Abstract
Immunosuppressive cytotoxic T lymphocyte associated antigen-4 immunoglobulin fusion proteins (CTLA4-Ig) block the CD28:CD80/86 costimulatory pathway. On a cellular level, CTLA4-Ig is understood to dampen T cell responses. As a mechanism, CTLA4-Ig has been reported to affect dendritic cell (DC) function via inducing the immunosuppressive indoleamine 2,3 dioxygenase (IDO) pathway and promoting a DC regulatory phenotype. We here probed cellular mechanisms of CTLA4-Ig immunoregulation in an allogeneic setting using C57BL/6 splenic or bone marrow derived DCs (BMDCs) as stimulators of allogeneic Balb/c derived T cells. To address whether CTLA4-Ig immunosuppression affected DCs, we pre-exposed C57BL/6 splenic or BMDCs to CTLA4-Ig and removed unbound CTLA4-Ig before co-culture with allogeneic T cells. CTLA4-Ig disappeared rapidly (within 4 h) from the cell membrane by combined internalization and dissociation. These CTLA4-Ig pre-exposed DCs were fully capable of stimulating allogeneic T cell proliferation, suggesting that CTLA4-Ig does not impair the DC stimulatory capacity. Only the presence of CTLA4-Ig during DC/T cell co-culture resulted in the expected inhibition of proliferation. C57BL/6 splenic or BMDCs exposed to CTLA4-Ig did not display IDO activity. We conclude that CTLA4-Ig immunosuppressive activity does not depend on a DC regulatory phenotype but on its presence during DC/T cell interaction.
Keywords: CTLA4-Ig, Abatacept, IFN-γ, IDO, Reverse signaling
Highlights
► CTLA4-Ig binding to CD80/86 on murine DC surface is short lived. ► CTLA4-Ig does not confer a DC regulatory phenotype ► CTLA4-Ig does not induce IDO ► CTLA4-Ig mediates immune inhibition by affecting DC/T cell interaction
1. Introduction
A major task in transplantation research, including solid organ transplantation (SOT) and hematopoietic stem cell transplantation (HSCT), is to rapidly achieve a state of allo-specific tolerance. T cells play a key role in regulating transplantation immunology. Allo-specific T cell tolerance implies that T cells do not mount pathogenic immune reactions towards allogeneic organs but preserve protective activity towards environmental pathogens. Thus, allo-specific tolerized T cells are critical to accomplishing transplantation tolerance.
T cells, in order to mount appropriate responses upon antigen recognition, need to receive costimulatory signals. Costimulatory signaling involves a multitude of costimulatory molecules present on antigen-presenting cells (APCs) interacting with their appropriate receptors on T cells to finally optimize T cell proliferation and cytokine secretion (reviewed in [1]). Previous studies have shown that antigen stimulation of T cells in the absence of costimulation will leave T cells anergic, i.e. unresponsive to subsequent stimulation by the same antigen [2–4]. The most extensively studied costimulatory pathway required for the induction of full T cell immune responses is the interaction of CD80 and CD86 molecules expressed on dendritic cells (DCs) with the CD28 molecule expressed on T cells, which provides signals for proliferation and survival. Cytotoxic T lymphocyte associated antigen-4 (CTLA-4) is expressed by activated T cells and mediates a T cell inhibitory signal to limit excess T cell stimulation. CTLA-4 immunoglobulin fusion proteins (CTLA4-Ig) have been pharmacologically engineered to block CD28-mediated costimulatory signaling to T cells to induce tolerance [5]. CTLA4-Ig consists of the extracellular binding domain of CTLA-4 linked to an Fc domain of IgG and possesses a high binding affinity to CD80 and CD86 (reviewed in [6,7]).
CTLA4-Ig pharmacological compounds (abatacept, belatacept) have been introduced to the clinic as a treatment of autoimmune disease, such as rheumatoid arthritis [8] and to prevent organ rejection in kidney transplantation [9]. The precise cellular mechanisms of tolerogenesis by CTLA4-Ig are still a matter of research. A recently proposed model suggested that CTLA4-Ig binding to CD80/CD86 molecules provided a ‘reverse’ signal to DCs resulting in the induction of the immunomodulatory enzyme indoleamine 2,3 dioxygenase (IDO) [10,11]. IDO is the major and rate limiting enzyme of tryptophan metabolism in mammals initiating tryptophan metabolism along the kynurenine pathway [12]. IDO augmented activity is preferentially expressed in antigen-presenting cells, such as DCs, and interferes with the T cell proliferative capacity [13,14] and promotes T cell apoptotic decline [15]. Thus, IDO activity in DCs has been proposed to possess powerful immune regulatory and tolerance inducing capacity [16,17]. Induction of IDO activity in DCs by CTLA4-Ig has been suggested to underlie the tolerogenic capacity of CTLA4-Ig [10,18].
In the present study, we set out to improve the understanding of the effects of CTLA4-Ig on a cellular level. Our findings, unexpectedly, provide evidence that immunoregulation by CTLA4-Ig does not require IDO activity or the induction of a DC regulatory phenotype; instead, CTLA4-Ig mediated regulation of allogeneic T cell proliferation by directly interfering with DC/T cell interaction.
2. Materials and methods
2.1. Mice and reagents
Female C57BL/6 (H-2b) and Balb/c (H-2d) mice (Charles River Laboratories, Sulzfeld, Germany), aged six to 10 weeks, were maintained under specific pathogen-free conditions at the Biomedical Research Institute, Medical University of Vienna (Austria). All experiments were approved by the institutional review board and followed the national and international guidelines of laboratory animal care.
CTLA4-Ig fusion protein (IgG1) (abatacept) [19,20] was kindly provided by Bristol-Meyers Squibb Pharmaceuticals (Princeton, NJ, USA).
2.2. Cell culture medium
All assays were performed in IMDM (Invitrogen, Carlsbad, CA, USA) containing 2 mM l-glutamin and 25 mM Hepes supplemented with 10% FBS (PAA Laboratories, Pasching, Austria), antibiotics and 50 μM 2-mercaptoethanol (Sigma, St. Louis, MO, USA), hereafter termed complete medium. Cell cultures were maintained in humidified air containing 5% CO2 at 37 °C.
2.3. Cell preparation
Splenic DCs were enriched from C57BL/6 mice following previously described protocols [10,21]. In brief, spleens were injected with collagenase (type IV, 100 U/ml, Sigma) and placed in collagenase IV (400 U/ml) solution for 30 min at 37 °C and made into single cell suspensions. Single cells were recovered in 50% isoosmotic Nycodenz solution (Sigma), and centrifuged at 450 g. The low density fraction was adhered for 2 h and non-adherent cells were removed. After further 18 h, the detached cells were recovered. Alternatively, DCs were enriched by magnetic cell sorting (MACS) using CD11c selection columns (Miltenyi Biotec, Bergisch-Gladbach, Germany).
