Abstract
Regeneration of the lens in newts is quite a unique process. The lens is removed in its entirety and regeneration ensues from the pigment epithelial cells of the dorsal iris via transdifferentiation. The same type of cells from the ventral iris are not capable of regenerating a lens. It is, thus, expected that differences between dorsal and ventral iris during the process of regeneration might provide important clues pertaining to the mechanism of regeneration. In this paper, we employed next generation RNA-seq to determine gene expression patterns during lens regeneration in Notophthalmus viridescens. The expression of more than 38,000 transcripts was compared between dorsal and ventral iris. Although very few genes were found to be dorsal- or ventral-specific, certain groups of genes were up-regulated specifically in the dorsal iris. These genes are involved in cell cycle, gene regulation, cytoskeleton and immune response. In addition, the expression of six highly regulated genes, TBX5, FGF10, UNC5B, VAX2, NR2F5, and NTN1, was verified using qRT-PCR. These graded gene expression patterns provide insight into the mechanism of lens regeneration, the markers that are specific to dorsal or ventral iris, and layout a map for future studies in the field.
Introduction
Amphibians, especially newts, possess regenerative capabilities that are missing in higher vertebrates. Newts can regenerate their limbs, brain, heart, tail with spinal cord and other tissues. Interestingly, newts can also regenerate the lens after its complete removal (lentectomy). This system provides many advantages for regenerative studies because the whole organ (lens) is being removed. Lens regeneration occurs from the iris by a process that involves the transdifferentiation of pigmented epithelial cells (PECs) to lens cells. Another interesting aspect of this process is that lens regeneration occurs only from the dorsal and never from the ventral iris. This allows the use of the ventral iris as a natural non-regenerative control in lens regeneration experiments [1], [2], [3].
Two major hallmarks of lens regeneration are the re-entry of the cell cycle 4 days post-lentectomy (dpl) and the formation of a dedifferentiated vesicle 8 dpl [4]. Interestingly, both dorsal and ventral iris cells re-enter the cell cycle [5]. In the past, limited expression studies, either using individual gene probes or small-scale microarray analysis have indicated that dorsal and ventral irises show no major differences in gene expression. In other words, most of the examined genes were expressed in both irises. Thus, to date no clear expression pattern has emerged to account for the ability of the dorsal iris to be the source of the regenerating lens. More recently a microarray analysis during early stages of lens regeneration was performed. In that study expression in 1, 3 and 5 dpl from the dorsal or the ventral iris was compared with the corresponding intact iris (0 day). While that study indicated regulation in genes related to DNA repair, extracellular matrix and redox homeostasis, direct comparisons between gene expression in dorsal and ventral iris could not be assessed. Thus, a direct comparison of transcriptomes was needed to delineate global gene expression differences between dorsal and ventral iris during lens regeneration in newts [6], [7].
Next-generation high-throughput techniques allow transcriptome analysis based on de novo assemblies, making them extremely useful for non-model systems like the newt. Here, we investigate transcriptional changes during newt lens regeneration in an attempt to identify patterns that provide clues for the ability of dorsal iris but not the ventral to transdifferentiate. We focused on 4 dpl and 8 dpl for both dorsal and ventral iris as these time points are crucial stages for lens regeneration because these time points encompass the events of cell cycle re-entry and dedifferentiation. For RNA-seq we used a de novo assembled trancriptome making use of short Illumina and longer 454 and Sanger reads [8].
Here we report and for the first time the expression of more than 38,000 annotated transcripts in dorsal and ventral iris during lens regeneration. The analysis has been focused on the quantitative and qualitative differences between dorsal and ventral iris of those transcripts. We found very few genes to be dorsal or ventral iris-specific. However, certain cohorts of genes grouped according to their function were found to be preferentially up-regulated in the dorsal iris. Genes involved in the cell cycle, transcriptional apparatus, cytoskeleton and immune response are among those with much higher expression in the dorsal than the ventral iris. This graded expression might provide robust regulation that allows the dorsal iris to “win” over the ventral iris.
Methods
Animals - Lentectomy
Handle and operations on Notophthalmus viridescens have been described previously [6]. Briefly, newts were purchased from Charles Sullivan Inc. Newt Farm. Newts were anesthetized in 0.1%(w/v) ethyl-3-aminobenzoate methanesulfonic acid (MS222; Sigma) in phosphate buffered saline. Lentectomy was performed using a scalpel to incise the cornea and tweezers to pull out the lens through the incision. For the present study newts were kept for 4 and 8 dpl before tissue harvest.
Ethics Statement
All procedures involving animals were approved by the University of Dayton Institutional Animal Care and Use Committee (IACUC; Protocol ID: 011-02). All surgical procedures were performed in anesthetized with MS222 newts. All appropriate procedures were used in order to alleviate pain and distress while working with newts.
Tissue Harvest and RNA Extraction for qRT-PCR
4 or 8 dpl newts were anesthetized in MS222. Whole eye balls were removed and placed in dishes filled with RNAlater® Solution (Applied Biosciences). Using fine scissors and tweezers, eye balls were dissected first by separating the anterior from the posterior part (Figure 1A) and then by removing remaining neural retina and the ciliary body from the anterior part (Figure 1C). Dorsal or ventral iris sectors were collected in approximately 135° of the whole iris leaving out a board area between dorsal and ventral side which has a black-colored pigmentation (Figure 1B). Dorsal and ventral sectors were then collected in microcentrifuge tubes filled with RNAlater® Solution. The tubes were briefly centrifuged and RNAlater® Solution was completely removed. RNA extraction was performed following TRIzol® Reagent protocol (Applied Biosciences) for 500 µl of reagent or the aqueous phase was transferred to RNA Clean & Concentrator™ (Zymo Research) columns and the recommended protocol was followed. Quality of isolated RNA was determined by Nanodrop 2000 spectrophotometer (Thermo Scientific). Good quality samples had A260/A280 ratio greater than 2 and a peak at 260 nm.
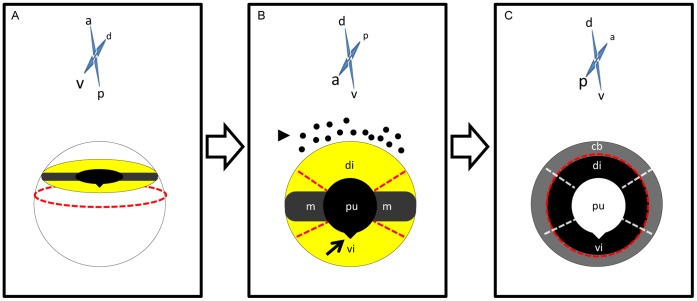
Figure 1. Diagram for collecting iris pieces.
A. Whole eye ball with anterior side facing up and ventral facing the screen. Iris appears in the anterior side. Red dashed line indicates the plane that anterior and posterior sides are separated. B. Anterior view of a newt’s anterior part separated previously. Arrow head indicates black pigments present in the dorsal side of the eye. Arrow indicates the v-shaped pupil in the ventral side. These marks are indicative of the dorsoventral axis of the iris. Red dash lines indicate the separation of dorsal and ventral iris pieces performed while in the anterior view of the eye. C. Posterior view of a newt’s anterior part separated previously. Red dash lines indicate the separation of ciliary body and iris performed in this view. Transparent white dash lines indicate the separation of dorsal and ventral iris sectors performed in the anterior view. di: Dorsal iris sectors that have been isolated for the experiment, vi: Ventral iris sectors that have been isolated for the experiment. m: pigmented midline, cb: ciliary body, pu: pupil. Orientation in each panel is indicated above the illustrated eye parts, a: anterior side, d: dorsal side, v: ventral side, p: posterior side.
Reverse Transcription Reaction (RT)
200 ng total RNA was used for RT reactions. First-strand cDNA synthesis kit (GE healthcare) was used following the recommended protocol for oligo(dt) primers. Half the volumes were used for negative RT reaction without using oligo(dt) primers and the samples were incubated for 5 min at 98°C for enzyme inactivation. All the samples had a clear band after Polymerase Chain Reaction (PCR) with RPL27 gene (housekeeping gene). Dorsal samples needed to be positive for TBX5 and negative for VAX2. Ventral samples needed to be positive for VAX2 and negative for TBX5.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
qRT-PCR was performed using iQ™ SYBR® Green Supermix (Bio-Rad) and Bio-Rad iCycler (Bio-Rad) following company’s protocol for 25 µl. Primer specificity was determined using melt curve analysis. An extra cycle of 6 sec was added to genes that were showing detectable signal from primer dimers and the temperature was determined by the melt curve (usually 2–4°C lower than the melting temperature; see methods below). Amplification cycles (Ct) of samples were compared to Ct of a standard curve created by the cDNA of the gene used. Gene expressions were then normalized to the expression of the reference gene (RPL27).
Primers, PCR and qRT-PCR Settings
For the present study the following primers were used (written from 5′ to 3′): TBX5 Forward: CTGCCATGCCAGGGCGGTTG. TBX5 Reverse: GGTCGTGGGCAGGAGGTCCT. VAX2 Forward: TGTGCCAGCGCCACCTAACC. VAX2 Reverse: AGGTCCCCAAGCCGTACCCC. FGF10 Forward: GCTGTGCGTCACCAACTACT. FGF10 Reverse: TTGCTTTCTACGCCCCTCAC. NR2F5 Forward: CGGAACCTGAGCTACACCTG. NR2F5 Reverse: GGGAGATGAACCCCGTCAAG. UNC5B Forward: AGTCCAACCGGGGTGATCCTG. UNC5B Reverse: CATCTCGCTCTTGCCCATCTCC. NTN1 Forward: GGTTGCTCCACCCACTACAG. NTN1 Reverse: ACCATTCTCCAGCCTTGTCAG. RPL27 Forward: ATTTATGAAACCCGGGAAGG. RPL27 Reverse: CCAGGGCATGACTGTAAGGT.
PCR (performed with Premix Taq™ DNA polymerase (TaKaRa)) settings for TBX5∶40 cycles including 95°C for 30 sec, 65°C for 30 sec and 72°C for 30 sec. VAX2∶40 cycles including 95°C for 30 sec, 64°C for 30 sec, 72°C for 30 sec. RPL27∶40 cycles including 95°C for 30 sec, 55°C for 30 sec, 72°C for 30 secs. Last extension was 72°C for 10 mins for all the genes.
qRT-PCR settings for TBX5∶95°C for 3 mins, 40 cycles of 95°C for 30 sec, 65°C for 30 sec, 72°C for 30 sec and 85.5°C for 6 sec. VAX2∶95°C for 3 mins, 40 cycles of 95°C for 30 sec, 64°C for 30 sec, 72°C for 30 sec and 86.5°C for 6 sec. FGF10∶95°C for 3 mins, 40 cycles of 95°C for 30 sec, 57°C for 30 sec, 72°C for 30 sec and 84.5°C for 6 sec. NR2F5∶95°C for 3 mins, 40 cycles of 95°C for 30 sec, 57°C for 30 sec, 72°C for 30 sec. UNC5B: 95°C for 3 mins, 40 cycles of 95°C for 30 sec, 59°C for 30 sec, 72°C for 30 sec and 82°C for 6 sec. NTN1∶95°C for 3 mins, 40 cycles of 95°C for 30 sec, 57°C for 30 sec, 72°C for 30 sec and 82.5°C for 6 sec. RPL27∶95°C for 3 mins, 40 cycles of 95°C for 30 sec, 55°C for 30 sec, 72°C for 30 sec.
Statistical Analysis for qRT-PCR Results
Statistical analysis was performed using two-way analysis of variance (ANOVA) and Student’s t-test for independed samples. Samples were run in triplicates (n = 3). Statistical significance was determined with 95% confidence (p<0.05). Equal variances for student’s t-test were assumed when Levene’s test p value was greater than 0.05.
Newt Transcriptome, Data Mining and Functional Annotation
The newt transcriptome [8] was annotated with the BLAST2GO tool [9] using the nr database. We used a cutoff of (e−10) for sequence assignments, collected annotations and corresponding GO terms [10]. Transcripts were selected depending on their expression and location in the iris as described in Figure 2 for comparing dorsal iris versus ventral iris groups and as described in Figure 4 for comparing day 4 and day 8 groups. Fisher’s exact test corrected for multiple selections (feature available in BLAST2GO tool) was used for the different groups and statistically significant enriched GO terms were identified (FDR <0.05). Transcripts assigned to enriched terms were selected. Human homologues of those transcripts were found using the BLAST tool [11].
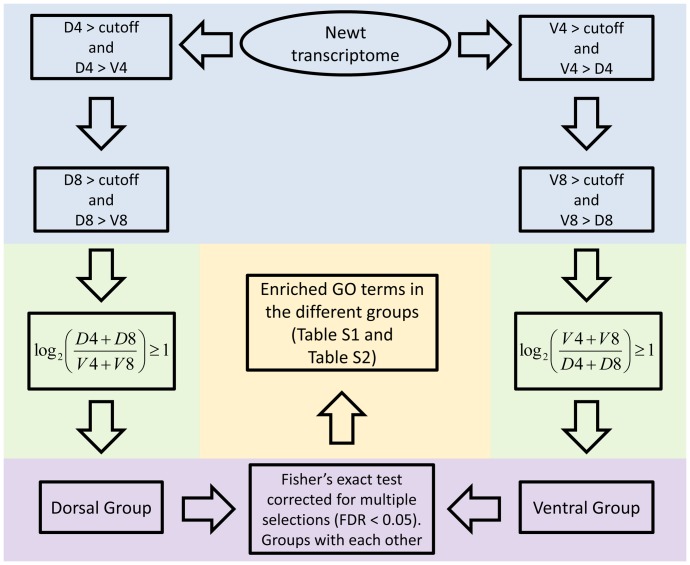
Figure 2. Workflow used to select transcripts for comparison of expression between the dorsal and ventral iris.
