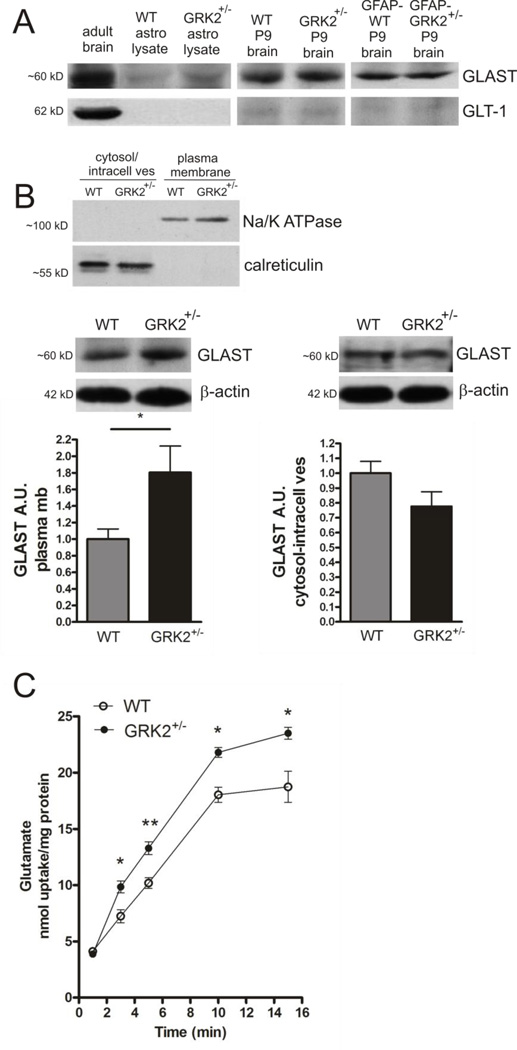
Figure 2. Functional consequences of low astrocyte GRK2 for plasma-membrane GLAST expression and glutamate uptake.
A: Western Blot examples showing expression of GLAST and not GLT-1 in total lysates of primary cultures of WT and GRK2-deficient astrocytes. Total protein samples of adult WT and P9 WT and GRK2+/− or P9 GFAP-WT and GFAP-GRK2+/− mouse brains are loaded as controls.
B: Upper part shows quality of subcellular fractionation of astrocyte lysates into plasma-membrane fraction and fraction containing cytosol and intracellular vesicular membranes (cytosol/intracell ves). Note exclusive presence of Na+/K+ ATPase in plasma-membrane fraction and exclusive presence of calreticulin, a marker for ER vesicles, in the cytosol/intracellular vesicle fraction.
Lower part shows GLAST expression in plasma-membrane (plasma mb) fraction (left) and cytosol/intracellular vesicle fraction (right) of WT and GRK2-deficient primary cultures of astrocytes analyzed by Western Blotting. Insets show representative Western Blot examples; β-actin is used as a loading control for both fractions. Ponceau S staining gave similar equal loading results as observed after probing with β-actin antibody. n=8 animals per genotype.
C: Glutamate uptake capacity of WT and GRK2-deficient astrocytes was tested by measuring 3H-labeled glutamate (100 µM) uptake over 15 minutes. Data are presented as nmol glutamate uptake per mg protein. n=9 per genotype per time point. A.U.: arbitrary units.
*p<0.05; **p<0.01 WT vs GRK2+/−.
Primary astrocyte cultures were fractionated into a plasma-membrane protein fraction and a cytosol/intracellular vesicle membrane protein fraction. The presence of Na+/K+ ATPase and the absence of calreticulin in the plasma membrane fraction confirms that the plasma membrane fraction is not contaminated with ER vesicles (Fig. 2B; upper part). Plasma-membrane GLAST levels were significantly higher in GRK2-deficient astrocytes as compared to WT astrocytes. GLAST levels in the cytosol/intracellular vesicle fraction seemed to be decreased in GRK2-deficient astrocytes compared to WT cells, but this did not reach statistical significance (Fig. 2B).
Next, we determined whether the increased expression of GLAST in GRK2-deficient astrocytes had functional consequences for glutamate uptake capacity. The capacity of both WT and GRK2-deficient astrocytes to take up glutamate was analyzed by using a 3H-glutamate uptake assay. Figure 2C shows that glutamate uptake was significantly increased in GRK2-deficient astrocytes as compared to WT astrocytes.
