Abstract
Undertaking behaviour is an essential activity in social insects. Corpses are often recognized by a postmortem change in a chemical signature. Reticulitermes flavipes responded to corpses within minutes of death. This undertaking behaviour did not change with longer postmortem time (24 h); however, R. flavipes exhibited distinctively different behaviours toward dead termites from various origins. Corpses of the congeneric species, Reticulitermes virginicus, were buried onsite by workers with a large group of soldiers guarding the burial site due to the risk of interspecific competition; while dead conspecifics, regardless of colony origin, were pulled back into the holding chamber for nutrient recycling and hygienic purposes. The burial task associated with congeneric corpses was coupled with colony defence and involved ten times more termites than retrieval of conspecific corpses. Our findings suggest elicitation of undertaking behaviour depends on the origin of corpses which is associated with different types of risk.
How to deal with the dead is a problem faced by all social animals. One of the major concerns associated with the deceased individuals is the risk of pathogen transmission, and this may be particularly true for eusocial animals due to their enclosed living quarters1,2. In eusocial insects, corpse management is an integral part of the behavioural repertoire for controlling disease. Termites, social bees, wasps, and ants, employ an array of behavioural adaptations (e.g., isolation of sick from healthy individuals3,4, corpse management5,6,7,8,9, and "social vaccination" of nestmates by pathogen-exposed individuals10,11) to manage diseases. To prevent further direct contact with corpses, social insects practice undertaking behaviour to segregate dead individuals from colony members. These behaviours include necrophobic (avoidance), necrophoric (removal), cannibalism, and burial behaviour5,6,9,12. Overlapping generations and cooperative brood care, two of the defining characteristics of eusociality, make social organisms more vulnerable to pathogens through extensive contact1. As a result, hygienic behaviours are consistently correlated with the evolution of eusociality. Most ant species dispose of dead colony members by discarding them away from the nests, placing them into refuse piles, or carrying them to special refuse chambers6,13,14. Honey bees remove nestmate corpses and drop them from the hive7,15. In contrast, termites exhibit complex undertaking behaviours to deal with the dead, including avoidance9, cannibalism3,12, removal, and burial behaviour4,9,16,17,18.
Distinguishing the dead from the living depends on accumulation or loss of chemical cues. Postmortem accumulation of unsaturated fatty acids (e.g., oleic or linoleic acid) is common among diverse taxa. Undertaking responses in two ant species (Pogonomyrmex badius and Solenopsis saevissima) were elicited by fatty acids, particularly oleic acid, produced after death5. This “fatty acid death cue” has been found in other ant species, honey bees, and termites5,6,7,17,18,19,20. Recently, the converse of a death cue was found in Argentine ants, Linepithema humile (formerly Iridomyrmex humilis), where the dissipation of vital chemical signs (dolichodial and iridomyrmecin) after death elicited the undertaking response14.
Undertaking behaviour in termites has been studied as a part of the efforts to refine pest management tactics3,9,21. Su and his colleagues investigated the foraging behaviour of Coptotermes formosanus, a subterranean termite and a devastating structural pest. Coptotermes formosanus corpses resulted from physical contacts with synthetic soil insecticides, including fipronil and thiamethoxam, were walled off by healthy workers to prevent future contacts9. Burial behaviour was also observed in a Reticulitermes species, when challenged with Metarhizium anisopliae, a generalist fungal pathogen and a well-studied biological control agent3. Recently, there has been renewed interests in undertaking behaviour17,18,22,23. In a fungus-growing Macrotermitinae species, Pseudacanthotermes spiniger, wingless primary reproductives buried other dealate corpses to prevent potential pathogen outbreak17. Ulyshen and Shelton reported that the presence of dead insects elicited building behaviour in R. virginicus, regardless of corpse types including nestmates, predators, and others18. Neoh et al.22 suggested that undertaking responses vary among species, associated with their feeding behaviour and nesting ecology, and undertaking behaviours are complex with different responses depending on the nature of corpses. In Coptotermes formosanus, when fungus (Metarhizium anisopliae) induced mortality was low, cannibalism was the primary undertaking response; however at higher levels, burial was predominant23.
Undertaking responses may also vary within species depending on the social context and experimental setup. In Florida harvester ant Pogonomyrmex badius, oleic acid served to release undertaking behaviour when the major activity in a colony was midden work or nest maintenance. In contrast, undertaking response was not elicited when the individuals were mainly involved in foraging or convening24. In Reticultermes, termites isolated fungus-infected cadavers in a dish assay3, whereas in a planar arena, termites opted to cannibalize corpses exposed to the same pathogen25.
Competition between two colonies can lead to injury and mortality of both colonies through aggression. As a result the encounter rate with dead individuals from the competing species may be high26. Interspecific corpses induced sand/soil deposition, and the resultant dead individuals were eventually incorporated into the tunnel building materials to block future contacts (e.g., Coptotermes formosanus and Coptotermes gestroi27, Cornitermes cumulans and Procornitermes araujoi28). The eastern subterranean termite, Reticulitermes flavipes, is one of the most common termite species in the continental United States29. A congeneric species, R. viginicus, is morphologically and ecologically similar to R. flavipes. In the field we observed that the two species sometimes nested adjacently and occasionally one nesting site was replaced by the other species, therefore territorial competition between the two species would lead to aggression and result in dead individuals of both species. Previous studies suggested that interspecific and intraspecific interactions differ in R. flavipes. Reticultermes flavipes has been reported to lack intraspecific aggression and as a result colony fusion occurs30, but to show agonistic behaviour toward R. virginicus in laboratory31. Given the fact that R. flavipes and R. virginicus have distinctly different chemical signatures32, and potentially represent different risks such as interspecific competition, we hypothesized that R. flavipes would respond differently toward congeneric and conspecific corpses. Similarly, each R. flavipes colony carries its unique chemical ID, which may be diet mediated33 and/or due to genetic variations34. We expected differential undertaking responses toward intraspecific corpses (nestmate and non-nestmate). To test these hypotheses, we presented R. flavipes with dead individuals from the same and different R. flavipes colonies, and corpses from a congeneric species, R. virginicus.