Bone marrow (BM) derived DCs (BMDCs) were generated from mouse BM as previously described [22]. DC populations were identified by co-expression of CD11c and CD11b; either procedure yielded ~ 90% CD11c+ cells.
For examining the effect of CTLA4-Ig, splenic DCs (1 × 106/ml per well) were plated in 24-well culture plates (IWAKI Europe, Willich, Germany) and exposed to CTLA4-Ig (40 μg/ml) [10] for 24 h. BMDCs were activated with LPS (Escherichia coli O111:B4, Calbiochem, San Diego, CA) and IFN-γ (BD Biosciences, San Diego, CA, USA) for 48 h as indicated. The DC phenotype was examined for MHC class I and class II, and CD80 and CD86 expression. T cells were enriched from Balb/c spleens using the Pan T Cell Isolation Kit (MACS; Miltenyi Biotec), routinely yielding > 95% CD3+ cells.
2.4. T cell stimulation and mixed lymphocyte reaction (MLR)
CD3+ T cells (1 × 105) were co-cultured with allogeneic DCs (1 × 104) for 3 to 6 d in 96-well round bottom plates (NUNC, Thermo Fisher, Rochester, NY, USA) in triplicates in 200 μl complete medium per well (MLR). For DC independent T cell proliferation assays, CD3+ T cells (1 × 105) were stimulated with 3 μg/ml immobilized anti-CD3 and 1 μg/ml anti-CD28 (BD Biosciences) for 48 h.
T cell proliferation was assessed by CFSE (Sigma) dilution as previously described [23]. Inhibition of proliferation was calculated as follows: Percent inhibition = [1 − (percent CFSE− T cells in co-cultures with CTLA4-Ig / percent CFSE− T cells in co-cultures without CTLA4-Ig)] × 100.
2.5. Flow cytometry
Flow cytometric examinations were performed using a FACSCalibur or a BD LSR II flow cytometer (BD Biosciences). List mode data were analyzed using either FACSDiva (BD Biosciences) or FlowJo (Tree Star, Ashland, OR, USA) software. The following Abs were used: unconjugated anti-CD16/32 (2.4G2), FITC-anti-H-2Db (KH95), PE-anti-I-Ab (AF6-120.1), PE-Cy7-anti-CD11c (HL3), APC-Cy7-anti-CD11b (M1/70), APC-anti-CD3 (145-2C11), PerCP-anti-CD4 (RM4-5), PE-Cy7-anti-CD25 (PC61) (all from BD Biosciences), PerCP/Cy5.5-anti-CD80 (16-10A1) and Alexa Fluor 700-anti-CD86 (PO3) (all from BioLegend, San Diego, CA, USA).
2.6. Enzyme linked immunosorbent assay (ELISA)
Splenic DCs or BMDCs (1 × 106/ml) were incubated with or without LPS (100 ng/ml), CTLA4-Ig (50 μg/ml) and/or human IgG1 (50 μg/ml) (Sigma) for 24 h. Interferon-gamma (IFN-γ) was measured in culture supernatants by ELISA (BD OptEIA mouse IFN-γ ELISA set, BD Biosciences), or mouse IFN-γ ELISA (Ready-SET-Go!, eBiosciences, San Diego, CA, USA). Optical densities were analysed using an EnSpire reader (PerkinElmer, Waltham, MA, USA).
2.7. Immunoblotting
IDO protein expression in DCs was investigated using a rabbit anti-mouse IDO polyclonal Ab kindly provided by O. Takikawa (National Institute for Longevity Sciences, National Center for Geriatrics and Gerontology, Japan) [24]. Mouse monoclonal anti-mouse GAPDH antibody (Ambion, Austin, TX, USA) was used as an internal control. Ab binding was visualized using the Odyssey Infrared Imaging System (Odyssey Classic, LI-COR Biosciences, Lincoln, NE, USA) and the respective fluorescent secondary Abs: goat anti-rabbit IgG, DyLight800 conjugated and goat anti-mouse IgG, DyLight680 conjugated (Pierce Biotechnology, Rockford, IL, USA). Densitometric analysis was done using the ImageJ freeware (NIH, Bethesda, MD, USA).
2.8. IDO mRNA detection
Expression levels of IDO transcript in DCs were determined by semiquantitative RT-PCR. In brief, total RNA was isolated from cells with the use of Trizol reagent (Invitrogen, Lofer, Austria). RNA was reversely transcribed with 200 Units Moloney-murine leukemia virus RT (Invitrogen) and 100 pmol random hexamers (GE Healthcare, Vienna, Austria) at 42 °C for 1 h. RT-PCR was performed using Hot Start Taq polymerase (Qiagen, Vienna, Austria) with an initial activation step at 95 °C for 14 min according to the manufacturer's instructions.
Cycling conditions were as follows: denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and elongation at 72 °C for 1 min. 35 cycles were followed by a final extension at 72 °C for 7 min.
Oligonucleotides (MWG Biotech AG, Ebersberg, Germany) used for amplification of the murine IDO or of the murine GAPDH were as follows: IDO, 5′-CGACATAGCTACCA GTCTGGAGAAAG-3′ and 5′-GCGAGGTGGAACTTTCTCACAGAG-3′; GAPDH, 5′-AC CACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. Amplification products were size-fractionated by agarose gel electrophoresis on a 1% agarose gel, stained with ethidium bromide and quantified by scanning densitometry (Gel-Doc 1000, Molecular Analyst Software, Biorad, Hercules, CA, USA).
2.9. Quantification of tryptophan, kynurenine and nitrite
IDO enzymatic activity was determined by measuring the levels of tryptophan and kynurenine in the cell culture supernatants by HPLC as described [25]. Synthesis of the stable NO metabolite nitrite (NO2−) was determined in the cell-free culture supernatants by the Griess reaction assay [26]. In brief, sulfanilamide was quantitatively converted to a diazonium salt through reaction with NO2− (present in samples) in acid (phosphoric acid) conditions. The diazonium salt was then coupled to N(1-naphthyl) ethylenediamine dihydrochloride (NED), forming an azo dye that was read at 540 nm in a spectrophotometer.
2.10. CTLA4-Ig internalization/dissociation
C57BL/6 BMDCs identified as CD11c+ DCs were incubated with CTLA4-Ig (10–100 μg/ml) for 30 min at 4 °C. After two wash procedures, DCs were incubated 2, 4 and 16 h at 37 °C and 4 °C to allow and dampen receptor turnover, respectively, thus estimating internalization and dissociation [27]. Subsequently, DC were thoroughly washed and incubated with a PE-conjugated AffiniPure F(ab′)2 fragment donkey anti-human IgG antibody (Jackson ImmunoResearch Lab Inc., West Grove, PA, USA) for 30 min at 4 °C and were examined by flow cytometry. The mean fluorescence intensity (MFI) of CTLA4-Ig-IgG (PE) was examined to quantitatively estimate the presence of CTLA4-Ig on the DC surface. In parallel, DCs were incubated with total human IgG (Beriglobin P, ZLB Behring GmbH, Vienna, Austria) and subjected to the same procedure to determine the extent of unspecific Fc binding [28].
