Abstract
NIH 3T3 cells infected with Moloney murine leukemia virus (MoMLV) express high levels of virus-specific RNA. To inhibit replication of the virus, we stably introduced chimeric tRNA genes encoding antisense templates into NIH 3T3 cells via a retroviral vector. Efficient expression of hybrid tRNA-MoMLV antisense transcripts and inhibition of MoMLV replication were dependent on the use of a particular type of retroviral vector, the double-copy vector, in which the chimeric tRNA gene was inserted in the 3' long terminal repeat. MoMLV replication was inhibited up to 97% in cells expressing antisense RNA corresponding to the gag gene and less than twofold in cells expressing antisense RNA corresponding to the pol gene. RNA and protein analyses suggest that inhibition was exerted at the level of translation. These results suggest that RNA polymerase III-based antisense inhibition systems can be used to inhibit highly expressed viral genes and render cells resistant to viral replication via intracellular immunization strategies.
Full text
PDF








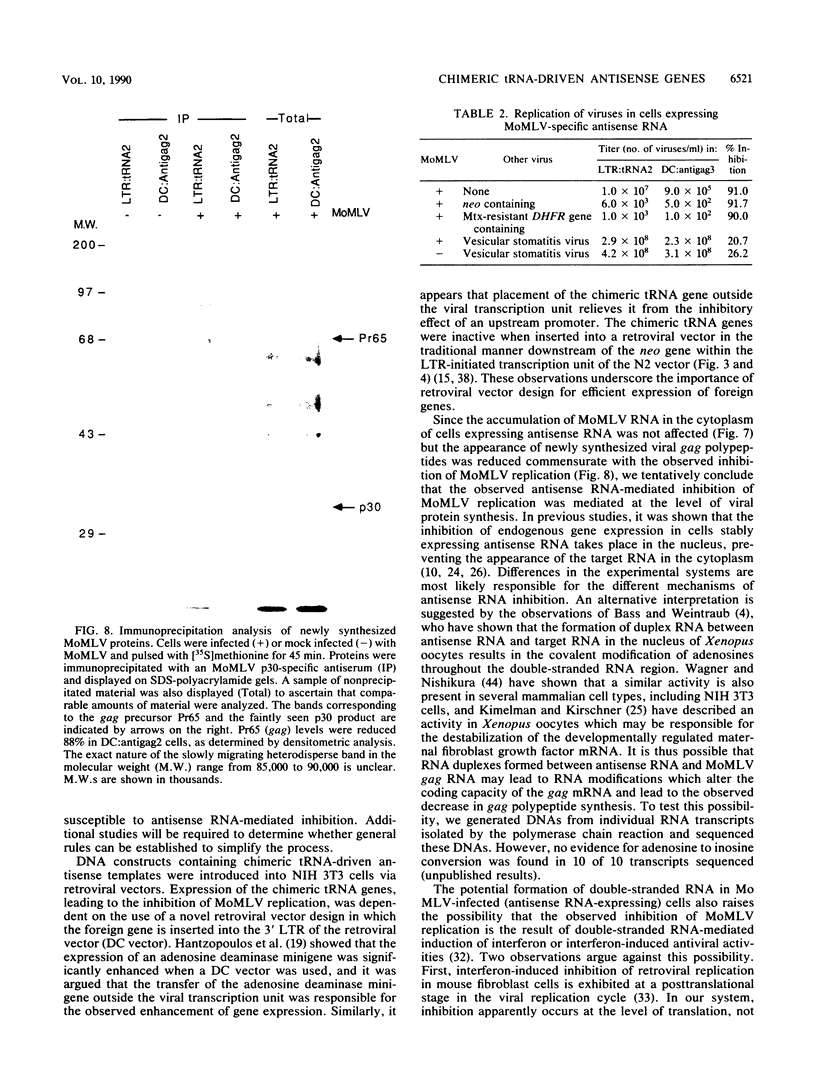


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeniyi-Jones S., Romeo P. H., Zasloff M. Generation of long read-through transcripts in vivo and in vitro by deletion of 3' termination and processing sequences in the human tRNAimet gene. Nucleic Acids Res. 1984 Jan 25;12(2):1101–1115. doi: 10.1093/nar/12.2.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988 Sep 29;335(6189):395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988 Dec 23;55(6):1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Chang L. J., Stoltzfus C. M. Gene expression from both intronless and intron-containing Rous sarcoma virus clones is specifically inhibited by anti-sense RNA. Mol Cell Biol. 1985 Sep;5(9):2341–2348. doi: 10.1128/mcb.5.9.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cicurel L., Lee J. C., Enjuanes L., Ihle J. N. Monoclonal antibodies to the envelope proteins of Moloney leukemia virus: characterization of recombinant viruses. Transplant Proc. 1980 Sep;12(3):394–397. [PubMed] [Google Scholar]
- Coleman J., Green P. J., Inouye M. The use of RNAs complementary to specific mRNAs to regulate the expression of individual bacterial genes. Cell. 1984 Jun;37(2):429–436. doi: 10.1016/0092-8674(84)90373-8. [DOI] [PubMed] [Google Scholar]
- Cotten M., Birnstiel M. L. Ribozyme mediated destruction of RNA in vivo. EMBO J. 1989 Dec 1;8(12):3861–3866. doi: 10.1002/j.1460-2075.1989.tb08564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T. E., Nellen W., Gomer R. H., Firtel R. A. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985 Dec;43(3 Pt 2):633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Gilboa E. Retrovirus vectors and their uses in molecular biology. Bioessays. 1986 Dec;5(6):252–257. doi: 10.1002/bies.950050605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Han J. H., Rooney R. J., Harding J. D. Structure and evolution of mammalian tRNA genes: sequence of a mouse tRNAiMet gene, the 5'-flanking region of which is homologous to a human gene. Gene. 1984 May;28(2):249–255. doi: 10.1016/0378-1119(84)90263-4. [DOI] [PubMed] [Google Scholar]
- Hantzopoulos P. A., Sullenger B. A., Ungers G., Gilboa E. Improved gene expression upon transfer of the adenosine deaminase minigene outside the transcriptional unit of a retroviral vector. Proc Natl Acad Sci U S A. 1989 May;86(10):3519–3523. doi: 10.1073/pnas.86.10.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Jennings P. A., Molloy P. L. Inhibition of SV40 replicon function by engineered antisense RNA transcribed by RNA polymerase III. EMBO J. 1987 Oct;6(10):3043–3047. doi: 10.1002/j.1460-2075.1987.tb02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesch T., Berg P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol Cell Biol. 1986 Jul;6(7):2593–2601. doi: 10.1128/mcb.6.