Abstract
Expression of NKX2-1 is required to specify definitive endoderm to respiratory endoderm. However, the transcriptional regulation of NKX2-1 is not fully understood. Here we demonstrate that aside from specifying undifferentiated human embryonic stem cell (hESC) to definitive endoderm, high concentrations of Activin-A are also necessary and sufficient to induce hESC-derived definitive endodermal progeny to a FOXA2/NKX2-1/GATA6/PAX9 positive respiratory epithelial fate. Activin-A directly mediates the induction of NKX2-1 by interacting with ALK4, leading to phosphorylation of SMAD2, which binds directly to the NKX2-1 promoter and activates its expression. Activin-A can be replaced by GDF11 but not transforming growth factor β1. Addition of Wnt3a, SHH, FGF2, or BMP4 failed to induce NKX2-1. These results suggest that direct binding of Activin-A–responsive SMAD2 to the NKX2-1 promoter plays essential role during respiratory endoderm specification.
Introduction
Expression of the homeodomain NKX2-1 transcription factor is the earliest indication of the establishment of respiratory progenitors as well as thyroid epithelium in the ventral foregut endoderm [1]. NKX2-1 is critical for the expression of many pulmonary specific genes, including surfactant proteins (SP)-A, -B, and -C and the Clara cell CC-10 protein, as well as NKX2-1 itself [1–5]. Inactivation of NKX2-1 causes tracheoesophageal fistulae and impairment of pulmonary branching, causing severe lung hypoplasia [1,6].
How NKX2-1 is regulated is not yet fully understood. Embryos lacking Wnt2/2b expression or where β-Catenin has been inactivated in the ventral foregut endoderm, exhibit complete lung agenesis without NKX2-1 expression and conditional expression of an activated form of β-Catenin leads to expansion of NKX2-1 into adjacent endoderm, including the stomach epithelium [7,8]. Explant culture studies show that NKX2-1 expression is upregulated in the cells closest to the cardiac mesoderm receiving the highest amount of fibroblast growth factor (FGF) signaling, suggesting a role for FGF2 in specification to pulmonary epithelium [9].
NKX2-1 can be induced in mouse (m) or human embryonic stem cell (hESC) in vitro [10–16]. Some studies have suggested that Activin-A [12,13] positively influences the differentiation ability of m/hESC towards NKX2-1 expressing pulmonary epithelium, whereas other studies suggest that pulmonary epithelium specification from the ventral foregut requires dual inactivation of BMP4/transforming growth factor (TGF) beta. Some but not all studies have demonstrated that high concentrations of FGF2 increase NKX2-1 expression in differentiating hESC [9,10,15]. Mou et al., found that BMP4 induces NKX2-1 expression in differentiating human induced pluripotent stem cells [16], but Rankin et al. found that suppression of BMP4 signaling is required for wnt/beta-catenin-mediated lung specification in Xenopus [17]. Thus, as is true for in vivo development of pulmonary epithelium from definitive endoderm (DE), the signals/growth factors that support specification of m/hESC- derived DE to the pulmonary lineage are not yet understood.
The promoter of NKX2-1 contains binding sites for the GATA6, HNF3, FOXP2, SP1, and SP3 transcription factors [18–22]. In addition, a number of SMAD binding elements (SBE) are present in the NKX2-1 promoter.
Here we demonstrate that Activin-A or GDF11 can specify hESC-derived DE towards NKX2-1 positive cells. We further demonstrate that in response to Activin-A signaling, SMAD2 binds to the NKX2-1 promoter and that SMAD2/3 activity enhances NKX2-1 promoter activity. These results establish a direct link between SMAD2/3 signaling and NKX2-1 expression, and demonstrate the importance of Activin-A signaling in respiratory endoderm specification.
Materials and Methods
Cell lines and maintenance
The human ESC lines H1 (passages 50–55) and H9 (passages 48–60) were both purchased from the NIH Stem Cell Bank. hESCs were maintained on plates coated with growth-factor-depleted matrigel (BD Biosciences) in mTesR1 (StemCell Technologies) in a 5% CO2/5% O2 environment. Cells were split at a 3 to 6 ratio every 4–5 days, and intermittently, an aliquot was examined by Reverse transcription quantitative polymerase chain reaction (RT-qPCR) for the expression of the endogenous pluripotency gene OCT3A as well as teratoma assay. The pulmonary epithelial cell line NCI-H441, was purchased from Lonza, Switzerland, and was maintained in RPMI (Invitrogen) containing 10% fetal bovine serum (Hyclone, Perbio France). Routine sterility checks, including screening for Mycoplasma were performed throughout the studies.