2.11. Statistical analysis
All statistical analyses were performed with the Student t test (paired, 2-tailed). A P-value < 0.05 was considered to indicate statistical significance.
3. Results
3.1. CTLA4-Ig binds to CD80/86 in C57BL/6 splenic and BMDCs
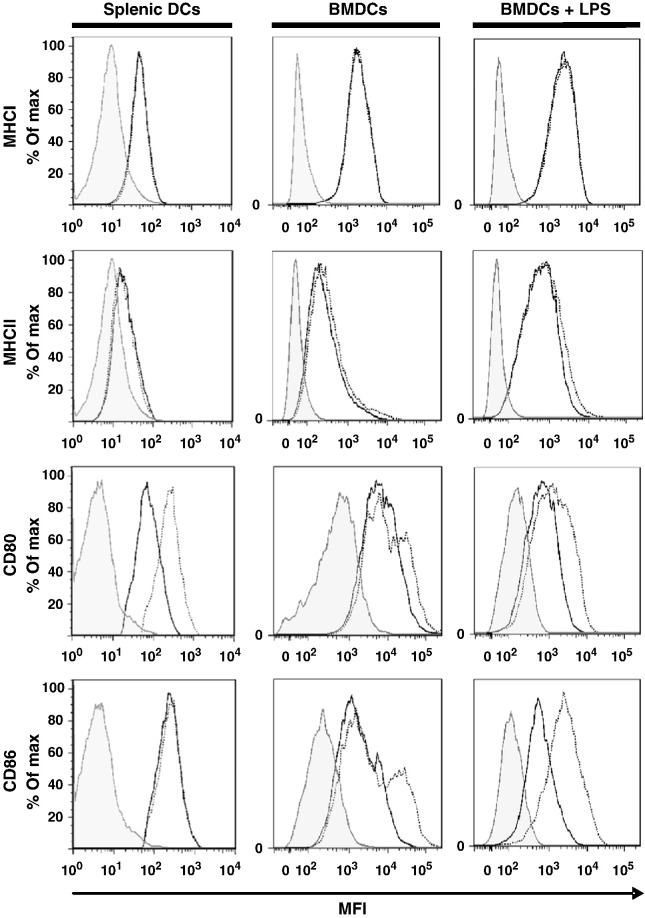
CD11c+ DCs enriched from C57BL/6 spleens displayed a mature phenotype, indicated by MHC class I/II expression and by constitutive expression of high levels of the costimulatory molecules CD80 and CD86 [29,30]. Exposure of these DCs to CTLA4-Ig reduced the detectability of costimulatory CD80 and CD86 molecules with the notion that CTLA4-Ig particularly bound to the CD80 epitope (Fig. 1, left panel) [31]. Consistent with the specificity of CTLA4-Ig, no effect was observed on the expression levels of MHC class I/II molecules.
Fig. 1.
Effect of CTLA4-Ig on costimulatory molecule expression in C57BL/6 splenic and BMDCs. Splenic DCs (left column) were incubated for 24 h with CTLA4-Ig (40 μg/ml). BMDCs (middle, right column) were incubated for 24 h without or with LPS stimulation (100 ng/ml) and were subsequently exposed to CTLA4-Ig (100 μg/ml, 2 h). After washing, DCs were stained for MHC class I and II, CD80 and CD86 and analyzed by flow cytometry. Shaded histograms, isotype control; open histograms, DCs without (dotted line) or with (black line) CTLA4-Ig. MFI, mean fluorescence intensity. One representative experiment of 3 experiments is shown.
The pattern of co-stimulatory molecule expression in BMDCs was different. In the unstimulated CD11c+ BMDC population only a fraction expressed CD80 and CD86 costimulatory molecules. As expected, stimulation with LPS increased the levels of MHC class I and class II expression and, in parallel, the CD11c+ population showed particularly increased levels of expression of both CD80 and CD86 costimulatory molecules. Exposing BMDCs to CTLA4-Ig reduced the detectability of CD80 and CD86 in unstimulated as well as in stimulated DCs, indicating that CTLA4-Ig effectively prevented CD80 and CD86 antibodies from binding to their epitopes (Fig. 1, middle and right panel).
3.2. Upon removal from the microenvironment, CTLA4-Ig rapidly disappears from DC surface
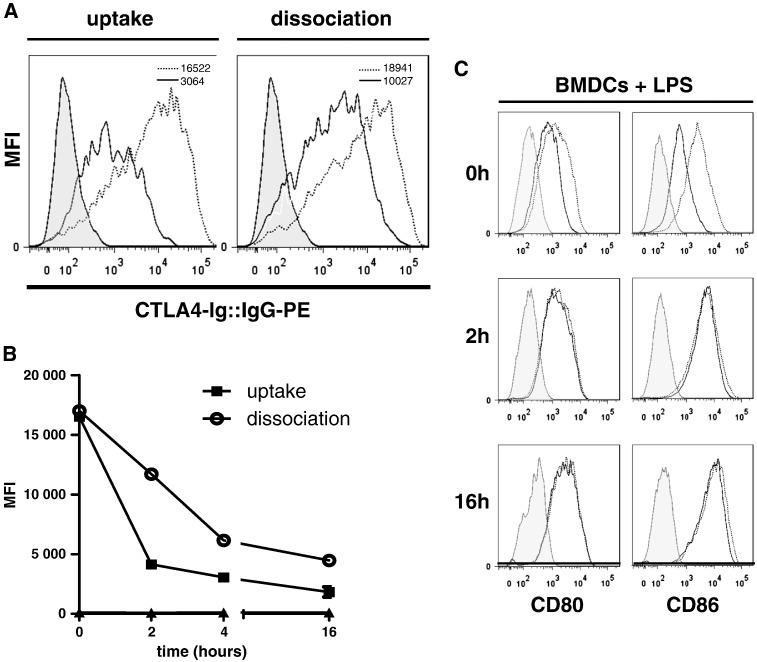
To assess whether after binding to CD80/86 CTLA4-Ig is stably present at the surface of DCs, we examined the time course of CTLA4-Ig's detectability on the DC membrane considering cellular uptake by receptor turnover and dissociation. To pursue this question, we devised an experiment, in which we incubated C57BL/6 BMDCs with CTLA4-Ig and either maintained DCs at 37 °C to estimate cellular uptake or exposed the DCs to the cold (4 °C) to dampen receptor turnover and estimate CTLA4-Ig dissociation [27]. At the indicated time points we assessed the quantity of CTLA4-Ig present at the DC surface by measuring the MFI of a PE labeled anti-IgG molecule by flow cytometry.