Only transcripts expressed above the cutoff (see methods, light blue) and up-regulated at least 2-fold (light green) were included. Fisher’s exact test corrected with multiple selections (FDR <0.05) was used to compare the GO of the two groups (light purple). Enriched GO terms were found (light yellow).
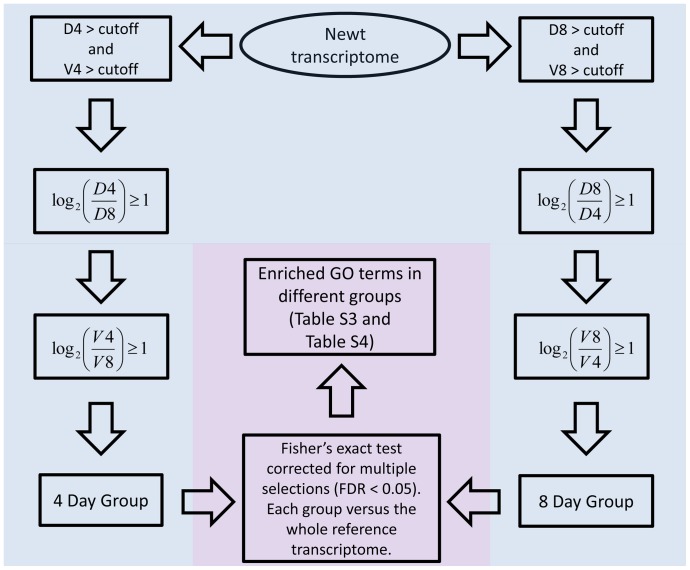
Figure 4. Workflow used to select transcripts for comparison of gene expression 4 and 8 days post-lentectomy.
Only transcripts expressed above the cutoff (see methods) in the day of interest and up-regulated more than 2-fold in both dorsal and ventral iris (light blue) were considered. Fisher’s exact corrected for multiple selections (FDR <0.05) was used to compare the GO of the groups versus the remaining transcripts of the transcriptome. Enriched GO terms were found (light purple).
RNA Expression Calculation
Illumina reads were mapped to the newt transcriptome using BWA [12]. Reads per kilobase per million mapped reads (RPKM) values were calculated for each transcript having at least one unique mapping read. Since intron/exon data are missing for the newt, we used a recently published microarray experiment for RPKM cutoff estimation [6]. We selected transcripts presented on the microarray having a valid spot structure (value for circularity >80%, intensity variation within the spot very small, flagged as valid) but lacking a significant signal during microarray analysis (signal to noise ratio (snr) <1, significant spots are used that have a snr ratio >3). We detected 101 spots to be valid for these parameters. Mapping of array coordinates to the transcriptome resulted in 81 non redundant individual transcripts. We assumed these candidates to be a good estimate for RPKM cutoff selection and calculated the average RPKM value for this transcript set for each investigated timepoint. We received a RPKM value of 0.64 for 4dv, 1.14 for 4dd, 1.13 for 8dv and 1.1 for 8dd. Illumina sequencing raw reads can be found in the NCBI sequence read archive under the accession: ERP001353 [8]. Assembled transcripts and annotation are located in the newtomics database [8], [13].
Results and Discussion
Transcriptome and Analysis Overview
The recently assembled newt transcriptome contains nearly 38,000 annotated genes [8]. After Illumina sequencing, the reads from the dorsal and ventral iris 4 and 8 dpl were mapped to the newt transcriptome and RPKM values were calculated. Since the newt genome is not yet available and reads that map to non-coding areas can not been found, we calculated the RPKM cutoff using micorarrays that were previously used during lens regeneration (see methods for more details). The cutoff in the RPKM values were 0.64 for ventral iris 4 dpl, 1.14 for dorsal iris 4 dpl, 1.13 for ventral iris 8 dpl and 1.1 for dorsal iris 8 dpl. Transcripts found to have RPKM more than the cutoff were considered to be expressed and were taken into account for the comparisons below.
Dorsal Iris Shows Enrichment of Up-regulated Genes Involved in Cell Cycle, Cytoskeleton, Gene Expression and Immune Response
Since the major purpose of this study is to identify patterns of gene expression in the dorsal and ventral iris that might correlate with the ability of the dorsal iris for transdifferentiation, we first looked into transcripts that were regulated either in the dorsal or in the ventral iris at both collection points (4 dpl and 8 dpl). Analysis of the results was performed as outlined in Figure 2.
Interestingly, we only saw a handful of genes that are either exclusively expressed (no reads were mapped to them) in the dorsal or the ventral iris. This finding was not surprising because in the past our laboratory had seen, using limited expression data, that the ventral and dorsal irises show similar patterns of gene expression (see below).
Next, we investigated potential patterns of up-regulated genes. Specifically we asked which genes are consistently up-regulated in the dorsal or the ventral iris (both at 4 and 8 dpl). Fisher’s exact test with multiple testing correction for Gene Ontology (GO) from transcripts that are up-regulated at least 2 fold in dorsal iris 4 and 8 dpl compared to the ventral iris, and transcripts that are up-regulated at least 2 fold in ventral iris 4 and 8 dpl compared to the dorsal iris are shown in Table S1. Interestingly, we found that more GO terms are enriched in the dorsal samples than in the ventral. In particular, GO terms related to cell cycle, regulation of gene expression, cytoskeleton and immune response were overrepresented. In contrast, ventral samples, which generally generated fewer enriched GO terms, primarily showed GO terms related to transposons like RNA-directed DNA polymerase activity, RNA-directed DNA replication and DNA integration.
Although we did not find major qualitative differences in the expression patterns between dorsal and ventral irises, a clear-cut difference in the expression level of several genes were evident: GO terms related to cell cycle (indicating proliferation), cytoskeleton (indicating cell migration and morphological changes), transport of molecules and cytokinesis (indicating altered gene regulation), and immune response (indicating responsiveness to injury) were enriched at the site where lens regeneration commences. Strikingly, in these gene categories 609 genes were up-regulated in the dorsal iris and only 66 in the ventral iris, which will provide much needed insights into the mechanisms that allow the dorsal to “win” over the ventral iris.
Cell Cycle-related Transcripts
Most of the factors related to cell cycle were up-regulated in the dorsal iris when compared to ventral iris (124/7) (Table 1). In detail:
Table 1. List of up-regulated (>2 times) transcripts related to cell cycle*.
| Function | Dorsal | Ventral | |||||
| Mitosis | CNTRL | PLK1 | CLASP1 | RAD21 | NUSAP1 | SMC4 | NCOR1 |
| KIF11 | Asun | STAG1 | KATNB1 | PARD3 | TPX2 | − | |
| TACC3 | CDCA8 | CEP120 | KIF2C | ZWILCH | UBE2E1 | − | |
| TUBB | CENPE | NCAPD2 | NDC80 | NEK3 | ASPM | − | |
| RAB35 | CENPF | NCAPG | SPC24 | SGOL1 | SMC3 | − | |
| ROCK1 | CEP192 | ERCC6L | SPC25 | DSCC1 | AURKB | − | |
| NUP43 | NEDD1 | NCAPG2 | KNTC1 | SKA1 | MAD2L1 | − | |
| NUF2 | NUMA1 | − | − | − | − | − | |
| tumor suppressor | PSMD10 | XRN1 | HBP1 | MDM4 | CENPF | MRPL41 | PTEN |
| CCAR1 | APC | LIN9 | E4F1 | LIN9 | TRRAP | − | |
| Interphase | MNAT1 | CUL4B | CDK2 | MCM3 | MCM7 | LIN9 | NR2F2 |
| CDC25A | CCNA2 | POLA1 | MCM4 | UHRF1 | HECTD3 | − | |
| CDK1 | CCNB1 | POLE | MCM5 | HEXIM1 | MRPL41 | − | |
| CENPF | CCNE2 | MCM2 | MCM6 | DLGAP5 | SMAD6 | − | |
| RCC1 | DSCC1 | E4F1 | APP | CHAF1A | NASP | − | |
| PLK4 | SMARCA4 | TAF2 | RINT1 | CHAF1B | − | − | |
| DNA repair | HERC2 | LIG1 | GTF2H1 | RAD1 | TLK1 | TOP2A | RNF8 |
| CLSPN | FANCI | H2AFX | CHEK1 | − | − | RAD50 | |
| APC/C complex | ANAPC1 | ANAPC7 | FZR1+ | CDC20 | UBE2C | UBE2S | FZR1+ |
| ANAPC13 | CDC27 | − | − | − | − | − | |
| proliferation | BOP1 | CGRRF1 | DST | HBP1 | PHIP | SMARCA2 | VASH1 |
| BMP2 | NR2F2 | FGF10 | GTPBP4 | PRDM4 | STRADA | − | |
| BAX | DTYMK | CDCA7 | − | − | − | − | |
GO:0007049 cell cycle; GO:0022403 cell cycle phase; GO:0000278 mitotic cell cycle; GO:0022402 cell cycle process; GO:0000087 M phase of mitotic cell cycle; GO:0051301 cell division; GO:0000279 M phase; GO:0007067 mitosis.
Transcript names are from their human homologs.
Potential isoforms.
Mitosis: Table 1 shows factors related to all steps of mitosis including spindle formation, microtubule-associated, chromosomal movement and mitosis progression, which are up-regulated in dorsal samples. Interestingly, proteins that act as complexes are concomitantly up-regulated including NDC80 complex, cohesin complex and chromosomal passenger complex (CPC). NDC80 complex is essential for chromosome segregation and spindle formation [14], and NDC80, SPC24, SPC25 and NUF2 are up-regulated. Cohesin complex is required for the packing of the chromosomes [15], and STAG1, RAD21, SMC3, SMC4, PLK1 and SGOL1 are up-regulated. CPC is related to centromere functions during mitosis [16], and AURKB and CDCA8 are up-regulated.
Anaphase promoting complex/cyclosome (APC/C) is a complex that is instrumental for mitosis progression and division [17]. There are 8 APC/C-related transcripts which are up-regulated in dorsal samples: ANAPC1, ANAPC13, ANAPC7, CDC27, FZR1, UBE2S, CDC20 and UBE2C.
Interphase: Factors related to all phases of the interphase are up-regulated in dorsal samples. Cyclins and cyclin-dependent kinases that play a key role in G1/S, G2/M, G1 and S phases are up-regulated including CCNA2 [18], CCNB1 [19], CCNE2 [20], CDK1 [21] and CDK2 [22]. Proteins that act upon cyclins and cyclin-dependent kinases are up-regulated too, including MNAT1 [23], CDC25A [24] and HEXIM1 [25]. Factors that play a role in DNA synthesis during the S phase are up-regulated including POLA1, POLE [26], all the MCM complex (MCM2–7) [27], NASP [28], DSCC1 [29], CHAF1A and CHAF1B [30].
Tumor suppression, proliferation-related, p53/TP53-associated and RB1-associated proteins are up-regulated in dorsal iris. These proteins promote or repress proliferation and cell cycle. It has been previously shown that newt muscle cells re-enter the cell cycle after inactivation of RB. After entering the S phase these cells were resting in G2 phase. So, it was hypothesized that factors should be expressed, which promote the G2/M checkpoint after phosphorylation of RB [31]. Our data suggests that transcripts that regulate the G2/M transition and thereby proliferation include CDC25A, CDK1, CCNB1, the APC/C complex and PLK1.
Factors playing a role in DNA repair need to be seen in the context of cell proliferation and cell cycle, since the failure to repair DNA damage will prevent proliferation. In previous studies using qRT-PCR, it was shown that rad1 is up-regulated at 3 and 5 dpl in the dorsal compared to ventral iris showing activation of robust DNA repair mechanism to prevent accumulation of mutations in dividing cells [6]. A similar pattern emerged in the current RNA-seq analysis: many factors that play a role in DNA repair are at least 2 fold up-regulated in the dorsal compared to the ventral iris. Among them RAD1 along with CHEK1, a kinase that has a key role in cell cycle arrest and apoptosis decisions after DNA damage [32].
