Abstract
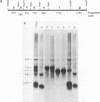
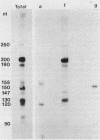
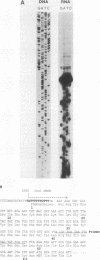
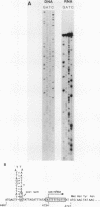
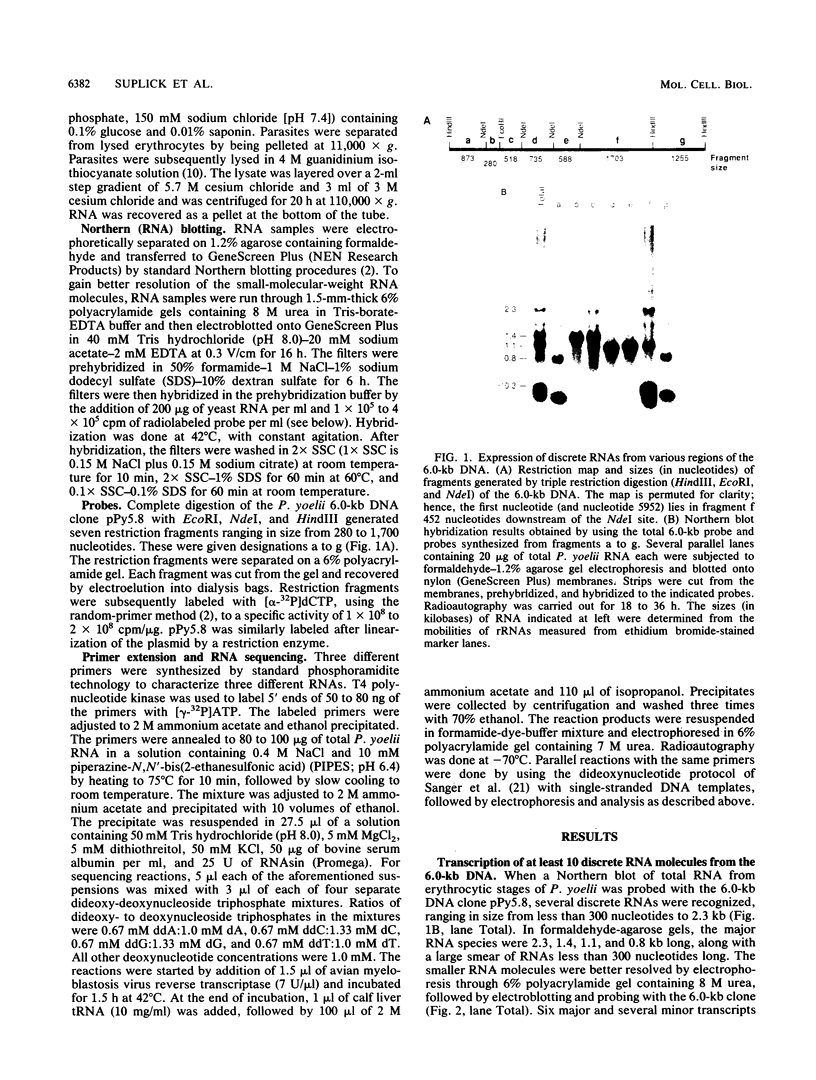
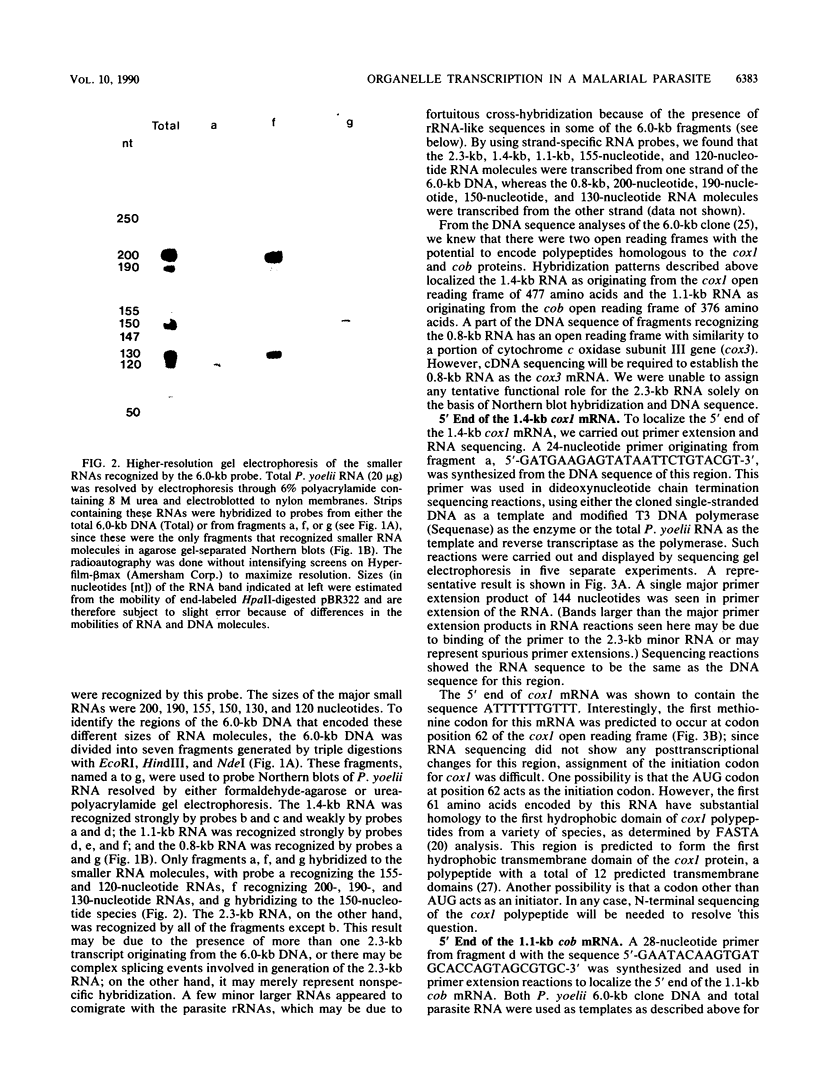
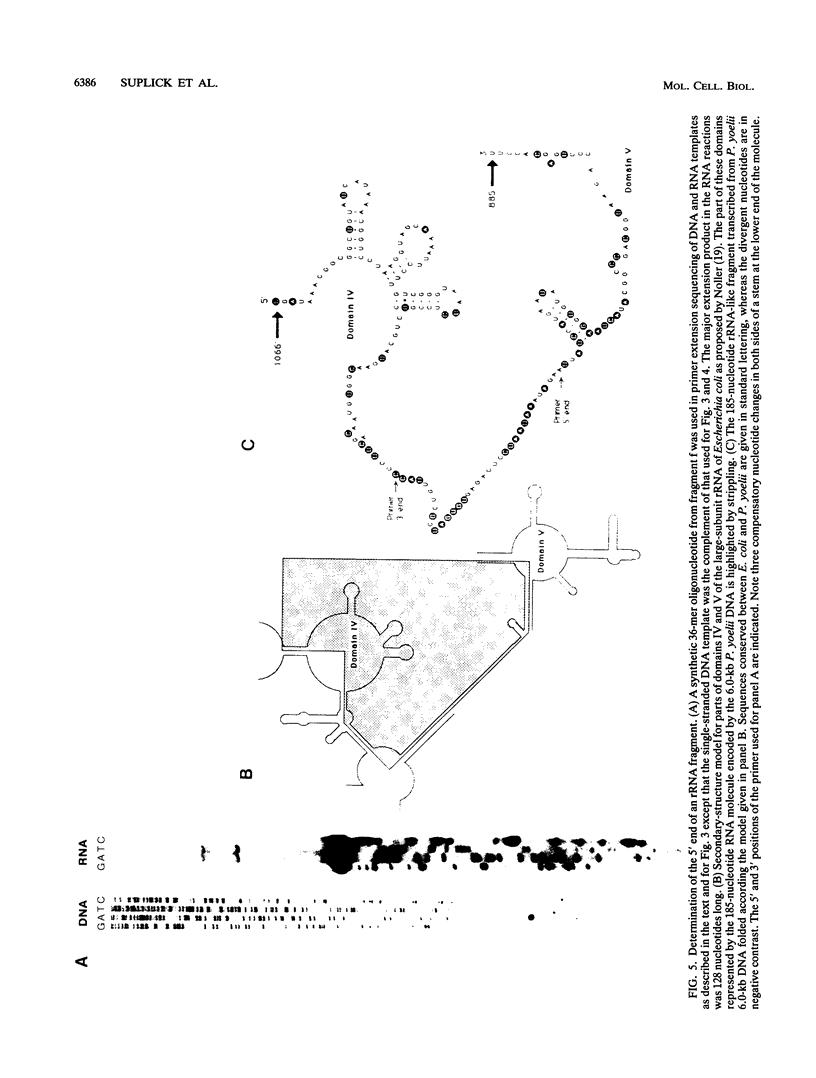
All tested members of genus Plasmodium contain tandemly arrayed, transcribed, extrachromosomal DNA with a unit length of 6.0 kb. This DNA contains two open reading frames with potential to encode cytochrome c oxidase subunit I (cox1) and cytochrome b (cob) as well as fragments of rRNA genes scattered on both strands. At least 10 discrete RNA molecules transcribed during erythrocytic stages of a rodent malarial parasite, Plasmodium yoelii, were recognized by the 6.0-kb DNA probes. The RNA molecules of 1.4 and 1.1 kb were identified as encoding cox1 and cob, respectively. Primer extension and RNA sequencing were used to locate and characterize 5' ends of these two RNAs, showing that an identical 12-nucleotide sequence, 5'-TATTTTT TGTTT-3', was present at these positions. This sequence may act as a promoter or as an RNA processing signal. A stem-loop structure signifying a possible transcription termination was present at the end of the cox1 open reading frame. At least six discrete RNA molecules of less than 250 nucleotides were recognized by different fractions of the 6.0-kb DNA. The largest of these, 200 nucleotides, was also characterized by primer extension and RNA sequencing. This molecule had a high homology to portions of the large-subunit rRNA domains IV and V. Other, small RNA molecules were recognized by regions of the 6.0-kb DNA that had homology to the highly conserved peptidyltransferase domain of large-subunit rRNA. These results show that the unusual compactly organized mitochondrionlike DNA of malarial parasites is transcribed in a complex pattern.