“Increased death cue”5,17,18 and “diminished vital sign”14 are the two reported mechanisms of corpse recognition. In both cases the changes should be dependent on time after death. To gain a better understanding of the cues eliciting undertaking process in termites, we determined the temporal profile of R. flavipes undertaking behaviour when exposed to nestmates from 0 to 24 h after death.
Results
Undertaking response to corpses with different postmortem time
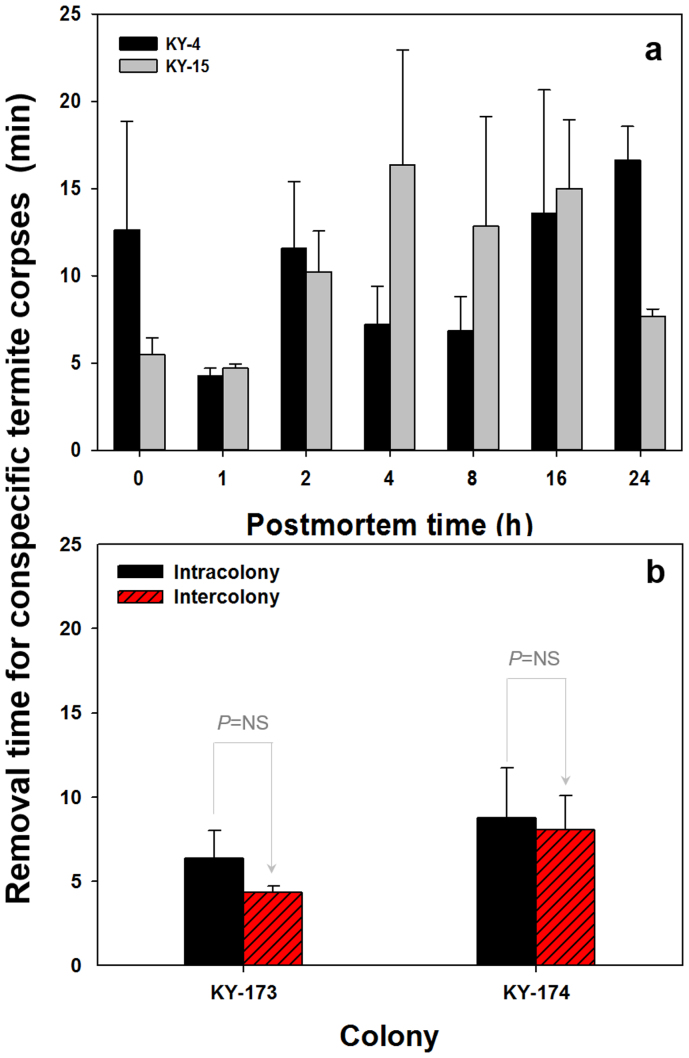
We examined the responses of R. flavipes workers to dead nestmates with various postmortem time by placing corpses at the holding chamber opening in a testing arena (Fig. 1). When a group of 10 dead nestmates were introduced, workers carried the corpses into the holding chamber irrespective of postmortem time. Total removal time did not differ significantly among treatments (F6,14 = 1.166 , P > 0.05 for colony KY-4; F6,14 = 1.416 , P > 0.05 for colony KY-15; 3 replications for each colony; Fig. 2a).
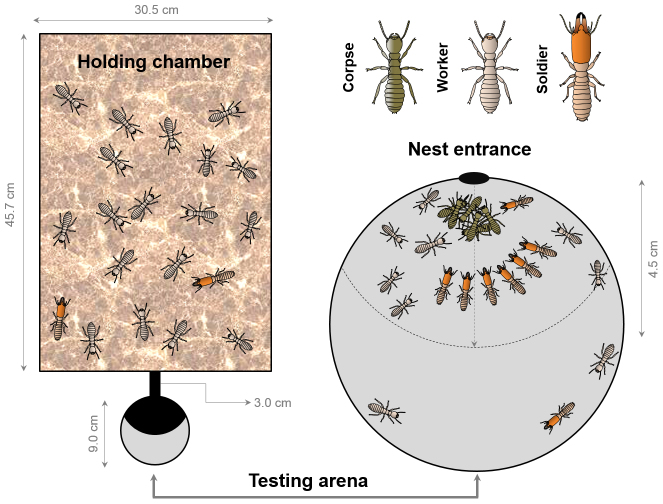
Figure 1. Schematic drawing of experimental set-up.
Colonies were maintained in holding chamber. A 9.0 cm-diameter testing arena was connected to the holding chamber with a 3.0 cm plastic tubing. The testing arena was covered with a lid to avoid disturbance by air movement. Corpses were placed in the vicinity of nest entrance, and the activities of termites in the testing arena were videotaped.
Figure 2. Corpse removal time by Reticulitermes flavipes.
(a) Removal time of dead nestmates with different postmortem time in two colonies, KY-4 and KY-15 (mean ± standard error). Unpaired t-test (P > 0.05) detected no significant difference among treatments with different postmortem time in both colonies, (b) Removal time of dead nestmates or inter-colony corpses in two colonies (mean ± standard error). NS represents no significant difference between removal times based on unpaired t-test, P > 0.05.
