Abstract
The Ca2+/voltage-gated K+ large conductance (BK) channel β1 subunit is particularly abundant in vascular smooth muscle. By determining their phenotype, BK β1 allows the BK channels to reduce myogenic tone, facilitating vasodilation. The endogenous steroid lithocholic acid (LCA) dilates cerebral arteries via BK channel activation, which requires recognition by a BK β1 site that includes Thr169. Whether exogenous nonsteroidal agents can access this site to selectively activate β1-containing BK channels and evoke vasodilation remain unknown. We performed a chemical structure database similarity search using LCA as a template, along with a two-step reaction to generate sodium 3-hydroxyolean-12-en-30-oate (HENA). HENA activated the BK (cbv1 + β1) channels cloned from rat cerebral artery myocytes with a potency (EC50 = 53 μM) similar to and an efficacy (×2.5 potentiation) significantly greater than that of LCA. This HENA action was replicated on native channels in rat cerebral artery myocytes. HENA failed to activate the channels made of cbv1 + β2, β3, β4, or β1T169A, indicating that this drug selectively targets β1-containing BK channels via the BK β1 steroid-sensing site. HENA (3–45 μM) dilated the rat and C57BL/6 mouse pressurized cerebral arteries. Consistent with the electrophysiologic results, this effect was larger than that of LCA. HENA failed to dilate the arteries from the KCNMB1 knockout mouse, underscoring BK β1’s role in HENA action. Finally, carotid artery-infusion of HENA (45 μM) dilated the pial cerebral arterioles via selective BK-channel targeting. In conclusion, we have identified for the first time a nonsteroidal agent that selectively activates β1-containing BK channels by targeting the steroid-sensing site in BK β1, rendering vasodilation.
Introduction
A widespread feature in ion channel organization in excitable tissues is the association of the ion channel–forming protein(s) with accessory subunits. These accessory proteins cannot form ion channels themselves but modify the ion current phenotype, including its pharmacology. Moreover, tissue-specific expression of channel subunits allows the resulting hetero-oligomeric channel complex to regulate physiology in a tissue-specific manner (Orio et al., 2002; Yan and Aldrich, 2012). Large conductance voltage- and Ca2+-gated K+ (BK) channels result from the association of four identical α (slo1) subunits, which are ubiquitously distributed (Ghatta et al., 2006; Salkoff et al., 2006). In most tissues, however, α homotetramers are accompanied by small, two transmembrane (TM2)-spanning accessory (β1–4) subunits that are encoded by four separate genes: KCNMB1–4 (Orio et al., 2002). Remarkably, KCNMB1 expression is highly tissue-specific, being abundant in the smooth muscle, including arterial myocytes (Brenner et al., 2000a,b; Orio et al., 2002).
BK channels are critical determinants of artery myogenic tone: upon activation, they generate outward currents that counteract depolarization-induced Ca2+ entry, limiting constriction and favoring artery dilation (Jaggar et al., 1998; Ghatta et al., 2006). BK β1 subunits increase the apparent Ca2+-sensitivity of the arterial smooth muscle BK channel, so this channel may be activated at voltage and Ca2+ levels reached in contracting myocytes, thus exerting its negative feedback on myocyte contraction (Jaggar et al., 1998; Rusch, 2009).
The critical role of BK β1 in controlling myogenic tone and vasomotion has been underscored by several basic research and clinical findings. For example, KCNMB1 K/O (knockout) mice are characterized by uncoupling between vasodilating, RyR-generated Ca2+-sparks and BK channel-generated spontaneous transient outward currents, which leads to increased myogenic tone, and systemic hypertension (Plüger et al., 2000). It should be mentioned, however, that KCNMB1 genetic ablation also results in K+ retention and hyperaldosteronism, major contributors to the increase in blood pressure found in the KCNMB1 K/O mouse (Holtzclaw et al., 2011). Also in a mouse model, downregulation of BK β1 by NFATc3 activation contributes to systemic hypertension (Nieves-Cintrón et al., 2007). In humans, the gain-of-function Glu65Lys substitution in BK β1 has been associated with low prevalence of diastolic (Fernández-Fernández et al., 2004; Nielsen et al., 2008) and systolic hypertension (Nielsen et al., 2008).
The central role of BK β1 in limiting vascular smooth muscle contraction, its scarce expression in tissues other than smooth muscle, and its poor identity with other membrane proteins have raised the expectation that ligands that selectively target this subunit could evoke effective vasodilation via smooth muscle BK channel activation while targeting neither BK channels in other tissues nor other receptors.
The quest for newer vasodilators acquires particular relevance when considering the cerebral circulation. Cerebral vasoconstriction underlies numerous pathologic conditions, including cerebral vasospasm, ischemia after aneurysmal subarachnoid hemorrhage (Jordan and Nyquist, 2010), the reversible cerebral vasoconstriction syndrome associated with nonaneurysmal subarachnoid hemorrhage, pregnancy, or exposure to certain drugs (Sattar et al., 2010), posttraumatic cerebral vasospasm after traumatic brain injury (Shahlaie et al., 2009), and abrupt-onset severe headaches (Ju and Schwedt, 2010). In addition, cerebral vasoconstriction and hypertension may coexist (Sekine et al., 2012). In spite of the high incidence and prevalence of cerebrovascular conditions associated with vasoconstriction, biomedical research has largely failed to provide effective and safe cerebrovascular dilators (Dorsch, 2011; Etminan et al., 2011).
A variety of physiologically relevant cholane steroids has been reported to activate smooth muscle BK channels (Dopico et al., 2002; Bukiya et al., 2007). Moreover, the most effective, lithocholic acid (LCA), evokes a robust cerebral artery in vitro dilation via activation of β1 subunit-containing BK channels (Bukiya et al., 2007). Recently, the LCA-recognition site was mapped to the BK β1 TM2 region. This steroid-recognition site includes Thr169 (Bukiya et al., 2011), a residue that is unique to BK β of type 1 (Brenner et al., 2000a), which likely explains why BK β2–4 fail to substitute for β1 in providing LCA sensitivity to BK channels (Bukiya et al., 2009a). Owing to their steroidal nature, LCA and related cholanes exert numerous biologic effects outside the vascular bed by interacting with many proteins, including the G-protein-coupled receptor BG37 (Maruyama et al., 2002), vitamin D (Makishima et al., 2002), ryanodine and inositol 1,4,5-trisphosphate receptors (Gerasimenko et al., 2006), cytosolic steroid-binding proteins, membrane transporters, and transcription factors (Modica and Moschetta, 2006). The resulting varied pharmacologic profile would likely cause widespread side effects were LCA and cholanes incorporated into clinical practice as vasodilators.
We hypothesized that LCA can be used as a template for the discovery of nonsteroidal selective activators of β1 subunit-containing BK channels and, thus, effective vasodilators. We have tested and supported this hypothesis by combining a chemical database similarity search with organic synthesis, computational modeling, point mutagenesis, patch-clamp electrophysiology on recombinant BK channels cloned from rat cerebral artery myocytes and their native counterparts in the myocyte membrane, the KCNMB1 K/O mouse model, and arterial diameter determinations both in isolated, pressurized arteries and in vivo, the latter using a closed cranial window.
Materials and Methods
Ethical Aspects of Research
The care of animals and experimental protocols were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, which is an institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Database Search and Computational Studies
A similarity search based on the LCA structure was performed throughout the Hit2Lead (https://www.hit2lead.com) database using a 70% similarity threshold. This screening rendered methyl 3-hydroxyolean-12-en-30-oate (compound ID 5808244) as a hit lead. Stochastic conformational search with the dielectric constant set to zero was performed to determine the lowest energy conformation of the LCA and hydrolyzed methyl 3-hydroxyolean-12-en-30-oate structures. Stochastic flexible alignment of hydrolyzed methyl 3-hydroxyolean-12-en-30-oate structure with the lowest energy conformation of LCA was performed. Both conformational search and flexible alignment were run using built-in functions in Molecular Operating Environment (MOE) 2006.08 (Chemical Computing Group, Montreal, QC, Canada).
Chemistry
We hydrolyzed 3′-hydroxy-olean-12-en-29-oic acid methyl ester (compound 1) to obtain 3′-hydroxy-olean-12-en-29-oic acid (compound 2) (Supplemental Fig. 1). Purified acid (2) was further converted to the corresponding sodium salt of 3′-hydroxy-olean-12-en-29-oic acid (compound 3) to improve solubility for probing of biologic activities.
Experimental Description.
We purchased compound 1 from Hit2Lead.com (ChemBridge Corporation, San Diego, CA) for the hydrolysis, and used it without further purification. The organic solvents used for reaction as well as purification were purchased from Sigma-Aldrich (St. Louis, MO) and were used without further purification. Characterization of compound 2 was conducted using proton [1H] and [13C] carbon NMR spectra (Varian 500-MHz spectrometer; Varian, Inc., Palo Alto, CA) along with mass spectral data (Brucker-HP Esquire-LC spectrometer; Bruker, Billerica, MA). Yields refer to purified products.
Preparation of Compound 2 (2S,4aS,6aS,6bR,8aR,10S,12aR,12bR,14bR)-10-Hydroxy-2,4a,6a,6b,9,9,12a-Heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-Icosahydropicene-2-Carboxylic Acid).
Compound 1 (0.085 mM, 40 mg) was dissolved in 10 ml of ethanol. After 300 mg of KOH dissolved in 2 ml of water had been added, the mix was refluxed for nearly 8 hours. The reaction mixture was then cooled and carefully acidified with diluted HCl. The mixture was extracted with chloroform. The chloroform layer was dried over anhydrous Na2SO4, then the chloroform was removed under reduced pressure. The crude residue was purified by flash chromatography (ethyl acetate:hexanes; 1:9).
Yield 34%; 1H NMR (CDCl3).
Compound 2 was obtained in 34% yield, its structure being confirmed by 1H NMR (CDCl3): δ 5.32 (t, J = 10.0 Hz, 1H), 3.25 (m, 1H, 3β-OCH), 1.78–2.05 (m, 6H), 1.72–1.48 (m, 7H), 1.46–1.13 (m, 14H, 2CH3 groups were merged in the multiplet), 1.08–0.93 (m, 10H, 3CH3 groups were merged in the multiplet), 0.90–0.72 (m, 8H, 2CH3 groups were merged in the multiplet); 13C NMR (CDCl3): δ 181.20, 143.68, 122.24, 78.10, 54.72, 47.54, 47.15, 43.46, 42.16, 41.03, 39.30, 38.24, 38.10, 37.78, 36.45, 32.17, 31.43, 30.62, 28.08, 27.56, 26.71, 26.46, 25.64, 25.43, 22.95, 17.84, 16.26, 15.01, 14.93. Compound 2 molecular weight was determined by mass spectrometry (electrospray ionization): m/z (mass-to-charge ratio) 455.1 [M–H]−; Anal. Calcd. (C30H48O3).
Preparation of Compound 3.
NaOH (1.93 mg) was moistened with a drop of water in a small vial. To this vial, 0.5 ml of methanol and 11 mg of pure acid (2) were added and stirred for 5 minutes. The methanol was evaporated under reduced pressure, and then the residue was finally dried over a high vacuum. The residue, sodium 3-hydroxyolean-12-en-30-oate, was referred to as “HENA” and was used to test biologic activities.
cDNA Cloning and Transcription
The cDNA cloning and functional characterization of rat cerebral artery myocyte BK channel-forming (cbv1) subunits (AY330293) are described elsewhere (Jaggar et al., 2005). Cbv1 cDNA inserted into the pBScMXT vector was linearized with SalI and transcribed using T3 (mMessage-mMachine; Ambion, Austin, TX).
