Abstract
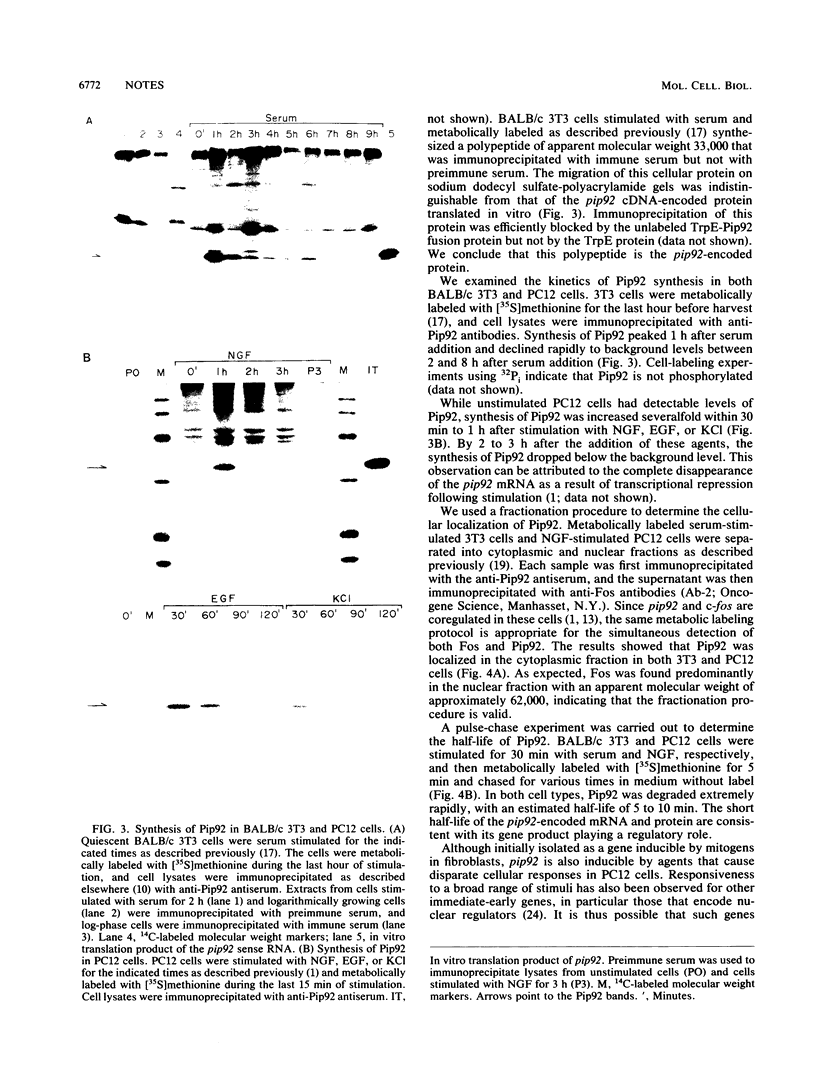
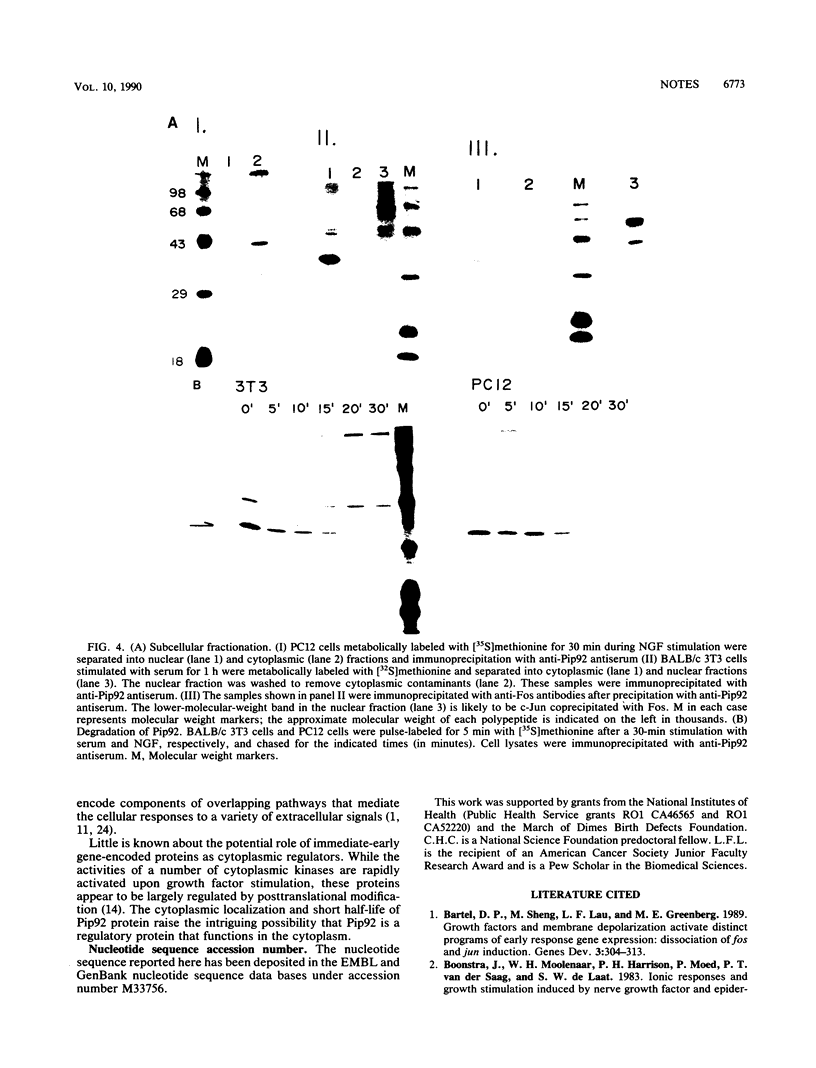
pip92 is a cellular immediate-early gene inducible by serum growth factors in fibroblasts. It is also induced in the rat pheochromocytoma cell line PC12 by agents that cause proliferation, neuronal differentiation, and membrane depolarization. We show that the pip92-encoded polypeptide is a proline-rich protein of 221 amino acids, has an extremely short half-life, and is localized in the cytoplasm. We hypothesize that Pip92 plays a role in mediating the cellular responses to a variety of extracellular signals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartel D. P., Sheng M., Lau L. F., Greenberg M. E. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989 Mar;3(3):304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleclough C., Kuhn L., Lefkovits I. Regulation of mRNA abundance in activated T lymphocytes: identification of mRNA species affected by the inhibition of protein synthesis. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1753–1757. doi: 10.1073/pnas.87.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann C. L., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem. 1985 Feb 10;260(3):1513–1520. [PubMed] [Google Scholar]