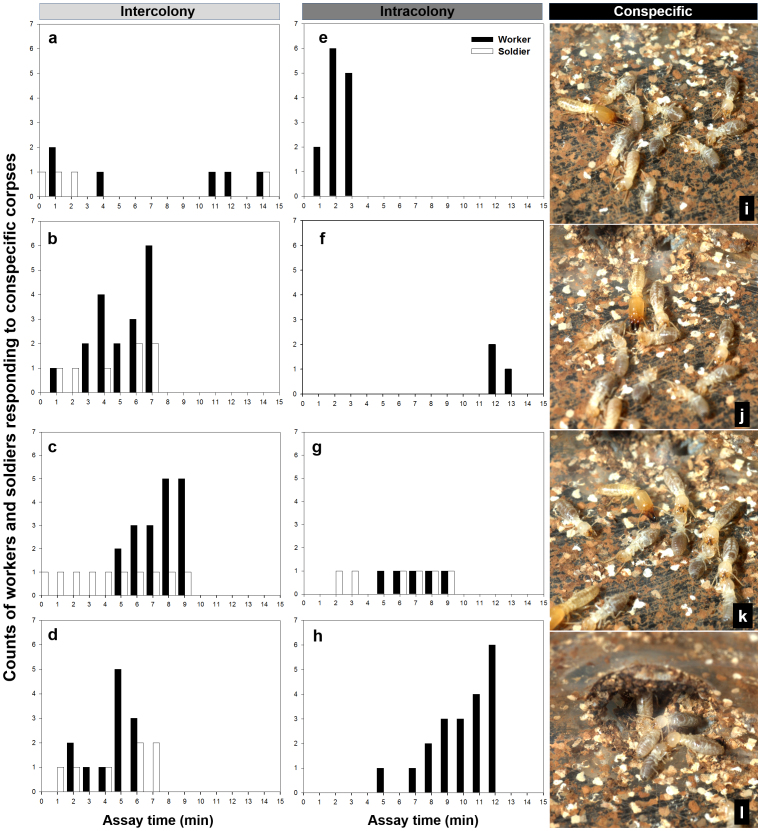
The undertaking response to dead nestmates started with the inspection of corpses by worker termites while one or more soldiers guarded the entrance. Typically, the first worker coming out of the holding chamber contacted a corpse with its antennae. Immediately after antennation, the worker grasped the corpse using its mandibles (Fig. 3j–k). Workers usually pulled the corpses straight to the entrance (Fig. 3l), but the removal path could be circuitous. Soldiers sometimes contacted corpses with their antennae, but there was no agonistic response toward conspecific corpses (Fig. 3i).
Figure 3. Undertaking responses of Reticulitermes flavipes to conspecific corpses.
(a)–(d) Counts of workers and soldiers which were recruited to inter-colony corpses. (a) and (b) represent two replications in colony KY-173, while (c) and (d) represent two replications in KY-174. (e)-(h) Counts of workers and soldiers which were recruited to intra-colony corpses. (e) and (f) represent two replications in colony KY-173, while (g) and (h) represent two replications in KY-174. (i) A soldier touching the corpse with antennae. (j) A worker attempting to grasp a corpse with its mandibles after antennation. (k) Three workers, each of them carrying a corpse, respectively. (l) Corpses being dragged into the holding chamber through the entrance by workers. (i)-(l) are the results of inter-colony treatments, but behaviours were the same with intra-colony treatments.
Undertaking response to corpses of different origin
The same undertaking behaviour pattern was observed toward intra- (nestmate) and inter-colony corpses 1 h postmortem. Conspecific corpses were removed from the testing arena and carried back to the holding chamber by workers, usually in less than 15 minutes (see Supplementary Video S1 online). Removal time between treatments with intra- and inter-colony corpses did not differ significantly (unpaired t-test: t8 = 1.170, P > 0.05 for colony KY-173; t8 = 0.200, P > 0.05 for colony KY-174; 5 replications per colony Fig. 2b).
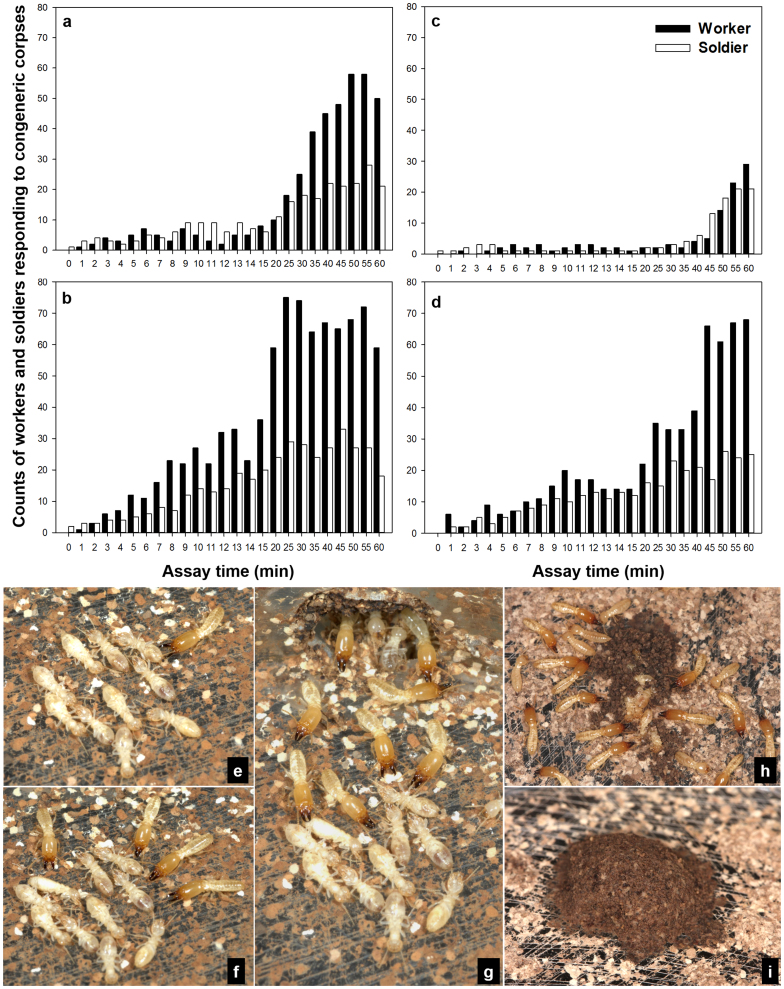
In contrast to conspecific corpses, corpses from the congeneric species, R. virginicus, triggered alarm behaviours by both soldiers and workers, aggression behaviour by soldiers, and burial behaviour by workers (see Supplementary Video S2, S3 online). A soldier from the holding chamber inspected the corpses with antennae and then immediately initiated an attack with its mandibles (Fig. 4e–f). More soldiers were recruited from the holding chamber (Fig. 4g). Workers that contacted R. virginicus corpses quickly retreated into the nest, and did not come out until more soldiers gathered around the corpses. Within ten minutes after the introduction of congeneric corpses, workers began to carry out soil from the holding chamber and place it onto the corpses, and some workers were observed to coat the soil with saliva at the burial site. The burial behaviour continued while a group of soldiers surrounded the pile of corpses (Fig. 4h). Within 12 h, the group of 10 R. virginicus corpses was buried by R. flavipes workers with soil particles, forming a compact and moist mound (Fig. 4i). Alarm behaviours characterized by rapid walking of agitated termites and/or vigorous vibrations of their bodies (as previously described by Crosland et al.35) were consistently observed during the 1 h observation period. In contrast, display of alarm behaviour was significantly less frequent in treatments with conspecific corpses (means of 36.75 ± 11.49 and 1.75 ± 1.44 for congeneric and intercolony corpses during first 15 min observation period; unpaired t-test: t6 = 3.022, P < 0.05; 2 replications per colony in KY-173 and -174). Alarm behaviour facilitated a rapid recruitment of both soldiers and workers during the first hour after the introduction of congeneric corpses (Fig. 4a–d), while termites involved in treatments of conspecific corpses were much fewer (Fig. 3a–h). Because of high variation in numbers between replicates, results are presented separately (Fig. 3a–h, 4a–d).