The cDNA of the rat cerebral artery myocyte BK β1 subunit (FJ154955) was cloned as described in our previous work (Bukiya et al., 2009b). The BK β2–4, and β4/β1 (β4TM21) chimeric cDNAs were generous gifts from Dr. Ligia Toro (University of California Los Angeles). Upon arrival, they were recloned into the pOx vector. A targeted T169A mutation was introduced into wild-type (WT) BK β1 cDNA inserted in the pOX using overlap-extension polymerase chain reaction and the Quickchange kit with pfu polymerase (Agilent Technologies, Santa Clara, CA) following the manufacturer’s instructions. Sequence of the chimeric cDNA constructs, presence of the targeted mutation, and absence of unintended mutations in the polymerase chain reaction-amplified regions of the β1–4 subunit constructs were verified by automated sequencing (Molecular Resource Center, University of Tennessee Health Science Center, Memphis, TN).
Beta1–4 cDNAs inserted into pOX vector were linearized and then transcribed in vitro using either T3 or T7 polymerases (mMessage-mMachine; Ambion, Austin, TX). The cRNA was dissolved in diethyl polycarbonate-treated water at 10 ng/µl (cbv1) and 30 ng/µl (β1, β2, β3, β4, β4TM21, or β1T169A); 1 μl aliquots were stored at −70°C.
Oocyte Extraction and cRNA Injection
Xenopus laevis females were purchased from Xenopus Express (Brooksville, FL) and maintained in artificial pond water on a 12-hour light/dark cycle. In this habitat, they do not show seasonal breeding behavior, so oocytes are available throughout the year. Stages V and VI oocytes were predominantly used because they transcribe mRNA into channels efficiently. Five frogs were used as oocyte donors. Before the surgery, the frogs were anesthetized by placement on ice after exposure to ethyl 3-aminobenzoate methanesulfonate salt (250 mg/l, pH 7.4). The oocytes were removed and kept in a Ca2+-free ND-96 solution (mM): 82.5 NaCl, 2 KCl, 1 MgCl2, 5 HEPES, pH 7.5, containing 2 mg/ml collagenase (type IV; Sigma-Aldrich), at room temperature on a shaker (60 oscillations per minute) for 15 minutes to remove the follicular layer. After defolliculation, the oocytes were transferred to Ca2+-containing ND-96 saline (mM): 82.5 NaCl, 2 KCl, 1.8 CaCl2, 5 HEPES, pH 7.4, supplemented with 2.5 mM sodium pyruvate and 2 mg/ml gentamicin. Injection of mRNA into the oocyte cytoplasm was conducted using a Drummond micropipette modified for microinjection (Drummond Scientific Co., Broomall, PA). The interval between cRNA injection and patch-clamping was 36 to 48 hours. During this time, the injected oocytes were kept at 15°C.
Rat Cerebral Artery Myocyte Isolation
Middle and basilar cerebral arteries were isolated from adult male Sprague-Dawley rats (≈250 g). Ten rats were used as myocyte donors. One animal per day was decapitated using a guillotine. Basilar and middle cerebral arteries were dissected out from each brain under a stereozoom microscope (Nikon C-PS, Tokio, Japan) and placed into ice-cold dissociation medium (DM) with the following composition (mM): 0.16 CaCl2, 0.49 EDTA, 10 HEPES, 5 KCl, 0.5 KH2PO4, 2 MgCl2, 110 NaCl, 0.5 NaH2PO4, 10 NaHCO3, 0.02 phenol red, 10 taurine, and 10 glucose. Each artery was cut into 1- to 2-mm long rings (up to 30 rings/experiment). Individual myocytes were enzymatically isolated. For this purpose, rat arterial rings were put in 3 ml DM containing 0.03% papain, 0.05% bovine serum albumin (BSA), and 0.004% dithiothreitol at 37°C for 15 minutes in a polypropylene tube and incubated in a shaking water bath at 37°C and 60 oscillations/min for 15 minutes. Then, the supernatant was discarded, and the tissue was transferred to a polypropylene tube with 3 ml of DM containing 0.06% soybean trypsin inhibitor, 0.05% BSA, and 2% collagenase (26.6 units/ml). The tube was incubated again in a shaking water bath at 37°C and 60 oscillations/min for 15 minutes. Finally, the artery tissue pellet was transferred into a tube with 3 ml of DM containing 0.06% soybean trypsin inhibitor. Tissue-containing DM was pipetted using a series of borosilicate Pasteur pipettes having fire-polished, diminishing internal diameter tips. The procedure rendered a cell suspension containing relaxed, individual myocytes (≥5 myocytes/field using a 20× objective) that could be identified under an Olympus IX-70 microscope (Olympus American Inc., Woodbury, NY). The cell suspension was stored in ice-cold DM containing 0.06% soybean trypsin inhibitor and 0.06% BSA. Myocytes were used for electrophysiology up to 4 hours after isolation.
Electrophysiology Data Acquisition and Analysis
Before the recordings, oocytes were placed into a dish containing a hypertonic solution (mM): 200 K+ aspartate, 20 KCl, 1 MgCl2, 10 EGTA, 10 HEPES, pH 7.4, for 10 minutes. With this treatment, the oocytes shrink, allowing the removal of the vitelline layer with forceps and exposing the oocyte membrane for subsequent patch-clamp recording. Then the oocytes were placed back into ND-96 saline (in this case without gentamicin; for composition, see previous explanation) for 10–15 minutes before ionic current recording. Currents were recorded from excised, inside-out (I/O) patches. For experiments with oocytes, both bath and electrode solutions contained (mM): 130 K+ gluconate, 5 EGTA, 2.28 MgCl2, 15 HEPES, and 1.6 N-(2-Hydroxyethyl) ethylenediaminetriacetic acid (HEDTA), pH 7.35. For experiments with myocytes, KCl substituted for K+ gluconate.
In all experiments, the free Ca2+ in solution was adjusted to the desired value by adding CaCl2. When the desired free Ca2+ did not exceed 1 μM, HEDTA was omitted from the solution, and the final MgCl2 was set to 1 mM. In all cases, the nominal free Ca2+ was calculated with MaxChelator Sliders (C. Patton, Stanford University, Stanford, CA) and was validated experimentally using Ca2+-selective electrodes (Corning Incorporated Science Products Division, Corning, NY) (Dopico, 2003).
Patch-clamp electrodes were pulled from glass capillaries (Drummond Scientific Co.). Immediately before recording, the tip of the electrode was fire-polished on a microforge (World Precision Instruments, Sarasota, FL) to give resistances of 8–10 MΩ when filled with extracellular solution (for composition, see previous explanation). An agar bridge with gluconate or chloride as the main anion was used as the ground electrode for oocyte and myocyte recordings, accordingly. After excision from the cell, the membrane patch was exposed to a stream of bath solution containing each agent at the final concentration. Solutions were applied onto the patch cytosolic side using a pressurized, automated DAD12 system (ALA Scientific Instruments, Farmingdale, NY) via a micropipette tip with an internal diameter of 100 μm. Experiments were performed at room temperature (20°–22°C).
The ionic current was recorded using an EPC8 amplifier (HEKA, Lambrecht, Germany) at 1 kHz. Data were digitized at 5 kHz using a Digidata 1320A A/D converter and pCLAMP 8.0 (Molecular Devices, Sunnyvale, CA). The product of number of channels in the patch (N) and channel open probability (Po) was used as an index of channel steady-state activity. NPo was obtained using a built-in option in Clampfit 9.2 (Molecular Devices) from ≥30 second of gap-free recording under each condition.
Macroscopic currents were evoked from a holding potential of 0 mV by 200 milliseconds long, 10 mV steps ranging from −150 to +150 mV. The current amplitude was averaged within 100–150 milliseconds after the start of the depolarizing step. Macroscopic conductance G/Gmax–V plots were fitted to a Boltzmann function of the type G(V) = Gmax/1 + exp[(−V + V1/2)/k]. Boltzmann fitting routines were run using the Levenberg-Marquardt algorithm to perform nonlinear least squares fits.
Cerebral Artery Diameter Measurement
Adult male Sprague-Dawley rats (≈250 g; 10 animals) and 8- to 12-week-old male KCNMB1 K/O (5 animals) and C57BL/6 control (6 animals) mice were decapitated using a guillotine and sharp scissors, respectively. Middle cerebral arteries were isolated on ice under microscope (Nikon SMZ645; Nikon) from the rat or mouse brains and cut into 1- to 2-mm-long segments. Endothelium was removed by passing an air bubble into the vessel lumen for 90 seconds before vessel cannulation. A segment was cannulated at each end in a temperature-controlled, custom-made perfusion chamber. Using a Dynamax RP-1 peristaltic pump (Rainin Instruments, Inc., Oakland, CA), the chamber was continuously perfused at a rate of 3.75 ml/min with physiologic saline solution (PSS) (mM): 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.6 CaCl2, 1.2 MgSO4, 0.023 EDTA, 11 glucose, and 24 NaHCO3. The PSS was equilibrated at pH 7.4 with a 21/5/74% mix of O2/CO2/N2 and maintained at 35–37°C. Arteries were observed with a CCD camera (Sanyo VCB-3512T; Sanyo Electric Co., Moriguchi, Japan) attached to an inverted microscope (Nikon Eclipse TS100; Nikon). The artery external wall diameter was measured using the automatic edge-detection function of IonWizard software (IonOptics, Milton, MA) and was digitized at 1 Hz using a personal computer.
Steady-state changes in intravascular pressure were achieved by elevating an attached reservoir filled with PSS and were monitored using a pressure transducer (Living Systems Instruments, Burlington, VT). Arteries were first incubated at an intravascular pressure of 10 mm Hg for 10 minutes. The intravascular pressure then was increased to 60 mm Hg and held steady throughout the experiment to develop and maintain the arterial myogenic tone. Drugs were dissolved to make stock solutions, diluted in PSS to their final concentration, and applied to the artery via chamber perfusion. The effect of a drug application was evaluated at the time it reached a maximal, steady level. Absence of endothelium was confirmed by the absence of vessel’s response to an endothelium-dependent (10 μM acetylcholine) vasodilator after artery pressurization (Liu et al., 2004; Bukiya et al., 2007).
Pial Arteriole Arteriolar Diameter Measurements
Adult male Sprague-Dawley rats (≈250–300 g; 20 animals) were anesthetized with ketamine hydrochloride (33 mg/kg i.m.) and acepromazine (3.3 mg/kg i.m.). Catheters inserted into the carotid artery were used to infuse the drugs of interest; the drugs were dissolved to a final concentration in sodium saline solution (SSS) (0.9% NaCl). Body temperature was maintained at 37°–38°C with a heating pad. The animals were equipped with a custom-built stainless steel cranial window that allowed 1) measurement of pial arteriolar diameter (PAD) with a videomicrometer coupled to a television camera, and 2) delivery of drugs directly to the brain surface through the ports of the window. The space under the window was filled with artificial cerebrospinal fluid (aCSF) that consisted of (mM): 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3. This solution was equilibrated with 6% CO2/6% O2/88% N2 to pH 7.3–7.35 at 37°C. Pial arterioles (50–100 µm in external diameter) were used to test vascular reactivity. Control PAD values were measured over a 10-minute period under basal conditions. LCA and HENA stock solutions were diluted in SSS to a final concentration of 45 μM and 1 ml of this solution was infused into the cerebral circulation via the carotid artery. A stock solution of paxilline in dimethyl sulfoxide (DMSO) was diluted in aCSF to a final concentration of 1 μM. Paxilline and 4-aminopyridine (4-AP) (0.8 mM in aCSF) were applied topically on the surface of the brain.
Chemicals
We purchased 5β-cholanic acid-3α-ol (lithocholic acid) from Steraloids (Newport, RI) and methyl 3-hydroxyolean-12-en-30-oate from Hit2Lead.com (ChemBridge Corporation). All other chemicals were purchased from Sigma-Aldrich. On the day of the experiment, LCA or HENA were initially dissolved in DMSO to render stock solutions of 333mM. Stocks were sonicated for 30 minutes and then further diluted with either electrophysiology bath recording, PSS or SSS solution to final concentration. In all experiments DMSO-containing solution was used as control perfusion. Concentration of DMSO in “control” matched the corresponding amount of DMSO in LCA- or HENA-containing solution.