Differentiation culture
Differentiations were performed in 60% Dulbecco's modified Eagle medium (DMEM)-low glucose (Gibco), 40% MCDB-201-water (Sigma), 1× Linoleic acid–Bovine serum albumin (Sigma), 1× Insulin-transferrin-selenium (Sigma), 0.1 mM Ascorbic Acid (Sigma), 55 mM 2-mercaptoethanol (Gibco). The following growth factors were used, human Activin-A, human BMP4, human TGF β1, human sonic hedgehog (SHH), human GDF11, human Wnt3a, human bFGF, SB-431542. All factors were purchased from R&D Systems, except SB-431542, which was from Tocris Ellisville USA. Medium changes were performed every other day.
Reverse transcription quantitative polymerase chain reaction
RNA was isolated using the RNeasy Mini-kit/Micro-kit (Qiagen), and DNAse treatment with the Turbo DNAse kit (Ambion). cDNA synthesis was performed from 1 μg of RNA with Superscript III First-Strand synthesis system (Invitrogen). Real time PCR was performed using the Platinum SYBR green qPCR Supermix-UDG (Invitrogen) and the Eppendorf realplex/ABI 7000 (Eppendorf, Applied Biosciences). Relative gene expression was calculated by the 2(-DDCt) method compared to undifferentiated cells (day 0), using GAPDH as housekeeping gene. The list of primers used can be found in Supplementary Table 1; Supplementary Data are available online at www.liebertonline.com/scd. Human total lung RNA (Clontech) was used as positive control.
Immunostaining
Differentiations were done in 4-well chamber slides (Nunc) or in 12-well plates (Corning). Cells were fixed using 10% Neutral Buffered Formalin (Fisher Scientific) for 15 min at room temperature (RT). Permeabilization was done for 10 min using a phosphate buffered saline (PBS) containing 0.1% Triton X-100 (PBST) (Acros Organics). PBST, containing 5% Normal Donkey Serum (Jackson), was used for blocking for 30 min at RT. The cells were then incubated with the mixture of primary antibodies diluted in PBS containing 5% donkey serum and incubated overnight at 4°C After 3 washes in PBS, the cells were incubated with the mixture of respective Alexa dye conjugated secondary antibodies (Invitrogen) and Hoechst dye (Sigma) for 30 min at RT. All dilutions were optimized on positive control cells (NCI-H441) and using the respective isotype control antibodies. The list of primary and secondary antibodies used can be found in Supplementary Table 1. To enumerate the percentage of cells that stained positive, cells were imaged using a Zeiss Axioskop microscope and AxioVision Version Rel 4.0 software was used to quantify the number of positive cells in 5 to 10 random areas per slide and per condition.
Western blot analysis
Cells were harvested and frozen at −80°C. Protein extracts were prepared in a RIPA buffer (Sigma) plus proteinase inhibitor and phosphatase inhibitor (both from Roche Diagnostics) and equal amounts of protein were separated on NuPAGE gels (Invitrogen). Proteins were transferred onto Immobilon-P transfer membranes (Millipore Corp.) and analyzed by Western blotting using antibodies recognizing the following proteins: SMAD2, phospho-SMAD2, beta-actin. All antibodies were purchased from Cell Signaling Technology.
Luciferase reporter assay
Luciferase reporter plasmids driven by the NKX2-1 distal promoter (named pNKX2-1-Luc) [22a] was constructed by inserting the −1736 to −307 NKX2-1 promoter sequence into the promoterless pGV-BM2 (Addgene). We generated mutated/deleted reporter vectors, pNKX2-1(mSBEs)-Luc/pNKX2-1(dSBEs)-Luc, by mutating/deleting the putative SBEs in NKX2-1 promoter with the Site-Directed Mutagenesis Kit (FINNZYMES). Mutations/deletions of nonspecific areas in the promoter region were used as control (pNKX2-1(mNC)-Luc/pNKX2-1(dNC)-Luc) (Supplementary Fig. 1). All mutations/deletions were confirmed by sequencing. Human NCI-441 cells were used to assess the promoter activation.