To ascertain that the PE labeled anti-IgG antibody specifically detects CTLA4-Ig bound to CD80 or CD86 molecules, cells were incubated with or without CTLA4-Ig or human immunoglobulin (Beriglobin, 100 μg/ml) at 37 °C and then labeled with the PE labeled anti-IgG antibody. Flow cytometric analyses revealed that staining with the PE labeled anti-IgG antibody only was barely detectable at the DC surface and after pre-exposure to total IgG was 50% lower than upon exposure to CTLA4-Ig, thus reassuring the detectability of specific binding (Supplementary Fig. S1).
Next, to evaluate internalization by receptor turnover, we incubated DCs with CTLA4-Ig for 30 min at 4 °C to allow binding and then washed the cells extensively. Subsequently, DCs were incubated at 37 °C for 4 h to allow CTLA4-Ig uptake by receptor turnover. Flow cytometric analyses revealed a rapid decrease of the MFI of PE-labeled anti-IgG antibody, which dropped by mean 52%, 63% and 80% after 2, 4 and 16 h of culture, respectively (n = 3), after removing CTLA4-Ig from the cellular microenvironment. This observation is consistent with a rapid internalization of a major proportion of CTLA4-Ig (Fig. 2A, B).
Fig. 2.
CTLA4-Ig is rapidly removed from the DC surface by internalization and dissociation.
(A) C57BL/6 BMDCs were incubated without (shaded histogram) or with 100 μg/ml CTLA4-Ig for 4 h, washed and stained with a phycoerythrin (PE) labeled anti-IgG antibody and analyzed by flow cytometry either immediately after CTLA4-Ig exposure (dotted line) or after a further 16-hour incubation period at 37 °C (left panel, black line, estimating internalization) or at 4 °C (right panel, black line, estimating dissociation). The inserts in the histograms depict the MFI. One representative experiment of 3 experiments is shown.
(B) C57BL/6 BMDCs were incubated with 100 μg/ml CTLA4-Ig as in (A), washed and incubated at 37 °C (filled rectangles) or at 4 °C (open circles) for increasing time periods, indicated as hours. Then, cells were stained with anti-human IgG-PE, as above. The level of expression was examined as the MFI of histogram analyses.
(C) LPS (100 ng/ml) stimulated C57BL/6 BMDCs (24 h), were incubated with CTLA4-Ig (100 μg/ml) for 2 h and washed. Subsequently, cells were cultured in complete medium without CTLA4-Ig for further 2 and 16 h and the expression levels of CD80 and CD86 were analysed by flow cytometry. Shaded histogram, isotype control; open histograms, DCs without (dotted line) or with (black line) CTLA4-Ig. One representative experiment of 2 experiments is shown. MFI, mean fluorescence intensity.
To examine a potential passive dissociation of CTLA4-Ig from the DC surface, DCs were exposed to CTLA4-Ig and extensively washed as above and incubated for 4 h at 4 °C. Since low temperatures are known to dampen receptor turnover, a reduction of the cell-surface located CTLA4-Ig reflects dissociation rather than uptake. In this setting, the MFI of bound PE-labeled anti-IgG antibody dropped by mean 22%, 45% and 75% at 2, 4 and 16 h, respectively (n = 3), after removing CTLA4-Ig (Fig. 2A, B).
Finally, to address the kinetics of re-expression of CD80 and CD86 after blockade, we exposed DCs to CTLA4-Ig and washed them as above and re-examined the levels of CD80 and CD86 expression at 2 and 16 h later. Remarkably, the levels of expression of CD80 and CD86 of CTLA4-Ig exposed DCs were comparable to DCs that had not been exposed to CTLA4-Ig already 2 h after removing CTLA4-Ig from the microenvironment, indicating that the expression of costimulatory molecules is rapidly restored once CTLA4-Ig is removed (Fig. 2C).
Taken together, the results indicated that (i) the major portion of bound CTLA4-Ig disappeared from the DC surface within 2–4 h after removal of unbound CTLA4-Ig from the cellular microenvironment by combined receptor turnover and dissociation and that (ii) the DCs rapidly recovered the costimulatory signaling apparatus.
3.3. CTLA4-Ig does not stimulate DCs to acquire IDO competence
So far, the findings suggested that binding of CTLA4-Ig to CD80/86 at the DC surface is short-lived. To explore a potential signaling effect of CTLA4-Ig to DCs [10,32], we tested whether CTLA4-Ig stimulated IFN-γ production [32] which was reported to be linked to expression and activity of the tryptophan metabolizing and immunosuppressive enzyme IDO [10] by DCs.
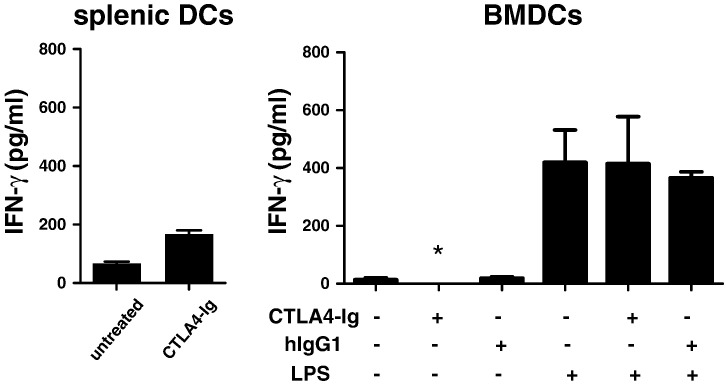
We first observed that a 24-hour exposure of splenic DCs to CTLA4-Ig resulted in a slight increase of IFN-γ secretion (~ 200 pg/ml, mean of 3 experiments), similar to a previous report [32] (Fig. 3, left panel). In contrast, in BMDCs, IFN-γ release was inducible only by exposure to LPS but remained undetectable when BMDCs were exposed to 50 μg/ml CTLA4-Ig (Fig. 3, right panel) and even when the dose of CTLA4-Ig was increased up to 100 μg/ml (not shown). As well, a human IgG1 isotype binding solely to DC Fc receptors was unable to stimulate DC IFN-γ production. Together, the findings indicate that the ability of CTLA4-Ig to stimulate low-level IFN-γ secretion was restricted to splenic DCs.
Fig. 3.
Effect of CTLA4-Ig on IFN-γ production in C57BL/6 splenic and BMDCs.
C57BL/6 splenic DCs (left panel) were incubated with CTLA4-Ig (40 μg/ml) for 24 h, C57BL/6 BMDCs (right panel) were incubated with or without LPS (100 ng/ml), CTLA4-Ig (50 μg/ml) and/or human IgG1 (50 μg/ml) for 24 h. Cell culture supernatants were examined for IFN-γ release by ELISA. *Below detection limit.