GRK2 deficiency increases GFAP expression
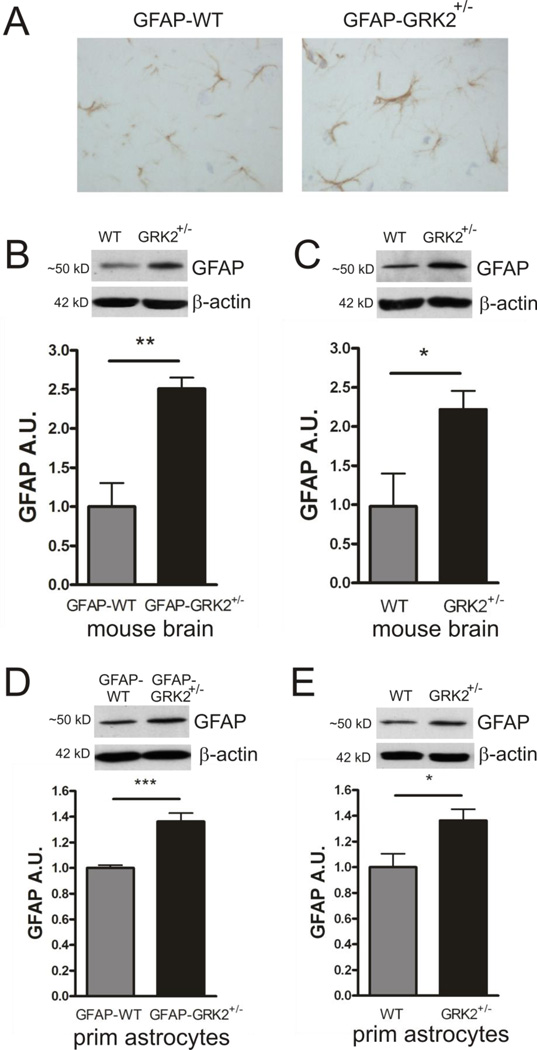
GFAP is involved in retaining GLAST in the plasma-membrane, thereby regulating glutamate uptake (Sullivan et al., 2007). We measured GFAP levels in the brains of GFAP-WT and GFAP-GRK2+/− mice and WT and GRK2+/− mice by immunohistochemistry and Western blot analysis and observed an increase in GFAP in GRK2-deficient animals as compared to WT littermates (Figs. 3A to C). To investigate whether increased GFAP levels in brains of GRK2+/− mice were associated with increased GFAP expression in astrocytes or with a higher amount of GFAP-positive cells in the GRK2+/− brain, we quantified GFAP levels in isolated GFAPGRK2 +/− astrocytes. GFAP levels in primary cultures of GFAP-GRK2+/− astrocytes were increased compared to astrocytes of GFAPWT littermates (Fig. 3D). Similarly, increased GFAP levels were observed in primary cultures of astrocytes from GRK2+/− mice, indicating that the increase in GFAP expression was independent of Cre transgene expression and was also not due to potential differences in the genetic background of the animals (Fig. 3E). These findings strongly indicate that increased GFAP levels in the brain of GFAP-GRK2+/− mice are due to a higher GFAP expression in GRK2-deficient astrocytes.
Figure 3. GRK2-deficiency is associated with increased GFAP in astrocytes.
A: Representative photographs of immunohistochemical staining of GFAP illustrating increased GFAP expression in parietal cortices of sham-control GFAP-GRK2+/− mice compared to GFAP-WT littermates.
B–C: GFAP expression in total brain homogenates of GFAP-WT and GFAP-GRK2+/− (B) or WT and GRK2+/− (C) mice analyzed by Western Blotting. Insets show representative Western Blot examples; β-actin is used as a loading control. n=5-6 animals per genotype.
D–E: GFAP expression in total lysates of primary cultures of GFAP-WT and GFAP-GRK2+/− (D)or WT and GRK2+/− (E) astrocytes analyzed by Western Blotting. Insets show representative Western Blot examples; β-actin is used as loading control. n=6-8 animals per genotype.
A.U.: arbitrary units. *p<0.05; ***p<0.001 WT vs GRK2+/− or GFAP-WT vs GFAP-GRK2+/−
GRK2 deficiency increases plasma-membrane GLAST expression via an ezrin-dependent mechanism
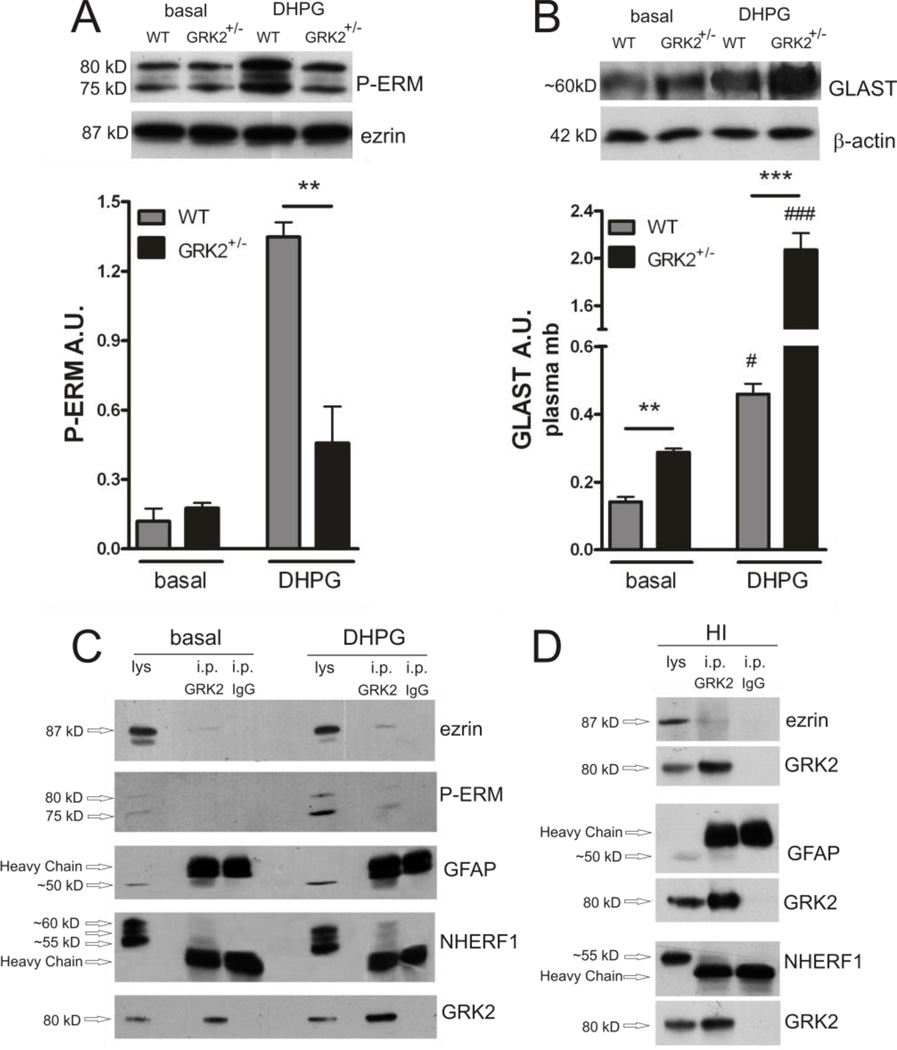
GFAP is thought to promote GLAST association with the plasma-membrane by forming a complex with ezrin and NHERF1 (Sullivan et al., 2007) and GRK2 is known to phosphorylate ezrin (Cant and Pitcher, 2005). Levels of phosphorylated ezrin (PERM) were strongly increased when WT astrocytes were stimulated with the group I mGluR-agonist DHPG (Fig. 4A). DHPG stimulation also rapidly and significantly increased plasma-membrane levels of GLAST in WT astrocytes (Fig. 4B). Figure 4B shows that the increase in GLAST expression in the astrocyte plasma-membrane was much more pronounced in GRK2-deficient astrocytes after DHPG stimulation compared to WT. In line with the fact that ezrin is a GRK2 substrate, the level of phosphorylated ezrin was significantly decreased in GRK2-deficient compared to WT astrocytes upon DHPG stimulation (Fig. 4A).