Human NCI-H441 cells (60,000/cm2) were transfected with each of the reporter vectors together with pRL-tk plasmid (Promega) (at 1/10 of the DNA amount for the test vector) and Renilla luciferase as an internal control, using FuGENE HD transfection reagent (Roche Applied Science). Cells were harvested 48 h after transfection and firefly and Renilla luciferase activities in the lysates tested using the Dual-Luciferase Reporter Assay System (Promega) on a Perkin Elmer/Wallac Victor 2 multilabel Counter (Internation Equipment Trading Ltd.). The ratio between Firefly and Renilla luciferase activity was obtained for each sample. Relative luciferase units were calculated via normalization of each of the ratios for all groups by the average ratio for the promoterless group.
Chromatin immunoprecipitation-qPCR
Chromatin isolation and IP were performed according to the instructions from the Transcription Factor ChIP kit (Diagenode). Chromatin was isolated from nearly 5×107 d8 differentiated human ESCs for each condition. Antibodies against SMAD2 (Cell Signaling Technology) or an isogenic antibody (BD, Pharmingen) were used at 2 μg per IP reaction. Purified DNA was used as template for qPCR using Platinum SYBR green (Invitrogen 11733-046) to amplify the NKX2-1 promoter with the forward primer sequence 5′- GCA GAC AGA CTG ACA GAC ACG and reverse primer sequence 5′- CAG TCG CCA ACA AAT GAG C. The PCR product size was 83 bp. The following PCR conditions were used, 3 min at 95o and 40 cycles of 15 s at 95o, 45 s at 60o and 1 min at 95o.
Statistics
Results are expressed as mean±SEM. Statistical significance was determined by student's t-test.
Results
Activin-A induces NKX2-1 expression
To commit hESC to lung epithelium, initial commitment to DE is required. In vivo this occurs by signaling via Nodal. As has been demonstrated by others and us [23,24], we here demonstrate that transcripts for the mesendoderm (ME)/DE marker genes, including BRACHYURY, GSC, EOMES, and SOX17 were expressed maximally on d4 of differentiation in the presence of Activin-A (n=3)(Supplementary Fig. 2B). In vivo, gastrulation and induction of foregut endoderm is also supported by canonical Wnt signaling [24,25]. Although addition of Wnt3a from d0 to d2 or d2 to d4, improved ME/DE marker gene expression, the differences with Activin-A alone were non-significant (n=3) (Fig. 1B; Supplementary Fig. 2B).
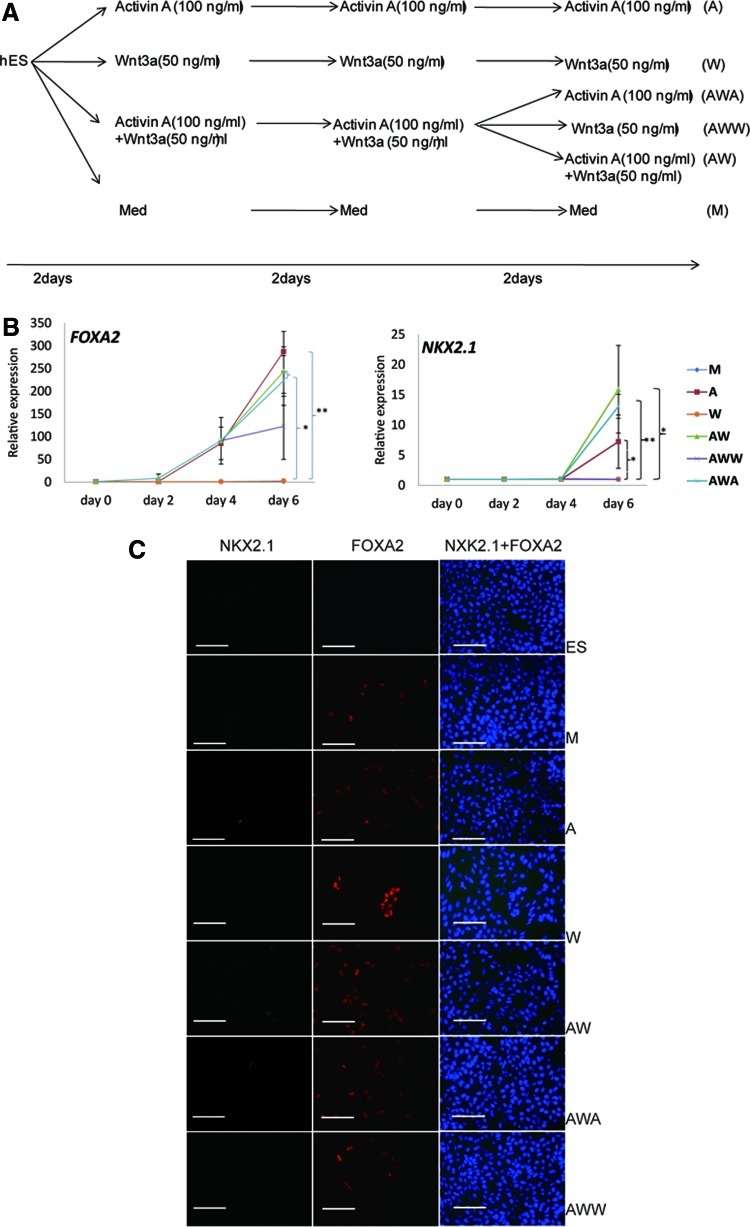
FIG. 1.