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldritt S. M., Joseph J. T., Wirth D. F. Sequence identification of cytochrome b in Plasmodium gallinaceum. Mol Cell Biol. 1989 Sep;9(9):3614–3620. doi: 10.1128/mcb.9.9.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas T. K., Ticho B., Getz G. S. In vitro characterization of the yeast mitochondrial promoter using single-base substitution mutants. J Biol Chem. 1987 Oct 5;262(28):13690–13696. [PubMed] [Google Scholar]
- Blum J. J., Yayon A., Friedman S., Ginsburg H. Effects of mitochondrial protein synthesis inhibitors on the incorporation of isoleucine into Plasmodium falciparum in vitro. J Protozool. 1984 Aug;31(3):475–479. doi: 10.1111/j.1550-7408.1984.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Boer P. H., Gray M. W. Scrambled ribosomal RNA gene pieces in Chlamydomonas reinhardtii mitochondrial DNA. Cell. 1988 Nov 4;55(3):399–411. doi: 10.1016/0092-8674(88)90026-8. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Yoza B. K. Accurate in vitro transcription of Xenopus laevis mitochondrial DNA from two bidirectional promoters. Mol Cell Biol. 1986 Jul;6(7):2543–2550. doi: 10.1128/mcb.6.7.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Precise assignment of the heavy-strand promoter of mouse mitochondrial DNA: cognate start sites are not required for transcriptional initiation. Mol Cell Biol. 1986 Sep;6(9):3262–3267. doi: 10.1128/mcb.6.9.