Figure 4. GRK2 deficiency decreases DHPG-induced P-ERM and increases DHPG-induced plasma-membrane GLAST association.
A: Phosphorylated ezrin (P-ERM) in plasma-membrane fractions of WT and GRK2+/− astrocytes after 10 min of stimulation with culture medium (basal) or DHPG (50 µM) analyzed by Western Blotting. Insets show representative Western Blot examples; total ezrin is used as a loading control. **p<0.01 WT vs GRK2+/−.
B: GLAST expression in plasma-membrane fractions of WT and GRK2+/− astrocytes after 10 min of stimulation with culture medium (basal) or DHPG (50 µM) analyzed by Western Blotting. Insets show representative Western Blot examples; β-actin is used as a loading control. # p<0.05, ### p<0.001: basal vs DHPG per genotype. **p<0.01; ***p<0.001: WT vs GRK2+/−. A/B : Data are from 3 independent experiments (n=3) performed in 8-fold.
C–D: GRK2 protein was immunoprecipitated (i.p. GRK2) in samples from unstimulated (basal) and DHPG-stimulated cultures of primary WT astrocytes (C) or in P9 mouse brain homogenates pooled from 5 WT animals at 0.5 h post-HI (D). The same amount of mouse IgG (IgG2a kappa) was used as a control (i.p. IgG). The immunoprecipitated products were analyzed by Western Blotting. Blots show co-immunoprecipitation of ezrin, phosphorylated ezrin (P-ERM) (after DHPG in cultured astrocytes), GFAP and NHERF1 with GRK2. Arrows indicate migration of the bands of interest and the heavy chain of the IgG around 50 kD. Total astrocyte lysate (lys; C) or total brain homogenate (lys; D) (10% of input) was loaded for comparison. GRK2 blots show increased levels of GRK2 after immunoprecipitation, indicating successful immunoprecipitation.
Co-immunoprecipitation studies in primary astrocytes show that GRK2 is found in the same complex as ezrin, GFAP and NHERF1 (Fig. 4C). Phosphorylated ezrin (P-ERM) was detected within the GRK2 containing protein complex only after exposure to DHPG in cultured astrocytes (Fig. 4C). Moreover, the interaction of GRK2 with ezrin, GFAP and NHERF1 was confirmed by co-immunoprecipication in brain samples obtained at 0.5 h post-HI in vivo (Fig. 4D). In sham-operated mouse brains, ezrin, GFAP and NHERF1 were not detectable in the complex with GRK2 (data not shown), indicating that HI promotes the formation of this complex in vivo.