Data Analysis
Final plotting, fitting, and statistical analysis of the data were conducted using Origin 8.5.1 (OriginLab, Northampton, MA) and InStat 3.0 (GraphPad, La Jolla, CA). Statistical analysis was conducted using either one-way analysis of variance and Bonferroni’s multiple comparison test or paired Student’s t test, according to the experimental design. P < 0.05 was considered statistically significant. Data are expressed as the mean ± S.E.M., and n = number of patches/arteries/pial arterioles. Each patch was obtained from a different oocyte/myocyte, and each pressurized artery/PAD measurement was obtained from a separate animal.
Results
HENA Is a Novel, Effective Nonsteroid Activator of β1 Subunit–Containing Recombinant BK Channels.
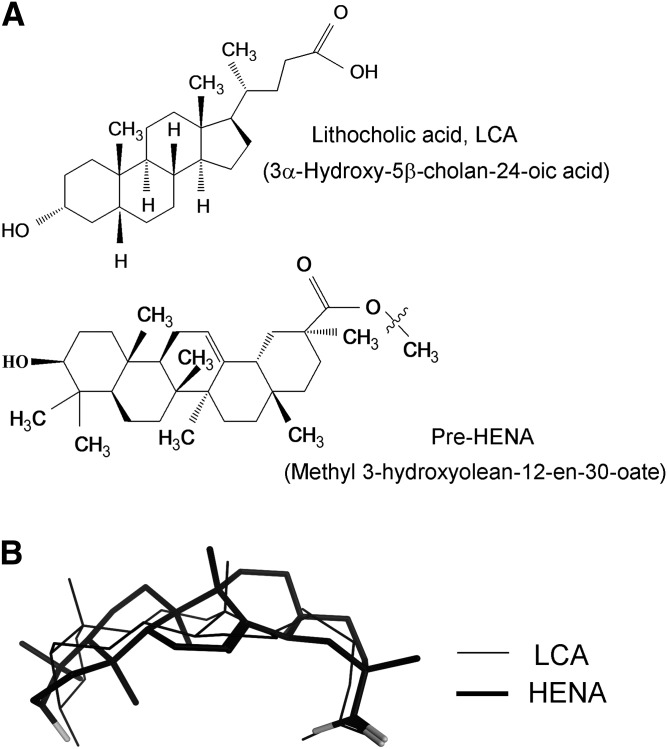
Among 18 cholane steroids tested, LCA was the most effective activator of β1-containing BK channels (Dopico et al., 2002; Bukiya et al., 2008a). Thus, in search of novel selective activators of these channels, we took the LCA molecule (Fig. 1A, top structure) as the template for a similarity search, and screened the Hit2Lead.com database of chemical compounds. The screening yielded methyl 3-hydroxyolean-12-en-30-oate (ID 5808244; Fig. 1A, bottom structure) as the lead compound. This compound is not a steroid, yet it contains all the structural features previously identified as necessary for cholane steroid activation of BK channels: C3-hydroxyl, hydrophobic nucleus and polar lateral chain (Dopico et al., 2002; Bukiya et al., 2008a, 2012). The highly hydrophobic methyl 3-hydroxyolean-12-en-30-oate required dissolution in pure DMSO before further dissolution in saline solutions for biologic evaluation. The resulting DMSO concentration in saline solution exceeded 0.5%, which is known to lead to apoptosis (Qi et al., 2008). Thus, to improve active compound solubility and bolster structural similarity between methyl 3-hydroxyolean-12-en-30-oate and LCA, we performed a two-step organic chemical reaction to render HENA (Fig. 1A, bottom structure; Supplemental Fig. 1). With a carboxylate similar to that of LCA (pKa ~5.5), the HENA molecule is expected to remain significantly ionized at physiologic pH ≈7.3–7.4. Thus, flexible alignment of ionized LCA and HENA molecules showed high degree of overlap at both their C3-hydroxyl and carboxyl ends (Fig. 1B). Most important, HENA was able to adopt a bean-like shape, which is critical for BK channel activation by LCA and related cholane steroids (Dopico et al., 2002; Bukiya et al., 2008a). Therefore, HENA exhibits all main structural features considered necessary for BK channel activation via the BK β1 subunit cholane steroid site (Bukiya et al., 2008a, 2011).
Fig. 1.
The nonsteroid HENA shows substantial structural similarity with lithocholic acid (LCA). (A) Chemical structures of LCA and HENA. In HENA, a zigzag line highlights the point of hydrolysis of methyl 3-hydroxyolean-12-en-30-oate to render HENA. (B) Flexible alignment of LCA and HENA in their ionized forms reveals close similarity in the overall shape of the molecules and overlap in critical functional groups. Oxygen atoms in the HENA structure are shown in light gray.
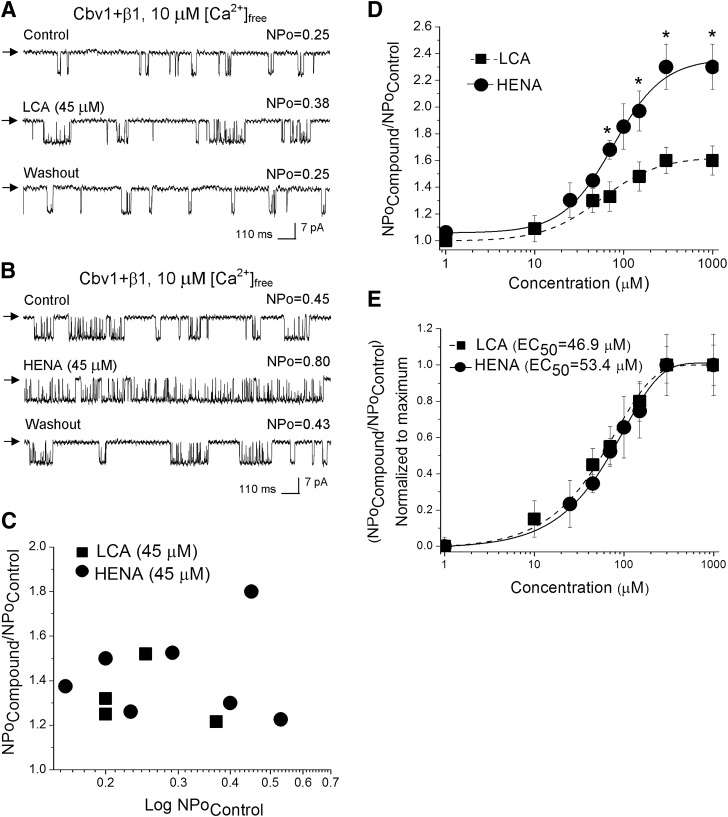
To determine HENA’s ability to activate BK channels, we first studied ligand action on recombinant cbv1 + β1 channels heterologously expressed in Xenopus oocytes. We cloned both the BK channel–forming cbv1 (AY330293) and accessory β1 (FJ154955) subunits from freshly isolated rat cerebral artery myocytes; the resulting recombinant BK complex represented an ideal model of the native BK channel (Bukiya et al., 2009b). In our experiments, we used I/O patches with the membrane potential and free Ca2+i set at values similar to those reported in the rat cerebrovascular myocyte during contraction (−40 to −30 mV and 10 μM), that is, when BK channel activity plays a critical role in limiting depolarization-induced Ca2+-entry and thus further contraction (Jaggar et al., 1998; Knot and Nelson, 1998; Pérez et al., 2001). After excision, each patch was exposed to the control (solvent-containing) solution, and BK activity was continuously recorded for no less than 30 seconds. Application of the LCA-containing (1–1,000 µM) solution reversibly increased NPo in a concentration-dependent fashion: EC50 = 46.9 ± 4.1 µM, Emax ~ 300 µM (Fig. 2, A, D, and E). These values are similar to those reported with LCA on this system and are attributed to a direct interaction between LC monomers and the BK channel proteins (Bukiya et al., 2007). Application of HENA-containing (1–1,000 µM) solution also reversibly increased NPo in a concentration-dependent manner, with potency similar to that of LCA: EC50 = 53.4 ± 3.9 µM and Emax ~ 300 µM (Fig. 2, B, D, and E). For both LCA and HENA, drug potentiation of the BK channel activity did not depend on basal, predrug channel activity (Fig. 2C). At every concentration tested, however, HENA was more effective than LCA in increasing BK NPo (Fig. 2D), with NPo in presence of HENA Emax reaching 2.5 times that of control.
Fig. 2.
HENA is more efficient than LCA in increasing the activity of cbv1 + β1 channels. Single-channel recordings from I/O patches excised from X. laevis oocytes expressing cbv1 + β1 channel complexes. Unitary current records depict BK channel activity before, during, and after 45 μM LCA (A) or 45 μM HENA application (B). Vm = −40 mV, [Ca2+]free = 10 μM. Here and in all other figures: channel openings are shown as downward deflections; arrows indicate the baseline; control and washout correspond to vehicle-containing bath solution. (C) HENA- and LCA-induced increase in BK channel NPo shows no correlation with the channel’s basal (predrug) activity. (D) concentration response curves for HENA and LCA. Each point represents the average of no less than three patches, each patch obtained from a different oocyte. *Different from LCA (P < 0.05). (E) Concentration-response curves where drug-induced BK channel activation at a given concentration is normalized to the drug’s maximal effect show that HENA and LCA have a similar EC50.