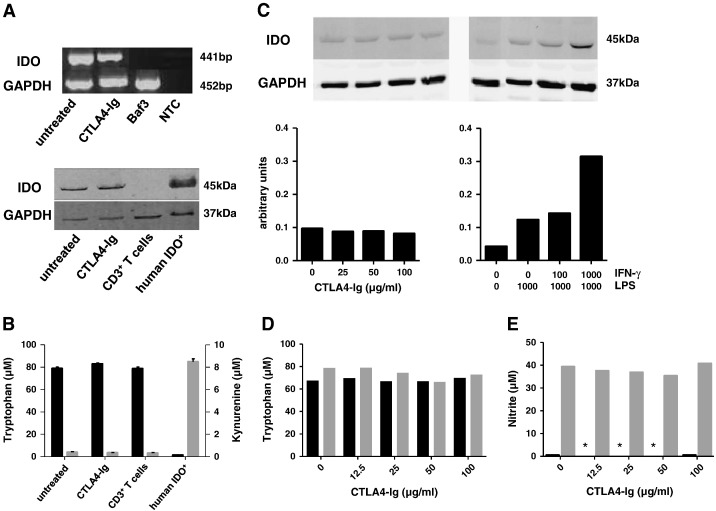
As our findings did not consistently support the concept of CTLA4-Ig stimulated DC IFN-γ production, in a next step, we straightforwardly investigated whether CTLA4-Ig induced IDO expression and activity. Our data did not support this concept. In splenic DCs, consistent with previous reports [33], some basal levels of expression of IDO mRNA were detected by RT-PCR even in the absence of CTLA4-Ig (Fig. 4A, upper panel). These baseline levels of IDO mRNA expression did not increase by exposure of DCs to CTLA4-Ig. Likewise, baseline levels of IDO protein expression detectable by immunoblotting [32] remained at the same level upon exposing DCs to CTLA4-Ig (Fig. 4A, lower panel). In consistency with these findings, CTLA4-Ig did not induce DC IDO activity. In fact, neither a decrease of the levels of tryptophan nor an increase of the tryptophan metabolite kynurenine was detectable in the supernatants of CTLA4-Ig exposed splenic DCs as compared to the untreated cells (Fig. 4B).
Fig. 4.
CTLA4-Ig does not induce IDO activity in C57BL/6 splenic and BMDCs.
(A) Upper panel: IDO mRNA expression in splenic DCs was analyzed by RT-PCR. Total RNA of murine Baf3 cells was used as negative control and GAPDH as internal standard; NTC, non template control. Lower panel: IDO protein expression was analyzed in the same DCs as described above. Human DCs stimulated by LPS/IFN-γ as described [25] served as positive and murine CD3 + T cells as negative controls. Internal standard, GAPDH. All samples were run on one gel. One representative of three experiments is shown.
(B) Mean concentrations of tryptophan (black bars, left scale) and kynurenine (gray bars, right scale) in cell culture supernatants of the same DCs were determined by HPLC (n = 3).
(C) Upper panel: BMDCs were left unstimulated or were exposed to increasing concentrations of CTLA4-Ig (left panel) or to LPS (1000 ng/ml) and increasing concentrations of IFN-γ (right panel, given as U/ml) for 24 h; expression of IDO protein was examined by immunoblotting as in (A); lower panel: The relative increase of IDO protein expression in relation to the internal standard, GAPDH, was calculated as arbitrary units using the ImageJ freeware. One representative of two experiments is shown.
(D) The concentrations of tryptophan in unstimulated (black bars) or LPS/IFN-γ stimulated (gray bars) cultures were determined in cell culture supernatants.
(E) In parallel to (D), cell culture supernatants were examined for the concentrations of nitrite. *Below detection limit.
In BMDCs, like in splenic DCs, a basal expression of IDO protein was detectable even in unstimulated cells. Similarly, when BMDCs were exposed to increasing concentrations of CTLA4-Ig (up to 100 μg/ml), IDO protein expression remained at the same level (Fig. 4C). Only upon activation with LPS and IFN-γ, IDO protein expression became detectable by immunoblotting (Fig. 4C). Remarkably, like in splenic DCs, despite IDO expression at the protein level, IDO enzymatic activity remained undetectable in BMDCs irrespective whether or not they were exposed to CTLA4-Ig or stimulated with LPS/IFN-γ, as depicted by unchanged tryptophan (Fig. 4D) and low kynurenine (not shown) concentrations in cell culture supernatants after 24 h of stimulation. Thus, in this model, CTLA4-Ig failed to induce IDO enzymatic activity in C57BL/6 splenic and BMDCs.
To address possible mechanisms for the divergence of IDO protein expression without IDO enzymatic activity, we examined the production of nitric oxide by BMDCs. Nitric oxide is known to inhibit IDO enzymatic activity [34] by interfering with the oxidation of the parent IDO protein. We found that nitric oxide, as determined by the concentration of nitrite, was enhanced in C57BL/6 BMDCs stimulated by LPS/IFN-γ, suggesting that IDO activity in LPS/IFN-γ stimulated DCs expressing IDO protein was inhibited by the IDO competitor nitric oxide (Fig. 4E).
3.4. CTLA4-Ig directly affects the interaction of stimulator and responder cells
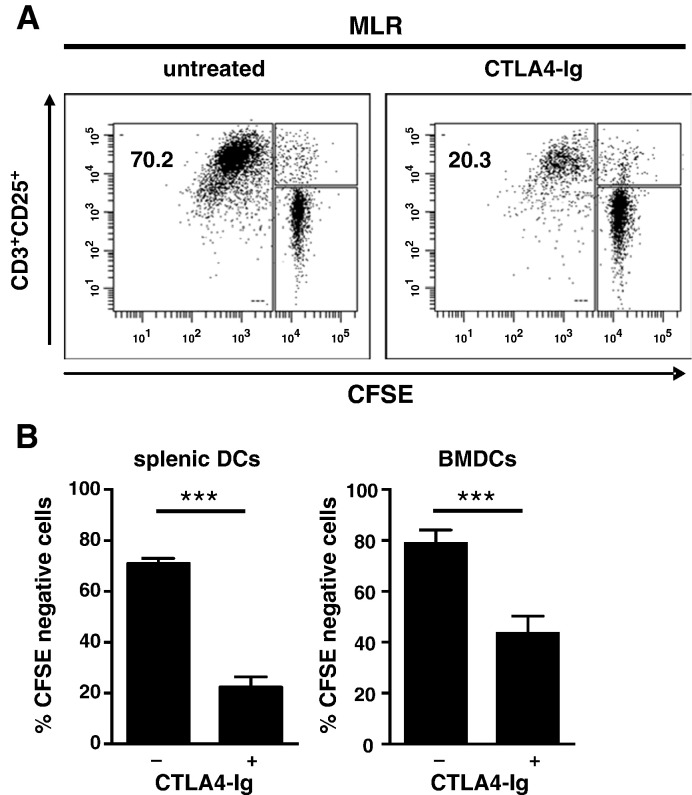
The above findings did not support the concept of CTLA4-Ig inducing a DC regulatory phenotype via IDO in this model. Therefore, in the next series of experiments, we explicitly addressed the cellular mechanism of CTLA4-Ig mediated immune regulatory activity by separating the interacting cell populations, DCs and T cells. First, we confirmed that C57BL/6 splenic as well as BMDCs were potent stimulators of allogeneic Balb/c T cells. In fact, co-culturing splenic or BMDCs with allogeneic highly enriched T cells in a 1:10 DC:T cell ratio resulted in a substantial proliferative T cells response indicated by > 70% CFSE negative T cells after a 6 day co-culture (Fig. 5A, B).