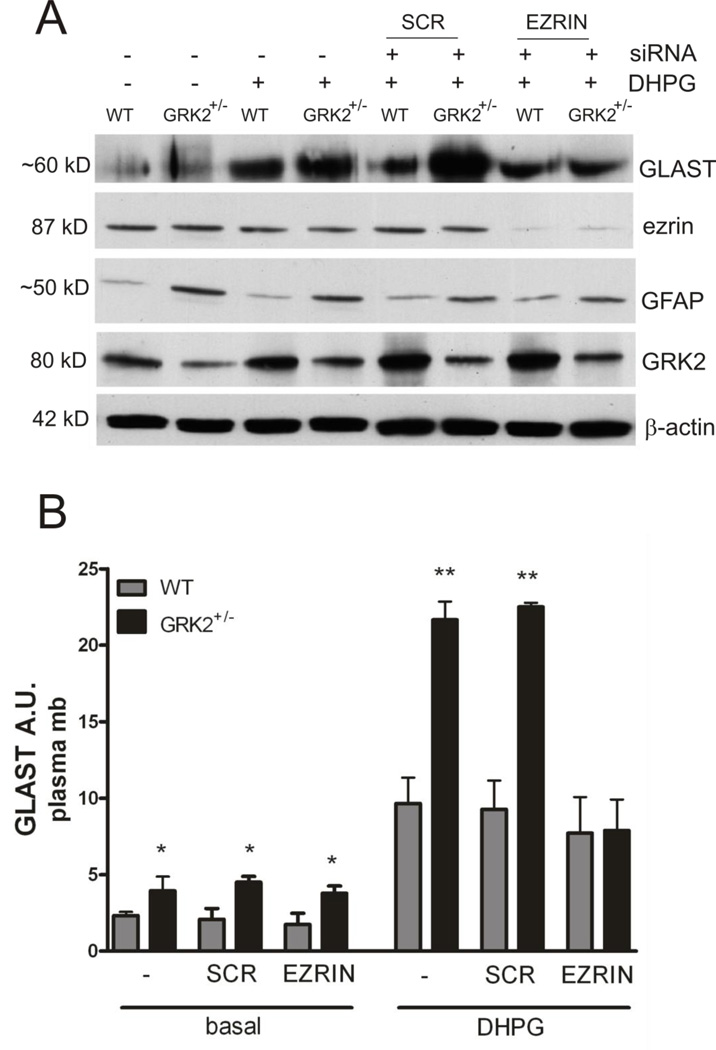
To test the hypothesis that ezrin is involved in GRK2-dependent regulation of plasma-membrane levels of GLAST, we used siRNA to silence ezrin in WT and GRK2-deficient astrocytes. Using this approach ezrin protein was reduced by >90% (Fig. 5A). Scrambled siRNA did not decrease ezrin protein level (Fig. 5A). As anticipated, silencing ezrin did not affect GFAP or GRK2 levels (Fig. 5A). Under basal (unstimulated) conditions silencing of ezrin did not affect GLAST levels in the plasma-membrane in GRK2-deficient astrocytes compared to WT astrocytes (Fig. 5B). However, silencing of ezrin completely abolished the increased DHPG-induced expression of plasma-membrane GLAST in GRK2-deficient astrocytes (Figs. 5A and B). The DHPG-induced increase in plasma-membrane GLAST in WT astrocytes was not affected after silencing of ezrin. These data indicate that ezrin is required for increased GLAST plasma-membrane localization after agonist stimulation in conditions of low GRK2.
Figure 5. Increased DHPG-induced GLAST association with the plasma-membrane in GRK2-deficient cells is dependent on ezrin.
A: Ezrin protein expression was silenced in WT and GRK2+/− astrocytes using siRNA. 48 h later, astrocytes were stimulated with culture medium (basal) or DHPG (50 µM for 10 min) and protein lysates were analyzed by Western Blotting. β-actin is used as a loading control. Ezrin protein level was reduced >90% after transfection with siRNA. Transfection with scrambled (SCR) siRNA did not have any effect on ezrin protein levels.
B: Quantification of GLAST expression in plasma-membrane fractions of WT and GRK2+/− astrocytes after siRNA transfection (ezrin or scrambled) and stimulation with culture medium (basal) or DHPG (see A).
Data are from 2-4 independent experiments performed in 4-fold. *p<0.05; **p<0.01
Discussion
Astrocytes are believed to act as endogenous protectors against excitotoxicity as they constitutively express high-affinity glutamate transporters which can clear excess glutamate and thereby prevent brain damage (Danbolt, 2001; Rossi et al., 2007). One important finding in our study is that GRK2-deficient astrocytes display higher plasma-membrane levels of GLAST than WT astrocytes. In addition, glutamate uptake is increased in GRK2-deficient astrocytes compared to WT astrocytes, indicating that increased GLAST in GRK2-deficient astrocytes has functional consequences for glutamate homeostasis. Moreover, the DHPG-induced increase in plasma-membrane GLAST was significantly higher in GRK2-deficient astrocytes compared to WT cells. The relevance of these in vitro data for the in vivo situation is attested by our findings showing that low astrocyte GRK2 reduces neonatal HI brain damage. Our data show a novel role of GRK2 in astrocytes and add to the growing knowledge how astrocytes can defend the brain against injury. We show that a 60% reduction in the protein level of one kinase, GRK2, can have crucial effects on the endogenous neuroprotective capacity of astrocytes.
Previously we described the effect of reduced GRK2 in all cells on ischemic cerebral damage. Mice with a 40–50% reduction in GRK2 protein (GRK2+/− mice) were more sensitive to neonatal ischemic brain injury (Nijboer et al., 2008). Using Cre-Lox technology, we demonstrated that low GRK2 in CamKIIα-positive (forebrain) neurons increased the extent of ischemic brain damage, whereas low GRK2 in microglia accelerated the onset of cerebral damage without changing the total amount of damage (Nijboer et al., 2010).
In the present study, we used GFAP-GRK2+/− mice to study the role of GRK2 in astrocytes. In contrast to the exacerbated HI brain damage that develops in mice with cell-specific reduction of GRK2 in forebrain neurons (CamKIIα-GRK2+/− mice) (Nijboer et al., 2010), we show here that reduction of GRK2 in astrocytes reduces cerebral infarct size after HI. These data point out that GRK2 has a cell-specific effect on HI-induced brain damage. Previously we showed that HI reduces cerebral GRK2 levels in vivo (Lombardi et al., 2004). Immunohistochemical analysis revealed that exposure to neonatal HI reduced neuronal GRK2 levels in cortex and hippocampus (Nijboer et al., 2008). Here we show that HI also reduces GRK2 levels in astrocytes in the brain in vivo.
During postnatal brain development, GFAP has also been described to be transiently expressed by neural progenitor cells (Fox et al., 2004; Imura et al., 2003), therefore we cannot completely rule out that a reduction in neuronal GRK2 contributes to the phenotype in GFAP-GRK2+/− animals. However, we described earlier that a reduced GRK2 level in neurons in fact exacerbates HI brain damage, which makes it unlikely that a potential reduction in neuronal GRK2 explains the reduced brain damage in our GFAP-GRK2+/− mice.