NKX2-1 expression can be induced by Activin-A. (A) Differentiation scheme used. (B) Reverse transcription quantitative polymerase chain reaction (RT-qPCR) measurement of FOXA2 and NKX2-1 mRNA levels in human embryonic stem cell (hESC)-H9 cultured under different conditions. Data are represented as mean±SEM from 3 independent experiments (*P<0.05; **P<0.01). (C) Immunostaining for NKX2-1 and FOXA2 in hESC on day 0 (ES) and day 6 for Medium (M), A, W, AW, AWA, and AWW. Scale bar, 100 μm. Percentage FOXA2 positive cells: M=5.8%±5.4%, A=16.3%±8.2%, W=7.2%±3.9%, AW=16.4%±11.3%, AWA=18%±14.7%, AWW=8.7%±4.3%; Percentage NKX2-1 positive cells: 0%–2% in A, AW and AWA conditions, all other conditions 0%. Color images available online at www.liebertpub.com/scd
Canonical Wnt signaling is important in patterning foregut endoderm to pulmonary endoderm in mice in vivo [7,8]. several studies [11,15] demonstrated that ESC-derived embryoid bodies could only be committed to pulmonary epithelium by blocking TGFβ family-mediated signaling with Noggin and an ALK4/5/7 inhibitor, SB431542, followed by stimulation with among others, Wnt3a. Therefore, we tested the effect of Activin-A and Wnt3a alone or combined between d4 and d6 on the expression of the endodermal marker gene, FOXA2, and the pulmonary epithelial master gene, NKX2-1. When cells exposed until d4 to Activin-A±Wnt3a were subsequently cultured with Activin-A±Wnt3a, FOXA2 transcripts increased significantly, whereas this was substantially less when cells were exposed between d4–d6 to Wnt3a alone (n=3) (Fig. 1B). Concomitant with the increased expression of FOXA2, we also detected a±10-fold increase in NKX2-1 expression when cells were cultured with Activin-A±Wnt3a between d4 and d6, but not with Wnt3a alone.
Immunostaining on d6 mirrored what was seen by RT-qPCR. Approximately 16% of cells cultured with Activin-A or Activin-A+Wnt3a for 6 days, and Activin-A+Wnt3a for 4 days followed by Activin-A alone for 2 more days, stained positive for FOXA2 (n=3) (Fig. 1C) and 1%–2% of cells stained for NKX2-1. By contrast, the percentage of cells cultured in differentiation medium alone, Wnt3a alone or Activin-A+Wnt3a for 4 days followed by Wnt3a alone, that stained positive for FOXA2 was significantly lower (5%–8%) (n=3) (Fig. 1C) and no NKX2-1 positive cells were found. These studies suggested that hESC could be fated to DE as well as pulmonary endoderm by Activin-A, but not Wnt3a.