Gene Regulation-related Transcripts
It is expected that the process of transdifferentiation is marked by activation and regulation of many genes of the transcriptional apparatus. Indeed, we do find many genes related to gene regulation. Such genes are listed and categorized in Table 2 depending on the level of gene expression that they regulate (transcription, histone modification, RNA, translation). Again the majority of these genes show up-regulation in the dorsal iris (314 transcripts) and only a few show up-regulation in the ventral (39 transcripts).These include a number of proteins that play a role in the general transcriptional apparatus that transcribe all types of RNA including POLR1A, POLR2A, POLR1B, POLR1C, POLR1D, POLR2J, ZNRD1, GTF2H1, GTF2H2, MED12, MED23, MED24, ESF1, BRF1, TWISTNB and some factors from the CCR4-NOT complex (CNOT6, CNOT6L, CNOT4, CHAF1A, CHAF1B) [33]. Transcriptional factors and factors that link them to the basal transcriptional apparatus to regulate specific types or families of genes include ATF6 and CREB3L2, which activate certain genes upon stress [34], [35]. Furthermore, MYCBP and CDCA7 regulate MYC activity [36], [37], CBY1 inhibits Wnt via beta-catenin [38], NR2F2, RARA and RXRA are involved in gene activation after binding to retinoic acid [39], [40], [41], NR2C2 is a receptor that represses retinoic acid receptors [42], CYLD, RELB and NFKB2 are related to NF-kappaB pathway [43], [44], [45], RNF20 is involved in Hox gene activation [46], SMAD6 is a TGF-beta signaling-induced inhibitor of BMP signaling [47], [48], TBX5 is involved in dorsal eye patterning and in limb regeneration [49], [50], NR4A1 is a receptor found to play a role in liver regeneration [51], MDM4 inhibits p53 [52], and TEAD1 is involved in hippo pathway [53], are all up-regulated in the dorsal iris. It is noteworthy that VAX2 is up-regulated in the ventral iris, which is a major player in formation of ventral eye axis during embryogenesis [54]. It is interesting to speculate that the differential regulation of TBX5 and VAX2 in the dorsal and ventral iris demarcates their regenerative ability. TBX5 is expressed over 32-fold higher in the dorsal and VAX2 is expressed 32 fold higher in the ventral iris (see also below).
Table 2. List of up-regulated (>2 times) transcripts related to gene regulation*.
| Function | Dorsal | Ventral | |||||
| Transcription | ATF6 | CNBP | POLR2A | FOXN2 | ZNF3 | SMAD6 | NR2F1 |
| APP | CBY1 | POLR2J | GATAD1 | KLF15 | MYCN | POLR1A+ | |
| ARNTL | NR2F1 | POLR1D | GTF2H1 | KLF7 | ZNFX1 | HIPK2 | |
| ARID4A | CBX1 | DPF2 | GTF2H2 | LANCL2 | NFKB2 | VAX2 | |
| KIAA2018 | CHD7 | E2F3 | RAD54L2 | LCOR | NR4A1 | BRWD1 | |
| BRMS1L | NR2F2 | RNF20 | HEXIM1 | LIMD1 | NR6A1 | MYPOP | |
| BAHD1 | CRTC2 | UHRF1 | HBP1 | LMX1B | NR1H2 | NR2F2 | |
| MYCBP | CREB3L2 | IKBKAP | HMX1 | MEF2B | NR2C2 | NFIB | |
| DENND4A | CYLD | ELP3 | HIPK3 | MED12 | NFYB | NFE2L1 | |
| MNAT1 | POLR1A+ | ELF2 | HIPK2 | MED23 | ESF1 | POLR2H | |
| CDCA7 | ZNRD1 | FOXJ2 | IFT57 | MED24 | NFXL1 | ZBED6 | |
| POLR1C | PUF60 | PHTF2 | PHF6 | PPARG | PWP1 | PRDM2 | |
| PRDM4 | PQBP1 | POLR1B | PHRF1 | PHF12 | PPARA | MLLT3 | |
| XAB2 | BUD31 | RCOR2 | SMARCA4 | RELB | BRF1 | ETS2 | |
| PFDN5 | LBH | RARA | TBX5 | TFAP2B | TCF4 | RAX | |
| CIAO1 | MDM4 | RXRA | BTAF1 | TFAP2C | TFB1M | RGMB | |
| PHB2 | RREB1 | STAT6 | TEAD1 | E4F1 | TAF12 | LEO1 | |
| ZEB1 | ZGPAT | TWISTNB | TRIM33 | TLE3 | TAF2 | SPEN | |
| ZFHX4 | ZBTB5 | WWTR1 | TP53BP1 | TLE4 | TADA1 | SOX6 | |
| ZKSCAN1 | ZIC1 | RLF | SLC30A9 | CNOT6 | CHAF1B | TAF9B | |
| CNOT6L | CNOT4 | CHAF1A | − | − | − | − | |
| HistoneModifications | BRMS1L | RNF20 | KAT5+ | SETDB1 | KDM2A | MLL3 | ARID1A+ |
| ARID4A | TOPORS | MYSM1 | SUV420H1 | KDM2B | NAA16 | KAT5+ | |
| BRPF1 | ENY2 | RBBP7 | KDM6B | KDM1B | NCOR1 | KAT6A | |
| CHD4 | EZH2 | MLL | ARID1A+ | MBTD1 | NCOA3 | MLL2 | |
| DMAP1 | KAT2B | WHSC1L1 | MLL5 | MTA3 | PHF10 | KDM5C | |
| WHSC1 | SMARCA2 | TRIM28 | VPS72 | PHF2 | PHF12 | NCOR1 | |
| RBBP5 | SMARCA4 | TADA3 | YEATS2 | ASXL3 | ZGPAT | − | |
| SIRT6 | SMARCC2 | TRRAP | YY1 | − | − | − | |
| RNA | XRN1 | CPSF3 | HEATR1 | INTS6 | WDR77 | PPWD1 | DHX29 |
| BOP1 | DDX23 | HNRNPAB | AQR+ | MBNL2 | PUF60 | AQR+ | |
| CARHSP1 | DDX46 | INTS1 | CLASRP | MBNL1 | PNPT1 | PPIL3 | |
| CSTF1 | EBNA1BP2 | INTS7+ | CDK11B | PDCD11 | PRPF8 | INTS7+ | |
| GEMIN4 | EXOSC10 | INTS9 | DBR1 | PPIH | XAB2 | − | |
| SF1 | SRSF11 | SRRM1 | RBM28 | RP9 | DDX41 | − | |
| SFSWAP | SNRPA1 | RSRC1 | RBM5 | NOP2 | UTP11L | − | |
| SCAF1 | UTP6 | SRSF12 | SARNP | RBFOX2 | PUM1 | − | |
| SNRPN | THOC2 | THOC6 | TFB1M | TFIP11 | MPHOSPH10 | − | |
| SMNDC1 | THOC5 | − | − | − | − | − | |
| translation | MRPS18B | MRPL14 | GFM1 | EIF2B5 | MRPL17 | GTPBP4 | EIF2B1+ |
| MRPS14 | RPS27L | EEF2K | QRSL1 | MRPL23 | RARS2 | RPS6KB2+ | |
| MRPS5 | CPEB2 | EEF1E1 | IARS | MRPL41 | PDCD4 | − | |
| MRPL15 | DUS3L | EIF2D | MRPL12 | MRPS9 | TRUB2 | − | |
| PUS10 | PUS7 | TRMT61A | TARS | GFM2 | SRP14 | − | |
| TRNAU1AP | RPL3 | NSUN2 | EIF2B1+ | EEFSEC | EIF2B2 | − | |
| DUS2L | RPS6KB2+ | TRMT2A | − | − | − | − | |
| miRNA | DICER1 | MOV10 | PNPT1 | EIF2C3 | TNRC6A | TNRC6C | − |
| Other | NEO1 | AHSA1 | AARSD1 | A2M | DYNC2H1 | SIRT5 | PRKDC |
| TACC3 | PPIH | BAX | AKT2 | EIF4ENIF1 | PCSK1 | HTT+ | |
| CENPF | PPWD1 | FGF10 | TNFSF13B | MYO6 | SERPINE1 | INPPL1+ | |
| C3 | PFDN5 | HTT+ | BMP2 | PEX1 | NDFIP2 | PTEN | |
| CCNA2 | CDK2 | MAPKAPK2 | CCAR1 | SORT1 | NLK | RPS6KA4 | |
| PSEN1 | DISP1 | RRAGC | RIPK1 | MTOR | PHIP | PCM1 | |
| EIF1AD | PPP3CB | RASSF8 | ATP6AP2 | TRIP11 | INPPL1+ | XPO5 | |
| TBL3 | TLR2 | − | − | − | − | − | |
GO: 0010467 gene expression; GO:0010468 regulation of gene expression; GO:0006350 transcription; GO:0045449 regulation of transcription.
Transcript names are from their human homologs.
Potential isoforms.
Factors that are involved in post-transcriptional regulation and in pre-mRNA maturation like splicing and alternative splicing and are up-regulated in the dorsal group include: GEMIN4 [55], DDX23, DDX41, AQR, PPWD1, XAB2, SRRM1, TFIP11 [56], DDX46 [57], CLASRP, CDK11B [58], DBR1 [59], WDR77 [60], MBNL2, MBNL1 [61], PPIH [62], PUF60 [63], PRPF8 [64], RBFOX2 [65], RBM28 [66], RBM5 [67], RSRC1 [68], SRSF12 [69], SRSF11 [70], SFSWAP [71], SCAF1 [72] and SMNDC1 [73], or other ways of pre-mRNA maturation using CSTF1 [74], CPSF3 [75] and EXOSC10 [76]. Factors that play a role in ribosomal RNA maturation include BOP1 [77], EBNA1BP2 [78], HEATR1 [79], PDCD11 [80], UTP11L, UTP6 [81] and MPHOSPH10 [82]. Factors that play role in the stability and transport of the RNA include CARHSP1 [83], PUM1 [84] and part of the TREX complex (THOC2, THOC5, and THOC6) [85].
Protein complexes and related factors involved in histone modifications and are up-regulated in dorsal group include the SIN3A/HDAC1 complex (BRMS1L, ARID4A, TOPORS, RBBP7, NCOR1 and SMARCC2), the NuRD complex (CHD4, RBBP7, MTA3, TRIM28 and ZGPAT) [86],the NuA4 complex (DMAP1, KAT5, TRRAP and VPS72) [87], the PRC2/EED-EZH2 complex (EZH2 and RBBP7) [88], the MLL1/MLL complex (MLL and RBBP5) [89], and the SWI/SNF complex (BAF complexes) (ARID1A, PHF10, SMARCA2, SMARCA4 and SMARCC2) [90], and are all up-regulated in the dorsal iris.
Likewise, most of the transcripts that were identified to act on translation (and miRNA processing and function) are up-regulated in the dorsal iris. Only 1 transcript, eIF2B, was found to be up-regulated in the ventral iris.
Cytoskeleton-related Transcripts
Table 3 shows transcripts that are up-regulated in dorsal iris at least 2 fold than the ventral iris and the opposite. Most of the transcripts are shown to be up-regulated in the dorsal iris (134/18).
Table 3. List of up-regulated (>2 times) transcripts related to cytoskeleton*.
| Function | Dorsal | Ventral | |||||
| microtubule | CNTRL | TUBA1A | CEP120 | DNAH7 | KIF22 | KNTC1 | CLIC5 |
| SSNA1 | BBS2 | CYLD | TUBGCP6 | KIF23 | DYNLT1 | MARK1 | |
| CHEK1 | TUBB | DYNC1I2 | HTT+ | KIF2C | AURKB | HTT+ | |
| CENPF | TUBB4B | DYNC2H1 | IFT57 | KIF14 | LYST | PCM1 | |
| KIF11 | CAMSAP3 | DYNC1LI1 | KATNB1 | KIF20A | MAP1B | KIF1B | |
| PLK1 | CEP170 | CLIP2 | KIAA1279 | KIF13A | MACF1 | KIAA0284 | |
| APC | CLASP1 | DNM2 | KIF13B | NDC80 | MAST2 | NCOR1 | |
| PSKH1 | SMC3 | CCNB1 | CDC27 | TUBB3 | PLK4 | − | |
| ARL2BP | RIF1 | MAD2L1 | CEP350 | TUBD1 | SNTB2 | − | |
| TPX2 | ASPM | NUP85 | CEP192 | ZNF415 | SKA1 | − | |
| CDCA8 | CENPE | RANGAP1 | CBX1 | NEDD1 | DCX | − | |
| NIN | NINL | NUMA1 | NUSAP1 | SHROOM2 | SHROOM3 | − | |
| RAB3IP | AURKA | − | − | − | − | − | |
| actin | WASH1 | CTNNA1 | MYO1E | MYO5A | PLEK2 | VASP | ACTA2 |
| ARPC1B | INTS6 | MYO9A | MYO7A | DIAPH1 | WDR1 | INPPL1+ | |
| ARPC5L | IQGAP1 | MTSS1 | PPP1R9A | SSH2+ | INPPL1+ | SSH2+ | |
| ACTR6 | KLHL3 | MYO1G | MYO1D | RAB3IP | SNTB2 | RDX | |
| SCIN | MYO10 | MYO6 | PDLIM5 | IQGAP2 | SYNE1 | TPM1 | |
| CNN2 | LANCL2 | MYO9B | MACF1 | ROCK1 | − | UTRN+ | |
| Other | MPP1 | GAN | LMNB1 | SYNM | NES | BAG1 | DES |
| FERMT3 | DLGAP5 | NF2 | UTRN+ | TNS1 | UBR4 | RAI14 | |
| GNE | RAPH1 | NF1 | CORO1A | ANK3 | SLC26A5 | SLC4A1 | |
| APP | FRMD6 | TRIB2 | PTPN14 | SLC30A9 | STOML2 | MICALL2 | |
| PSEN1 | − | − | − | − | − | − | |
GO:0005856 cytoskeleton; GO:0015630 microtubule cytoskeleton; GO:0044430 cytoskeletal part.
Transcript names are from their human homologs.
Potential isoforms.