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Precise assignment of the light-strand promoter of mouse mitochondrial DNA: a functional promoter consists of multiple upstream domains. Mol Cell Biol. 1986 Sep;6(9):3253–3261. doi: 10.1128/mcb.6.9.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Precise identification of individual promoters for transcription of each strand of human mitochondrial DNA. Cell. 1984 Mar;36(3):635–643. doi: 10.1016/0092-8674(84)90343-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Divo A. A., Geary T. G., Jensen J. B., Ginsburg H. The mitochondrion of Plasmodium falciparum visualized by rhodamine 123 fluorescence. J Protozool. 1985 Aug;32(3):442–446. doi: 10.1111/j.1550-7408.1985.tb04041.x. [DOI] [PubMed] [Google Scholar]
- Dore E., Frontali C., Forte T., Fratarcangeli S. Further studies and electron microscopic characterization of Plasmodium berghei DNA. Mol Biochem Parasitol. 1983 Aug;8(4):339–352. doi: 10.1016/0166-6851(83)90080-4. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Levens D., Rabinowitz M. Analysis of transcriptional initiation of yeast mitochondrial DNA in a homologous in vitro transcription system. Cell. 1982 Dec;31(2 Pt 1):337–346. doi: 10.1016/0092-8674(82)90127-1. [DOI] [PubMed] [Google Scholar]
- Eling W. Ficoll fractionation for the separation of parasitized erythrocytes from malaria infected blood. Bull World Health Organ. 1977;55(1):105–114. [PMC free article] [PubMed] [Google Scholar]
- Gardner M. J., Bates P. A., Ling I. T., Moore D. J., McCready S., Gunasekera M. B., Wilson R. J., Williamson D. H. Mitochondrial DNA of the human malarial parasite Plasmodium falciparum. Mol Biochem Parasitol. 1988 Oct;31(1):11–17. doi: 10.1016/0166-6851(88)90140-5. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Divo A. A., Geary T. G., Boland M. T., Jensen J. B. Effects of mitochondrial inhibitors on intraerythrocytic Plasmodium falciparum in in vitro cultures. J Protozool. 1986 Feb;33(1):121–125. doi: 10.1111/j.1550-7408.1986.tb05570.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., Dave D., Richards W. H. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979 Feb 1;582(3):390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Circular mitochondrial DNA from the avian malarial parasite Plasmodium lophurae. Biochim Biophys Acta. 1975 May 16;390(3):276–284. doi: 10.1016/0005-2787(75)90348-2. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suplick K., Akella R., Saul A., Vaidya A. B. Molecular cloning and partial sequence of a 5.8 kilobase pair repetitive DNA from Plasmodium falciparum. Mol Biochem Parasitol. 1988 Sep;30(3):289–290. doi: 10.1016/0166-6851(88)90098-9. [DOI] [PubMed] [Google Scholar]
- Tanabe K. Staining of Plasmodium yoelii-infected mouse erythrocytes with the fluorescent dye rhodamine 123. J Protozool. 1983 Nov;30(4):707–710. doi: 10.1111/j.1550-7408.1983.tb05347.x. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Arasu P. Tandemly arranged gene clusters of malarial parasites that are highly conserved and transcribed. Mol Biochem Parasitol. 1987 Jan 15;22(2-3):249–257. doi: 10.1016/0166-6851(87)90056-9. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Wilson R. J., Bates P. A., McCready S., Perler F., Qiang B. U. Nuclear and mitochondrial DNA of the primate malarial parasite Plasmodium knowlesi. Mol Biochem Parasitol. 1985 Feb;14(2):199–209. doi: 10.1016/0166-6851(85)90038-6. [DOI] [PubMed] [Google Scholar]