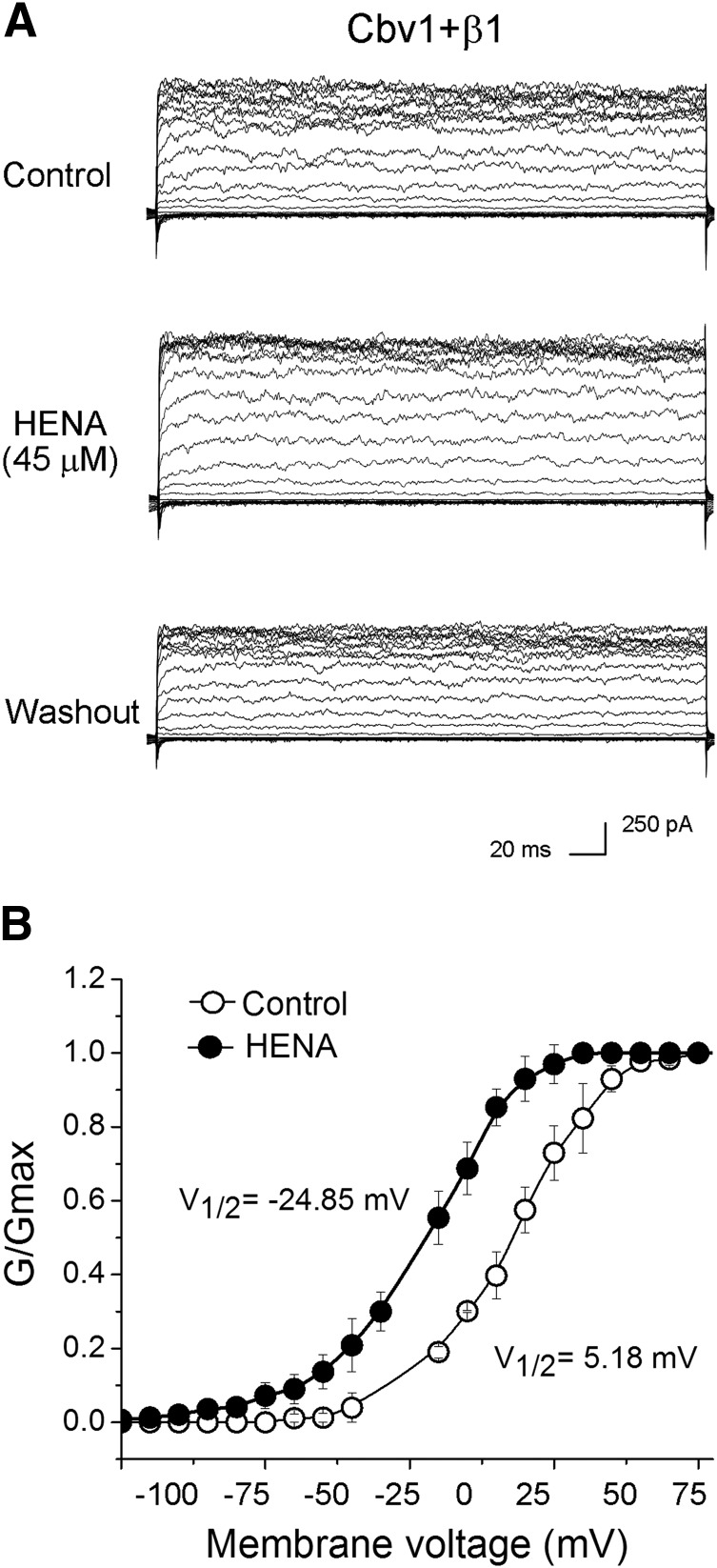
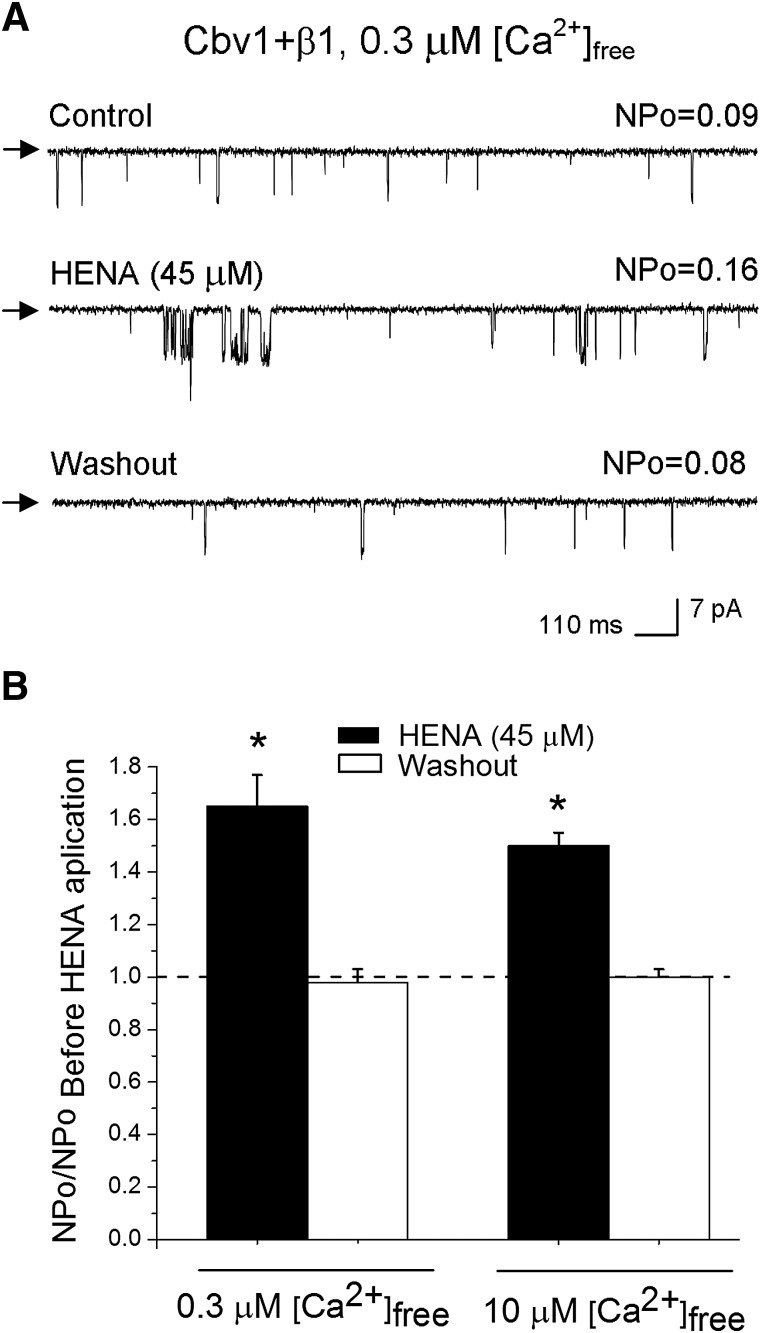
It is noteworthy that HENA-induced BK channel activation remained fairly constant within a wide voltage range (−150 to +150 mV), as documented by a parallel leftward shift in G/Gmax plots from macroscopic cbv1 + β1 currents in the presence versus absence of HENA (Fig. 3, A and B). On the other hand, HENA-induced BK channel activation was identical at submicromolar (0.3 μΜ) and micromolar (10 μΜ) levels of free Ca2+i (Fig. 4, A and B). Collectively, our results demonstrate that HENA-induced BK channel activation is voltage independent and observed at levels of Ca2+i found in the resting and contracting cerebral artery myocyte (Pérez et al., 2001).
Fig. 3.
HENA activates BK channels within a wide voltage range. (A) HENA (45 μΜ) increases macroscopic current mediated by cbv1 + β1 subunits expressed in X. laevis I/O membrane patches. Voltage steps of 200 millisecond duration were applied from −150 to +150 mV in 10 mV increments from Vholding = 0 mV; [Ca2+]free = 10 μM. (B) G/Gmax–V curves from cbv1 + β1 channels at 10 μM [Ca2+]free show a parallel leftward shift in the presence of 45 μM HENA when compared with control. V1/2 denotes the transmembrane voltage (Vm) at which G/Gmax = 0.5. Each data point represents the averaged from ≥4 patches.
Fig. 4.
HENA-induced BK channel activation is observed at physiologically relevant Ca2+i levels. (A) Single-channel records from cbv1 + β1 complexes expressed in X. laevis oocytes show an increase in NPo in response to 45 μM HENA. Vm = −40 mV, [Ca2+]free = 0.3 μM. (B) Averaged responses of cbv1 + β1 channels to 45 μM HENA, obtained at 0.3 and 10 μM [Ca2+]free. Each data point was obtained from no less than four patches; each patch was obtained from a different cell. A horizontal dashed line defines a lack of HENA effect. *Different from pre-HENA NPo (P < 0.05).
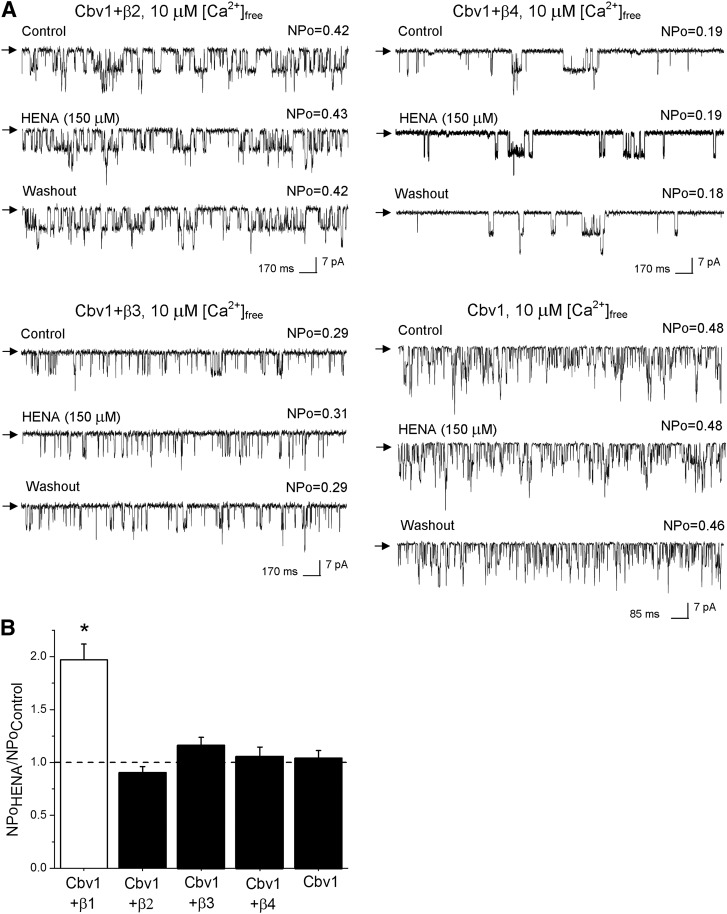
Considering that 1) HENA has a high structural similarity with LCA (Fig. 1), and 2) LCA selectively activates β1 subunit–containing BK channels (Bukiya et al., 2009a), we decided to determine whether HENA-induced BK channel activation required the presence of BK β1 subunits. We performed electrophysiologic recordings in I/O membrane patches from Xenopus oocytes expressing heteromeric cbv1 + β2, β3, or β4 subunits, or homomeric cbv1 channels under identical conditions of transmembrane voltage (Vm = −40 mV) and Ca2+i (10 μM). In all cases, the resulting ion current phenotype was confirmed by characteristic BK current kinetics (Supplemental Fig. 2) or distinctive pharmacologic features, as we previously described in detail elsewhere (Bukiya et al., 2009a). In contrast to their cbv1 + β1 counterparts, homomeric cbv1 channels were consistently resistant to HENA, even when this compound was tested at 150 μΜ (Fig. 5, A, bottom right panel, and B), which corresponds to EC90 for HENA activation of β1-containing BK complexes. Thus, as previously shown for the steroid LCA (Bukiya et al., 2009a), sensitivity of BK channels to the nonsteroid HENA requires the presence of functional BK β1 subunits.
Fig. 5.
BK βs 2–4 fail to substitute for β1 in providing HENA sensitivity to the BK complex. (A) Unitary current records obtained in I/O patches from X. laevis oocytes that express BK channels of different subunit composition. Vm = −40 mV, [Ca2+]free = 10 μM. (B) Average increase in BK activity (NPo) in the presence of 150 μM HENA. Each point was obtained from no less than 4–5 patches. *Different from cbv1 (P < 0.05).
Moreover, heteromers made of cbv1 + β2, β3, or β4 subunits were all HENA-resistant (Fig. 5, A, bottom left and top panels, and B). Thus, as previously found for LCA (Bukiya et al., 2009a), BK β2, β3, and β4 failed to substitute for β1 in conferring HENA sensitivity to BK channels. Collectively, the qualitative and quantitative similarities between HENA and LCA action on recombinant cerebrovascular BK channels seem to indicate that BK β1 behaves as a specific sensor of HENA, likely involving the cholane steroid-recognizing site recently identified in this protein (Bukiya et al., 2011).
HENA Activates BK Channels via Cholane Steroid-Sensing Site on BK β1 Subunit TM2 Domain.