Microtubules-associated: In this category some of the transcripts are related to the cell cycle like in spindle formation and chromosome movement and we have discussed them previously. Other transcripts that are up-regulated in the dorsal group have a role during signal transduction: APC negatively regulates Wnt signaling [91], MACF1 positively regulates Wnt signaling [92], CYLD and MAST2 positively regulates NF-kappaB pathway [93]. Transcripts involved in microtubule organization and stability include CAMSAP3 [94], CEP170 [95], CLASP1 [96], KATNB1 [97], KIAA1279 [98], MAP1B [99], NIN [100], NINL [101] and NUSAP1 [102]. Transcripts involved in transport of molecules include DYNC1I2 [103], DYNC1LI1 [104], DYNC2H1 [105], DNM2 [106], HTT [107], LYST [108], DYNLT1 [109] and PSKH1 [110], in cilia formation include BBS2 [111], DNAH7 [112] and IFT57 [113], in cell shape and movement include ELMO2 [114] and GAN [115].
Actin-related: Transcripts up-regulated in the dorsal group and included in this category play roles in actin polymerization and organization in order to support cell shape, movement and cell adhesion with the extracellular matrix, and the linkage of actin with other proteins in order to facilitate transport or contraction. WASH1 [116], ARPC1B, ARPC5L [117], SSH2 [118] SCIN [119], IQGAP1 [120], PPP1R9A [121] and DIAPH1 [122] are playing roles in actin polymerization and organization. CNN2, MYO10, MYO1E, MYO9A, MYO1G, MYO6, MYO9B, MYO7A, MYO1D and ROCK1 are involved in contraction [123], [124]. CTNNA1 and MTSS1 are related to cell adhesion with the extracellular matrix and cell-cell contact [125], [126]. KLHL3 and MYO5A are playing roles in molecular transport [127], [128]. PLEK2 and VASP are related to cell movement [129], [130] and SYNE1 and WDR1 are linking cytoskeleton with other proteins [131], [132].
In addition to factors that are actin or tubulin-related, Table 3 shows other proteins that are related to cell adhesion, movement and linkage of plasma membrane with cytoskeleton that are up-regulated in the dorsal iris. For lens regeneration, cell adhesion and locomotion is very important since PECs need to change their environmental behavior to transdifferentiate and change their cell fate. Previous studies have found that extracellular matrix is being remodeled and matrix metalloproteinases are up-regulated already 1 day post-lentectomy to prepare the environment for the onset of lens regeneration [6]. Our data clearly show changes in the molecules that determine the interaction of PECs with the environment and remodeling of cytoskeleton components and networks of PECs. Another interesting aspect is that many factors involved in tumor metastasis are up-regulated in the dorsal iris which indicates a role of these molecules at the onset of cell locomotion.
Immunity-related Transcripts
Most of the transcripts related to immune response are up-regulated in the dorsal iris samples (Table 4). Only 2 transcripts were up-regulated in the ventral iris versus 37 that were up-regulated in the dorsal iris. Factors in this category regulate NF-kappaB activity among others and include SIVA1 [133], CHUK, NFKB2, TLR7 [134] and RELB. Factors involved in immune cells activation and migration include TNFSF13B [135], CD97 [136], GPR183 [137], ENPP2 [138], DCLRE1C [139] and TLR2 [140]. Complement component -related transcripts include C1S, C3, C1QB and C1QBP and other factors involved in cytokine secretion and inflammation include CCL5, DDX58, STAT6 and ZEB1.
Table 4. List of up-regulated (>2 times) transcripts related to immune response*.
| Dorsal | Ventral | |||||||
| A2M | TNFSF13B | TUBB4B | CD97 | CCL5 | C3 | C1QBP | DDR1 | TIMM50 |
| SIVA1 | TUBB | CTNNBL1 | CADM1 | C1S | C1QB | CHUK | TOPORS | INPPL1+ |
| RELB | ENPP2 | STAT6 | PPP3CB | INPPL1+ | NCF2 | GAB2 | SMAD6 | − |
| SYK | FCN1 | TLR2 | RARA | PSEN1 | NFKB2 | LYST | DDX58 | − |
| ZEB1 | GPR183 | TLR7 | INPP5D | DCLRE1C | − | − | − | − |
GO:0006955 immune response.
Transcript names are from their human homologs.
Potential isoforms.
The role of immune response and its involvement in the initiation of regeneration has been extensively investigated in the past [141], [142]. It has been hypothesized that molecules involved in the regulation of the immune response have a novel role in regeneration or that the immune response itself is crucial for regeneration. The issue has not been settled yet. Nevertheless, complement components seem to be important for liver regeneration [143] and have also found to be expressed in limb and lens regeneration [144], [145]. The present results provide strong evidence of a crucial role of injury response in regeneration, which needs to be investigated further.
Transposon-related Transcripts
Interestingly, transposons are the only transcripts that are enriched in ventral compared to dorsal samples (Table S2). Transposons have many types and they do not have an assigned biological function. They can be transcribed, reverse-transcribed and integrated back to the genome (retrotransposons) or not. So far, we have no specific role of transposons in regeneration (or rather, inhibition of it?). Since transposon-related transcripts are enriched in ventral samples it would be interesting to learn more about a specific role in repressing specific programs.
Highly Regulated Transcripts
Summing up, we have identified patterns of gene expression that are predominant in the dorsal iris. Genes that are involved in cell cycle, gene regulation, cytoskeleton and immune response show a graded expression along the dorsal/ventral iris. Thus, our study is the first to show how the dorsal iris differs from the ventral iris, and how specific patterns of gene expression correlates with the dorsal iris’ regenerative ability. Comparisons comprised two critical time points, 4 and 8 dpl. Interestingly, comparison of gene expression patterns at each time point separately, recapitulates our primary finding that most transcripts of these gene categories are up-regulated at 4 dpl in the dorsal iris in comparison to 4 dpl in the ventral iris, or in 8 dpl in the dorsal iris in relation to 8 dpl in the ventral iris. In Tables 5 and 6 we show a selected group of genes to exemplify this point. The tables also allow a view on the top regulated transcripts. It becomes clear that a few of them are either dorsal-specific or ventral-specific. Only 3 transcripts were found to be exclusively present in the dorsal iris. These transcripts correspond to protein-1 like (ras associated and pleckstrin domains-containing), transmembrane protein 185A-like (TMEM family) and to chromatin assembly factor 1 (CAF1). Other transcripts that show very high expression in dorsal iris were TBX5, TMEM185A, E3 ubiquitin-protein ligase HERC2-like (HERC2) (>32 times), TMEM116, ephrin–B2, and netrin receptor (UNC5B) (>16 times). In the ventral iris, except transposons, we find that netrin-1 (NTN1), nuclear receptor 2F5-like (NR2F5), and VAX2 are expressed 32 times higher than in the dorsal iris. The function of TBX5 and VAX2 were discussed above, but it is interesting to note here that they might provide a dorsal or ventral identity to the adult iris. Currently, it is not known to what extent these genes control regeneration but functional assays will settle this issue. Nevertheless, TBX5 and VAX2 can be used as markers for dorsal and ventral iris, respectively. Interestingly, we find NTN1 in the ventral iris but its receptor (UNC5B) in the dorsal iris. Likewise, we find ephrin-B2 in the dorsal and its receptor in the ventral iris, which might reveal a so-far unsuspected communication between dorsal and ventral iris. Despite its role in axon guidance, NTN1 has also been shown to inhibit leukocyte migration. Thus, NTN1 up-regulation might protect injured tissues [146]. UNC5B is responsible for apoptosis and because NTN1 is up-regulated by p53 it is considered as an oncogene [147]. Ephrin receptors activated by ephrins have been shown to inhibit signaling by oncogenes. The case of TMEM proteins is interesting as well. Even though not much is known for TMEM185A and TMEM116, TMEM16F is known to form a Ca2+-activated channel, which plays a role in blood coagulation that is also mediated by thrombin activation [148]. In turn, blood coagulation has been implicated in the induction of lens regeneration from the dorsal iris [142].
Table 5. A selected list of 50 transcripts highly up-regulated in the dorsal iris.
| transcript ID | Annotation (Blastx against nr) | v4 | d4 | v8 | d8 | log2Fc4 | log2Fc8 | log2Fc |
| transcript114225 | LOW QUALITY PROTEIN: ras-associated and pleckstrin homology domains-containing protein 1-like [Xenopus (Silurana) tropicalis]# | 0.000 | 2.987 | 0.000 | 1.296 | #DIV/0! | #DIV/0! | ###### |
| transcript89311 | transmembrane protein 185A-like [Anolis carolinensis]# | 0.000 | 6.490 | 0.000 | 1.292 | #DIV/0! | #DIV/0! | ###### |
| transcript53947 | similar to Chromatin assembly factor 1 subunit B [Canis familiaris]# | 0.000 | 5.182 | 0.000 | 1.986 | #DIV/0! | #DIV/0! | ###### |
| transcript75898 | pG1 protein [Lactobacillus jensenii 269-3] # | 0.331 | 5.828 | 0.151 | 19.812 | 4.140 | 7.038 | 5.735 |
| transcript41516 | LOW QUALITY PROTEIN: probable E3 ubiquitin-protein ligase HERC2-like [Xenopus (Silurana) tropicalis]# | 0.000 | 4.070 | 0.193 | 2.527 | #DIV/0! | 3.713 | 5.098 |
| transcript28206 | regeneration blastema forelimb-specific Tbx [Notophthalmus viridescens]# | 0.272 | 4.549 | 0.248 | 12.007 | 4.062 | 5.595 | 4.990 |
| transcript14449 | similar to TMEM116 protein [Gallus gallus]# | 0.335 | 1.492 | 0.485 | 20.092 | 2.156 | 5.373 | 4.719 |
| transcript12545 | hypothetical protein LOC432274 [Xenopus laevis] | 0.000 | 3.858 | 0.253 | 1.988 | #DIV/0! | 2.976 | 4.532 |
| transcript63962 | hypothetical protein LOC495396 [Xenopus laevis] | 0.290 | 4.756 | 0.000 | 1.765 | 4.038 | #DIV/0! | 4.493 |
| transcript80306 | ephrin-B2 [Taeniopygia guttata]# | 0.302 | 4.602 | 0.358 | 8.955 | 3.930 | 4.645 | 4.361 |
| transcript87396 | programmed cell death 2 [Xenopus laevis] | 0.140 | 2.293 | 0.127 | 3.168 | 4.038 | 4.637 | 4.354 |
| transcript89253 | transmembrane protein 116-like [Meleagris gallopavo]# | 0.163 | 1.691 | 0.653 | 14.078 | 3.374 | 4.430 | 4.272 |
| transcript59308 | DEP domain-containing protein 1B-like isoform 1 [Nomascus leucogenys]# | 0.106 | 3.145 | 0.194 | 2.270 | 4.886 | 3.548 | 4.172 |
| transcript15984 | nuclear pore membrane glycoprotein 210 precursor [Mus musculus]# | 0.000 | 1.977 | 0.183 | 1.155 | #DIV/0! | 2.658 | 4.097 |
| transcript88594 | similar to Thimet oligopeptidase [Canis familiaris]# | 0.000 | 3.005 | 0.298 | 1.991 | #DIV/0! | 2.740 | 4.068 |
| transcript82136 | WD repeat and FYVE domain-containing protein 3 isoform 1 [Monodelphis domestica]# | 0.000 | 2.541 | 0.252 | 1.684 | #DIV/0! | 2.740 | 4.068 |
| transcript92162 | netrin receptor UNC5B-like [Xenopus (Silurana) tropicalis]# | 0.000 | 2.333 | 0.476 | 5.587 | #DIV/0! | 3.552 | 4.056 |
| transcript89179 | apoptosis-inducing factor 2-like [Anolis carolinensis]# | 0.000 | 4.743 | 0.387 | 1.246 | #DIV/0! | 1.686 | 3.951 |
| transcript22057 | dihydroxyacetone kinase 2 [Taeniopygia guttata]# | 0.000 | 2.147 | 0.282 | 2.094 | #DIV/0! | 2.892 | 3.911 |
| transcript88286 | lysophospholipid acyltransferase 5-like [Xenopus (Silurana) tropicalis]# | 0.132 | 2.776 | 0.181 | 1.922 | 4.394 | 3.412 | 3.909 |
| transcript103122 | LOW QUALITY PROTEIN: laminin subunit alpha-4-like [Xenopus (Silurana) tropicalis]# | 0.000 | 5.539 | 0.513 | 2.030 | #DIV/0! | 1.985 | 3.884 |
| transcript57122 | calmodulin-regulated spectrin-associated protein 3 [Danio rerio]# | 0.000 | 4.118 | 0.497 | 2.953 | #DIV/0! | 2.570 | 3.830 |
| transcript65968 | biphenyl hydrolase-like (serine hydrolase) [Xenopus laevis]# | 0.000 | 3.044 | 0.470 | 3.573 | #DIV/0! | 2.928 | 3.817 |