Fig. 5.
CTLA4-Ig inhibits the allogeneic response stimulated by C57BL/6 splenic DCs or BMDCs.
(A) C57BL/6 splenic DCs were co-cultured with allogeneic CFSE labeled Balb/c T cells at a 1:10 DC:T cell ratio in the absence or presence of CTLA4-Ig (100 μg/ml) for 6 days. T cells were identified as CD3+ cells and were analyzed for CD25 expression and CFSE dilution (i.e. proliferation). Inserted numbers indicate the fraction of CD25+CFSE− T cells.
(B) Summary (mean ± SEM) of three independently performed experiments (as in A) in which splenic DCs (left) or BMDCs (right) were used as stimulator cells. ***P < 0.001.
Next, to demonstrate the inhibitory activity of CTLA4-Ig, we added 100 μg/ml CTLA4-Ig to co-cultures and, as expected, observed a robust inhibition of proliferation of 68% ± 5% (mean ± SEM) in splenic DCs (P < 0.001) (Fig. 5B, left) [35] and a slightly smaller inhibition (45 ± 5%) in BMDCs (P < 0.001) (Fig. 5B, right).
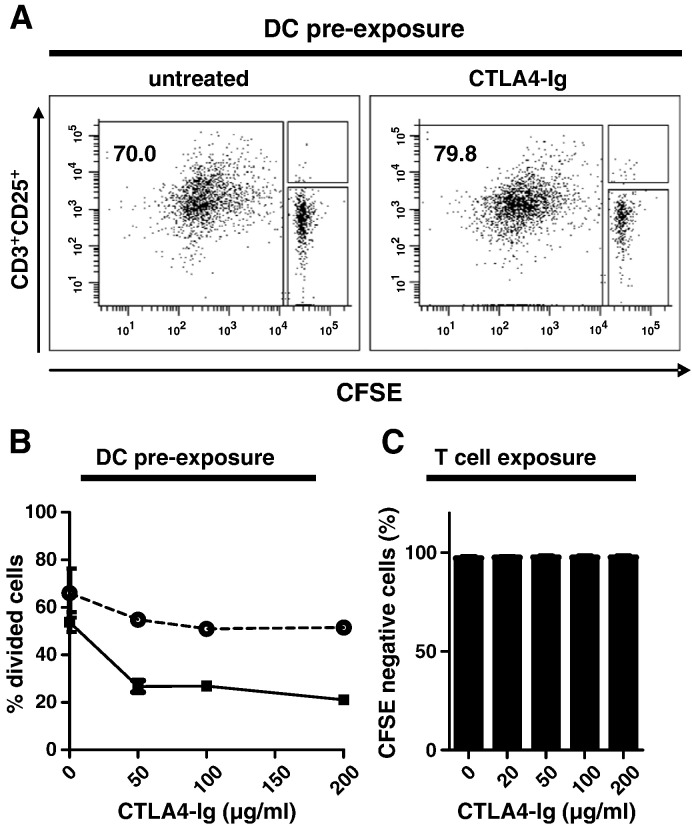
To address whether CTLA4-Ig specifically affects the stimulatory function of DCs, we devised an experiment in which the DC population was pre-exposed to CTLA4-Ig before being used for stimulating allogeneic T cells. In detail, C57BL/6 splenic DCs were exposed to CTLA4-Ig for 24 h and thoroughly washed to remove unbound CTLA4-Ig; thereafter, DCs were co-cultured with Balb/c T cells in the absence of CTLA4-Ig. Noticeably, a pre-exposure of DCs to CTLA4-Ig did not affect their subsequent stimulatory capacity. Indeed, C57BL/6 splenic DCs induced a comparable allogeneic T cell proliferative response (> 70% CFSE negative T cells), irrespective of whether DCs were left untreated or pre-exposed to CTLA4-Ig (Fig. 6A). The same was observed when BMDCs were pre-exposed to CTLA4-Ig and washed prior to co-culture with allogeneic T cells (Fig. 6B, dotted line). Only when CTLA4-Ig was present throughout DC/T cell co-cultures, the proliferative T cell response to allogeneic stimulation was inhibited (Fig. 6B, solid line).
Fig. 6.
CTLA4-Ig does not confer a DC regulatory phenotype but acts at the level of DC/T cell interaction.
(A) C57BL/6 splenic DCs were exposed to CTLA4-Ig (100 μg/ml, 24 h) or maintained in medium only and washed prior to co-culture with allogeneic Balb/c T cells. T cells were analyzed after 6 days of DC/T cell co-culture in the absence of CTLA4-Ig. One representative of three experiments is shown.
(B) BMDCs were exposed to increasing concentrations of CTLA4-Ig for 24 h. Thereafter, DCs were washed and co-cultured with allogeneic Balb/c T cells in the presence (black squares, solid line) or absence (open circles, dashed line) of CTLA4-Ig for 6 days. T cells were analyzed as in (A). One representative of three experiments is shown.
(C) Purified Balb/c CD3+ T cells were stimulated with plate bound anti-CD3/anti-CD28 for 48 h in the absence or presence of increasing concentrations of CTLA4-Ig. T-cell proliferation was assessed by CFSE dilution. One representative of two experiments is shown.
To exclude that CTLA4-Ig affected the T cell response independently of binding to DCs, we performed DC independent T cell proliferation assays by stimulating T cells with plate-bound anti-CD3/anti-CD28 in the presence of increasing concentrations of CTLA4-Ig. In these experiments, CTLA4-Ig did not reduce T cell proliferation even at high concentrations (200 μg/ml) of CTLA4-Ig (Fig. 6C). Furthermore, CTLA4-Ig did not have a toxic effect on T cells as assessed by DAPI/Annexin V staining (not shown). To examine whether CTLA4-Ig induced IDO activity during the course of the DC/T cell co-culture, we examined the concentrations of tryptophan and kynurenine in cell culture supernatants at the end of the MLR. The data did not indicate any tryptophan consumption or kynurenine accumulation (Supplementary Table 1, S2), thus corroborating that immunoregulatory activity of CTLA4-Ig does not depend on IDO activity.
In summary, our data indicated that CTLA4-Ig (i) rapidly disappeared from the DC surface once removed from the microenvironment, (ii) failed to induce IDO activity and (iii) did not elicit a DC regulatory phenotype, but (iv) exerted suppressor activity at the level of DC/T cell interaction.
4. Discussion
The present study focused particularly on the relevance of reverse signaling for better understanding the immune regulatory mechanism of CTLA4-Ig. In composite, our data do not support the view that reverse signaling is critical to CTLA4-Ig mediated immune regulation.