In several studies using murine primary astrocytes, it has been shown that upon a rise in extracellular glutamate, astrocytes can acutely (within seconds to minutes) upregulate glutamate transporters in the plasma-membrane (Duan et al., 1999; Munir et al., 2000; Vermeiren et al., 2005). We show here that GRK2-deficient astrocytes display a higher level of plasma-membrane GLAST than WT cells under both basal as well as stimulated conditions. We propose that this increase in plasma-membrane GLAST that may occur both at synatic as well as extrasynaptic sites, is responsible for the increased glutamate uptake by GRK2- deficient astrocytes in vitro and the observed neuroprotection in vivo. We could not detect GLT-1 in our primary astrocytes cultures, but GLT-1 was expressed at low levels in the P9 mouse brain. Therefore we can not exclude that a potential effect of GRK2 on GLT-1 subcellular distribution or activity contributes to the observed protective effect of astrocyte GRK2-deficiency in neonatal HI brain injury in vivo.
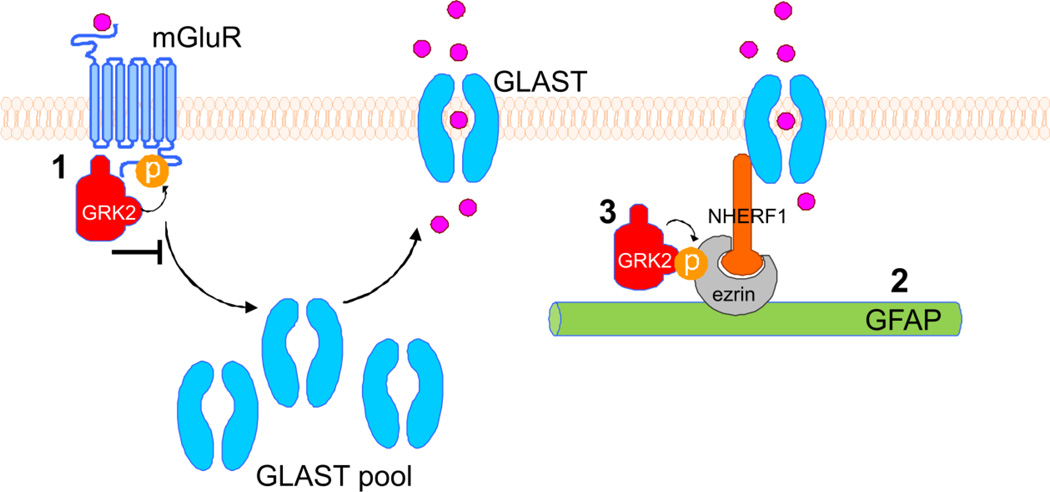
Figure 6 summarizes the possible routes via which reduction in astrocytic GRK2 can lead to enhanced GLAST plasma-membrane association. First, GRK2 is known to regulate termination of group I-mGluR signaling in response to agonist-binding by phosphorylation of these receptors (Fig. 6; point 1) (Dale et al., 2000; Sorensen and Conn, 2003). Interestingly, group I mGluRs are essential in regulating glutamate transporter levels in rat astrocytes.Vermeiren et al. (2005) showed that stimulation of group I-mGluRs with DHPG rapidly increased aspartate uptake in astrocytes, which could be blocked by a mGluRantagonist or a specific glutamate transporter blocker. Additionally,Gegelashvili et al. (2000) showed that astrocyte mGluRs are crucial in regulating GLAST plasma-membrane expression. We show here that the mGluR agonist-induced increase in plasmamembrane GLAST levels is larger in GRK2-deficient astrocytes as compared to WT astrocytes. Therefore, it is conceivable that impaired GRK2-mediated phosphorylation and desensitization of mGluR is one of the mechanisms via which low GRK2 enhances GLAST translocation to the plasma-membrane and subsequent glutamate uptake.
Figure 6. Diagram showing possible regulatory mechanisms of GRK2 on GLAST plasma-membrane localization.
Left part of diagram: upon binding of glutamate or a mGluR agonist, mGluRs are activated and start signaling. As a consequence, GLAST is redistributed from cytoplasmic pools to the plasma-membrane, where the GLAST transporters take up glutamate. Our results show that GRK2-deficient astrocytes express higher levels of plasma-membrane GLAST under basal and stimulated (mGluR-agonist DHPG) conditions. Several mechanisms for a regulatory role of GRK2 can be proposed:
1: GRK2 phosphorylates group I mGluRs, leading to termination of signaling downstream of these receptor and receptor internalization (inhibitory arrow). GRK2-deficiency leads to enhanced signaling via the mGluR and might directly regulate GLAST distribution.
2: A crucial role for GFAP intermediate filaments has been shown for retainment of GLAST in the plasma-membrane (Sullivan et al., 2007). GRK2-deficient astrocytes show enhanced GFAP expression, which might lead to enhanced linking of GLAST in the plasma-membrane.
3: GFAP regulates GLAST plasma-membrane localization via linker proteins ezrin and NHERF1. Ezrin is a substrate of GRK2. Stimulation of the mGluR induces phosphorylation of ezrin. GRK2-deficient astrocytes show reduced phospho-ezrin levels. Silencing of ezrin completely abolished the increase GLAST plasma-membrane expression in GRK2-deficient astrocytes. Our data show that DHPG-induced regulation of GLAST transport to the plasma-membrane becomes dependent on ezrin only when GRK2 is low.