| transcript87765 | ras and Rab interactor 2-like [Meleagris gallopavo]# | 0.278 | 4.391 | 0.127 | 1.320 | 3.979 | 3.378 | 3.816 |
| transcript115898 | serine/threonine-protein kinase PLK4 [Xenopus (Silurana) tropicalis]# | 0.000 | 3.900 | 0.467 | 2.426 | #DIV/0! | 2.378 | 3.761 |
| transcript105338 | epithelial cell transforming sequence 2 oncogene [Xenopus (Silurana) tropicalis]# | 0.000 | 2.945 | 0.303 | 1.124 | #DIV/0! | 1.892 | 3.748 |
| transcript55938 | hypothetical protein [Gallus gallus]# | 0.000 | 7.267 | 0.811 | 3.277 | #DIV/0! | 2.015 | 3.701 |
| transcript23291 | LOW QUALITY PROTEIN: serine/threonine-protein kinase WNK1-like [Anolis carolinensis]# | 0.210 | 3.866 | 0.240 | 1.850 | 4.201 | 2.949 | 3.668 |
| transcript93294 | 39S ribosomal protein L15, mitochondrial precursor [Xenopus laevis]# | 0.155 | 4.783 | 0.353 | 1.574 | 4.948 | 2.155 | 3.645 |
| transcript11364 | glycerol kinase [Candidatus Liberibacter americanus]# | 4.046 | 7.288 | 0.580 | 50.265 | 0.849 | 6.436 | 3.637 |
| transcript111723 | adenylate cyclase type 6 isoform 2 [Equus caballus]# | 0.285 | 7.856 | 0.519 | 1.350 | 4.786 | 1.378 | 3.517 |
| transcript63503 | coiled-coil domain-containing protein 85C-like [Anolis carolinensis]# | 0.000 | 3.369 | 0.567 | 3.051 | #DIV/0! | 2.428 | 3.501 |
| transcript56063 | hypothetical protein RAYM_09754 [Riemerella anatipestifer RA-YM]# | 1.421 | 22.491 | 0.678 | 1.115 | 3.984 | 0.719 | 3.492 |
| transcript63410 | signal peptide, CUB and EGF-like domain-containing protein 3 [Monodelphis domestica]# | 0.000 | 2.531 | 0.479 | 2.609 | #DIV/0! | 2.445 | 3.423 |
| transcript59917 | cell cycle regulator Mat89Bb homolog [Sus scrofa]# | 0.000 | 8.811 | 1.121 | 3.103 | #DIV/0! | 1.469 | 3.409 |
| transcript95772 | hypothetical protein [Gallus gallus]# | 0.113 | 2.902 | 0.413 | 2.646 | 4.679 | 2.679 | 3.398 |
| transcript23822 | UPF0679 protein C14orf101-like [Sus scrofa]# | 0.000 | 6.341 | 0.880 | 2.745 | #DIV/0! | 1.641 | 3.368 |
| transcript109593 | mesoderm development candidate 1 [Taeniopygia guttata]# | 0.261 | 3.091 | 0.298 | 2.522 | 3.564 | 3.081 | 3.327 |
| transcript84524 | unnamed protein product [Tetraodon nigroviridis]# | 0.000 | 1.879 | 0.356 | 1.497 | #DIV/0! | 2.073 | 3.246 |
| transcript25525 | fas apoptotic inhibitory molecule 1-like [Anolis carolinensis]# | 0.000 | 7.291 | 0.973 | 1.763 | #DIV/0! | 0.857 | 3.218 |
| transcript47004 | LOW QUALITY PROTEIN: matrix-remodeling-associated protein 5-like [Pongo abelii]# | 0.000 | 4.089 | 0.690 | 2.278 | #DIV/0! | 1.722 | 3.205 |
| transcript83773 | hypothetical protein [Ornithorhynchus anatinus]# | 0.205 | 6.858 | 0.933 | 3.327 | 5.066 | 1.833 | 3.162 |
| transcript80809 | HIV-1 tat interactive protein [Danio rerio]# | 0.221 | 3.551 | 0.402 | 2.017 | 4.009 | 2.325 | 3.160 |
| transcript101040 | LOW QUALITY PROTEIN: protein NEDD1-like [Anolis carolinensis]# | 0.000 | 2.837 | 0.525 | 1.755 | #DIV/0! | 1.740 | 3.128 |
| transcript83315 | ras GTPase-activating-like protein IQGAP2 [Xenopus (Silurana) tropicalis]# | 0.000 | 3.094 | 0.537 | 1.424 | #DIV/0! | 1.407 | 3.073 |
| transcript48784 | 30S ribosomal protein S14 [Chryseobacterium gleum ATCC 35910]# | 0.761 | 10.280 | 0.644 | 1.509 | 3.756 | 1.228 | 3.069 |
| transcript19175 | hyaluronan synthase 2-like [Anolis carolinensis]# | 0.205 | 7.412 | 1.975 | 10.789 | 5.175 | 2.450 | 3.062 |
| transcript90710 | vaccinia related kinase 1 [Xenopus laevis]# | 0.311 | 2.960 | 0.213 | 1.315 | 3.252 | 2.629 | 3.030 |
| transcript119283 | neurobeachin-like protein 1-like [Anolis carolinensis]# | 0.000 | 5.855 | 0.889 | 1.320 | #DIV/0! | 0.570 | 3.013 |
| transcript104553 | exocyst complex component 1-like isoform 1 [Anolis carolinensis]# | 0.29 | 4.137 | 0.47 | 1.884 | 3.8202 | 2.011 | 2.985 |
v4: RPKM value of ventral iris 4 dpl, d4: RPKM value of dorsal iris 4 dpl, v8: RPKM value of ventral 8 dpl, d8: RPKM value of dorsal iris 8 dpl, log2Fc4: fold expression at 4 dpl between dorsal and ventral iris, log2Fc8: fold expression at 8 dpl between dorsal and ventral iris, log2Fc: fold expression between dorsal and ventral iris at both the days.
Table 6. A selected list of 50 transcripts highly up-regulated in the ventral iris.
| transcript ID | Annotation (Blastx against nr) | v4 | d4 | v8 | d8 | log2Fc4 | Log2Fc8 | Log2Fc |
| transcript31815 | netrin-1-like [Xenopus (Silurana) tropicalis]# | 5.052 | 0.141 | 7.897 | 0.066 | −5.166 | −6.892 | −5.965 |
| transcript93602 | retrotransposable element Tf2 155 kDa protein type 1-like, partial [Xenopus (Silurana) tropicalis]# | 2.200 | 0.000 | 3.177 | 0.124 | #NUM! | −4.678 | −5.437 |
| transcript32521 | ventral anterior homeobox 2a-like [Xenopus (Silurana) tropicalis]# | 6.383 | 0.271 | 9.860 | 0.122 | −4.560 | −6.336 | −5.371 |
| transcript26555 | nuclear receptor subfamily 2 group F member 5-like [Xenopus (Silurana) tropicalis]# | 6.299 | 0.378 | 14.165 | 0.158 | −4.057 | −6.482 | −5.253 |
| transcript73289 | hypothetical protein LOC100486110 [Xenopus (Silurana) tropicalis]# | 8.643 | 0.196 | 2.039 | 0.404 | −5.464 | −2.336 | −4.155 |
| transcript99097 | hypothetical protein LOC100485130 [Xenopus (Silurana) tropicalis]# | 19.132 | 0.951 | 3.079 | 0.708 | −4.331 | −2.121 | −3.744 |
| transcript45528 | ORF2-encoded protein [Danio rerio]# | 9.423 | 0.436 | 1.574 | 0.449 | −4.434 | −1.808 | −3.635 |
| transcript14061 | similar to reverse transcriptase-like protein [Strongylocentrotus purpuratus]# | 10.385 | 0.315 | 2.696 | 0.811 | −5.043 | −1.732 | −3.538 |
| transcript66096 | retrotransposable element Tf2 155 kDa protein type 1-like [Danio rerio]# | 6.047 | 0.567 | 3.939 | 0.292 | −3.413 | −3.752 | −3.538 |
| transcript97196 | ATP-binding cassette, sub-family G (WHITE), member 2 [Xenopus laevis]# | 4.516 | 0.424 | 2.059 | 0.218 | −3.413 | −3.237 | −3.356 |
| transcript19896 | CR1-3 [Lycodichthys dearborni]# | 8.640 | 0.481 | 3.807 | 0.744 | −4.167 | −2.356 | −3.345 |
| transcript106073 | sorting nexin-25 [Monodelphis domestica]# | 2.248 | 0.246 | 3.760 | 0.381 | −3.191 | −3.304 | −3.261 |
| transcript67285 | zinc finger protein 850-like [Monodelphis domestica]# | 1.431 | 0.209 | 1.305 | 0.081 | −2.776 | −4.015 | −3.240 |
| transcript8700 | similar to ORF2-encoded protein [Strongylocentrotus purpuratus]# | 6.676 | 0.000 | 3.119 | 1.103 | #NUM! | −1.500 | −3.151 |
| transcript116527 | retrotransposable element Tf2 155 kDa protein type 1-like [Anolis carolinensis]# | 4.011 | 0.465 | 3.874 | 0.479 | −3.109 | −3.015 | −3.062 |
| transcript8488 | hypothetical protein LOC100494670 [Xenopus (Silurana) tropicalis]# | 8.977 | 0.985 | 2.830 | 0.515 | −3.188 | −2.459 | −2.977 |
| transcript44589 | reverse transcriptase [Anguilla japonica]# | 14.281 | 2.024 | 6.449 | 0.616 | −2.819 | −3.387 | −2.973 |
| transcript41350 | hypothetical protein LOC100496475 [Xenopus (Silurana) tropicalis]# | 15.178 | 1.601 | 2.794 | 0.731 | −3.245 | −1.935 | −2.946 |
| transcript56731 | hypothetical protein LOC100494422 [Xenopus (Silurana) tropicalis]# | 12.385 | 1.216 | 3.181 | 0.819 | −3.349 | −1.957 | −2.935 |
| transcript15059 | reverse transcriptase [Danio rerio]# | 16.797 | 1.881 | 3.116 | 0.724 | −3.159 | −2.106 | −2.934 |
| transcript30275 | cleavage stimulation factor subunit 2-like [Homo sapiens]# | 7.765 | 0.900 | 2.351 | 0.442 | −3.109 | −2.411 | −2.914 |
| transcript3811 | pol protein [Salamandra salamandra]# | 15.064 | 1.665 | 5.586 | 1.192 | −3.178 | −2.229 | −2.854 |
| transcript19227 | similar to reverse transcriptase-like protein, partial [Strongylocentrotus purpuratus]# | 8.679 | 0.292 | 2.690 | 1.281 | −4.891 | −1.070 | −2.853 |
| transcript41219 | hypothetical protein LOC100493707 [Xenopus (Silurana) tropicalis]# | 7.539 | 0.842 | 1.994 | 0.485 | −3.162 | −2.040 | −2.845 |
| transcript21599 | SPARC-related modular calcium-binding protein 1-like [Anolis carolinensis]# | 5.688 | 1.029 | 13.814 | 1.709 | −2.467 | −3.015 | −2.832 |
| transcript67066 | phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 2-like [Anolis carolinensis]# | 8.278 | 0.680 | 3.421 | 0.964 | −3.606 | −1.828 | −2.832 |
| transcript40028 | reverse transcriptase-like protein [Salmo salar]# | 8.686 | 0.839 | 4.019 | 0.951 | −3.372 | −2.079 | −2.827 |
| transcript84492 | similar to Family with sequence similarity 116, member A [Ornithorhynchus anatinus]# | 6.781 | 0.431 | 1.496 | 0.741 | −3.975 | −1.015 | −2.820 |
| transcript18114 | Pro-Pol-dUTPase polyprotein [Mus musculus]# | 3.825 | 0.234 | 3.326 | 0.783 | −4.032 | −2.087 | −2.814 |
| transcript29937 | reverse transcriptase [Chironomus tentans]# | 10.035 | 0.840 | 2.872 | 0.999 | −3.578 | −1.523 | −2.811 |
| transcript5182 | similar to LReO_3 [Strongylocentrotus purpuratus]# | 20.890 | 2.839 | 6.570 | 1.098 | −2.879 | −2.582 | −2.802 |
| transcript71146 | putative nuclease HARBI1 [Xenopus (Silurana) tropicalis]# | 47.020 | 5.281 | 10.514 | 2.979 | −3.154 | −1.820 | −2.800 |
| transcript1139 | sushi, von Willebrand factor type A, EGF and pentraxin domain-containing protein 1-like [Xenopus (Silurana) tropicalis]# | 27.032 | 2.851 | 7.238 | 2.099 | −3.245 | −1.786 | −2.791 |
| transcript57867 | endonuclease/reverse transcriptase [Sus scrofa]# | 5.817 | 0.249 | 1.211 | 0.771 | −4.545 | −0.652 | −2.785 |
| transcript78186 | reverse transcriptase-like protein-like [Saccoglossus kowalevskii]# | 15.112 | 1.917 | 2.519 | 0.670 | −2.979 | −1.910 | −2.768 |
| transcript2222 | reverse transcriptase-like protein [Paralichthys olivaceus]# | 14.076 | 1.403 | 3.805 | 1.237 | −3.327 | −1.622 | −2.760 |
| transcript5593 | Myb protein P42POP, isoform CRA_a [Mus musculus]# | 13.400 | 1.539 | 3.506 | 0.967 | −3.122 | −1.858 | −2.754 |
| transcript899 | hypothetical protein TcasGA2_TC015886 [Tribolium castaneum]# | 10.490 | 0.880 | 2.036 | 0.982 | −3.576 | −1.051 | −2.750 |
| transcript51721 | hypothetical protein LOC100488716 [Xenopus (Silurana) tropicalis]# | 4.344 | 0.000 | 1.585 | 0.882 | #NUM! | −0.845 | −2.748 |
| transcript7725 | hypothetical protein LOC734400 [Xenopus laevis]# | 9.043 | 0.907 | 2.479 | 0.815 | −3.317 | −1.605 | −2.742 |
| transcript108620 | hypothetical protein LOC100497892 [Xenopus (Silurana) tropicalis]# | 17.822 | 2.260 | 3.565 | 0.953 | −2.980 | −1.904 | −2.735 |
| transcript70652 | hypothetical protein LOC100492542 [Xenopus (Silurana) tropicalis]# | 7.011 | 0.825 | 2.004 | 0.532 | −3.087 | −1.915 | −2.733 |
| transcript15129 | similar to transposase [Strongylocentrotus purpuratus]# | 7.680 | 0.917 | 4.192 | 0.886 | −3.066 | −2.242 | −2.718 |
| transcript1001 | hypothetical protein LOC100490320 [Xenopus (Silurana) tropicalis]# | 44.292 | 5.312 | 9.908 | 2.944 | −3.060 | −1.751 | −2.715 |
| transcript7879 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 4 b [Xenopus laevis]# | 1521.4 | 153.4 | 130.6 | 98.732 | −3.310 | −0.403 | −2.712 |
| transcript112029 | similar to CR1 Danio rerio 2 reverse transcriptase isoform 3 [Strongylocentrotus purpuratus]# | 17.674 | 1.804 | 3.208 | 1.394 | −3.293 | −1.202 | −2.707 |
| transcript68620 | hypothetical protein LOC100497926 [Xenopus (Silurana) tropicalis]# | 5.412 | 0.790 | 3.565 | 0.611 | −2.776 | −2.545 | −2.680 |
| transcript1148 | hypothetical protein LOC100493982 [Xenopus (Silurana) tropicalis]# | 17.683 | 2.600 | 5.544 | 1.056 | −2.766 | −2.392 | −2.667 |
| transcript338 | reverse transcriptase-like protein [Takifugu rubripes]# | 364.357 | 50.283 | 86.144 | 20.794 | −2.857 | −2.051 | −2.664 |
| transcript1216 | hypothetical protein LOC100495475, partial [Xenopus (Silurana) tropicalis]# | 8.246 | 0.355 | 2.898 | 1.419 | −4.537 | −1.030 | −2.651 |
v4: RPKM value of ventral iris 4 dpl, d4: RPKM value of dorsal iris 4 dpl, v8: RPKM value of ventral 8 dpl, d8: RPKM value of dorsal iris 8 dpl, log2Fc4: fold expression at 4 dpl between dorsal and ventral iris, log2Fc8: fold expression at 8 dpl between dorsal and ventral iris, log2Fc: fold expression between dorsal and ventral iris at both the days.