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. W. An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell. 1989 Nov 17;59(4):687–696. doi: 10.1016/0092-8674(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Lewis E. D., Manley J. L. Polyadenylylation of an mRNA precursor occurs independently of transcription by RNA polymerase II in vivo. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8555–8559. doi: 10.1073/pnas.83.22.8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988 Dec;167(2):400–406. [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. Inhibition of heat shock protein synthesis by heat-inducible antisense RNA. Proc Natl Acad Sci U S A. 1986 Jan;83(2):399–403. doi: 10.1073/pnas.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Daugherty B. L., Jung V., Hotta K., Pestka R. K. Anti-mRNA: specific inhibition of translation of single mRNA molecules. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7525–7528. doi: 10.1073/pnas.81.23.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Rowe W. P., Oxman M. N. Effect of interferon on exogenous, endogenous, and chroniv murine leukemia virus infection. Virology. 1976 Apr;70(2):324–338. doi: 10.1016/0042-6822(76)90275-0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986 Aug 7;322(6079):562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- Santos T., Zasloff M. Comparative analysis of human chromosomal segments bearing nonallelic dispersed tRNAimet genes. Cell. 1981 Mar;23(3):699–709. doi: 10.1016/0092-8674(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Sollner-Webb B., Cleveland D. W. Specificity of RNA maturation pathways: RNAs transcribed by RNA polymerase III are not substrates for splicing or polyadenylation. Mol Cell Biol. 1987 Oct;7(10):3602–3612. doi: 10.1128/mcb.7.10.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. L., Stuhlmann H., Jähner D., Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To R. Y., Booth S. C., Neiman P. E. Inhibition of retroviral replication by anti-sense RNA. Mol Cell Biol. 1986 Dec;6(12):4758–4762. doi: 10.1128/mcb.6.12.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevor K., Linney E., Oshima R. G. Suppression of endo B cytokeratin by its antisense RNA inhibits the normal coexpression of endo A cytokeratin. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1040–1044. doi: 10.1073/pnas.84.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. W., Nishikura K. Cell cycle expression of RNA duplex unwindase activity in mammalian cells. Mol Cell Biol. 1988 Feb;8(2):770–777. doi: 10.1128/mcb.8.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Lim B., Spooncer E., Longtine J., Dexter T. M. Restriction of expression of an integrated recombinant retrovirus in primary but not immortalized murine hematopoietic stem cells. Blood. 1988 Jun;71(6):1738–1743. [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Santos T., Romeo P., Rosenberg M. Transcription and precursor processing of normal and mutant human tRNAiMet genes in a homologous cell-free system. J Biol Chem. 1982 Jul 10;257(13):7857–7863. [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- de la Peña P., Zasloff M. Enhancement of mRNA nuclear transport by promoter elements. Cell. 1987 Aug 14;50(4):613–619. doi: 10.1016/0092-8674(87)90034-1. [DOI] [PubMed] [Google Scholar]
- van der Krol A. R., Mol J. N., Stuitje A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques. 1988 Nov-Dec;6(10):958–976. [PubMed] [Google Scholar]
- von Rüden T., Gilboa E. Inhibition of human T-cell leukemia virus type I replication in primary human T cells that express antisense RNA. J Virol. 1989 Feb;63(2):677–682. doi: 10.1128/jvi.63.2.677-682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]