Neither TGFβ1, BMP4, FGF2, or SHH alone can specify hESC towards NKX2-1 expressing cells in vitro, which is Activin-A (and GDF11) specific
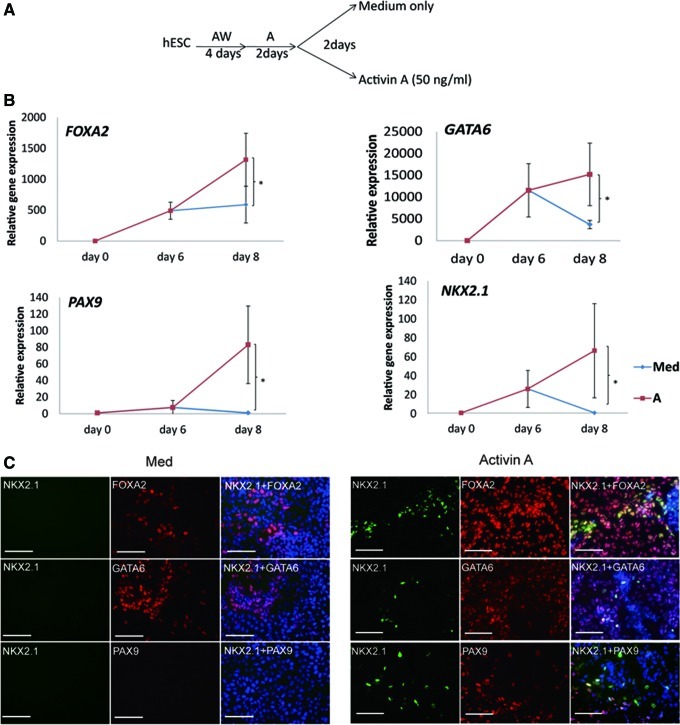
We next tested if longer exposure to Activin-A would commit additional hESC to DE cells and to NKX2-1 positive pulmonary epithelium-fated cells. As progeny from hESC cultured for 4 days with Activin-A+Wnt3a and Activin-A alone between d4 and d6 expressed aside from FOXA2 also NKX2-1, this culture condition was used for all subsequent studies. When d6 hESC progeny were exposed for an additional 2 days to 50 ng/mL Activin-A, a further significant increase in FOXA2 and NKX2-1 transcripts occurred by d8 (n=6) (Fig. 2B). Similar effects of Activin-A were seen when the hESC line H1 was used (n=5) (Supplementary Fig. 3). We also detected a significant increase in transcript levels for other pulmonary epithelial transcription factors in hESC-H9 progeny, including GATA6 and PAX9 [21,26,27], but not PAX8, expressed by thyroid epithelium [28,29]. Immunostaining demonstrated that >80% of hESC-H9 progeny stained positive for FOXA2, of which approximately 6% co-stained with antibodies against NKX2-1 (Fig. 2C). Most PAX9 positive cells were also NKX2-1 positive, whereas of the GATA6 positive cells, also expressed in other endodermal cells, only a fraction stained positive for NKX2-1. These studies demonstrate in 2 independent hESC lines, that Activin-A specifies a fraction of DE cells to a pulmonary epithelial fate.
FIG. 2.
Expression of NKX2-1, PAX9 and GATA6 is induced by Activin-A. (A) Differentiation scheme. (B) RT-qPCR measurement of FOXA2, GATA6, PAX9, and NKX2-1 mRNA in cells cultured in the different culture conditions. Data are represented as mean±SEM from 6 independent experiments (*P<0.05). (C) Immunostaining for NKX2-1, FOXA2, GATA6 and PAX9 in day hESC-H9 progeny cultured from d6 with medium alone or with Activin-A. Scale bar, 100 μm. Percentage FOXA2 positive cells: M=65.6%±8.5%, A=89%±2.8%; Percentage NKX2-1 positive cells: M=0%; A=6.1%±4.2% (n=3). Color images available online at www.liebertpub.com/scd
As Activin-A signals via SMAD2/3 we tested other TGFβ family members that signal via these receptor SMADs, including TGFβ1 and GDF11. Replacing Activin-A with 250 ng/mL GDF11 in all steps of the differentiation process, mimicked the effect of Activin-A: a gradual induction of FOXA2 and significant induction of NKX2-1 from d6 onwards, as shown by RT-qPCR (n=3). Immunostaining also demonstrated that culture with GDF11 resulted in >70% FOXA2 positive and ∼4% NKX2-1 positive cells on d8 (n=2) (Supplementary Fig. 4D).