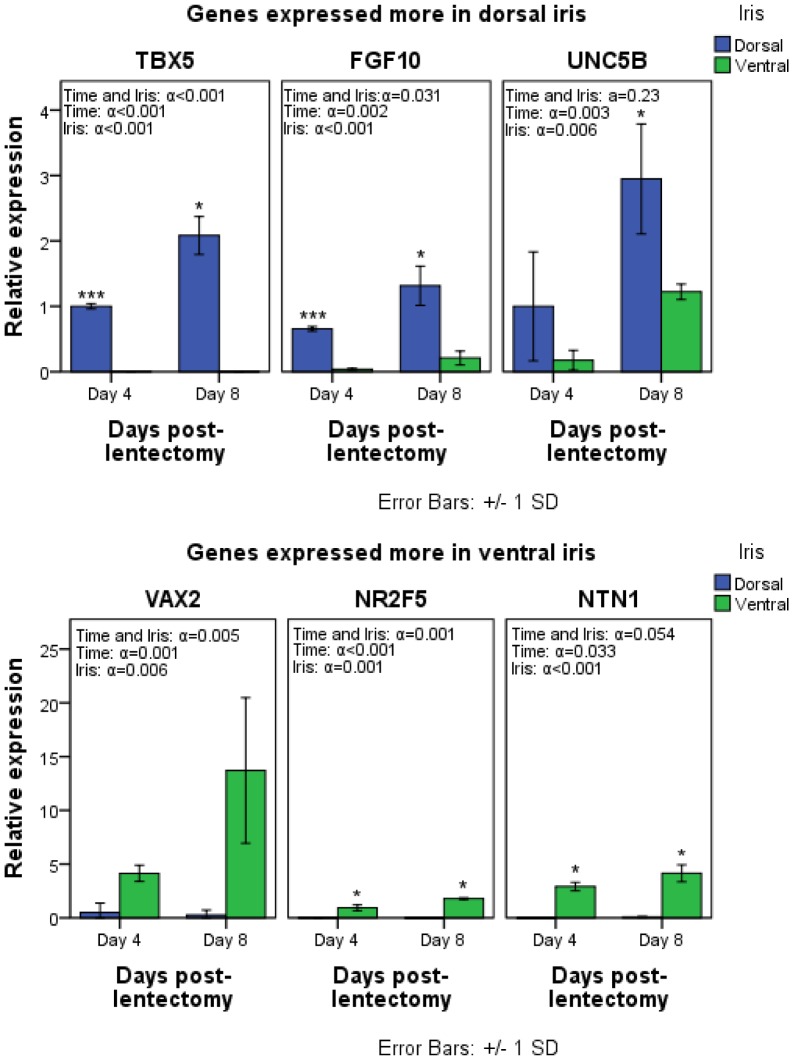
We have also verified some of these patterns via qRT-PCR, which confirm this remarkable difference in expression along the dorsal/ventral iris. The up-regulation of TBX5, FGF10 and UNC5B in the dorsal iris and the up-regulation of VAX2, NR2F5 and NTN1 in the ventral iris are shown in Figure 3. Interestingly, the expression of these genes is also dependent on time, suggesting a potential role of those genes during regeneration (ANOVA; α<0.05). In addition, we further verified the up-regulation of NTN1 in the ventral iris and its receptor (UNC5B) in the dorsal iris.
Figure 3. qRT-PCR expression validation of TBX5, FGF10, UNC5B, VAX2, NR2F5 and NTN1.
Expression of the different genes at the RNA level is indicated as relative expression. Bars indicate standard deviation. Statistical test was performed with two-way ANOVA and Student’s t-test. Asterisks above the bars indicate statistical significance (*: p<0.05, ***: p<0.001) between dorsal and ventral iris samples of the same day.
What Genes are Regulated Specifically at 4 or 8 dpl?
We showed only minor qualitative differences in gene expression between the dorsal and ventral iris. Quantitative changes are dominant and seem to correlate with regenerative abilities. To answer the question whether there are any differences at day 4 (both dorsal and ventral) versus day 8 (dorsal and ventral), which might uncover the importance of timing rather than spatial regulation, we compared the 4 day group with the 8 day group as shown in Figure 4.
Fisher’s exact test with multiple testing corrections for GO from transcripts that are up-regulated in both dorsal and ventral iris 4 dpl compared to 8 dpl versus the whole reference transcripts reveals that DNA polymerase activity and nucleotidyltransferase activity GO terms to be significantly enriched (Table S3). This indicates the initiation of the cell cycle re-entry which has been found to be the major event 4 dpl [149].
Fisher’s exact test with multiple testing corrections for GO from transcripts that are up-regulated in both dorsal and ventral iris 8 dpl compared to 4 dpl versus the whole reference transcripts revealed many interesting patterns (Table S4): as expected GO terms in the cellular component category and related to extracellular matrix are over-represented in the group. Furthermore over-represented terms include extracellular matrix structural constituents and metalloendopeptidase activity in the molecular function category (Table S4). Collagen catabolic process, collagen metabolic process, cell adhesion, extracellular matrix and structure organization, and peptide secretion are over-represented in the biological process category (Table S4). In addition, many of the GO terms in the biological process category related to differentiation, movement, development and patterning are over-represented in the group. Finally, many GO terms that are over-represented in the group are related to macromolecule transport and synthesis. These results indicate active remodeling, transcription and metabolism at day 8, which was expected because the process of dedifferentiation and specification of the lens vesicle peaks at this time point.
Conclusion
The transcriptome analysis during lens regeneration revealed much needed and useful information. First, we were able to identify quantitative patterns of gene expression that create gradients along the dorsal/ventral iris. This finding is of particular importance since it establishes a molecular framework that drives the ability of the dorsal iris for lens regeneration. Second, our analysis identified genes that might be critical for the induction of lens regeneration. For the first time, we now know factors that can be studied in functional assays, such as in trangenesis or knockdown [150], [151], to establish their potency in inducing/inhibiting regeneration. In addition, this knowledge allows us to perform comprehensive comparisons to other animal models that lack the ability for lens regeneration, which might unveil fundamental differences and similarities between regenerating and non-regenerating species.
Supporting Information
GO terms that are over-represented in dorsal iris 4 and 8 dpl versus ventral iris 4 and 8 dpl.
(XLSX)
GO terms that are over-represented in ventral iris 4 and 8 dpl versus dorsal iris 4 and 8 dpl.
(XLSX)
GO terms that are over-represented in transcripts commonly up-regulated (>2 fold) in dorsal and ventral iris at 4 dpl compared to 8 dpl versus whole reference transcriptome.
(XLSX)
GO terms that are over-represented in transcripts that are commonly up-regulated (>2 fold) in dorsal and ventral iris 8 dpl compared to 4 dpl versus whole reference transcriptome.
(XLSX)
Funding Statement
This work was supported by National Institutes of Health grant EY10540. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Grogg MW, Call MK, Okamoto M, Vergara MN, Del Rio-Tsonis K, et al. (2005) BMP inhibition-driven regulation of six-3 underlies induction of newt lens regeneration. Nature 438: 858–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Henry JJ, Tsonis PA (2010) Molecular and cellular aspects of amphibian lens regeneration. Prog Retin Eye Res 29: 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baddour JA, Sousounis K, Tsonis PA (2012) Organ repair and regeneration: an overview. Birth Defects Res C Embryo Today 96: 1–29. [DOI] [PubMed] [Google Scholar]
- 4.Eguchi G (1963) Electon microscopic studies on lens regeneration I mechanism of depigmentation of the iris. Embryologia: 45–62.