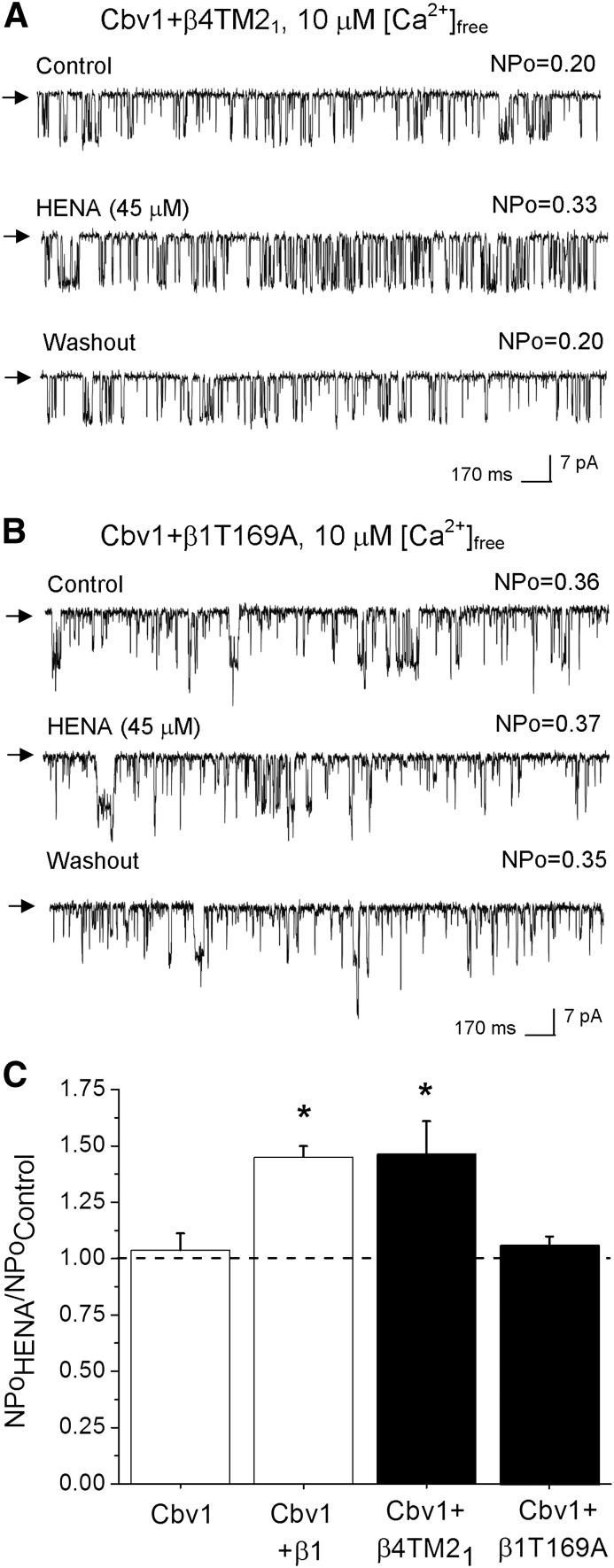
Based on the fact that the β1, but not β4, subunit confers HENA sensitivity to the BK channel, and that LCA exerts its effect via the TM2 of β1, we used chimeric β1/β4 subunits to identify the BK β1 protein region targeted by HENA. In the chimeric construct termed β4TM21, the BK β4 TM2 domain was substituted by the TM2 region from BK β1. The presence of functional β4TM21 coexpressed with cbv1 was confirmed by the resistance of the current to iberiotoxin (100 nM) block, which is due to the presence of the BK β4 extracellular loop region (Meera et al., 2000; Bukiya et al., 2008b).
Under experimental conditions similar to those used to probe HENA on WT β1 + cbv1, 45 μM HENA applied to the cytosolic side of I/O patches evoked a robust and reversible increase in BK NPo in all cases (Fig. 6, A and C). Remarkably, this effect was identical to that obtained with HENA and cbv1 + WT β1 channels (Fig. 2, B, D, and E), indicating that β1 TM2 is the channel region that senses the HENA presence, rendering the activation of BK channels.
Fig. 6.
Threonine 169 within BK β1 TM2 confers HENA sensitivity to the BK complex. (A) Channels resulting from cbv1 and engineered β4 subunits containing the β1 TM2 domain (cbv1 + β4TM21) retain sensitivity to 45 μM HENA. (B) Currents from cbv1 + β1T169A are resistant to HENA. In A and B: Vm = −40 mV, [Ca2+]free = 10 μM. (C) Averaged responses to 45 μM HENA from BK complexes containing modified β1 subunits. HENA-resistant cbv1 homomers and HENA-sensitive cbv1 + β1 heteromers serve as negative and positive controls, respectively. Each point was obtained from 4–5 patches. *Different from cbv1 (P < 0.05).
The cholane-sensing site in the BK β1 TM2 domain includes Thr169, a residue that is distinct to BK β of type 1 (Brenner et al., 2000a). Computational modeling shows that this residue forms a hydrogen bond with the LCA C3-hydroxyl, and thus the T169A substitution fully abolishes LCA sensitivity of BK channels (Bukiya et al., 2008a, 2011). Next, we tested whether HENA recognition by BK β1 also required Thr169. Thus, we coexpressed cbv1 with BK β1T169A and evaluated HENA action under experimental conditions identical to those used to evaluate the nonsteroidal agent on cbv1 + β4TM21 and cbv1 + WT β1 channels (see previous discussion). In all cases, application of HENA, whether at 45 μΜ (Fig. 6, B and C) or 150 μΜ (unpublished data), onto I/O membrane patches expressing cbv1 + β1T169A failed to modify BK NPo (n = 4 for each HENA concentration). These negative data indicate that Thr169, a residue unique to BK β subunits of type 1 and critical for providing cholane steroid sensitivity to β1-containing BK channels, is also necessary for HENA activation of these channels. This fact strongly suggests that HENA activates β1-containing BK channels via specific interaction with the BK cholane steroid site (see Discussion).
HENA Activates Native Cerebral Artery Myocyte BK Channels.
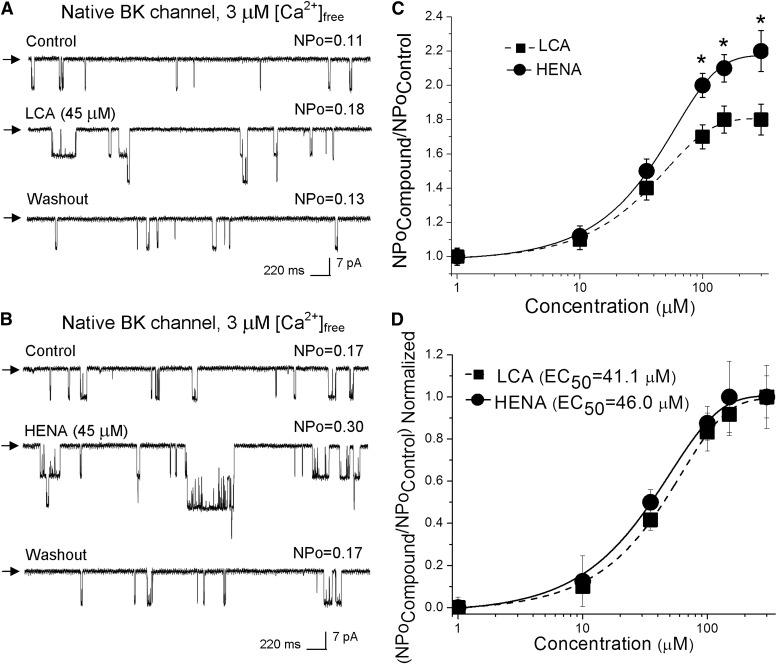
To determine whether HENA was able to target β1-containing BK channels when expressed in their natural membrane, we studied drug action on native BK channels in I/O patches excised from freshly isolated rat cerebral artery myocytes while comparing HENA action to that of LCA (Fig. 7). Membrane potential and Ca2+i were set at values within the range found in cerebrovascular myocytes during contraction: −40 to −30 mV and 3 μM free Ca2+i (Knot and Nelson, 1998; Pérez et al., 2001). After excision, the patch was exposed to control (vehicle-containing) solution, and BK NPo was recorded for no less than 30 seconds. Application of HENA-containing (1–300 µM) solution reversibly increased NPo in a concentration-dependent fashion, with EC50 = 46 ± 6 µM and Emax ~ 300 µM (Fig. 7, B and C). These values are similar to those reported for HENA activation of recombinant cbv1 + β1 channels expressed in amphibian membranes (Fig. 2E) and LCA activation of rat cerebrovascular smooth muscle BK channels (Fig. 7) (Bukiya et al., 2007). At HENA Emax, however, NPo reached 220% of control, which is significantly higher than that reached at LCA Emax (Fig. 7, B and C). Thus, as found for recombinant channels, HENA is more efficacious than LCA in activating native cerebrovascular myocyte BK channels.
Fig. 7.
HENA increases the activity of rat cerebral artery myocyte BK channels more effectively than LCA. (A and B) Single BK channel recordings obtained in I/O patches excised from freshly isolated rat cerebral artery myocytes. Records depict native BK channel activity before, during, and after 45 μM LCA (A) or 45 μM HENA application (B). Vm = −40 mV, [Ca2+]free = 3 μM. (C) concentration response curves for HENA and LCA. Each point represents the average of no less than three patches, each patch excised from a different oocyte. *Different from LCA (P < 0.05). (D) Concentration-response curves where the drug-induced BK channel activation at a given concentration is normalized to the drug’s maximal effect show that HENA and LCA have a similar EC50.
It is noteworthy that the HENA-induced increase in NPo was observed in membrane patches that were excised from the myocyte >5 minutes before applying the drug under continuous bath perfusion in the absence of nucleotides. Therefore, HENA action does not require cell integrity or the continuous presence of cytosolic messengers to activate native BK channels from freshly isolated rat cerebral artery myocytes. Overall, the qualitative and quantitative similarities in HENA action on recombinant versus native BK channels suggest that the likely different proteolipid environments in amphibians versus mammalian cell membranes do not affect HENA activation of β1-contaning BK channels, underscoring that potentiation of BK channel activity is due to a direct interaction between the HENA molecule and the BK β1 protein itself.
HENA Evokes Dilation of Smooth Muscle BK β1 Subunit–Containing Cerebral Arteries.
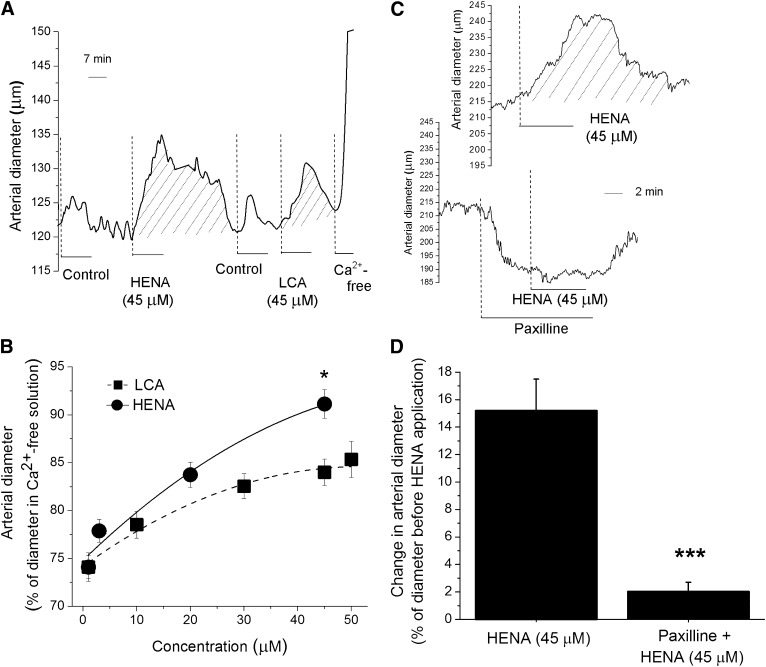
To determine the impact of smooth muscle BK channel targeting by HENA on organ function, we evaluated drug action on the diameter of pressurized middle cerebral arteries from rats. The vessel dissection, de-endothelization, and pressurization are described in Materials and Methods. After myogenic tone development at 60 mm Hg, the arteries were sequentially probed with the control (DMSO-containing) and HENA- or LCA-containing solutions. At the end of each experiment, the artery was perfused with Ca2+-free PSS to assess the passive arterial diameter.