A second mechanism that may contribute to increased plasma-membrane GLAST levels in GRK2-deficient astrocytes is the increased GFAP level (Fig. 6; point 2) that we observed in primary cultures of GRK2-deficient astrocytes as well as in GFAPGRK2 +/− and GRK2+/− mouse brains.Sullivan et al. (2007) have demonstrated an essential role for GFAP in regulating plasmamembrane localization of GLAST and aspartate uptake. Moreover, trafficking of glutamate transporters to the plasmamembrane is disrupted in GFAP−/− astrocytes (Hughes et al., 2004). Thus, it is likely that increased GFAP in GRK2-deficient astrocytes increases GLAST localization ot the plasma-membrane. It remains to be determined how GRK2 regulates GFAP expression in astrocytes. One possibility could be that the increased GFAP expression in GRK2-deficient astrocytes is associated with an increased activation state of these cells already under baseline conditions.
A third possible mechanism via which reduced GRK2 expression in astrocytes could influence GLAST localization at the plasma-membrane is via regulation of cytoskeleton-associated linker proteins such as ezrin and NHERF1 (Na+/H+ exchanger regulatory factor 1) (Fig. 6; point 3). The rapid increase in plasma-membrane GLAST expression after glutamate stimulation requires an intact cytoskeleton (Duan et al., 1999; Poitry-Yamate et al., 2002; Shin et al., 2009). In addition, GLAST upregulation at the plasma-membrane occurs much more rapidly than the time required for protein synthesis (Munir et al., 2000).Sullivan et al. (2007) showed that GLAST is present in the same protein complex as GFAP and the linker proteins NHERF1 and ezrin. When we immunoprecipitated GRK2 from astrocyte lysates or from the brain after HI, we observed GRK2 is also present in complex with the cytoskeleton-associated proteins ezrin, NHERF1 and GFAP. Interestingly, it has been shown that GRK2 phosphorylates ezrin (Cant and Pitcher, 2005) and other cytoskeleton-associated proteins (Kahsai et al, 2010; Pitcher et al., 1998;). GRK2-mediated ezrin phosphorylation is promoted by GPCR activation as has been shown for the β2-adrenergic receptor (Cant and Pitcher, 2005). Accordingly, we show here that stimulation of type I-mGluR by DHPG induces ezrin phosphorylation and that DHPG-induced ezrin phosphorylation is attenuated in GRK2-deficient astrocytes. Notably, reduced P-ERM in GRK2-deficient astrocytes was associated with a stronger increase in plasma-membrane GLAST in response to DHPG stimulation compared to WT. Additionally, P-ERM appeared in the protein complex with GRK2 after DHPG stimulation of primary cultured astrocytes. Furthermore, in the brain in vivo the interaction of GRK2 and ezrin was promoted by HI as GRK2 and ezrin were not observed in the same protein complex in brains of sham-operated control mice. These findings together indicate that stimulation of mGluRs by either DHPG in vitro or HI in vivo promoted the interaction between GRK2 and ezrin and possibly the subsequent phosphorylation of ezrin. We therefore propose that the increased DHPG-induced plasma-membrane association of GLAST in GRK2-deficient astrocytes is at least partially dependent on an interaction between ezrin and GRK2. Supporting this hypothesis, when ezrin protein expression was reduced by means of siRNA, the increased DHPG-induced plasma-membrane GLAST association in GRK2-deficient astrocytes was completely abolished. Notably, silencing of ezrin did not affect the increased plasma-membrane GLAST association under basal conditions in GRK2-deficient astrocytes. Moreover, in WT astrocytes silencing of ezrin did not influence DHPG-induced GLAST expression in the plasma-membrane. These results together indicate that ezrin phosphorylation is not directly responsible for GLAST upregulation in WT cells after DHPG stimulation (or under basal conditions in GRK2-deficient astrocytes), but show that DHPG-induced regulation of GLAST transport to the plasma-membrane becomes dependent on ezrin only when GRK2 is low. One possibility is that ezrin can only affect plasma-membrane GLAST localization when GFAP levels are high, which might be a limiting step in WT astrocytes.
To conclude, reduced levels of GRK2 in astrocytes may well promote the translocation of GLAST to the plasma-membrane via enhanced signaling of the mGluR due to impaired receptor phosphorylation in combination with increased formation of the GFAP-ezrin-NHERF1 complex of proteins which are responsible for GLAST anchoring in the plasma-membrane.
In the current study we describe mechanisms by which GRK2 levels in astrocytes regulate the trafficking of the glutamate transporter GLAST and thereby glutamate uptake in vitro. We propose that these mechanisms contribute to the observed reduction in HI brain damage in GFAP-GRK2+/− in vivo. It may well be possible, however, that additional astrocyte-dependent mechanisms, including potential effects on cerebral blood flow or scar formation might contribute to the observed effects on HI brain damage in vivo. However, we have shown in a previous study that the HI-induced reduction in cerebral blood flow was not different between WT and GRK2+/− animals that have low GRK2 in all cells including astrocytes (Nijboer et al., 2008).
More and more research in the field of brain injury indicates an important role for astrocytes in protecting neurons. Here, we highlight this endogenous neuroprotective role of astrocytes in the newborn brain and demonstrate for the first time that a downregulation of GRK2 is key in the protective capacity of astrocytes. Our data elucidate that reduction of only one kinase has crucial effects on the capacity of astrocytes to regulate association of GLAST with the astrocytic plasma-membrane, a critical step in regulation of glutamate homeostasis by astrocytes.
Highlights.