CTLA4-Ig has been originally developed based on the concept that specific binding to CD80/CD86 molecules expressed on DCs interrupts CD28 mediated signaling to T cells (reviewed in [4,6]) and thus can propagate the emergence of anergy [36]. This effect of CTLA4-Ig has been solidly evidenced in numerous in vitro and in vivo studies [2,37,38]. The recently reported aspect of CTLA4-Ig activity – claiming that CTLA4-Ig binding to CD80/86 transmits a signal back to DCs to ultimately result in activation of the immunomodulatory IDO pathway [10,11] – laid the basis for conceptualizing immunoregulatory CTLA4-Ig to promote a DC regulatory phenotype in an IDO dependent fashion. In their study, Grohmann et al. [10] found that IDO competence was induced in DCs upon exposure to a CTLA4-Ig preparation in which the CTLA-4 molecule was fused with IgG3 immunoglobulin tail in a JAK-STAT1 dependent manner involving IFN-γ [32]. In a subsequent study, Munn et al. found that IDO induction by CTLA4-Ig was restricted to the B220+CD8α+ DC subset and did not require IFN-γ for IDO induction [11]. Crediting CTLA4-Ig with the capability of inducing IDO via reverse signaling as a general mechanism has stimulated a controversial debate. Pree et al. showed that CTLA4-Ig, while being essential for the development of mixed chimerism in a murine model of non-myeloablative bone marrow transplantation, did not involve tryptophan metabolism and its effect was not reversed by inhibition of IDO [39]. In addition, several further murine and human studies reported that the tolerogenic effect of CTLA4-Ig occurred without the involvement of IDO [20,40]. Finally, microarray gene analysis of human immature and mature DCs exposed to two clinically probed CTLA4-Ig compounds, abatacept or belatacept, failed to reveal any significant changes in gene expression, thus questioning the relevance of reverse signaling of CTLA4-Ig to DCs [41].
In the present study, two lines of evidence stringently argue against the concept of CTLA4-Ig inducing a DC regulatory phenotype via IDO. (1) DCs exposed to CTLA4-Ig and washed prior to co-culture with allogeneic T cells were fully capable of stimulating T cell proliferation. Upon removal from the microenvironment, CTLA4-Ig disappeared rapidly from the DC membrane and thereafter had no enduring effect on the DC stimulatory capacity, which would be expected if DCs acquired regulatory activity from CTLA4-Ig mediated reverse signaling. (2) The explicit examination of DC IDO activity failed to generate evidence that CTLA4-Ig induced tryptophan breakdown. The only slight induction of IFN-γ by CTLA4-Ig in splenic DCs [32] was not reproducible in BMDCs. Apparently, CTLA4-Ig was unable to induce IDO activity or any other immune regulatory effect in both DC populations. Of note, the pharmacologically designed CTLA4-Ig fusion protein, abatacept, containing a human IgG1 Fc-portion was used throughout the hereby presented experiments. In addition, the testing of a recombinant mouse cytolytic CTLA4-Ig (IgG2a, Sigma) fusion protein gave the same results (data not shown). This indicates that the absence of reverse signaling by CTLA4-Ig in this study was not related to the different IgG portions (IgG1, IgG2a) of the CTLA4-Ig fusion protein.
The problem of discrepant findings in the various studies addressing an association of CTLA4-Ig and IDO induction remains unsolved at this point. States of IDO expression on an mRNA and protein level without concomitant tryptophan metabolizing activity have been described. These states are particularly associated with alterations of the post-translational modifications, i.e. the oxidation of the ferric to the ferrous form of the heme moiety of IDO which is essential to initiate IDO enzymatic activity [17,34]. Nitric oxide is known as an important inhibitor of IDO enzymatic activity and may contribute to the failure of murine LPS/IFN-γ-stimulated C57BL/6 CD11c+ DCs to exhibit IDO enzymatic activity as we observed in this study [34]. As well, seemingly trivial differences in experimental procedures or CTLA4-Ig preparations or in utilizing different DC:T cell ratios in the MLR may underlie the discrepancies, as recently discussed [25,42,43].
From our findings, we conclude that CTLA4-Ig is not effective in generating regulatory DCs but exerts potent immunoregulatory activity by affecting the DC/T cell crosstalk. This conclusion has a major implication for designing immunoregulatory cell therapies. CTLA4-Ig apparently has no durable effect on DCs, implying that CTLA4-Ig exposed DCs cannot be expected to display immune regulatory activity upon adoptive transfer. Rather, the efficacy of CTLA4-Ig blocking the CD28:CD80/86 pathway for the generation of T cell anergy can be exploited by co-culturing T cells with APCs in the continuous presence of CTLA4-Ig ex vivo prior to adoptive transfer of anergic T cells, as has been recently described [44].
Disclosure
The authors of this manuscript have no conflicts of interest to disclose.
Acknowledgment
This work was supported by a grant from the Austrian Science Fund (FWF # 19865 to AH) and by the St. Anna Kinderkrebsforschung e.V.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.intimp.2013.02.007.
Appendix A. Supplementary data
Supplementary materials.
