Abstract
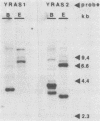
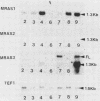
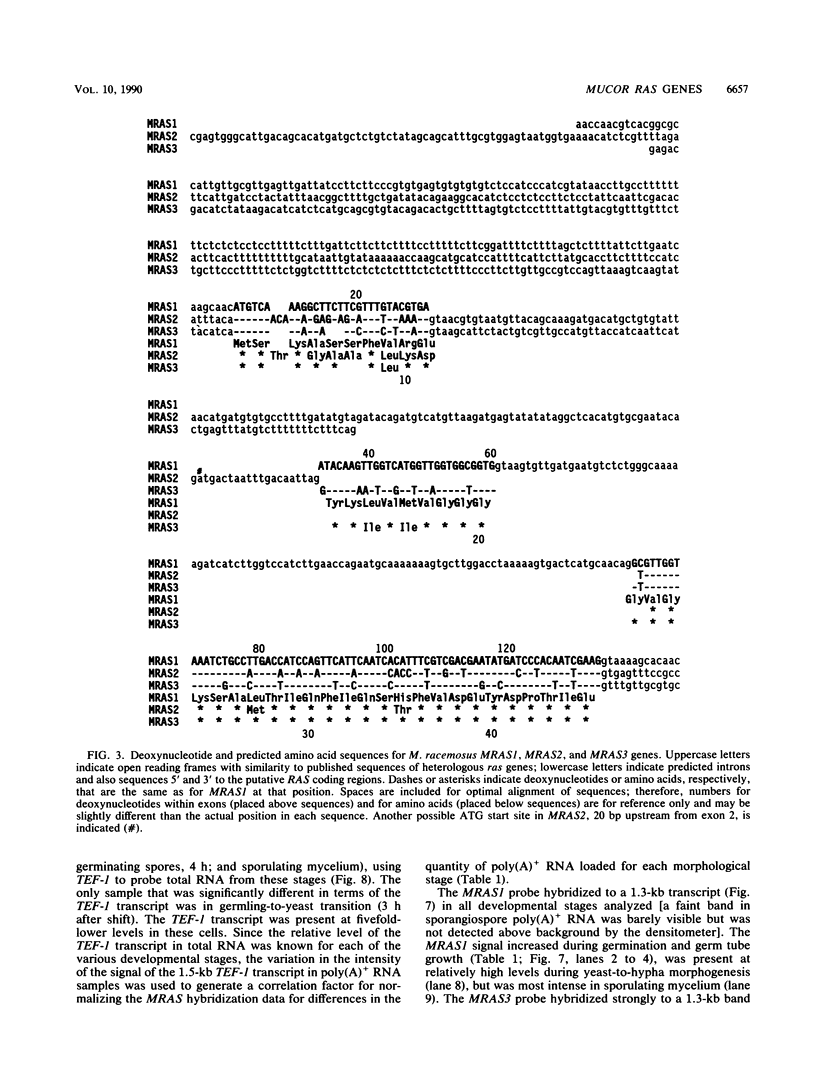
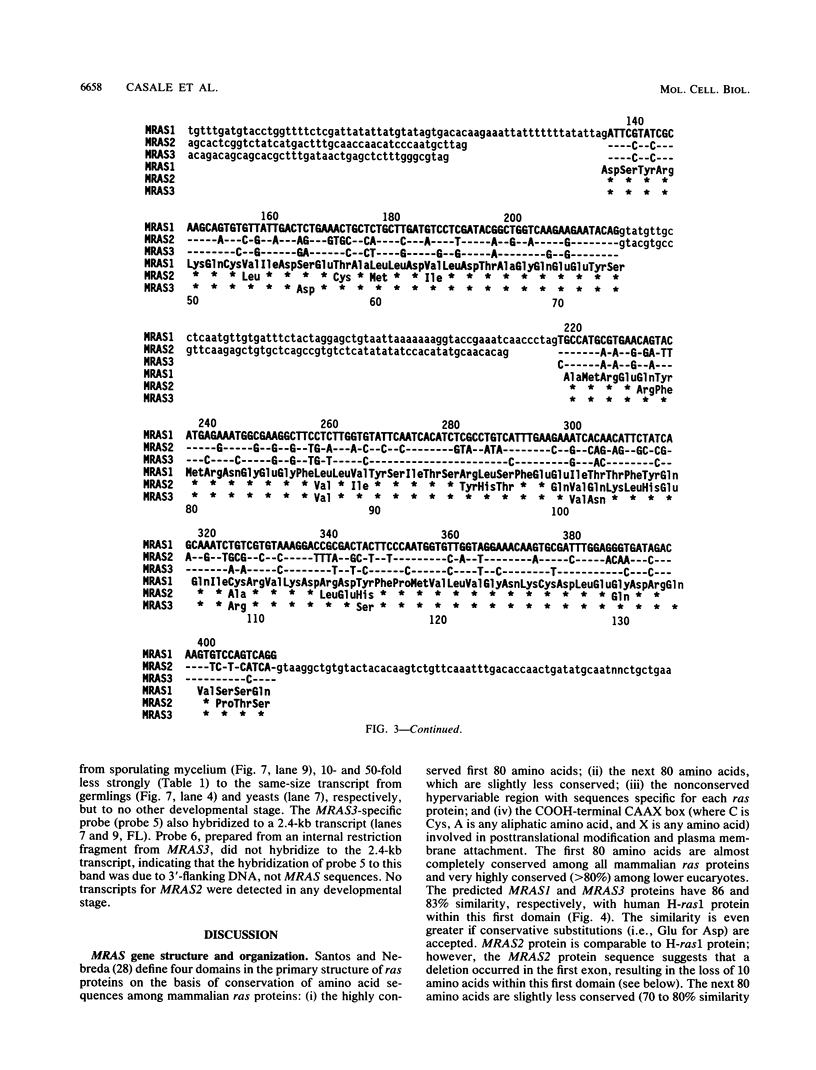
Sporulation, spore germination, and yeast-hypha dimorphism in the filamentous fungus Mucor racemosus provide useful model systems to study cell development in eucaryotic cells. Three RAS genes (MRAS1, MRAS2, and MRAS3) from M. racemosus have been cloned, and their nucleotide sequences have been determined. The predicted amino acid sequences and the sizes of the three MRAS proteins exhibit a high degree of similarity with other ras proteins, including that encoded by H-ras, which have been implicated in regulation of proliferation and development in eucaryotic cells by mediating signal transduction pathways. The MRAS proteins show conservation of functional domains proposed for ras proteins, including guanine nucleotide interaction domains, an effector domain, a binding epitope for neutralizing antibody Y13-259, and the COOH-terminal CAAX box, which is a site of thiocylation and membrane attachment. Amino acid sequences unique to each MRAS protein occur adjacent to the CAAX box, consistent with the location of the hypervariable region in other ras proteins. Northern (RNA) analysis was used to study expression of the three MRAS genes in relation to cell development. Gene-specific probes for two of these genes, MRAS1 and MRAS3, hybridized to different 1.3-kb mRNA transcripts. The accumulation of these transcripts depended on the developmental stage, and this pattern was different between the two MRAS genes. No transcript for MRAS2 was detected in the developmental stages examined. The unique patterns of MRAS transcript accumulation suggest that individual MRAS genes and proteins may play distinct roles in cell growth or development.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bos J. L. The ras gene family and human carcinogenesis. Mutat Res. 1988 May;195(3):255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Cihlar R. L., Sypherd P. S. The organization of the ribosomal RNA genes in the fungus Mucor racemosus. Nucleic Acids Res. 1980 Feb 25;8(4):793–804. [PMC free article] [PubMed] [Google Scholar]