By contrast, when TGFβ1 was added instead of Activin-A from d6 onwards we still detected the DE markers, FOXA2/GATA6, albeit at decreased levels, but not the pulmonary epithelial markers NKX2-1/PAX9 (n=5) (Fig. 3A). This was reversed when 50 ng/mL Activin-A was added together with TGFβ1 (n=5) (Fig. 3A). We also tested if BMP4, which activates SMAD1/5/8, could induce NKX2-1 expression. Addition of 100 ng/mL BMP4 between d6 and d8 did not induce NKX2-1 expression, which again could be reversed when Activin-A was also added (n=5) (Supplementary Fig. 2C).
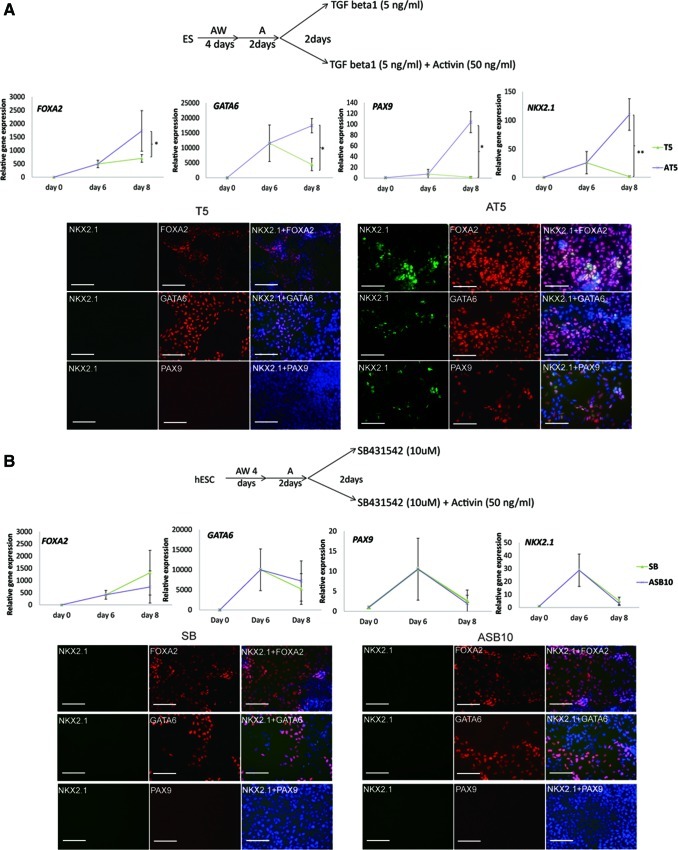
FIG. 3.
Induction of NKX2-1 expression is Activin-A specific. (A) RT-qPCR measurement and immunostaining for NKX2-1, FOXA2, GATA6, and PAX9 of hESC-H9 cells treated with transforming growth factor (TGF)β1 and TGFβ1+Activin-A between d6 and d8. Data are represented as mean±SEM from 5 independent experiments (*P<0.05; **P<0.01). Scale bar, 100 μm. Percentage FOXA2 positive cells: T5=72.3%±8.4%, AT5=79.5%±11.8%; Percentage NKX2-1 positive cells: T5=0%; AT5=17%±11.6% (n=3). (B) RT-qPCR measurement and immunostaining for NKX2-1, FOXA2, GATA6 and PAX9 of hESC-H9 cells treated with SB431542 and SB431542+Activin-A between d6 and d8. Data are represented as mean±SEM from 5 independent experiments. Scale bar, 100 μm. Percentage FOXA2 positive cells: SB=78.1%±5%, ASB=86.9%±7.9%; Percentage NKX2-1 positive cells=0% (n=2). Color images available online at www.liebertpub.com/scd
Activin-A binds initially to Activin-A receptors type II (ActRIIA or ActRIIB) and then recruits Activin-A receptor like kinase (ALK)-4. ALK-4 then interacts with and phosphorylates the receptor SMADs, SMAD2 and SMAD3 [31–33]. To further delineate the Activin-A mediated specification of DE towards NKX2-1 positive cells, we cultured hESC progeny from d6 to d8 with SB431542, a specific inhibitor of ALK4/5/7. When d6 hESC progeny were cultured with SB431542 or Activin-A+SB431542 for 2 additional days, expression of FOXA2 and GATA6 transcripts/protein decreased. In addition, NKX2-1 and PAX9 transcripts/protein could no longer be detected (n=5) (Fig. 3B).