- 5. Eguchi G, Shingai R (1971) Cellular analysis on localization of lens forming potency in the newt iris epithelium. Dev Growth Differ 13: 337–349. [DOI] [PubMed] [Google Scholar]
- 6. Sousounis K, Michel CS, Bruckskotten M, Maki N, Borchardt T, et al. (2013) A microarray analysis of gene expression patterns during early phases of newt lens regeneration. Mol Vis 19: 135–145. [PMC free article] [PubMed] [Google Scholar]
- 7. Makarev E, Call MK, Grogg MW, Atkinson DL, Milash B, et al. (2007) Gene expression signatures in the newt irises during lens regeneration. FEBS Lett 581: 1865–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Looso M, Preussner J, Sousounis K, Bruckskotten M, Michel CS, et al. (2013) A de novo assembly of the newt transcriptome combined with proteomic validation identifies new protein families expressed during tissue regeneration. Genome Biol 14: R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, et al. (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676. [DOI] [PubMed] [Google Scholar]
- 10. Myhre S, Tveit H, Mollestad T, Laegreid A (2006) Additional gene ontology structure for improved biological reasoning. Bioinformatics 22: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 11. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 12. Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruckskotten M, Looso M, Reinhardt R, Braun T, Borchardt T (2012) Newt-omics: a comprehensive repository for omics data from the newt Notophthalmus viridescens. Nucleic Acids Res 40: D895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tooley J, Stukenberg PT (2011) The Ndc80 complex: integrating the kinetochore's many movements. Chromosome Res 19: 377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta GD, Rizvi SM, Ghosh SK (2012) Cohesin: A guardian of genome integrity. Biochim Biophys Acta 1823: 1324–1342. [DOI] [PubMed] [Google Scholar]
- 16. van der Waal MS, Hengeveld RC, van der Horst A, Lens SM (2012) Cell division control by the Chromosomal Passenger Complex. Exp Cell Res 318: 1407–1420. [DOI] [PubMed] [Google Scholar]
- 17. McLean JR, Chaix D, Ohi MD, Gould KL (2011) State of the APC/C: organization, function, and structure. Crit Rev Biochem Mol Biol 46: 118–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henglein B, Chenivesse X, Wang J, Eick D, Brechot C (1994) Structure and cell cycle-regulated transcription of the human cyclin A gene. Proc Natl Acad Sci U S A 91: 5490–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pines J, Hunter T (1989) Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58: 833–846. [DOI] [PubMed] [Google Scholar]
- 20. Lauper N, Beck AR, Cariou S, Richman L, Hofmann K, et al. (1998) Cyclin E2: a novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene 17: 2637–2643. [DOI] [PubMed] [Google Scholar]
- 21. Draetta G, Beach D (1988) Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell 54: 17–26. [DOI] [PubMed] [Google Scholar]
- 22. Gu Y, Rosenblatt J, Morgan DO (1992) Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J 11: 3995–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tassan JP, Jaquenoud M, Fry AM, Frutiger S, Hughes GJ, et al. (1995) In vitro assembly of a functional human CDK7-cyclin H complex requires MAT1, a novel 36 kDa RING finger protein. EMBO J 14: 5608–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galaktionov K, Beach D (1991) Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell 67: 1181–1194. [DOI] [PubMed] [Google Scholar]
- 25. Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, et al. (2003) Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol Cell 12: 971–982. [DOI] [PubMed] [Google Scholar]
- 26. Rytkonen AK, Vaara M, Nethanel T, Kaufmann G, Sormunen R, et al. (2006) Distinctive activities of DNA polymerases during human DNA replication. FEBS J 273: 2984–3001. [DOI] [PubMed] [Google Scholar]
- 27. Bochman ML, Schwacha A (2009) The Mcm complex: unwinding the mechanism of a replicative helicase. Microbiol Mol Biol Rev 73: 652–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Rand MG, Richardson RT, Zimmerman LJ, Widgren EE (1992) Sequence and localization of human NASP: conservation of a Xenopus histone-binding protein. Dev Biol 154: 37–44. [DOI] [PubMed] [Google Scholar]
- 29. Merkle CJ, Karnitz LM, Henry-Sanchez JT, Chen J (2003) Cloning and characterization of hCTF18, hCTF8, and hDCC1. Human homologs of a Saccharomyces cerevisiae complex involved in sister chromatid cohesion establishment. J Biol Chem 278: 30051–30056. [DOI] [PubMed] [Google Scholar]
- 30. Kaufman PD, Kobayashi R, Kessler N, Stillman B (1995) The p150 and p60 subunits of chromatin assembly factor I: a molecular link between newly synthesized histones and DNA replication. Cell 81: 1105–1114. [DOI] [PubMed] [Google Scholar]
- 31. Tanaka EM, Gann AA, Gates PB, Brockes JP (1997) Newt myotubes reenter the cell cycle by phosphorylation of the retinoblastoma protein. J Cell Biol 136: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, et al. (2000) Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev 14: 1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 33. Collart MA, Panasenko OO (2012) The Ccr4–not complex. Gene 492: 42–53. [DOI] [PubMed] [Google Scholar]
- 34. Ye J, Rawson RB, Komuro R, Chen X, Dave UP, et al. (2000) ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6: 1355–1364. [DOI] [PubMed] [Google Scholar]
- 35. Kondo S, Saito A, Hino S, Murakami T, Ogata M, et al. (2007) BBF2H7, a novel transmembrane bZIP transcription factor, is a new type of endoplasmic reticulum stress transducer. Mol Cell Biol 27: 1716–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guo Q, Xie J, Dang CV, Liu ET, Bishop JM (1998) Identification of a large Myc-binding protein that contains RCC1-like repeats. Proc Natl Acad Sci U S A 95: 9172–9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prescott JE, Osthus RC, Lee LA, Lewis BC, Shim H, et al. (2001) A novel c-Myc-responsive gene, JPO1, participates in neoplastic transformation. J Biol Chem 276: 48276–48284. [DOI] [PubMed] [Google Scholar]
- 38. Takemaru K, Yamaguchi S, Lee YS, Zhang Y, Carthew RW, et al. (2003) Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature 422: 905–909. [DOI] [PubMed] [Google Scholar]
- 39. Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, et al. (2008) Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol 6: e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giguere V, Ong ES, Segui P, Evans RM (1987) Identification of a receptor for the morphogen retinoic acid. Nature 330: 624–629. [DOI] [PubMed] [Google Scholar]
- 41. Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM (1990) Nuclear receptor that identifies a novel retinoic acid response pathway. Nature 345: 224–229. [DOI] [PubMed] [Google Scholar]
- 42. Hirose T, Apfel R, Pfahl M, Jetten AM (1995) The orphan receptor TAK1 acts as a repressor of RAR-, RXR- and T3R-mediated signaling pathways. Biochem Biophys Res Commun 211: 83–91. [DOI] [PubMed] [Google Scholar]
- 43. Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, et al. (2003) CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 424: 793–796. [DOI] [PubMed] [Google Scholar]
- 44. Ruben SM, Klement JF, Coleman TA, Maher M, Chen CH, et al. (1992) I-Rel: a novel rel-related protein that inhibits NF-kappa B transcriptional activity. Genes Dev 6: 745–760. [DOI] [PubMed] [Google Scholar]
- 45. Schmid RM, Perkins ND, Duckett CS, Andrews PC, Nabel GJ (1991) Cloning of an NF-kappa B subunit which stimulates HIV transcription in synergy with p65. Nature 352: 733–736. [DOI] [PubMed] [Google Scholar]
- 46. Zhu B, Zheng Y, Pham AD, Mandal SS, Erdjument-Bromage H, et al. (2005) Monoubiquitination of human histone H2B: the factors involved and their roles in HOX gene regulation. Mol Cell 20: 601–611. [DOI] [PubMed] [Google Scholar]
- 47. Afrakhte M, Moren A, Jossan S, Itoh S, Sampath K, et al. (1998) Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members. Biochem Biophys Res Commun 249: 505–511. [DOI] [PubMed] [Google Scholar]
- 48. Hata A, Lagna G, Massague J, Hemmati-Brivanlou A (1998) Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev 12: 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leconte L, Lecoin L, Martin P, Saule S (2004) Pax6 interacts with cVax and Tbx5 to establish the dorsoventral boundary of the developing eye. J Biol Chem 279: 47272–47277. [DOI] [PubMed] [Google Scholar]
- 50. Khan P, Linkhart B, Simon HG (2002) Different regulation of T-box genes Tbx4 and Tbx5 during limb development and limb regeneration. Dev Biol 250: 383–392. [PubMed] [Google Scholar]
- 51. Scearce LM, Laz TM, Hazel TG, Lau LF, Taub R (1993) RNR-1, a nuclear receptor in the NGFI-B/Nur77 family that is rapidly induced in regenerating liver. J Biol Chem 268: 8855–8861. [PubMed] [Google Scholar]
- 52. Chen L, Gilkes DM, Pan Y, Lane WS, Chen J (2005) ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage. EMBO J 24: 3411–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao B, Ye X, Yu J, Li L, Li W, et al. (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22: 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barbieri AM, Lupo G, Bulfone A, Andreazzoli M, Mariani M, et al. (1999) A homeobox gene, vax2, controls the patterning of the eye dorsoventral axis. Proc Natl Acad Sci U S A 96: 10729–10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Charroux B, Pellizzoni L, Perkinson RA, Yong J, Shevchenko A, et al. (2000) Gemin4. A novel component of the SMN complex that is found in both gems and nucleoli. J Cell Biol 148: 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jurica MS, Licklider LJ, Gygi SR, Grigorieff N, Moore MJ (2002) Purification and characterization of native spliceosomes suitable for three-dimensional structural analysis. RNA 8: 426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Will CL, Urlaub H, Achsel T, Gentzel M, Wilm M, et al. (2002) Characterization of novel SF3b and 17S U2 snRNP proteins, including a human Prp5p homologue and an SF3b DEAD-box protein. EMBO J 21: 4978–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu D, Mayeda A, Trembley JH, Lahti JM, Kidd VJ (2003) CDK11 complexes promote pre-mRNA splicing. J Biol Chem 278: 8623–8629. [DOI] [PubMed] [Google Scholar]
- 59. Kim JW, Kim HC, Kim GM, Yang JM, Boeke JD, et al. (2000) Human RNA lariat debranching enzyme cDNA complements the phenotypes of Saccharomyces cerevisiae dbr1 and Schizosaccharomyces pombe dbr1 mutants. Nucleic Acids Res 28: 3666–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Friesen WJ, Wyce A, Paushkin S, Abel L, Rappsilber J, et al. (2002) A novel WD repeat protein component of the methylosome binds Sm proteins. J Biol Chem 277: 8243–8247. [DOI] [PubMed] [Google Scholar]
- 61. Ho TH, Charlet BN, Poulos MG, Singh G, Swanson MS, et al. (2004) Muscleblind proteins regulate alternative splicing. EMBO J 23: 3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Teigelkamp S, Achsel T, Mundt C, Gothel SF, Cronshagen U, et al. (1998) The 20 kD protein of human [U4/U6.U5] tri-snRNPs is a novel cyclophilin that forms a complex with the U4/U6-specific 60 kD and 90 kD proteins. RNA 4: 127–141. [PMC free article] [PubMed] [Google Scholar]
- 63. Page-McCaw PS, Amonlirdviman K, Sharp PA (1999) PUF60: a novel U2AF65-related splicing activity. RNA 5: 1548–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Luo HR, Moreau GA, Levin N, Moore MJ (1999) The human Prp8 protein is a component of both U2- and U12-dependent spliceosomes. RNA 5: 893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang SC, Ou AC, Park J, Yu F, Yu B, et al. (2012) RBFOX2 promotes protein 4.1R exon 16 selection via U1 snRNP recruitment. Mol Cell Biol 32: 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Damianov A, Kann M, Lane WS, Bindereif A (2006) Human RBM28 protein is a specific nucleolar component of the spliceosomal snRNPs. Biol Chem 387: 1455–1460. [DOI] [PubMed] [Google Scholar]
- 67. Kotlajich MV, Hertel KJ (2008) Death by splicing: tumor suppressor RBM5 freezes splice-site pairing. Mol Cell 32: 162–164. [DOI] [PubMed] [Google Scholar]
- 68. Cazalla D, Newton K, Caceres JF (2005) A novel SR-related protein is required for the second step of Pre-mRNA splicing. Mol Cell Biol 25: 2969–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cowper AE, Caceres JF, Mayeda A, Screaton GR (2001) Serine-arginine (SR) protein-like factors that antagonize authentic SR proteins and regulate alternative splicing. J Biol Chem 276: 48908–48914. [DOI] [PubMed] [Google Scholar]
- 70. Chaudhary N, McMahon C, Blobel G (1991) Primary structure of a human arginine-rich nuclear protein that colocalizes with spliceosome components. Proc Natl Acad Sci U S A 88: 8189–8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sarkissian M, Winne A, Lafyatis R (1996) The mammalian homolog of suppressor-of-white-apricot regulates alternative mRNA splicing of CD45 exon 4 and fibronectin IIICS. J Biol Chem 271: 31106–31114. [DOI] [PubMed] [Google Scholar]
- 72. Scorilas A, Kyriakopoulou L, Katsaros D, Diamandis EP (2001) Cloning of a gene (SR-A1), encoding for a new member of the human Ser/Arg-rich family of pre-mRNA splicing factors: overexpression in aggressive ovarian cancer. Br J Cancer 85: 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Neubauer G, King A, Rappsilber J, Calvio C, Watson M, et al. (1998) Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat Genet 20: 46–50. [DOI] [PubMed] [Google Scholar]
- 74. Takagaki Y, Manley JL (1992) A human polyadenylation factor is a G protein beta-subunit homologue. J Biol Chem 267: 23471–23474. [PubMed] [Google Scholar]
- 75. Ryan K, Calvo O, Manley JL (2004) Evidence that polyadenylation factor CPSF-73 is the mRNA 3' processing endonuclease. RNA 10: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schilders G, van Dijk E, Pruijn GJ (2007) C1D and hMtr4p associate with the human exosome subunit PM/Scl-100 and are involved in pre-rRNA processing. Nucleic Acids Res 35: 2564–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rohrmoser M, Holzel M, Grimm T, Malamoussi A, Harasim T, et al. (2007) Interdependence of Pes1, Bop1, and WDR12 controls nucleolar localization and assembly of the PeBoW complex required for maturation of the 60S ribosomal subunit. Mol Cell Biol 27: 3682–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wade C, Shea KA, Jensen RV, McAlear MA (2001) EBP2 is a member of the yeast RRB regulon, a transcriptionally coregulated set of genes that are required for ribosome and rRNA biosynthesis. Mol Cell Biol 21: 8638–8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Azuma M, Toyama R, Laver E, Dawid IB (2006) Perturbation of rRNA synthesis in the bap28 mutation leads to apoptosis mediated by p53 in the zebrafish central nervous system. J Biol Chem 281: 13309–13316. [DOI] [PubMed] [Google Scholar]