- Domek D. B., Borgia P. T. Changes in the rate of chitin-plus-chitosan synthesis accompany morphogenesis of Mucor racemosus. J Bacteriol. 1981 Jun;146(3):945–951. doi: 10.1128/jb.146.3.945-951.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano O., Taparowsky E., Fiddes J., Wigler M., Goldfarb M. Sequence and structure of the coding region of the human H-ras-1 gene from T24 bladder carcinoma cells. J Mol Appl Genet. 1983;2(2):173–180. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hattori S., Clanton D. J., Satoh T., Nakamura S., Kaziro Y., Kawakita M., Shih T. Y. Neutralizing monoclonal antibody against ras oncogene product p21 which impairs guanine nucleotide exchange. Mol Cell Biol. 1987 May;7(5):1999–2002. doi: 10.1128/mcb.7.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E. T., Cihlar R. L., Inderlied C. B. Lipid synthesis during morphogenesis of Mucor racemosus. J Bacteriol. 1982 Nov;152(2):880–887. doi: 10.1128/jb.152.2.880-887.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen A. D., Sypherd P. S. Cyclic adenosine 3',5'-monophosphate and morphogenesis in Mucor racemosus. J Bacteriol. 1974 Feb;117(2):432–438. doi: 10.1128/jb.117.2.432-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz J. E., Orlowski M. Differential gene expression during aerobic germination of Mucor racemosus sporangiospores. J Bacteriol. 1984 Sep;159(3):965–972. doi: 10.1128/jb.159.3.965-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz J. E., Orlowski M. Regulation of gene expression during aerobic germination of Mucor racemosus sporangiospores. J Gen Microbiol. 1987 Jan;133(1):141–148. doi: 10.1099/00221287-133-1-141. [DOI] [PubMed] [Google Scholar]
- Linz J. E., Orlowski M. Stored mRNA in sporangiospores of the fungus Mucor racemosus. J Bacteriol. 1982 Jun;150(3):1138–1144. doi: 10.1128/jb.150.3.1138-1144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz J. E., Sypherd P. S. Expression of three genes for elongation factor 1 alpha during morphogenesis of Mucor racemosus. Mol Cell Biol. 1987 May;7(5):1925–1932. doi: 10.1128/mcb.7.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. S., Gibbs J. B., Scolnick E. M., Sigal I. S. An adenylate cyclase from Saccharomyces cerevisiae that is stimulated by RAS proteins with effector mutations. Mol Cell Biol. 1988 Jan;8(1):52–61. doi: 10.1128/mcb.8.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M. Changing pattern of cyclic AMP-binding proteins during germination of Mucor racemosus sporangiospores. Biochem J. 1979 Aug 15;182(2):547–554. doi: 10.1042/bj1820547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M. Cyclic adenosine 3',5'-monophosphate and germination of sporangiospores from the fungus Mucor. Arch Microbiol. 1980 Jun;126(2):133–140. doi: 10.1007/BF00511218. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Ross J. F. Relationship of internal cyclic AMP levels, rates of protein synthesis and mucor dimorphism. Arch Microbiol. 1981 Jul;129(5):353–356. doi: 10.1007/BF00406461. [DOI] [PubMed] [Google Scholar]