A number of additional factors have been identified that may commit DE to lung epithelium, including SHH and FGF2 [9,30]. Addition of 200 ng/mL FGF2 (n=6) or 500 ng/mL SHH (n=4) between d6 and d8 failed to induce expression of NKX2-1 transcripts/protein on d8 (Supplementary Fig. 2A, B). However, NKX2-1 transcripts/protein were induced significantly when 50 ng/mL Activin-A was combined with either FGF2 (n=6) or SHH (n=4), further demonstrating the importance of Activin-A in pulmonary epithelial commitment (Supplementary Fig. 2A, B).
Activin-A and GDF11 activate NKX2-1 by phosphorylating SMAD2, which induces NKX2-1 expression by binding to SMAD binding sites in the NKX2-1 promoter
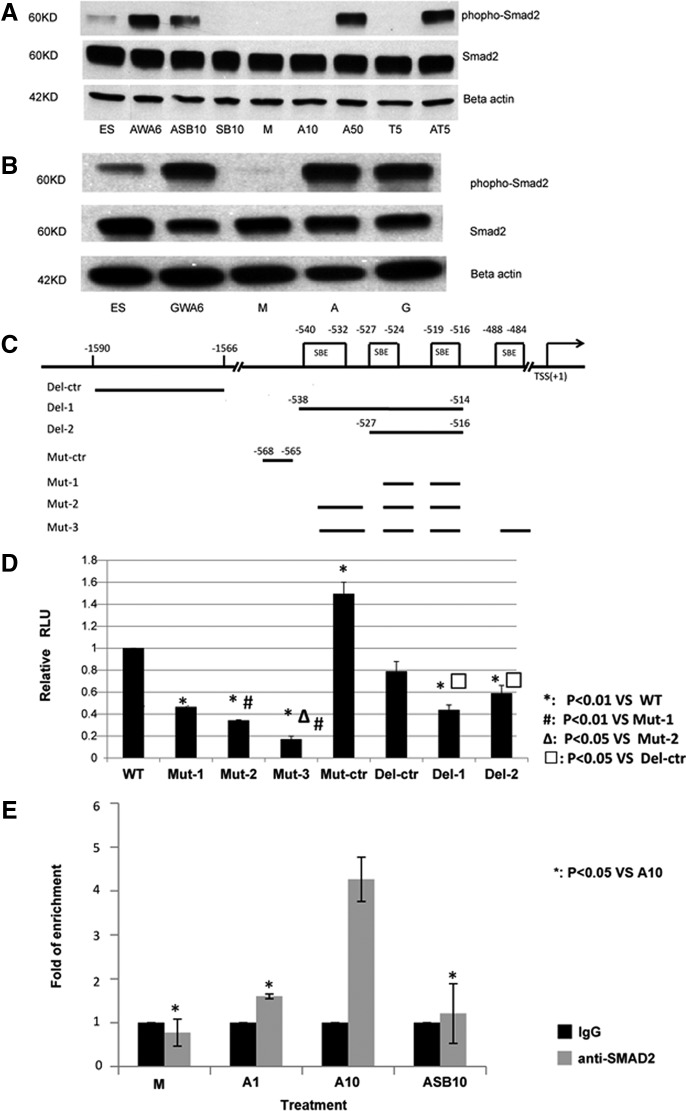
Activin-A and GDF11 phosphorylate SMAD2 and SMAD3, which bind to SMAD4, forming a complex that translocates to the nucleus where it binds to SBE in the promoter regions of target genes. We evaluated the phosphorylation state of SMAD2 in undifferentiated hESC and hESC progeny cultured under different conditions (Fig. 4 A, B). Low-level phosphorylation of SMAD2 was found in undifferentiated hESC, consistent with the notion that maintenance of hESC is Activin-A dependent [34]. hESC progeny from cultures exposed sequentially to Activin-A+Wnt3a for 4 days, followed by 2 days of Activin-A, contained phosphorylated SMAD2. When cells were cultured between d6 and d8 with either 10 or 50 ng/mL Activin-A, SAMD2 was phosphorylated only in cells exposed to 50 ng/mL Activin-A (n=3). When cells were cultured between d6 and d8 with TGFβ1, which also signals via SMAD2, no or low level phosphorylation of SMAD2 was found; however, combined addition of TGFβ1 and Activin-A caused SMAD2 phosphorylation (n=3) (Fig. 4A). When cells were cultured with Activin-A and SB431542, phosphorylation of SMAD2 was decreased (n=3, P=0.038). Consistent with the effects seen on NKX2-1 transcript and protein expression, culture of d6 hESC progeny with GDF11 also led to phosphorylation of SMAD2 (Fig. 4B). We next determined if SMAD2 binds directly to the NKX2-1 promoter by chromatin immunoprecipitation (ChIP) followed by qPCR (Fig. 4E). The promoter region was