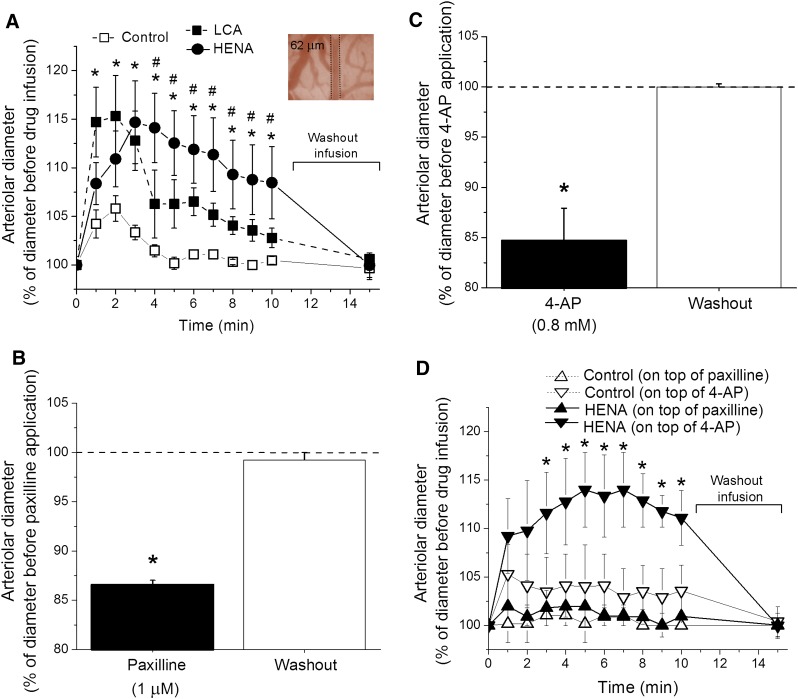
As previously reported elsewhere (Bukiya et al., 2007), the presence of vehicle in the chamber caused a mild and transient dilation of the arteries. This effect disappeared upon washout with solvent-free PSS (Fig. 8A). After artery perfusion with PSS, brief (≤10 minute) application of HENA (3–45 μΜ) produced an immediate increase in arterial diameter, which was sustained as far as HENA was present in the perfusing solution; it fully recovered to pre-HENA values after washout of HENA with compound-free PSS (Fig. 8A). This HENA action was concentration dependent, with maximal artery dilation being observed in the presence of 45 μΜ HENA: 95% of passive arterial diameter and an averaged 15% increase in artery diameter from pre-HENA diameter values (Fig. 8, A and B). This HENA action is expected to evoke a marked increase in cerebral blood flow (~45%) because changes in artery diameter are related to changes in cerebral blood flow by a factor of ~3 (Gourley and Heistad, 1984).
Fig. 8.
HENA dilates pressurized cerebral arteries via activation of BK channels and independently of an intact endothelium. (A) Diameter trace from de-endothelized, pressurized cerebral artery. After development of myogenic tone, acute application of 45 μM HENA causes reversible dilation, which is larger and lasts longer than dilation by 45 μM LCA. (B) concentration response curves for HENA and LCA on artery diameter. Each point represents the average of ≥3 arteries, each artery harvested from a different rat. *Different from LCA (P < 0.05). (C) Artery diameter traces showing that HENA action is ablated by selective BK channel block with 1 μM paxilline. (D) Average change in artery diameter evoked by 45 μM HENA in absence (n = 3) and presence (n = 3) of 1 μM paxilline. ***Different from HENA-induced dilation in the absence of paxilline (P < 0.001).
Finally, HENA-induced in vitro dilation of cerebral arteries was prevented by selective block of the BK channels with 1 μΜ paxilline (Fig. 8, C and D) (Strøbaek et al., 1996). Collectively: 1) this outcome, 2) with the arteries being de-endothelized before pressurization, and 3) with smooth muscle cells being estimated to account for up to 70% of de-endothelized arterial tissue (Lee, 1995) led us to conclude that HENA-induced cerebral artery dilation is primarily due to activation of BK channels present in the vascular smooth muscle. Consistent with this interpretation, the HENA-induced increase in arterial diameter was significantly higher than that evoked by LCA (Fig. 8, A and B), paralleling the HENA versus LCA differential efficacies of smooth muscle BK channel activation (Fig. 7). In spite of their marked structural similarities (Fig. 1), HENA and LCA differed in their ability to increase overall Ca2+ levels in cerebral artery smooth muscle, with 45 µM LCA being effective and HENA not (Supplemental Fig. 3; Supplemental Methods) (see Discussion).
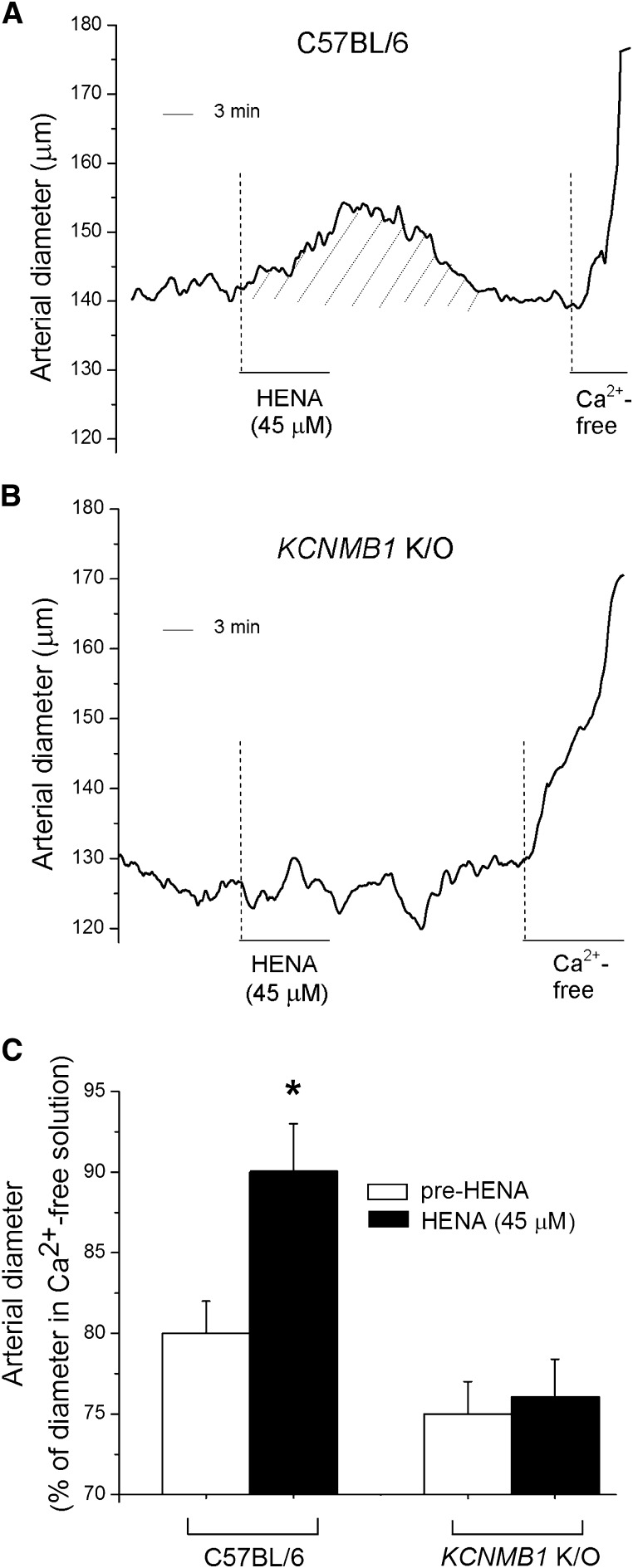
To determine whether HENA-induced artery dilation resulted from arterial smooth muscle BK β1 subunit targeting by this ligand, we evaluated HENA action on the arterial diameter of de-endothelized, pressurized cerebral arteries from β1 subunit-lacking (KCNMB1 K/O) versus β1 subunit-containing (WT C57BL/6) mice. As found with rat cerebral arteries, 45 μM HENA caused a sustained yet fully reversible increase in the diameter of WT mouse cerebral arteries (≈10% from diameter before HENA application) (Fig. 9, A and C). In sharp contrast, HENA consistently failed to dilate the arteries from KCNMB1 K/O mice (n = 5) (Fig. 9, B and C), indicating that the presence of smooth muscle BK β1 subunits in cerebral arteries is essential for HENA-induced vasodilation.
Fig. 9.
HENA fails to dilate pressurized arteries from BK β1 subunit-lacking (KCNMB1 K/O) mice. Cerebral artery diameter traces show that acute application of 45 μM HENA causes sustained diameter increase (dilation) in arteries from BK β1 subunit-containing C57BL/6 mouse (A) but not in arteries from KCNMB1 K/O mouse (B). (C) Average diameter data in response to HENA (dark bars) (n = 4) compared with pre-HENA levels (hollow bars). *Different from diameter before HENA application (P < 0.05).
HENA Causes Pial Arteriolar Dilation In Vivo via Selective Activation of BK Channels.
To test whether HENA can produce in vivo vasodilation, we used a closed cranial window on anesthetized rats. Our technique allows visual in vivo monitoring of pial arterioles (50–100 μm external diameter) that arise from major cerebral (including middle) arteries and are the principal vascular conduit for maintaining continuous blood flow to the brain (Baumbach and Heistad, 1985). Control (DMSO-containing) and HENA- or LCA-containing solutions were infused into the cerebral circulation via catheter in the carotid artery. As observed with control perfusion of pressurized cerebral arteries in vitro, infusion of vehicle-containing SSS (1 ml) caused a mild (≤5%) and transient arteriolar dilation, which fully disappeared within 4 minutes after infusion (Fig. 10A). In contrast, 45 μΜ of LCA or HENA rendered up to a ≥10% additional dilation (i.e., on top of that evoked by vehicle). LCA- and HENA-induced dilations were sustained throughout 10 minutes of diameter monitoring after continuous drug infusion. Notably, HENA-induced arteriolar dilation was significantly larger than that evoked by LCA during the 4th through the 10th minutes after drug infusion (Fig. 10A). Moreover, the effect of each compound was reversible, gradually diminishing until fully gone within 5 minutes of washout by infusion of 1 ml of drug-free SSS. Finally, evaluation of systemic blood pressure by catheterization of the femoral artery indicated that intracarotid infusion of HENA failed to modify mean arterial blood pressure: 71 ± 2.5 versus 69 ± 4.1 mm Hg before HENA intracarotid infusion and during 4–6th minutes after HENA intracarotid infusion, respectively. This result indicates that pial dilation in response to intracarotid HENA is not related to—or, at least, is not largely caused by—a fall in systemic blood pressure. Rather, HENA-induced cerebral artery dilation observed in vivo during and immediately after HENA intracarotid infusion represents a true dilator response to HENA of the cerebral circulation.
Fig. 10.
HENA dilates pial arterioles in vivo through selective activation of BK channels. (A) Pial arteriole dilation upon LCA or HENA infusion as function of time. Insert: snapshot from the surface of the brain via a cranial window depicting computer detection of arteriole external diameter before application of 45 μM LCA or HENA infusion via the carotid artery. (B) Averaged pial arteriolar diameter (as percentage of diameter before channel blocker) in response to topical application of the selective BK channel blocker paxilline (1 μM) on the brain surface. (C) Averaged pial arteriolar diameter (as percentage of diameter before blocker) in response to topical application of 4-AP (0.8 mM), which blocks Kv channels other than BK. In panels B and C, each data point represents an average from no less than three animals; a horizontal dashed line defines the arteriolar diameter before blocker application. *Different from preblocker level (P < 0.05). (D) Pial arteriole dilation upon HENA infusion in presence of paxilline or 4-AP as function of time. *Different from diameter upon vehicle-containing bath solution infusion (P < 0.05). #Different from LCA-induced pial arteriolar dilation (P < 0.05).
To determine the involvement of BK channels in HENA-induced pial arteriolar dilation, we used the selective BK channel blocker paxilline, which was topically applied at 1 μΜ on the brain surface. Paxilline application caused robust constriction of arterioles, with up to 15% reduction in diameter from prepaxilline values (Fig. 10B). This result underscores the key role of BK channels in limiting vessel constriction. When compared with control (DMSO-containing SSS), carotid artery infusion of 45 μΜ HENA in presence of paxilline failed to cause any change in arteriolar diameter throughout 10 minutes of continuous diameter monitoring (Fig. 10D). This lack of response to HENA infusion in the presence of paxilline points at BK channels as molecular targets of HENA-driven pial arteriolar in vivo dilation. Finally, washout of paxilline from the brain surface caused a rebound increase in arterial diameter back to prepaxilline levels (Fig. 10B), documenting arteriole viability after the several experimental manipulations.