- Paveto C., Epstein A., Passeron S. Studies on cyclic adenosine 3' ,5'-monophosphate levels, Adenylate cyclase and phosphodiesterase activities in the dimorphic fungus Mucor rouxii. Arch Biochem Biophys. 1975 Aug;169(2):449–457. doi: 10.1016/0003-9861(75)90187-3. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Reymond C. D., Gomer R. H., Mehdy M. C., Firtel R. A. Developmental regulation of a Dictyostelium gene encoding a protein homologous to mammalian ras protein. Cell. 1984 Nov;39(1):141–148. doi: 10.1016/0092-8674(84)90199-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Nebreda A. R. Structural and functional properties of ras proteins. FASEB J. 1989 Aug;3(10):2151–2163. doi: 10.1096/fasebj.3.10.2666231. [DOI] [PubMed] [Google Scholar]
- Sundstrom P., Lira L. M., Choi D., Linz J. E., Sypherd P. S. Sequence analysis of the EF-1 alpha gene family of Mucor racemosus. Nucleic Acids Res. 1987 Dec 10;15(23):9997–10006. doi: 10.1093/nar/15.23.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Tamanoi F. Yeast RAS genes. Biochim Biophys Acta. 1988 Aug 3;948(1):1–15. doi: 10.1016/0304-419x(88)90002-9. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Suard Y., Fasano O., Shimizu K., Goldfarb M., Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982 Dec 23;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- Tong L. A., de Vos A. M., Milburn M. V., Jancarik J., Noguchi S., Nishimura S., Miura K., Ohtsuka E., Kim S. H. Structural differences between a ras oncogene protein and the normal protein. Nature. 1989 Jan 5;337(6202):90–93. doi: 10.1038/337090a0. [DOI] [PubMed] [Google Scholar]
- Uno I., Mitsuzawa H., Tanaka K., Oshima T., Ishikawa T. Identification of the domain of Saccharomyces cerevisiae adenylate cyclase associated with the regulatory function of RAS products. Mol Gen Genet. 1987 Dec;210(2):187–194. doi: 10.1007/BF00325683. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Tong L., Milburn M. V., Matias P. M., Jancarik J., Noguchi S., Nishimura S., Miura K., Ohtsuka E., Kim S. H. Three-dimensional structure of an oncogene protein: catalytic domain of human c-H-ras p21. Science. 1988 Feb 19;239(4842):888–893. doi: 10.1126/science.2448879. [DOI] [PubMed] [Google Scholar]