References
- 1.Nurieva R.I., Liu X., Dong C. Yin–Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev. 2009;229:88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gribben J.G., Guinan E.C., Boussiotis V.A., Ke X.Y., Linsley L., Sieff C. Complete blockade of B7 family-mediated costimulation is necessary to induce human alloantigen-specific anergy: a method to ameliorate graft-versus-host disease and extend the donor pool. Blood. 1996;87:4887–4893. [PubMed] [Google Scholar]
- 3.Larsen C.P., Knechtle S.J., Adams A., Pearson T., Kirk A.D. A new look at blockade of T-cell costimulation: a therapeutic strategy for long-term maintenance immunosuppression. Am J Transplant. 2006;6:876–883. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 4.Wekerle T., Kurtz J., Bigenzahn S., Takeuchi Y., Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol. 2002;14:592–600. doi: 10.1016/s0952-7915(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 5.Linsley P.S., Nadler S.G. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009;229:307–321. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone J.A., St Clair E.W., Turka L.A. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Vincenti F. Costimulation blockade in autoimmunity and transplantation. J Allergy Clin Immunol. 2008;121:299–306. doi: 10.1016/j.jaci.2008.01.002. [quiz 7–8] [DOI] [PubMed] [Google Scholar]
- 8.Herrero-Beaumont G., Martinez Calatrava M.J., Castaneda S. Abatacept mechanism of action: concordance with its clinical profile. Reumatol Clin. 2012;8:78–83. doi: 10.1016/j.reuma.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Su V.C., Harrison J., Rogers C., Ensom M.H. Belatacept: a new biologic and its role in kidney transplantation. Ann Pharmacother. 2012;46:57–67. doi: 10.1345/aph.1Q537. [DOI] [PubMed] [Google Scholar]
- 10.Grohmann U., Orabona C., Fallarino F., Vacca C., Calcinaro F., Falorni A. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 11.Munn D.H., Sharma M.D., Mellor A.L. Ligation of B7-1/B7-2 by human CD4(+) T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 12.Moffett J.R., Namboodiri M.A. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 13.Munn D.H., Shafizadeh E., Attwood J.T., Bondarev I., Pashine A., Mellor A.L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanzinger M., Jurgens B., Hainz U., Dillinger B., Raberger J., Fuchs D. Ambivalent effects of dendritic cells displaying prostaglandin E2-induced indoleamine 2,3-dioxygenase. Eur J Immunol. 2012;42:1117–1128. doi: 10.1002/eji.201141765. [DOI] [PubMed] [Google Scholar]
- 15.Fallarino F., Grohmann U., Vacca C., Bianchi R., Orabona C., Spreca A. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 16.Mellor A.L., Munn D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 17.Heitger A. Regulation of expression and function of IDO in human dendritic cells. Curr Med Chem. 2011;18:2222–2233. doi: 10.2174/092986711795656018. [DOI] [PubMed] [Google Scholar]
- 18.Alegre M.L., Fallarino F. Mechanisms of CTLA-4-Ig in tolerance induction. Curr Pharm Des. 2006;12:149–160. doi: 10.2174/138161206775193046. [DOI] [PubMed] [Google Scholar]
- 19.Linsley P.S., Wallace P.M., Johnson J., Gibson M.G., Greene J.L., Ledbetter J.A. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 20.Davis P.M., Nadler S.G., Stetsko D.K., Suchard S.J. Abatacept modulates human dendritic cell-stimulated T-cell proliferation and effector function independent of IDO induction. Clin Immunol. 2008;126:38–47. doi: 10.1016/j.clim.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Mellor A.L., Chandler P., Baban B., Hansen A.M., Marshall B., Pihkala J. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 22.Huttner K.G., Breuer S.K., Paul P., Majdic O., Heitger A., Felzmann T. Generation of potent anti-tumor immunity in mice by interleukin-12-secreting dendritic cells. Cancer Immunol Immunother. 2005;54:67–77. doi: 10.1007/s00262-004-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons A.B., Parish C.R. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S., Tone S., Takikawa O., Kubo T., Kohno I., Minatogawa Y. Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem J. 2001;355:425–429. doi: 10.1042/0264-6021:3550425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jurgens B., Hainz U., Fuchs D., Felzmann T., Heitger A. Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood. 2009;114:3235–3243. doi: 10.1182/blood-2008-12-195073. [DOI] [PubMed] [Google Scholar]
- 26.Giustarini D., Dalle-Donne I., Colombo R., Milzani A., Rossi R. Adaptation of the Griess reaction for detection of nitrite in human plasma. Free Radic Res. 2004;38:1235–1240. doi: 10.1080/10715760400017327. [DOI] [PubMed] [Google Scholar]
- 27.Holzl M.A., Hofer J., Kovarik J.J., Roggenbuck D., Reinhold D., Goihl A. The zymogen granule protein 2 (GP2) binds to scavenger receptor expressed on endothelial cells I (SREC-I) Cell Immunol. 2011;267:88–93. doi: 10.1016/j.cellimm.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis P.M., Abraham R., Xu L., Nadler S.G., Suchard S.J. Abatacept binds to the Fc receptor CD64 but does not mediate complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity. J Rheumatol. 2007;34:2204–2210. [PubMed] [Google Scholar]
- 29.Anjuere F., Martin P., Ferrero I., Fraga M.L., del Hoyo G.M., Wright N. Definition of dendritic cell subpopulations present in the spleen, Peyer's patches, lymph nodes, and skin of the mouse. Blood. 1999;93:590–598. [PubMed] [Google Scholar]
- 30.Shortman K., Liu Y.J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 31.Linsley P.S., Greene J.L., Brady W., Bajorath J., Ledbetter J.A., Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 32.Vacca C., Fallarino F., Perruccio K., Orabona C., Bianchi R., Gizzi S. CD40 ligation prevents onset of tolerogenic properties in human dendritic cells treated with CTLA-4-Ig. Microbes Infect. 2005;7:1040–1048. doi: 10.1016/j.micinf.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Fallarino F., Vacca C., Orabona C., Belladonna M.L., Bianchi R., Marshall B. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14:65–68. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 34.Thomas S.R., Terentis A.C., Cai H., Takikawa O., Levina A., Lay P.A. Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J Biol Chem. 2007;282:23778–23787. doi: 10.1074/jbc.M700669200. [DOI] [PubMed] [Google Scholar]
- 35.Linhart B., Bigenzahn S., Hartl A., Lupinek C., Thalhamer J., Valenta R. Costimulation blockade inhibits allergic sensitization but does not affect established allergy in a murine model of grass pollen allergy. J Immunol. 2007;178:3924–3931. doi: 10.4049/jimmunol.178.6.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fathman C.G., Lineberry N.B. Molecular mechanisms of CD4 + T-cell anergy. Nat Rev Immunol. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 37.Gimmi C.D., Freeman G.J., Gribben J.G., Gray G., Nadler L.M. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci U S A. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenschow D.J., Zeng Y., Thistlethwaite J.R., Montag A., Brady W., Gibson M.G. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 39.Pree I., Bigenzahn S., Fuchs D., Koporc Z., Nierlich P., Winkler C. CTLA4Ig promotes the induction of hematopoietic chimerism and tolerance independently of Indoleamine-2,3-dioxygenase. Transplantation. 2007;83:663–667. doi: 10.1097/01.tp.0000255594.23445.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ierino F.L., Mulley W., Dodge N., Li Y.Q., Mouhtouris E., Christiansen D. Dendritic cells expressing soluble CTLA4Ig prolong antigen-specific skin graft survival. Immunol Cell Biol. 2010;88:846–850. doi: 10.1038/icb.2010.58. [DOI] [PubMed] [Google Scholar]
- 41.Carman J.A., Davis P.M., Yang W.P., Zhu J., Chang H., He A. Abatacept does not induce direct gene expression changes in antigen-presenting cells. J Clin Immunol. 2009;29:479–489. doi: 10.1007/s10875-009-9282-z. [DOI] [PubMed] [Google Scholar]
- 42.Terness P., Chuang J.J., Bauer T., Jiga L., Opelz G. Regulation of human auto- and alloreactive T cells by indoleamine 2,3-dioxygenase (IDO)-producing dendritic cells: too much ado about IDO? Blood. 2005;105:2480–2486. doi: 10.1182/blood-2004-06-2103. [DOI] [PubMed] [Google Scholar]
- 43.Munn D.H., Mellor A.L., Rossi M., Young J.W. Dendritic cells have the option to express IDO-mediated suppression or not. Blood. 2005;105:2618. doi: 10.1182/blood-2005-01-0122. [DOI] [PubMed] [Google Scholar]
- 44.Davies J.K., Yuk D., Nadler L.M., Guinan E.C. Induction of alloanergy in human donor T cells without loss of pathogen or tumor immunity. Transplantation. 2008;86:854–864. doi: 10.1097/TP.0b013e3181861b6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.