Figure 4. Undertaking responses of Reticulitermes flavipes to R. virginicus corpses.
(a)–(d) Counts of workers and soldiers which were recruited to congeneric corpses. (a) and (b) represent two replications in colony KY-173, while (c) and (d) represent two replications in KY-174. (e) A soldier touching the corpse with antennae and attacking it with open mandibles. (f) Soldiers attacking the corpses. (g) More soldiers being recruited. (h) Corpses being buried by workers while a group of soldiers guarding the burial site. (i) Ten R. virginicus corpses were completely buried.
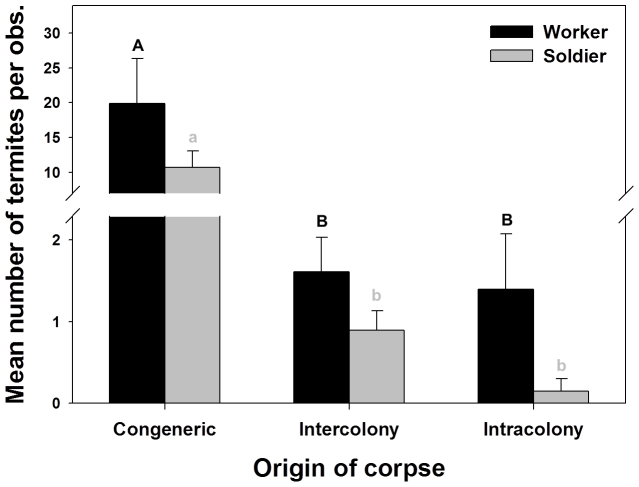
There was no significant effect of block or colony on the mean number of workers per 1 h observation period (F1,7 = 0.15, P > 0.05 and F1,7 = 0.30, P > 0.05). The source of the dead termites had a significant effect on number of workers observed (F2,7 = 11.80, P < 0.01) (Fig. 5). Recruitment of workers was greater with the R. virginicus corpses than with intra- or inter-colony R. flavipes corpses (means of 19.9, 1.4, 1.6 workers per observation, respectively; Tukey HSD Comparison Test). There was no significant effect of block or colony on the mean number of soldiers per observation period (F1,7 = 0.24, P > 0.05 and F1,7 = 0.03, P > 0.05). The source of the dead termites had a significant impact on the recruitment of soldiers (F2,7 = 37.88, P < 0.01). Many more soldiers were recruited to dead R. virginicus than dead intra or inter-colony R. flavipes (means of 10.7, 0.15, 0.89 soldiers per observation, respectively; Tukey HSD Comparison Test). There was no significant effect of block or colony on the proportion of soldiers observed (F1,7 = 1.48, P > 0.05 and F1,7 = 1.98, P > 0.05); however, the source of the dead termites had a significant effect (F2,7 = 5.94, P < 0.05). It is worth noting that in treatments with congeneric and inter-colony corpses, the soldier caste accounts for ~40% of the total number of termites in the testing arena, which is substantially more than the natural level in a field colony (4–5%)36, indicating a greater recruitment of soldiers in the undertaking process.
Figure 5. Number of termites involved in treatments with corpses of different origin.
Mean number of termites per observation in treatments with congeneric (R. virginicus), inter-colony, and intra-colony corpses. For each treatment, two colonies were used (KY-173 and KY-174) with 2 replications. Error bars represent standard error of 4 replications. Means between groups denoted with same letters were not significantly different (P > 0.05, Tukey HSD Comparison Test).
Discussion
Chemical cues have been attributed to the undertaking behaviour in both Hymenoptera and Isoptera. The involvement of tactile cues (e.g. shape and texture) in the undertaking process in R. flavipes is currently under investigation, however, the possible mechanism for death recognition has already been confirmed in a congeneric species, R. virginicus18, suggesting the recognition of death in termites involves multi-channel signalling, including chemical and tactile cues. Recognition of a “fatty acid death cue” has been studied extensively over the past 50 years and showed phylogenetic conservation or convergence across diverse taxa. Oleic acid and linoleic acid are the two major compounds of fatty acid -based necromone to induce undertaking behaviour in ants5,6,19 and to lead to avoidance in a wide range of arthropods including terrestrial Isopoda, Collembola, cockroaches, and social caterpillars20. In this study, the brief latency between corpse introduction and release of behaviour in both conspecific treatments and congeneric treatment suggests a chemical change has already occurred. However, this study alone does not discriminate between “increased death cue”5 and “diminished vital sign”14 hypotheses.
The disposal of dead colony members is a characteristic behaviour in ants and honey bees5,7, and it is true in R. flavipes. Workers showed rapid recognition and response to nestmate corpses within 1 h after death. This is consistent with honey bees7 and ants6,14, confirming undertaking response toward dead nestmates evolved convergently in eusocial insects. However, unlike ants5,14,37, which take corpses away from the nest and deposit them into the refuse pile; and in honey bees7,38, which remove corpses out of the hive; R. flavipes carried dead nestmates back into the holding chamber. The fate of those corpses was not examined in the current study. However, based on previous reports3,22 and our on-going investigation, the conspecific corpses were likely cannibalized (QS and XZ, unpublished data). Cannibalism/necrophagy has been used by many termites to recycle nutrients3,39, which is, in part, due to their nutritionally poor cellulosic diet40. Cannibalism also functions as a hygienic tactic, and the cannibalism of fungus-killed nestmates was found in R. flavipes25. Consuming corpses destroys the source of potential epidemic pathogens, and by ingesting the dead body it can also inhibit the growth of existing entomopathogens due to antimicrobial activity in the gut41.