Reduced GRK2 in astrocytes protects against ischemic brain damage in vivo
High glutamate transporter GLAST and GFAP levels in GRK2-deficient astrocytes
GRK2-deficient astrocytes show increased glutamate take up in vitro
GRK2 regulates GLAST localization via an ezrin-dependent route
A critical role for astrocyte GRK2 regulation in dampening ischemic brain injury
Acknowledgements
The study was supported by the European Commission: Sixth Framework Program, contract no. LSHM-CT-2006-036534: NEOBRAIN & Seventh Framework Program, contract no. HEALTH-F2-2009-241778: NEUROBID), by Fondation Leducq and by NIH grants RO1 NS073939 and RO1 NS074999.
We thank Dr. David Pow (University of Queensland, Australia), Dr. Chris Yun (Emory University School of Medicine, Atlanta) and Dr. J. Koenderink (UMC St. Radboud, Nijmegen, the Netherlands) for the generous gifts of GLAST, NHERF1 and Na+/K+ ATPase antibodies, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cora H. Nijboer, Email: C.Nijboer@umcutrecht.nl.
Cobi J. Heijnen, Email: C.Heijnen@umcutrecht.nl.
Vincent Degos, Email: degosv@anesthesia.ucsf.edu.
Hanneke L.D.M. Willemen, Email: H.L.D.M.Willemen@umcutrecht.nl.
Pierre Gressens, Email: pierre.gressens@inserm.fr.
Annemieke Kavelaars, Email: AKavelaars@mdanderson.org.
References
- Beaulé C, Swanstrom A, Leone MJ, Herzog ED. Circadian modulation of gene expression, but not glutamate uptake, in mouse and rat cortical astrocytes. PLoS One. 2009;4:e7476. doi: 10.1371/journal.pone.0007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant SH, Pitcher JA. G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol Biol Cell. 2005;16:3088–3099. doi: 10.1091/mbc.E04-10-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Anborgh PH, Murdoch B, Bhatia M, Nakanishi S, Ferguson SS. G protein-coupled receptor kinasemediated desensitization of metabotropic glutamate receptor 1A protects against cell death. J Biol Chem. 2000;275:38213–38220. doi: 10.1074/jbc.M006075200. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Stein BA, Swanson RA. Glutamate induces rapid upregulation of astrocyte glutamate transport and cellsurface expression of GLAST. J Neurosci. 1999;19:10193–10200. doi: 10.1523/JNEUROSCI.19-23-10193.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Heijnen CJ, Willemen HL, Deumens R, Joosten EA, Kleibeuker W, den Hartog I, van Velthoven CT, Nijboer C, Nassar MA, Dorn GW, Wood JN, Kavelaars A. GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J Neurosci. 2010a;30:2138–2149. doi: 10.1523/JNEUROSCI.5752-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Wang H, Garza-Carbajal A, Willemen HL, Zwartkruis FJ, Wood JN, Dantzer R, Kelley KW, Heijnen CJ, Kavelaars A. Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, protein kinase Cepsilon, and MEK/ERK. J Neurosci. 2010b;30:12806–12815. doi: 10.1523/JNEUROSCI.3142-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana AC, de Oliveira BR, Wojewodzic MW, Ferreira Dos SW, Coutinho-Netto J, Grutle NJ, Watts SD, Danbolt NC, Amara SG. Enhancing glutamate transport: mechanism of action of Parawixin1, a neuroprotective compound from Parawixia bistriata spider venom. Mol Pharmacol. 2007;72:1228–1237. doi: 10.1124/mol.107.037127. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Paucar AA, Nakano I, Mottahedeh J, Dougherty JD, Kornblum HI. Developmental expression of glial fibrillary acidic protein mRNA in mouse forebrain germinal zones�implications for stem cell biology. Brain Res Dev Brain Res. 2004;153:121–125. doi: 10.1016/j.devbrainres.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Dehnes Y, Danbolt NC, Schousboe A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem Int. 2000;37:163–170. doi: 10.1016/s0197-0186(00)00019-x. [DOI] [PubMed] [Google Scholar]
- Guillet BA, Velly LJ, Canolle B, Masmejean FM, Nieoullon AL, Pisano P. Differential regulation by protein kinases of activity and cell surface expression of glutamate transporters in neuron-enriched cultures. Neurochem Int. 2005;46:337–346. doi: 10.1016/j.neuint.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML. Loss of glial fibrillary acidic protein results in decreased glutamate transport and inhibition of PKA-induced EAAT2 cell surface trafficking. Brain Res Mol Brain Res. 2004;124:114–123. doi: 10.1016/j.molbrainres.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of betaadrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai AW, Zhu S, Fenteany G. G protein-coupled receptor kinase 2 activates radixin, regulating membrane protrusion and motility in epithelial cells. Biochim Biophys Acta. 2010;1803:300–310. doi: 10.1016/j.bbamcr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavelaars A, Eijkelkamp N, Willemen HL, Wang H, Carbajal AG, Heijnen CJ. Microglial GRK2: a novel regulator of transition from acute to chronic pain. Brain Behav Immun. 2011;25:1055–1060. doi: 10.1016/j.bbi.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Kleibeuker W, Jurado-Pueyo M, Murga C, Eijkelkamp N, Mayor F, Jr, Heijnen CJ, Kavelaars A. Physiological changes in GRK2 regulate CCL2-induced signaling to ERK1/2 and Akt but not to MEK1/2 and calcium. J Neurochem. 2008;104:979–992. doi: 10.1111/j.1471-4159.2007.05023.x. [DOI] [PubMed] [Google Scholar]