Besides BK, cerebrovascular smooth muscle tone is also controlled by purely voltage-gated K+ (KV) channels (Faraci and Sobey, 1998). To determine the selectivity of BK channel involvement in HENA-induced dilation, we evaluated HENA action in the presence of 4-AP. At submillimolar to low millimolar concentrations, AP blocks most KV but not BK channels in rat cerebral arteries (Liu et al., 2004). Topical application of 0.8 mM 4-AP on the surface of the brain caused an immediate decrease in diameter up to 15% from pre-4-AP level (Fig. 10C). In contrast to data obtained in the presence of HENA + paxilline, carotid infusion of HENA in the presence of 4-AP caused arteriolar dilation that was significantly higher compared with infusion of vehicle-containing SSS (Fig. 10D). Moreover, HENA-induced dilation reached 10% on top of the control value, which was identical to the HENA-induced dilation determined in the absence of 4-AP (Fig. 10, A and D).
These results indicate that KV channels other than BK do not play a major role in HENA-induced in vivo dilation of pial arterioles. Rather, the HENA effect is attributed to selective targeting of BK channels by the nonsteroidal ligand. Finally, washout of 4-AP from the brain surface caused a rebound increase in artery diameter back to pre-4-AP levels (Fig. 10C), documenting the arteriole viability after several experimental manipulations.
Discussion
It has been consistently documented that β subunits of type 1 are particularly abundant in smooth muscle in general and arterial myocytes in particular (Orio et al., 2002; Brenner et al., 2000a). Moreover, this subunit plays a central role in promoting smooth muscle relaxation and vasodilation (Brenner et al., 2000b). These previous findings raised hope that selective targeting of BK β1 subunits by small agents could be an effective mechanism to evoke vasodilation while barely targeting BK channels in tissues other than smooth muscle or other receptors. Our present data reveal for the first time that HENA, an exogenous and nonsteroidal agent, evokes a robust dilation of resistance size in cerebral arteries both in vitro and in vivo by smooth muscle BK channel activation. The strong quantitative similarities between the HENA in vitro dilation of isolated, de-endothelized arteries and the agent-induced dilation of cerebral arteries when administered via carotid artery to the intact animal strongly suggest that neither circulating nor endothelial factors exert a major regulatory effect on HENA targeting of smooth muscle BK channels. Moreover, this action involves HENA recognition by the recently discovered steroid-sensing site present in the BK β1 protein (Bukiya et al., 2011). Therefore, our study demonstrates that LCA and related steroids can be used as a template for the search, design, and development of nonsteroidal vasodilators that selectively target β1-containing BK channels.
Before our discovery of HENA, a few ligands have been claimed to require the presence of BK β1 subunits to increase BK channel activity. Among physiologic, endogenous ligands, 17β-estradiol at low micromolar levels has been reported to increase the activity of recombinant BK constructs made of hslo α (from human myometrium) + β1 subunits while failing to activate homomeric hslo α channels. This study also showed [3H]17β-estradiol-specific binding and bovine serum albumin–bound fluorescent 17β-estradiol labeling in membranes expressing BK α and β1 subunits but not in membranes expressing solely α subunits (Valverde et al., 1999). A later study, however, demonstrated that submicromolar levels of 17β-estradiol modulated BK activity in absence of BK β1 (Korovkina et al., 2004).
Also regarding physiologically relevant steroids, the vasoactive properties of LCA and other bile acids have been known for several decades (Bomzon and Ljubuncic, 1995), and BK channel activation by LCA and related cholanes has been demonstrated in both vascular and nonvascular smooth muscle (Dopico et al., 2002; Bukiya et al., 2007). In particular, our group has demonstrated that myocyte BK channel activation by LCA results in cerebral artery dilation, a steroid action that requires selective ligand recognition by a steroid-recognition site identified in the BK β1 TM2 (Bukiya et al., 2007, 2011). Due to their steroidal character, LCA and related cholanes exert numerous biologic effects within and outside the vascular bed involving interactions with multiple membrane and cytosolic proteins and transcription factors (Makishima et al., 2002; Maruyama et al., 2002; Gerasimenko et al., 2006; Modica and Moschetta, 2006).
As found for taurolithocholic acid 3-sulfate in pancreatic acinar cells (Gerasimenko et al., 2006), we showed here that μM LCA raised overall cytosolic Ca2+i in endothelium-free cerebral artery, an effect not observed with HENA (Supplemental Fig. 3). Thus, any possible use of LCA as a vasodilator might require additional interventions to reduce a bile-acid-induced increase in Ca2+i, as this increase would oppose smooth muscle relaxation and vasodilation. More generally, the widespread side effects secondary to cholane steroid-recognition by off-target proteins (i.e., other than β1-containing BK channels) would severely limit the use of LCA and related cholanes as vasodilators in clinical practice. A similar limitation would likely apply to other physiologic steroids. The targeting of multiple BK subunits and tissues by physiologically relevant steroids is reviewed elsewhere (Dopico et al., 2012).
Regarding ligands exogenous to the animal organism, the glycoside dehydrosoyasaponin-I (DHS-I), derived from the medicinal herb Desmodium adscendens, has been shown to activate β1 subunit–containing BK channels at nanomolar concentrations (McManus et al., 1993, 1995). Unfortunately, DHS-I is only effective in activating BK channels when applied from the intracellular side of the membrane (McManus et al., 1995), which drastically limits the delivery of DHS-I to myocyte BK channels via circulation. This limitation does not apply to HENA, as demonstrated by our present data (Figs. 8–10).
Other known BK channel activators do not discriminate between the different types of BK β subunits or do not require them at all. For example, the voltage-sensitive oxonol dye and related analogs can activate β1-containing and neuronally abundant, β4-containing BK channels (Morimoto et al., 2007). The benzimidazolone NS1619 [4-(trifluoromethyl)-2-[5-(trifluoromethyl)-2,3-dihydrobenzimidazol-1-yl]phenol] (Papassotiriou et al., 2000), the benzofuroindole CTBIC [4-chloro-7-(trifluoromethyl)-10H-benzofuro[3,2-b]indole-1-carboxylic acid], and the tetrahydroquinoline termed compound Z [(3aR,4S,9bS)-4-(Naphthalen-1-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-carboxylic acid] (Lee et al., 2012; Ponte et al., 2012) all induce BK channel activation in absence of BK β1, with drug action being likely mediated by the ubiquitously distributed BK α subunit. It remains unknown whether NS1619 amido derivatives promote relaxation of KCl-precontracted isolated thoracic aortic rings via BK β1 subunits (Calderone et al., 2008). Likewise, it remains unknown whether NS11021 [1-(3,5-bis-trifluoromethyl-phenyl)-3-[4-bromo-2-(1H-tetrazol-5-yl)-phenyl]-thiourea] and related anthraquinones activate BK channels (Roy et al., 2012) via BK β1 and, if so, whether other BK βs can substitute for β1 in mediating drug action. Therefore, to our knowledge, HENA is the only known nonsteroidal BK channel activator and vasodilator that 1) can be delivered from the extracellular side of the membrane, and 2) selectively targets β1-containing BK channels. From a basic research standpoint, HENA can be used as a pharmacologic tool alternative to LCA for detecting β1-containing BK channels and targeting the BK β1 subunit steroid-sensing site in biologic preparations.
Consideration of HENA and related compounds in clinical settings, however, requires some additional discussion. HENA belongs to a large family of naturally occurring pentacyclic triterpenes (PT) derived from herbal sources. Several derivatives having the PT skeleton are antitumoral agents and inhibitors of glycogen phosphorylase and Na+/K+-ATPase (Kaneda et al., 1992; Terasawa et al., 1992; Liang et al., 2011). Although a current CAPLUS database search on PTs results in over 100 reports, none of these reports points at vasoactive properties of PT-related compounds. Thus, HENA may possess unique chemical features that restrict its actions to the vascular smooth muscle and, in particular, BK channels. Indeed, our data show that HENA not only discriminates between BK β1 and other BK βs (Fig. 5), but also fails to functionally interact with smooth muscle KV TM6 channels other than BK (Fig. 10D). These outcomes may be explained by the low degree of similarity between the HENA-sensing BK β1 subunit and other known proteins. Indeed, Thr169, shown here as critical for HENA action, is unique to BK channels of the β1 type. The selectivity of HENA on BK β1–containing BK channels is critical from a drug development point of view, as a good drug development candidate should act on a minimal set of targets to evoke few side effects.
A fundamental feature that determines the clinical utility of a drug candidate is its efficacy on the tissue of interest. We show here that 45 μΜ HENA whether applied in vitro to a de-endothelized artery or in vivo through intracarotid infusion increases cerebral artery diameter by ~15% (Fig. 10). This change is expected to evoke a robust increase in cerebral blood flow (by 45%) (Gourley and Heistad, 1984), and it would rescue cerebral protein synthesis and likely prevent the extracellular accumulation of glutamate and lactate that are found upon a 20–50% reduction of cerebral blood flow (Hossmann, 1994).
The identification of the LCA-steroid site as mediating HENA action on BK channels, followed by further computational modeling and organic chemical synthesis would lead to the design of β1 subunit-containing BK channel antagonists that could be used to reduce HENA-induced vasodilation should this effect exceed a desired set point. Therefore, our study raises the hypothesis that HENA is an attractive drug development candidate for treating conditions associated with cerebral artery constriction such as cerebral vasospasm and ischemia. Although HENA efficacy on cerebral artery diameter is impressive, its in vitro potency is not optimal (EC50 = 45 µM). Thus, further medicinal chemistry efforts are likely needed to optimize the HENA structure and yield more potent while similarly effective vasodilators without compromising selectivity toward the BK β1 subunit. In conclusion, our study has demonstrated that selective targeting of the steroid site in β1-containing BK channels by a nonsteroidal, exogenous and novel ligand is an effective way to evoke vasodilation.
Supplementary Material
Acknowledgments
The authors thank Robert Brenner (University of Texas HSC at San Antonio) and Richard Aldrich (Univerity of Texas at Austin) for their generous gift of KCNMB1 K/O mice, and Maria Asuncion-Chin (University of Tennessee HSC) for excellent technical assistance. A.P. thanks the Chemical Computing Group for the MOE program.
Abbreviations
- aCSF
artificial cerebral spinal fluid
- 4-AP
4-aminopyridine
- BK
large conductance
- Ca2+/voltage-gated K+ compound 1
3′-hydroxy-olean-12-en-29-oic acid methyl ester
- compound 2
3′-hydroxy-olean-12-en-29-oic acid
- compound 3
sodium salt of 3′-hydroxy-olean-12-en-29-oic acid
- compound Z
(3aR,4S,9bS)-4-(Naphthalen-1-yl)-3a,4,5,9b-tetrahydro-3Hcyclopenta[c]quinoline-8-carboxylic acid
- CTBIC
4-chloro-7-(trifluoromethyl)-10H-benzofuro[3,2-b]indole-1-carboxylic acid
- DHS-I
dehydrosoyasaponin-I
- DM
dissociation medium
- DMSO
dimethyl sulfoxide
- HENA
sodium 3-hydroxyolean-12-en-30-oate
- I/O
inside-out
- K/O
knockout
- KV
voltage-gated K+
- LCA
lithocholic acid
- N
number of functional channels in the membrane patch
- NS1619
4-(trifluoromethyl)-2-[5-(trifluoromethyl)-2,3-dihydrobenzimidazol-1-yl]phenol
- PAD
pial arteriolar diameter
- Po
channel open probability
- PSS
physiologic saline solution
- PT
pentacyclic triterpenes
- SSS
sodium saline solution
- TM
transmembrane
- WT
wild type
Authorship Contributions
Participated in research design: Bukiya, McMillan, Miller, Parrill, Dopico.
Conducted experiments: Bukiya, McMillan, Fedinec.
Contributed new reagents or analytic tools: Patil, Miller.
Performed data analysis: Bukiya, McMillan.