As hypothesized, results from this study indicate that R. flavipes have differential responses to congeneric and conspecific corpses; however, conspecific corpses of nestmates and non-nestmates were disposed in the same manner under laboratory conditions. Introduction of dead R. virginicus induced an intense response from members of the R. flavipes colony, including rapid recruitment of soldiers and workers via alarm vibration exhibited by both castes, aggressive behaviours by soldier caste, and burial behaviour by workers. Nest soil consisting of chewed mulch and feces was used by R. flavipes workers to bury congeneric corpses. The propensity of termites for tunnel building plays an important role in their burial behaviour (compared with ants and bees). The use of fecal material and nest soil for burial provide antifungal components17,21,42. Burial behaviour has been observed in other species. For example, in Coptotermes formosanus, a large number of corpses were sealed off by their nestmates9,23, and in Pseudacanthotermes spiniger and R. virginicus, burial or building behaviour induced by dead nestmates were reported17,18. Unlike cannibalism, burial behaviour isolates corpses from other nest members to prevent the spread of pathogens. An increased risk of infection is possible when encountering dead foreign species that may carry a pathogen not tolerated by R. flavipes, given the fact that disease resistance varies among species. For example, susceptibility to the soil fungal pathogen, Metarhizium anisopliae, varies among seven termite species from six families. Specifically, Mastotermes darwiniensis, Hodotermopsis sjoestedti and Nasutitermes voeltzkowi are highly tolerant, Rhinotermitidae species, R. flavipes and Prorhinotermes canalifrons, are moderately susceptible, whereas Kalotermes flavicollis and Hodotermes mossambicus are highly vulnerable43.
In addition to a hygienic function, burial behaviour is also a colony defence mechanism. A previous report demonstrated that Coptotermes formosanus and Coptotermes gestroi sealed corpses, respectively, after interspecific aggression and avoided reopening the tunnel where two species encountered each other27. In this study, R. flavipes isolated alien corpses with thick layers of mud, and building behaviour at the nest opening utilizing mud was frequently observed. Consequently, burying corpses of congeneric species by R. flavipes may facilitate colony defence against competitors. Aggressive responses by the soldier caste is typical in colony defence44, however, when treated with dead R. virginicus, aggressive behaviour exhibited by soldiers is also an integral part of corpse management. The absence of worker aggression to R. virginicus in this study is likely the result of the presence of large number of soldiers. This is consistent with the damp-wood termite, Hodotermopsis sjostedti, in which the aggression level of workers was modulated by the presence of other castes. Specifically, the presence of reproductives aggravated the aggression level of workers, whereas the presence of soldier caste neutralized worker aggression45. Therefore, aggression and guarding behaviour exhibited by soldiers may ensure that workers focus on burial tasks during the undertaking process. Inter-colony aggression was not observed, which was similar to other studies with R. flavipes30,31.
Reticulitermes workers distinguish conspecific and congeneric individuals primarily by cuticular hydrocarbons46. When presented with congeneric corpses, R. flavipes soldier exhibited strong aggression behaviour, indicating that they are capable of nestmate recognition, but might not be able to recognize the dead. Similarly, the soldier caste of fungus-growing ant, Atta mexicana, is not sensitive to oleic acid, the death cue, but responds to an alarm pheromone47. Interestingly, Choe et al.14 found that triglycerides, which are common chemical components in insects, elicited both aggressive and undertaking behaviour in Argentine ants. If this is true in Reticulitermes, it is possible that additional chemicals in R. flavipes inhibit aggressive responses by conspecific individuals, or, elicit aggression to interspecifics. Further quantitative analysis of chemical cues is warranted to examine these hypotheses. In addition, we predict that workers and soldiers have different sensory or central processing of these cues.
Our results showed that R. flavipes distinguish between their own corpses and those of a congener, eliciting corpse retrieval behaviour or burial behaviour, respectively. Depending on the origin of corpses, undertaking behaviour functions as a hygienic tactic as shown in response to nestmate corpses, and as both hygienic and colony defensive strategy as shown in response to dead congenerics. The ability to switch between behavioural repertoires in R. flavipes is similar to the undertaking behaviours observed in an ant species Temnothorax lichtensteini, in which newly deceased congeneric individuals were buried while aged corpses of nestmates were removed outside the nest48. The differential response in termites to corpses of different origins can be advantageous. Burial is extremely costly in terms of time, energy, and resources committed to this form of disposal, but may be warranted when the risk of exogenous pathogens is high, or if the workers perceive a lingering risk of interspecific aggression. In addition to recycling nutrients, corpse retrieval requires less time, energy and resources than burial. However, inoculation of the colony with exogenous pathogens or the presence of intruders may have direct costs. A differential response to corpses of different origins may serve to mitigate the risks and costs associated with corpses and their disposal.
Methods
Termites
Reticulitermes flavipes colonies (KY-4, −15, −173, and −174) and a congeneric R. virginicus colony (KY-18) were collected from The Arboretum, a 100-acre botanical garden located on the University of Kentucky campus (Lexington, KY). Colonies were obtained using termite trapping stations filled with spirally coiled cardboard during spring and summer. Colony KY-4, KY-15 and KY-18 were collected in 2010, while Colony KY-173 and KY-174 were collected in 2011. Reticulitermes colonies were maintained in sealed plastic boxes in complete darkness (L:D = 0:24), at 27 ± 1°C, 80 ± 1% RH, and were provisioned with pine wood and hardwood mulch. Intercolony individuals were obtained from colony OH-A8, which was a gift from Dr. Susan Jones (The Ohio State University). It was originally collected from Columbus, OH, and has been maintained in the laboratory for 9 years. The KY- and OH- colonies were collected from two different states and at least 7 years apart and therefore should have a low degree of genetic relatedness. The identity of colonies as R. flavipes and R. virginicus, respectively, were verified by a combination of soldier morphology and 16 s mitochondrial ribosomal gene sequencing49. Termites were considered workers if they did not possess any sign of wing buds or distended abdomens, and had pronotal widths wider than mesonotal widths50.