- 80. Sweet T, Yen W, Khalili K, Amini S (2008) Evidence for involvement of NFBP in processing of ribosomal RNA. J Cell Physiol 214: 381–388. [DOI] [PubMed] [Google Scholar]
- 81. Champion EA, Lane BH, Jackrel ME, Regan L, Baserga SJ (2008) A direct interaction between the Utp6 half-a-tetratricopeptide repeat domain and a specific peptide in Utp21 is essential for efficient pre-rRNA processing. Mol Cell Biol 28: 6547–6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Westendorf JM, Konstantinov KN, Wormsley S, Shu MD, Matsumoto-Taniura N, et al. (1998) M phase phosphoprotein 10 is a human U3 small nucleolar ribonucleoprotein component. Mol Biol Cell 9: 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pfeiffer JR, McAvoy BL, Fecteau RE, Deleault KM, Brooks SA (2011) CARHSP1 is required for effective tumor necrosis factor alpha mRNA stabilization and localizes to processing bodies and exosomes. Mol Cell Biol 31: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Spassov DS, Jurecic R (2002) Cloning and comparative sequence analysis of PUM1 and PUM2 genes, human members of the Pumilio family of RNA-binding proteins. Gene 299: 195–204. [DOI] [PubMed] [Google Scholar]
- 85. Katahira J (2012) mRNA export and the TREX complex. Biochim Biophys Acta 1819: 507–513. [DOI] [PubMed] [Google Scholar]
- 86. Denslow SA, Wade PA (2007) The human Mi-2/NuRD complex and gene regulation. Oncogene 26: 5433–5438. [DOI] [PubMed] [Google Scholar]
- 87. Doyon Y, Cote J (2004) The highly conserved and multifunctional NuA4 HAT complex. Curr Opin Genet Dev 14: 147–154. [DOI] [PubMed] [Google Scholar]
- 88. Cao R, Zhang Y (2004) The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev 14: 155–164. [DOI] [PubMed] [Google Scholar]
- 89. Ansari KI, Mandal SS (2010) Mixed lineage leukemia: roles in gene expression, hormone signaling and mRNA processing. FEBS J 277: 1790–1804. [DOI] [PubMed] [Google Scholar]
- 90. Euskirchen G, Auerbach RK, Snyder M (2012) SWI/SNF Chromatin-remodeling Factors: Multiscale Analyses and Diverse Functions. J Biol Chem 287: 30897–30905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Su LK, Vogelstein B, Kinzler KW (1993) Association of the APC tumor suppressor protein with catenins. Science 262: 1734–1737. [DOI] [PubMed] [Google Scholar]
- 92. Chen HJ, Lin CM, Lin CS, Perez-Olle R, Leung CL, et al. (2006) The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes Dev 20: 1933–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhou H, Xiong H, Li H, Plevy SE, Walden PD, et al. (2004) Microtubule-associated serine/threonine kinase-205 kDa and Fc gamma receptor control IL-12 p40 synthesis and NF-kappa B activation. J Immunol 172: 2559–2568. [DOI] [PubMed] [Google Scholar]
- 94. Meng W, Mushika Y, Ichii T, Takeichi M (2008) Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell 135: 948–959. [DOI] [PubMed] [Google Scholar]
- 95. Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, et al. (2005) The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol Biol Cell 16: 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, et al. (2001) Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 104: 923–935. [DOI] [PubMed] [Google Scholar]
- 97. McNally KP, Bazirgan OA, McNally FJ (2000) Two domains of p80 katanin regulate microtubule severing and spindle pole targeting by p60 katanin. J Cell Sci 113 (Pt 9): 1623–1633. [DOI] [PubMed] [Google Scholar]
- 98. Wozniak MJ, Melzer M, Dorner C, Haring HU, Lammers R (2005) The novel protein KBP regulates mitochondria localization by interaction with a kinesin-like protein. BMC Cell Biol 6: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ding J, Liu JJ, Kowal AS, Nardine T, Bhattacharya P, et al. (2002) Microtubule-associated protein 1B: a neuronal binding partner for gigaxonin. J Cell Biol 158: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stillwell EE, Zhou J, Joshi HC (2004) Human ninein is a centrosomal autoantigen recognized by CREST patient sera and plays a regulatory role in microtubule nucleation. Cell Cycle 3: 923–930. [PubMed] [Google Scholar]
- 101. Casenghi M, Meraldi P, Weinhart U, Duncan PI, Korner R, et al. (2003) Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev Cell 5: 113–125. [DOI] [PubMed] [Google Scholar]
- 102. Raemaekers T, Ribbeck K, Beaudouin J, Annaert W, Van Camp M, et al. (2003) NuSAP, a novel microtubule-associated protein involved in mitotic spindle organization. J Cell Biol 162: 1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Vaughan KT, Vallee RB (1995) Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol 131: 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sivaram MV, Wadzinski TL, Redick SD, Manna T, Doxsey SJ (2009) Dynein light intermediate chain 1 is required for progress through the spindle assembly checkpoint. EMBO J 28: 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Merrill AE, Merriman B, Farrington-Rock C, Camacho N, Sebald ET, et al. (2009) Ciliary abnormalities due to defects in the retrograde transport protein DYNC2H1 in short-rib polydactyly syndrome. Am J Hum Genet 84: 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thompson HM, Skop AR, Euteneuer U, Meyer BJ, McNiven MA (2002) The large GTPase dynamin associates with the spindle midzone and is required for cytokinesis. Curr Biol 12: 2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pardo R, Molina-Calavita M, Poizat G, Keryer G, Humbert S, et al. (2010) pARIS-htt: an optimised expression platform to study huntingtin reveals functional domains required for vesicular trafficking. Mol Brain 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Faigle W, Raposo G, Tenza D, Pinet V, Vogt AB, et al. (1998) Deficient peptide loading and MHC class II endosomal sorting in a human genetic immunodeficiency disease: the Chediak-Higashi syndrome. J Cell Biol 141: 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Vlach J, Lipov J, Rumlova M, Veverka V, Lang J, et al. (2008) D-retrovirus morphogenetic switch driven by the targeting signal accessibility to Tctex-1 of dynein. Proc Natl Acad Sci U S A 105: 10565–10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Brede G, Solheim J, Prydz H (2002) PSKH1, a novel splice factor compartment-associated serine kinase. Nucleic Acids Res 30: 5301–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, et al. (2007) A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213. [DOI] [PubMed] [Google Scholar]
- 112. Zhang YJ, O'Neal WK, Randell SH, Blackburn K, Moyer MB, et al. (2002) Identification of dynein heavy chain 7 as an inner arm component of human cilia that is synthesized but not assembled in a case of primary ciliary dyskinesia. J Biol Chem 277: 17906–17915. [DOI] [PubMed] [Google Scholar]
- 113. Krock BL, Perkins BD (2008) The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci 121: 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, et al. (2001) CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 107: 27–41. [DOI] [PubMed] [Google Scholar]
- 115. Ding J, Allen E, Wang W, Valle A, Wu C, et al. (2006) Gene targeting of GAN in mouse causes a toxic accumulation of microtubule-associated protein 8 and impaired retrograde axonal transport. Hum Mol Genet 15: 1451–1463. [DOI] [PubMed] [Google Scholar]
- 116. Gomez TS, Billadeau DD (2009) A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell 17: 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Welch MD, DePace AH, Verma S, Iwamatsu A, Mitchison TJ (1997) The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J Cell Biol 138: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T (2002) Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell 108: 233–246. [DOI] [PubMed] [Google Scholar]
- 119. Chumnarnsilpa S, Lee WL, Nag S, Kannan B, Larsson M, et al. (2009) The crystal structure of the C-terminus of adseverin reveals the actin-binding interface. Proc Natl Acad Sci U S A 106: 13719–13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hart MJ, Callow MG, Souza B, Polakis P (1996) IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J 15: 2997–3005. [PMC free article] [PubMed] [Google Scholar]
- 121. Schuler H, Peti W (2008) Structure-function analysis of the filamentous actin binding domain of the neuronal scaffolding protein spinophilin. FEBS J 275: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bai SW, Herrera-Abreu MT, Rohn JL, Racine V, Tajadura V, et al. (2011) Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biol 9: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Riento K, Ridley AJ (2003) Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4: 446–456. [DOI] [PubMed] [Google Scholar]
- 124. Dantzig JA, Liu TY, Goldman YE (2006) Functional studies of individual myosin molecules. Ann N Y Acad Sci 1080: 1–18. [DOI] [PubMed] [Google Scholar]
- 125. Woodings JA, Sharp SJ, Machesky LM (2003) MIM-B, a putative metastasis suppressor protein, binds to actin and to protein tyrosine phosphatase delta. Biochem J 371: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Smythe WR, Williams JP, Wheelock MJ, Johnson KR, Kaiser LR, et al. (1999) Cadherin and catenin expression in normal human bronchial epithelium and non-small cell lung cancer. Lung Cancer 24: 157–168. [DOI] [PubMed] [Google Scholar]
- 127.Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, et al.. (2012) KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44: 456–460, S451–453. [DOI] [PubMed]
- 128. Strom M, Hume AN, Tarafder AK, Barkagianni E, Seabra MC (2002) A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem 277: 25423–25430. [DOI] [PubMed] [Google Scholar]
- 129. Yoshinaga S, Ohkubo T, Sasaki S, Nuriya M, Ogawa Y, et al. (2012) A Phosphatidylinositol Lipids System, Lamellipodin, and Ena/VASP Regulate Dynamic Morphology of Multipolar Migrating Cells in the Developing Cerebral Cortex. J Neurosci 32: 11643–11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hamaguchi N, Ihara S, Ohdaira T, Nagano H, Iwamatsu A, et al. (2007) Pleckstrin-2 selectively interacts with phosphatidylinositol 3-kinase lipid products and regulates actin organization and cell spreading. Biochem Biophys Res Commun 361: 270–275. [DOI] [PubMed] [Google Scholar]
- 131. Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D (2008) Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp Cell Res 314: 1892–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Oh SH, Adler HJ, Raphael Y, Lomax MI (2002) WDR1 colocalizes with ADF and actin in the normal and noise-damaged chick cochlea. J Comp Neurol 448: 399–409. [DOI] [PubMed] [Google Scholar]
- 133. Gudi R, Barkinge J, Hawkins S, Chu F, Manicassamy S, et al. (2006) Siva-1 negatively regulates NF-kappaB activity: effect on T-cell receptor-mediated activation-induced cell death (AICD). Oncogene 25: 3458–3462. [DOI] [PubMed] [Google Scholar]
- 134. Yu N, Zhang S, Zuo F, Kang K, Guan M, et al. (2009) Cultured human melanocytes express functional toll-like receptors 2–4, 7 and 9. J Dermatol Sci 56: 113–120. [DOI] [PubMed] [Google Scholar]
- 135. Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, et al. (1999) BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 285: 260–263. [DOI] [PubMed] [Google Scholar]
- 136. Gray JX, Haino M, Roth MJ, Maguire JE, Jensen PN, et al. (1996) CD97 is a processed, seven-transmembrane, heterodimeric receptor associated with inflammation. J Immunol 157: 5438–5447. [PubMed] [Google Scholar]
- 137. Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, et al. (2011) Oxysterols direct immune cell migration via EBI2. Nature 475: 524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, et al. (1992) Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem 267: 2524–2529. [PubMed] [Google Scholar]
- 139. Moshous D, Callebaut I, de Chasseval R, Corneo B, Cavazzana-Calvo M, et al. (2001) Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell 105: 177–186. [DOI] [PubMed] [Google Scholar]
- 140. Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173: 3916–3924. [DOI] [PubMed] [Google Scholar]
- 141. Godwin JW, Liem KF Jr, Brockes JP (2010) Tissue factor expression in newt iris coincides with thrombin activation and lens regeneration. Mech Dev 127: 321–328. [DOI] [PubMed] [Google Scholar]
- 142. Imokawa Y, Brockes JP (2003) Selective activation of thrombin is a critical determinant for vertebrate lens regeneration. Curr Biol 13: 877–881. [DOI] [PubMed] [Google Scholar]
- 143. Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD (2001) A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol 166: 2479–2486. [DOI] [PubMed] [Google Scholar]
- 144. Kimura Y, Madhavan M, Call MK, Santiago W, Tsonis PA, et al. (2003) Expression of complement 3 and complement 5 in newt limb and lens regeneration. J Immunol 170: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 145. Del Rio-Tsonis K, Tsonis PA, Zarkadis IK, Tsagas AG, Lambris JD (1998) Expression of the third component of complement, C3, in regenerating limb blastema cells of urodeles. J Immunol 161: 6819–6824. [PubMed] [Google Scholar]
- 146. Khan JA, Cao M, Kang BY, Liu Y, Mehta JL, et al. (2011) Systemic human Netrin-1 gene delivery by adeno-associated virus type 8 alters leukocyte accumulation and atherogenesis in vivo. Gene Ther 18: 437–444. [DOI] [PubMed] [Google Scholar]
- 147. Wang H, Ozaki T, Shamim Hossain M, Nakamura Y, Kamijo T, et al. (2008) A newly identified dependence receptor UNC5H4 is induced during DNA damage-mediated apoptosis and transcriptional target of tumor suppressor p53. Biochem Biophys Res Commun 370: 594–598. [DOI] [PubMed] [Google Scholar]
- 148. Yang H, Kim A, David T, Palmer D, Jin T, et al. (2012) TMEM16F Forms a Ca(2+)-Activated Cation Channel Required for Lipid Scrambling in Platelets during Blood Coagulation. Cell 151: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yamada T (1977) Control mechanisms in cell-type conversion in newt lens regeneration. Monogr Dev Biol 13: 1–126. [PubMed] [Google Scholar]
- 150. Tsonis PA, Haynes T, Maki N, Nakamura K, Casco-Robles MM, et al. (2011) Controlling gene loss of function in newts with emphasis on lens regeneration. Nat Protoc 6: 593–599. [DOI] [PubMed] [Google Scholar]
- 151. Casco-Robles MM, Yamada S, Miura T, Nakamura K, Haynes T, et al. (2011) Expressing exogenous genes in newts by transgenesis. Nat Protoc 6: 600–608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GO terms that are over-represented in dorsal iris 4 and 8 dpl versus ventral iris 4 and 8 dpl.
(XLSX)
GO terms that are over-represented in ventral iris 4 and 8 dpl versus dorsal iris 4 and 8 dpl.
(XLSX)
GO terms that are over-represented in transcripts commonly up-regulated (>2 fold) in dorsal and ventral iris at 4 dpl compared to 8 dpl versus whole reference transcriptome.
(XLSX)
GO terms that are over-represented in transcripts that are commonly up-regulated (>2 fold) in dorsal and ventral iris 8 dpl compared to 4 dpl versus whole reference transcriptome.
(XLSX)