- Dixon S. J., Stewart D., Grinstein S., Spiegel S. Transmembrane signaling by the B subunit of cholera toxin: increased cytoplasmic free calcium in rat lymphocytes. J Cell Biol. 1987 Sep;105(3):1153–1161. doi: 10.1083/jcb.105.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R. Extracellular signals, transcriptional responses and cellular specificity. Trends Biochem Sci. 1989 Nov;14(11):455–458. doi: 10.1016/0968-0004(89)90105-9. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R. W., Varnum B. C., O'Brien T. G., Herschman H. R. Induction of tumor promotor-inducible genes in murine 3T3 cell lines and tetradecanoyl phorbol acetate-nonproliferative 3T3 variants can occur through protein kinase C-dependent and -independent pathways. Mol Cell Biol. 1989 Apr;9(4):1790–1793. doi: 10.1128/mcb.9.4.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Lau L. F., Christy B., Hartzell S., Nakabeppu Y., Ryder K. Genomic response to growth factors. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):893–900. doi: 10.1101/sqb.1988.053.01.102. [DOI] [PubMed] [Google Scholar]
- O'Brien T. P., Yang G. P., Sanders L., Lau L. F. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990 Jul;10(7):3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Stanton L., Schwab M., Bishop J. M. Human proto-oncogene N-myc encodes nuclear proteins that bind DNA. Mol Cell Biol. 1986 Dec;6(12):4450–4457. doi: 10.1128/mcb.6.12.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Stiles C. D. Serum-inducible genes. Adv Cancer Res. 1989;53:1–32. doi: 10.1016/s0065-230x(08)60277-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]