Experimental set up
Termites used in the following experiments had been acclimated under the laboratory conditions for at least 3 months from the time of field collection. Each laboratory colony contains at least 5000 termites. Our experimental setup includes a holding chamber, a sealed plastic box (45.7 × 30.6 × 15.2 cm, Pioneer Plastics, Inc., Dixon, KY) containing the original stock colony, and a testing arena, a covered 9 cm-diameter Petri dish (1.5 cm height, VWR Inc, Radnor, PA) where observations of undertaking were made. The bottom surface of the dish was scratched with No. 7 insect pins to facilitate movement. Behavioural experiments began at least two weeks after the Petri dish was connected, when the bottom of the testing arena was covered with nest material by workers. An acrylic tube (inner diameter: 0.7 cm; length: 3 cm) connects the testing arena to the holding chamber (Fig. 1). The entire experimental setup was held in environmental chambers under the above mentioned rearing conditions. A video camcorder (Sony DCR-SR60, Tokyo, Japan) was placed directly above the testing arena.
Preparation of corpses
Undertaking responses toward corpses with different postmortem times were tested in two colonies (KY-4 and KY-15). Seven groups of workers from the same KY R. flavipes colony were frozen to death at −20°C for 15 min and kept in environmental chambers at 27 ± 1°C, 80 ± 1% RH, for 0, 1, 2, 4, 8, 16, and 24 h, respectively, in covered Petri dishes before subjecting to the behavioural bioassay. The 0 h old (freshly killed) corpses were left at room temperature for 5 min before they were placed in the arena. To discern the behavioural responses toward corpses of different origin, two colonies were tested (KY-173 and KY-174) with three types of corpses introduced: (a) intracolony corpses: dead workers collected from the same KY R. flavipes colony; (b) intercolony corpses: dead workers collected from a OH R. flavipes colony (OH-A8); and (c) congeneric corpses: dead workers collected from a KY R. virginicus colony (KY-18). Based on the result from the postmortem time experiment, we opted to use 1 h postmortem corpses as the age of corpses for the differential response study. Corpses were prepared by freezing as described above.
Undertaking behavioural bioassay
Behavioural assays were conducted at room temperature, and the experimental setup including a holding chamber and a testing arena was acclimated for 30 min before experiments. In each bioassay, the lid of testing arena was gently removed, 10 corpses were then placed into the arena using a pair of feather-weight forceps (BioQuip Products Inc., Gardena, CA). Corpses were placed in the vicinity of the nest entrance, and the arena was gently covered with a clear Petri dish lid to avoid disturbance by the air movement. Video recording was initiated immediately after the introduction of corpses to the testing arena.
For different postmortem times, behavioural bioassays were replicated 3 times in both KY-4 and KY-15 colonies. For corpses with different origins, bioassays were replicated 5 times in KY-173 and KY-174 colonies. Based on the length of undertaking processes toward different types of corpses, we empirically determined a 15 and 60 min recording time, respectively, for the assays with conspecific and congeneric corpses. Undertaking processes toward conspecific corpses (body removal) are concluded within a 15 min time frame. Undertaking process toward congeneric corpses (burial behaviour), however, usually lasts for 5 h or more. Nevertheless, the entire behavioural repertoire including antennation, alarm, recruitment, aggression, burial, and guarding was clearly displayed within the first 60 min of the undertaking process. Behavioural bioassays on the same colony were separated by at least 5 h to avoid the potential influence of short-term memory. To analyze the involvement of worker and soldier castes in the undertaking process, the number of workers and soldiers appearing under the viewing area were recorded, respectively. Picture frames were paused every minute during the observation period, and termites within the viewing area (a radius of 4.5 cm from the nest entrance in the testing arena, Fig. 1) were counted. To analyze the intensity of alarm behaviour, the frequency of alarm vibration was counted within a 15 min observation period immediately after the introduction of corpses.
Data analysis
Results from the experiments with different postmortem time were analyzed by ANOVA. Removal time was recorded from the time that the first termite contacted a corpse until all 10 corpses were removed. For the experiments of corpses with different origins, removal time toward conspecific (inter- and intra-colony) corpses, and frequency of alarm vibration toward congeneric and conspecific (inter-colony) corpses, were analyzed by unpaired t-test at significance level of 5%. Finally, the counts of workers or soldiers observed were summed over all observation periods. The numbers of observation periods varied because the task of removing dead workers was completed in minutes (within 15 min), while burial behaviour lasted hours (beyond the 60 min recording time). The mean number of workers or soldiers per observation was calculated. These means were log transformed (log (n+1)) prior to analysis of variance, which was conducted with block, colony, and treatment (intra-colony, inter-colony, or congeneric R. virginicus) as factors. The mean proportion of soldiers was arcsin transformed (arcsin (square root (p)) prior to analysis of variance. All statistical analyses were conducted using Statistix 9.0 (Analytical Software, Tallahassee, FL).
Author Contributions
Conceived and designed the experiments: Q.S. and X.Z. Performed the experiments: Q.S. Analyzed the data: Q.S., K.F.H. and X.Z. Contributed reagents/materials/analysis tools: X.Z. Wrote the paper: Q.S., K.F.H. and X.Z. All authors read and approved the final manuscript.
Supplementary Material
Supplementary Information
Video S1. Time lapse video stream showing corpse removal process by Reticulitermes flavipes toward dead nestmates.
Video S2. Video clip showing aggression and alarm behaviour by Reticulitermes flavipes toward R. virginicus corpses.
Video S3. Time lapse video stream showing burial process by Reticulitermes flavipes toward R. virginicus corpses.