- van der Kooij MA, Ohl F, Arndt SS, Kavelaars A, van Bel F, Heijnen CJ. Mild neonatal hypoxia-ischemia induces long-term motorand cognitive impairments in mice. Brain Behav Immun. 2010;24:850–856. doi: 10.1016/j.bbi.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JW, Okihara KL, Van de Pol M, Ochsmann S, Pawlak C, Schmidt RE, Heijnen CJ. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J. 1999;13:715–725. doi: 10.1096/fasebj.13.6.715. [DOI] [PubMed] [Google Scholar]
- Lombardi MS, van den Tweel E, Kavelaars A, Groenendaal F, van Bel F, Heijnen CJ. Hypoxia/ischemia modulates G proteincoupled receptor kinase 2 and beta-arrestin-1 levels in the neonatal rat brain. Stroke. 2004;35:981–986. doi: 10.1161/01.STR.0000121644.82596.7e. [DOI] [PubMed] [Google Scholar]
- Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW. Cardiacspecific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- Munir M, Correale DM, Robinson MB. Substrate-induced up-regulation of Na(+)-dependent glutamate transport activity. Neurochem Int. 2000;37:147–162. doi: 10.1016/s0197-0186(00)00018-8. [DOI] [PubMed] [Google Scholar]
- Nijboer CH, Groenendaal F, Kavelaars A, Hagberg HH, van Bel F, Heijnen CJ. Gender-specific neuroprotection by 2-iminobiotin after hypoxia-ischemia in the neonatal rat via a nitric oxide independent pathway. J Cereb Blood Flow Metab. 2007;27:282–292. doi: 10.1038/sj.jcbfm.9600342. [DOI] [PubMed] [Google Scholar]
- Nijboer CH, Kavelaars A, Vroon A, Groenendaal F, van Bel F, Heijnen CJ. Low endogenous G-protein-coupled receptor kinase 2 sensitizes the immature brain to hypoxia-ischemia-induced gray and white matter damage. J Neurosci. 2008;28:3324–3332. doi: 10.1523/JNEUROSCI.4769-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijboer CH, Heijnen CJ, Willemen HL, Groenendaal F, Dorn GW, van Bel F, Kavelaars A. Cell-specific roles of GRK2 in onset and severity of hypoxic-ischemic brain damage in neonatal mice. Brain Behav Immun. 2010;24:420–426. doi: 10.1016/j.bbi.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peregrin S, Jurado-Pueyo M, Campos PM, Sanz-Moreno V, Ruiz-Gomez A, Crespo P, Mayor F, Jr, Murga C. Phosphorylation of p38 by GRK2 at the docking groove unveils a novel mechanism for inactivating p38MAPK. Curr Biol. 2006;16:2042–2047. doi: 10.1016/j.cub.2006.08.083. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Hall RA, Daaka Y, Zhang J, Ferguson SS, Hester S, Miller S, Caron MG, Lefkowitz RJ, Barak LS. The G proteincoupled receptor kinase 2 is a microtubule-associated protein kinase that phosphorylates tubulin. J Biol Chem. 1998;273:12316–12324. doi: 10.1074/jbc.273.20.12316. [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Vutskits L, Rauen T. Neuronal-induced and glutamate-dependent activation of glial glutamate transporter function. J Neurochem. 2002;82:987–997. doi: 10.1046/j.1471-4159.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 2001;21:1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M, Alexander RT, Darborg BV, Mobjerg N, Hoffmann EK, Kapus A, Pedersen SF. Osmotic cell shrinkage activates ezrin/radixin/moesin (ERM) proteins: activation mechanisms and physiological implications. Am J Physiol Cell Physiol. 2008;294:C197–C212. doi: 10.1152/ajpcell.00268.2007. [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes HM, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Shin JW, Nguyen KT, Pow DV, Knight T, Buljan V, Bennett MR, Balcar VJ. Distribution of glutamate transporter GLAST in membranes of cultured astrocytes in the presence of glutamate transport substrates and ATP. Neurochem Res. 2009;34:1758–1766. doi: 10.1007/s11064-009-9982-z. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;19:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SD, Conn PJ. G protein-coupled receptor kinases regulate metabotropic glutamate receptor 5 function and expression. Neuropharmacology. 2003;44:699–706. doi: 10.1016/s0028-3908(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Sullivan SM, Lee A, Bjorkman ST, Miller SM, Sullivan RK, Poronnik P, Colditz PB, Pow DV. Cytoskeletal anchoring of GLAST determines susceptibility to brain damage: an identified role for GFAP. J Biol Chem. 2007;282:29414–29423. doi: 10.1074/jbc.M704152200. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ikegaya Y, Matsuura S, Kanai Y, Endou H, Matsuki N. Transient upregulation of the glial glutamate transporter GLAST in response to fibroblast growth factor, insulin-like growth factor and epidermal growth factor in cultured astrocytes. J Cell Sci. 2001;114:3717–3725. doi: 10.1242/jcs.114.20.3717. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- Takano T, Oberheim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40:S8–S12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares RG, Tasca CI, Santos CE, Alves LB, Porciuncula LO, Emanuelli T, Souza DO. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int. 2002;40:621–627. doi: 10.1016/s0197-0186(01)00133-4. [DOI] [PubMed] [Google Scholar]
- Vermeiren C, Najimi M, Vanhoutte N, Tilleux S, de H, I, Maloteaux JM, Hermans E. Acute up-regulation of glutamate uptake mediated by mGluR5a in reactive astrocytes. J Neurochem. 2005;94:405–416. doi: 10.1111/j.1471-4159.2005.03216.x. [DOI] [PubMed] [Google Scholar]
- Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]