Wrote or contributed to the writing of the manuscript: Bukiya, McMillan, Patil, Leffler, Parrill, Dopico.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung and Blood Institute [Grants AA011560, HL104631, HL34059, and HL042851].
Preliminary data were previously presented as a poster presentation: Bukiya A, McMillan J, Fedinec A, Leffler C, Parrill A, Dopico A (2012) Sodium 3-hydroxyolean-12-en-30-oate is a novel and selective activator of b1 subunit-containing BK channel and thus cerebral artery dilator. 2012 Annual Meeting of the Biophysical Society; 2012 Feb 25–29; San Diego, CA.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Baumbach GL, Heistad DD. (1985) Regional, segmental, and temporal heterogeneity of cerebral vascular autoregulation. Ann Biomed Eng 13:303–310 [DOI] [PubMed] [Google Scholar]
- Bomzon A, Ljubuncic P. (1995) Bile acids as endogenous vasodilators? Biochem Pharmacol 49:581–589 [DOI] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. (2000a) Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275:6453–6461 [DOI] [PubMed] [Google Scholar]
- Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. (2000b) Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407:870–876 [DOI] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Dopico AM. (2009b) The BK channel accessory beta1 subunit determines alcohol-induced cerebrovascular constriction. FEBS Lett 583:2779–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Toro L, Dopico AM. (2007) Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol 72:359–369 [DOI] [PubMed] [Google Scholar]
- Bukiya AN, McMillan J, Parrill AL, Dopico AM. (2008a) Structural determinants of monohydroxylated bile acids to activate beta 1 subunit-containing BK channels. J Lipid Res 49:2441–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Patil SA, Li W, Miller DD, Dopico AM. (2012) Calcium- and voltage-gated potassium (BK) channel activators in the 5β-cholanic acid-3α-ol analogue series with modifications in the lateral chain. ChemMedChem 7:1784–1792 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Singh AK, Parrill AL, Dopico AM. (2011) The steroid interaction site in transmembrane domain 2 of the large conductance, voltage- and calcium-gated potassium (BK) channel accessory β1 subunit. Proc Natl Acad Sci USA 108:20207–20212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Vaithianathan T, Toro L, Dopico AM. (2008b) The second transmembrane domain of the large conductance, voltage- and calcium-gated potassium channel β(1) subunit is a lithocholate sensor. FEBS Lett 582:673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Vaithianathan T, Toro L, Dopico AM. (2009a) Channel β2–4 subunits fail to substitute for beta1 in sensitizing BK channels to lithocholate. Biochem Biophys Res Commun 390:995–1000 [DOI] [PubMed] [Google Scholar]
- Calderone V, Fiamingo FL, Amato G, Giorgi I, Livi O, Martelli A, Martinotti E. (2008) New amido derivatives as potential BKCa potassium channel activators. XI. Eur J Med Chem 43:792–799 [DOI] [PubMed] [Google Scholar]
- Dopico AM. (2003) Ethanol sensitivity of BK(Ca) channels from arterial smooth muscle does not require the presence of the beta 1-subunit. Am J Physiol Cell Physiol 284:C1468–C1480 [DOI] [PubMed] [Google Scholar]
- Dopico AM, Bukiya AN, Singh AK. (2012). Differential contribution of BK subunits to nongenomic regulation of channel function by steroids, in Choesterol Regulaiton of Ion Channels and Receptors Levitan I, Barrantes F. eds) pp 109-134, Wiley, New York [Google Scholar]
- Dopico AM, Walsh JV, Jr, Singer JJ. (2002) Natural bile acids and synthetic analogues modulate large conductance Ca2+-activated K+ (BKCa) channel activity in smooth muscle cells. J Gen Physiol 119:251–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch N. (2011) A clinical review of cerebral vasospasm and delayed ischaemia following aneurysm rupture. Acta Neurochir Suppl (Wien) 110:5–6 [DOI] [PubMed] [Google Scholar]
- Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL. (2011) Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab 31:1443–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, Sobey CG. (1998) Role of potassium channels in regulation of cerebral vascular tone. J Cereb Blood Flow Metab 18:1047–1063 [DOI] [PubMed] [Google Scholar]
- Fernández-Fernández JM, Tomás M, Vázquez E, Orio P, Latorre R, Sentí M, Marrugat J, Valverde MA. (2004) Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest 113:1032–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Flowerdew SE, Voronina SG, Sukhomlin TK, Tepikin AV, Petersen OH, Gerasimenko OV. (2006) Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors. J Biol Chem 281:40154–40163 [DOI] [PubMed] [Google Scholar]
- Ghatta S, Nimmagadda D, Xu X, O’Rourke ST. (2006) Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacol Ther 110:103–116 [DOI] [PubMed] [Google Scholar]
- Gourley JK, Heistad DD. (1984) Characteristics of reactive hyperemia in the cerebral circulation. Am J Physiol 246:H52–H58 [DOI] [PubMed] [Google Scholar]
- Holtzclaw JD, Grimm PR, Sansom SC. (2011) Role of BK channels in hypertension and potassium secretion. Curr Opin Nephrol Hypertens 20:512–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA. (1994) Viability thresholds and the penumbra of focal ischemia. Ann Neurol 36:557–565 [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. (2005) Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res 97:805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, et al. (1998) Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand 164:577–587 [DOI] [PubMed] [Google Scholar]
- Jordan JD, Nyquist P. (2010) Biomarkers and vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am 21:381–391 [DOI] [PubMed] [Google Scholar]
- Ju YE, Schwedt TJ. (2010) Abrupt-onset severe headaches. Semin Neurol 30:192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda N, Pezzuto JM, Kinghorn AD, Farnsworth NR, Santisuk T, Tuchinda P, Udchachon J, Reutrakul V. (1992) Plant anticancer agents, L. cytotoxic triterpenes from Sandoricum koetjape stems. J Nat Prod 55:654–659 [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. (1998) Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korovkina VP, Brainard AM, Ismail P, Schmidt TJ, England SK. (2004) Estradiol binding to maxi-K channels induces their down-regulation via proteasomal degradation. J Biol Chem 279:1217–1223 [DOI] [PubMed] [Google Scholar]
- Lee BC, Lim HH, Kim S, Youn HS, Lee Y, Kim YC, Eom SH, Lee KW, Park CS. (2012) Localization of a site of action for benzofuroindole-induced potentiation of BKCa channels. Mol Pharmacol 82:143–155 [DOI] [PubMed] [Google Scholar]
- Lee RM. (1995) Morphology of cerebral arteries. Pharmacol Ther 66:149–173 [DOI] [PubMed] [Google Scholar]
- Liang Z, Zhang L, Li L, Liu J, Li H, Zhang L, Chen L, Cheng K, Zheng M, Wen X, et al. (2011) Identification of pentacyclic triterpenes derivatives as potent inhibitors against glycogen phosphorylase based on 3D-QSAR studies. Eur J Med Chem 46:2011–2021 [DOI] [PubMed] [Google Scholar]
- Liu P, Xi Q, Ahmed A, Jaggar JH, Dopico AM. (2004) Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci USA 101:18217–18222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. (2002) Vitamin D receptor as an intestinal bile acid sensor. Science 296:1313–1316 [DOI] [PubMed] [Google Scholar]
- Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. (2002) Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298:714–719 [DOI] [PubMed] [Google Scholar]
- McManus OB, Harris GH, Giangiacomo KM, Feigenbaum P, Reuben JP, Addy ME, Burka JF, Kaczorowski GJ, Garcia ML. (1993) An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry 32:6128–6133 [DOI] [PubMed] [Google Scholar]
- McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. (1995) Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 14:645–650 [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Toro L. (2000) A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97:5562–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica S, Moschetta A. (2006) Nuclear bile acid receptor FXR as pharmacological target: are we there yet? FEBS Lett 580:5492–5499 [DOI] [PubMed] [Google Scholar]
- Morimoto T, Sakamoto K, Sade H, Ohya S, Muraki K, Imaizumi Y. (2007) Voltage-sensitive oxonol dyes are novel large-conductance Ca2+-activated K+ channel activators selective for beta1 and beta4 but not for beta2 subunits. Mol Pharmacol 71:1075–1088 [DOI] [PubMed] [Google Scholar]
- Nielsen T, Burgdorf KS, Grarup N, Borch-Johnsen K, Hansen T, Jørgensen T, Pedersen O, Andersen G. (2008) The KCNMB1 Glu65Lys polymorphism associates with reduced systolic and diastolic blood pressure in the Inter99 study of 5729 Danes. J Hypertens 26:2142–2146 [DOI] [PubMed] [Google Scholar]
- Nieves-Cintrón M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. (2007) Activation of NFATc3 down-regulates the β1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem 282:3231–3240 [DOI] [PubMed] [Google Scholar]
- Orio P, Rojas P, Ferreira G, Latorre R. (2002) New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci 17:156–161 [DOI] [PubMed] [Google Scholar]
- Papassotiriou J, Köhler R, Prenen J, Krause H, Akbar M, Eggermont J, Paul M, Distler A, Nilius B, Hoyer J. (2000) Endothelial K(+) channel lacks the Ca(2+) sensitivity-regulating beta subunit. FASEB J 14:885–894 [PubMed] [Google Scholar]
- Pérez GJ, Bonev AD, Nelson MT. (2001) Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol 281:C1769–C1775 [DOI] [PubMed] [Google Scholar]
- Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. (2000) Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res 87:E53–E60 [DOI] [PubMed] [Google Scholar]
- Ponte CG, McManus OB, Schmalhofer WA, Shen DM, Dai G, Stevenson A, Sur S, Shah T, Kiss L, Shu M, et al. (2012) Selective, direct activation of high-conductance, calcium-activated potassium channels causes smooth muscle relaxation. Mol Pharmacol 81:567–577 [DOI] [PubMed] [Google Scholar]
- Qi W, Ding D, Salvi RJ. (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res 236:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Morayo Akande A, Large RJ, Webb TI, Camarasu C, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA. (2012) Structure-activity relationships of a novel group of large-conductance Ca(2+)-activated K(+) (BK) channel modulators: the GoSlo-SR family. ChemMedChem 7:1763–1769 . [DOI] [PubMed] [Google Scholar]
- Rusch NJ. (2009) BK channels in cardiovascular disease: a complex story of channel dysregulation. Am J Physiol Heart Circ Physiol 297:H1580–H1582 [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. (2006) High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7:921–931 [DOI] [PubMed] [Google Scholar]
- Sattar A, Manousakis G, Jensen MB. (2010) Systematic review of reversible cerebral vasoconstriction syndrome. Expert Rev Cardiovasc Ther 8:1417–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T, Ikeda K, Hirayama T, Suzuki A, Iwasaki Y. (2012) Transient splenial lesion after recovery of cerebral vasoconstriction and posterior reversible encephalopathy syndrome: a case report of eclampsia. Intern Med 51:1407–1411 [DOI] [PubMed] [Google Scholar]
- Shahlaie K, Boggan JE, Latchaw RE, Ji C, Muizelaar JP. (2009) Posttraumatic vasospasm detected by continuous brain tissue oxygen monitoring: treatment with intraarterial verapamil and balloon angioplasty. Neurocrit Care 10:61–69 [DOI] [PubMed] [Google Scholar]
- Strøbaek D, Christophersen P, Holm NR, Moldt P, Ahring PK, Johansen TE, Olesen SP. (1996) Modulation of the Ca(2+)-dependent K+ channel, hslo, by the substituted diphenylurea NS 1608, paxilline and internal Ca2+. Neuropharmacology 35:903–914 [DOI] [PubMed] [Google Scholar]
- Terasawa T, Okada T, Hara T, Itoh K. (1992) Glycyrrhetinic acid derivatives as potent inhibitors of Na+-, K+-ATPase. Synthesis and structure-activity relationships. Eur J Med Chem 27:345–351 DOI: 10.1016/0223-5234(92)90147-S. [Google Scholar]
- Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. (1999) Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science 285:1929–1931 [DOI] [PubMed] [Google Scholar]
- Yan J, Aldrich RW. (2012) BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc Natl Acad Sci USA 109:7917–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.