Acknowledgments
The authors are grateful to anonymous reviewers and editor for their constructive criticisms. Special thanks go to Drs. Susan Jones (Department of Entomology, The Ohio State University) for providing a Reticulitermes flavipes colony, John Obrycki (Department of Entomology, University of Kentucky) for his comments on an earlier draft, and Ric Bessin (Department of Entomology, University of Kentucky) for his assistance with statistical analysis. This research was supported by a start-up fund from the University of Kentucky, the NSF-EPSCoR Research Scholars Program, Kentucky Initiative in Ecological Genomics (Award Agreement No. NSF/EPSCoR RII Grant EPS-0814194), and a grant from the Kentucky Commercialization Fund Program, Kentucky Science and Technology Corporation (Award Agreement No. KSTC-144-401-09-034). The granting agencies have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The information reported in this paper (No. 13-08-025) is part of a project of the Kentucky Agricultural Experiment Station and is published with the approval of the Director.
References
- Cremer S., Armitage S. A. & Schmid-Hempel P. Social immunity. Curr. Biol. 17, 693–702 (2007). [DOI] [PubMed] [Google Scholar]
- Babayan S. A. & Schneider D. S. Immunity in society: Diverse solutions to common problems. PLoS Biol. 10, e1001297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramm K. R., West D. F. & Rockenbach P. G. Termite pathogens: Transfer of the entomopathogen Metarhizium anisopliae between Reticulitermes sp. termites. J. Invertebr. Pathol. 40, 1–6 (1982). [Google Scholar]
- Myles T. G. Alarm, aggregation, and defense by Reticulitermes flavipes in response to a naturally occurring isolate of Metarhizium anisopliae. Soiobiology 40, 13 (2002). [Google Scholar]
- Wilson E. O., Durlach N. I. & Roth L. M. Chemical releaser of necrophoric behavior in ants. Psyche 65, 108–114 (1958). [Google Scholar]
- Howard D. F. & Tschinkel W. R. Aspects of necrophoric behavior in the red imported fire ant, Solenopsis invicta. Behaviour 56, 157–180 (1976). [Google Scholar]
- Visscher P. K. The honey bee way of death: Necrophoric behaviour in Apis mellifera colonies. Anim. Behav. 31, 1070–1076 (1983). [Google Scholar]
- Milner R. J., Staples J. A. & Lutton G. G. The selection of an isolate of the hyphomycete fungus, Metarhizium anisopliae, for control of termites in Australia. Biol. Control. 11, 240–247 (1998). [Google Scholar]
- Su N.-Y. Response of the Formosan subterranean termites (Isoptera: Rhinotermitidae) to baits or nonrepellent termiticides in extended foraging arenas. J. Econ. Entomol. 98, 2143–2152 (2005). [DOI] [PubMed] [Google Scholar]
- Konrad M. et al. Social transfer of pathogenic fungus promotes active immunisation in ant colonies. PLoS Biol. 10, e1001300 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traniello J. F. A., Rosengaus R. B. & Savoie K. The development of immunity in a social insect: Evidence for the group facilitation of disease resistance. Proc. Natl. Acad. Sci. USA. 99, 6838–6842 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengaus R. & Traniello J. Disease susceptibility and the adaptive nature of colony demography in the dampwood termite Zootermopsis angusticollis. Behav. Ecol. Sociobiol. 50, 546–556 (2001). [Google Scholar]
- Holldobler B. & Wilson E. O. The Ants. (Harvard University Press, Cambridge, 1990). [Google Scholar]
- Choe D. H., Millar J. G. & Rust M. K. Chemical signals associated with life inhibit necrophoresis in Argentine ants. Proc. Natl. Acad. Sci. USA. 106, 8251–5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbo S. T., Huang Z.-Y. & Robinson G. E. Division of labor between undertaker specialists and other middle-aged workers in honey bee colonies. Behav. Ecol. Sociobiol. 41, 151–163 (1997). [Google Scholar]
- Zoberi M. H. Metarhizium anisopliae, a fungal pathogen of Reticulitermes flavipes (Isoptera: Rhinotermitidae).Mycologia 87, 354–359 (1995). [Google Scholar]
- Chouvenc T., Robert A., Semon E. & Bordereau C. Burial behaviour by dealates of the termite Pseudacanthotermes spiniger (Termitidae, Macrotermitinae) induced by chemical signals from termite corpses. Insectes Soc. 59, 119–125 (2012). [Google Scholar]
- Ulyshen M. D. & Shelton T. G. Evidence of cue synergism in termite corpse response behavior. Naturwissenschaften 99, 89–93 (2012). [DOI] [PubMed] [Google Scholar]
- Haskins C. P. & Haskins E. F. Notes on necrophoric behavior in the archaic ant Myrmecia vindex (Formicidae: Myrmeciinae). Psyche 81, 258–267 (1974). [Google Scholar]
- Yao M. et al. The ancient chemistry of avoiding risks of predation and disease. Evol. Biol. 36, 267–281 (2009). [Google Scholar]
- Chouvenc T. & Su N.-Y. Apparent synergy among defense mechanisms in subterranean termites (Rhinotermitidae) against epizootic events: Limits and potential for biological control. J. Econ. Entomol. 103, 1327–1337 (2010). [DOI] [PubMed] [Google Scholar]
- Neoh K. B., Yeap B. K., Tsunoda K., Yoshimura T. & Lee C. Y. Do termites avoid carcasses? Behavioral responses depend on the nature of the carcasses. PLoS ONE 7, e36375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouvenc T. & Su N.-Y. When subterranean termites challenge the rules of fungal epizootics. PLoS ONE 7, e34484 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. M. Dependence of necrophoric response to oleic acid on social context in the ant, Pogonomyrmex badius. J. Chem. Ecol. 9, 105–111 (1983). [DOI] [PubMed] [Google Scholar]
- Chouvenc T., Su N.-Y. & Elliott M. L. Interaction between the subterranean termite Reticulitermes flavipes (Isoptera: Rhinotermitidae) and the entomopathogenic fungus Metarhizium anisopliae in foraging arenas. J. Econ. Entomol. 101, 885–893 (2008). [DOI] [PubMed] [Google Scholar]
- Thorne B. L. & Haverty M. I. A review of intercolony, intraspecific and interspecific agonism in termites. Sociobiology 19, 115–145 (1991). [Google Scholar]
- Li H. F., Yang R. L. & Su N.-Y. Interspecific competition and territory defense mechanisms of Coptotermes formosanus and Coptotermes gestroi (Isoptera: Rhinotermitidae). Environ. Entomol. 39, 1601–7 (2010). [DOI] [PubMed] [Google Scholar]
- Jost C., Haifig I., Camargo-Dietrich C. R. R. & Costa-Leonardo A. M. A comparative tunnelling network approach to assess interspecific competition effects in termites. Insectes Soc. 59, 369–379 (2012). [Google Scholar]
- Su N.-Y., Scheffrahn R. H. & Cabrera B. J. Native subterranean termites: Reticulitermes flavipes (Kollar), Reticulitermes virginicus (Banks), Reticulitermes hageni Banks (Insecta: Isoptera: Rhinotermitidae). (University of Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, EDIS., 2001).
- Bulmer M. S. & Traniello J. F. A. Lack of aggression and spatial association of colony members in Reticulitermes flavipes. J. Insect Behav. 15, 121–126 (2002). [Google Scholar]
- Polizzi J. M. & Forschler B. T. Intra- and interspecific agonism in Reticulitermes flavipes (Kollar) and R. virginicus (Banks) and effects of arena and group size in laboratory assays. Insectes Soc. 45, 43–49 (1998). [Google Scholar]
- Haverty M. I., Forschler B. T. & Nelson L. J. An assessment of the taxonomy of Reticulitermes (Isoptera: Rhinotermitidae) from the southeastern United States based on cuticular hydrocarbons. Sociobiology 28, 287–318 (1996). [Google Scholar]
- Florane C., Bland J., Husseneder C. & Raina A. Diet-mediated inter-colonial aggression in the formosan subterranean termite Coptotermes formosanus. J. Chem. Ecol. 30, 2559–2574 (2004). [DOI] [PubMed] [Google Scholar]
- Jenkins T. et al. Correlation of mitochondrial haplotypes with cuticular hydrocarbon phenotypes of sympatric Reticulitermes Species from the Southeastern United States. J. Chem. Ecol. 26, 1525–1542 (2000). [Google Scholar]
- Crosland M. W. J. & Traniello J. F. A. Behavioral plasticity in division of labor in the lower termite Reticulitermes fukienensis. Naturwissenschaften 84, 208–211 (1997). [Google Scholar]
- Zhou X. G., Oi F. M. & Scharf M. E. Social exploitation of hexamerin: RNAi reveals a major caste-regulatory factor in termites. Proc. Natl. Acad. Sci. USA. 103, 4499–4504 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian G. E. & Cahan S. Undertaking specialization in the desert leaf-cutter ant Acromyrmex versicolor. Anim. Behav. 58, 437–442 (1999). [DOI] [PubMed] [Google Scholar]
- Morse R. A. Environmental control in the beehive. Sci. Am. 226, 92–98 (1972). [Google Scholar]
- Moore B. P. Biochemical studies in termites. in Biology of Termites (eds. Krishna, K. & Weesner, F. M.) 233–282 (Academic Press, New York, 1969). [Google Scholar]
- Rosengaus R. B., Traniello J. F. A. & Bulmer M. S. Ecology, behavior and evolution of disease resistance in termites. in Biology of Termites: A Modern Synthesis (eds. Bignell, E. D., Roisin, Y. & Lo, N.) 165–191 (Springer, New York, 2011). [Google Scholar]
- Chouvenc T., Su N. Y. & Robert A. Inhibition of Metarhizium anisopliae in the alimentary tract of the eastern subterranean termite Reticulitermes flavipes. J. Invertebr. Pathol. 101, 130–6 (2009). [DOI] [PubMed] [Google Scholar]
- Hamilton C., Lay F. & Bulmer M. S. Subterranean termite prophylactic secretions and external antifungal defenses. J. Insect Physiol. 57, 1259–66 (2011). [DOI] [PubMed] [Google Scholar]
- Chouvenc T., Su N. Y. & Robert A. Susceptibility of seven termite species (Isoptera) to the entomopathogenic fungus Metarhizium anisopliae. Sociobiology 54, 723–748 (2009). [Google Scholar]
- Traniello J. F. A. & Beshers S. N. Species-specific alarm/recruitment responses in a neotropical termite. Naturwissenschaften 72, 491–492 (1985). [Google Scholar]
- Ishikawa Y. & Miura T. Hidden aggression in termite workers: plastic defensive behaviour dependent upon social context. Anim. Behav. 83, 737–745 (2012). [Google Scholar]
- Bagneres A. G., Killian A., Clement J. L. & Lange C. Interspecific recognition among termites of the genus Reticulitermes: Evidence for a role for the cuticular hydrocarbons. J. Chem. Ecol. 17, 2397–2420 (1991). [DOI] [PubMed] [Google Scholar]
- López-Riquelme G. O., Malo E. A., Cruz-López L. & Fanjul-Moles M. L. Antennal olfactory sensitivity in response to task-related odours of three castes of the ant Atta mexicana (hymenoptera: formicidae). Physiol. Entomol. 31, 353–360 (2006). [Google Scholar]
- Renucci M., Tirard A. & Provost E. Complex undertaking behavior in Temnothorax lichtensteini ant colonies: from corpse-burying behavior to necrophoric behavior. Insectes Soc. 58, 9–16 (2010). [Google Scholar]
- Wang C. et al. Survey and identification of termites (Isoptera: Rhinotermitidae) in Indiana. Ann. Entomol. Soc. Am. 102, 1029–1036 (2009). [Google Scholar]
- Lainé L. & Wright D. The life cycle of Reticulitermes spp. (Isoptera: Rhinotermitidae): What do we know? Bull. Entomol. Res. 93, 267–278 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Video S1. Time lapse video stream showing corpse removal process by Reticulitermes flavipes toward dead nestmates.
Video S2. Video clip showing aggression and alarm behaviour by Reticulitermes flavipes toward R. virginicus corpses.
Video S3. Time lapse video stream showing burial process by Reticulitermes flavipes toward R. virginicus corpses.