significantly enriched after anti-SMAD2 ChIP in hESC progeny cultured between d6 and d8 with 100 ng/mL Activin-A compared with medium only. This was not seen in cells cultured in 10 ng/mL Activin-A or 100 ng/mL Activin-A+10 μM SB431542 (n=3). To further demonstrate that SMAD2 is responsible for the activation of the NKX2-1 gene, we performed luciferase promoter assays. We cloned the intact NKX2-1 promoter in the Luc plasmid. We also cloned promoter sequences where the SBEs located between −540/−532, −527/−524, −519/−516, and −488/−484 were mutated or deleted in the Luc-plasmid (Fig. 4D; Supplementary Fig. 4). We also created control promoter plasmids by mutation and deletion in regions outside the SBE containing region. These plasmids together with the pGV vector as internal control were transfected in the H441 lung carcinoma cell line. Compared to H441 cells transfected with the wild-type promoter-luciferase reporter plasmid, the luciferase activity in cells transfected with plasmids containing SBEs mutation or deletion was significantly reduced (n=3, P<0.01) and was similar to cells transfected with the control plasmid (Fig. 4D).
FIG. 4.
NKX2-1 expression by the Activin-A is dependent on SMAD2 activation. (A–C) Western blot analysis of phospho-SMAD2 and total SMAD2 in whole-cell lysates. (A) Undifferentiated hESC-H9=ES; hESC-H9 differentiated with Activin-A+Wnt3a d0–d4 and Activin-A between d4–6=AWA6; hESC-H9 differentiated between d6–d8 with Activin-A+SB431542 (ASB10), SB431542 alone (SB10), medium (M), 10 ng/mL Activin-A (A10), 50 ng/mL Activin-A (A50), 5 ng/mL TGFβ1 (T5), Activin-A+TGFβ1 (AT5). (B) Undifferentiated hESC-H9=ES; hESC-H9 differentiated with 250 ng/mL GDF11+Wnt3a between d0–d4 and GDF11 between d4–6 (GWG6); between d6–d8 with medium (M), 50 ng/mL Activin-A (A) or 250 ng/mL GDF11 (G). (C) Schematic representation of NKX2-1 distal promoter region (not to scale). TSS: transcription start site; SBE: SMAD binding element. (D) NCI-H441 cells were transfected with wild-type pNKX2-1-luc(WT), pNKX2-1(mSBE1)-Luc(Mut-1), pNKX2-1(mSBE2)-Luc(Mut-2), pNKX2-1(mSBE3)-Luc(Mut-3), pNKX2-1(mSBEctr)-Luc(Mut-ctr), pNKX2-1(dSBE1)-Luc(Del-1), pNKX2-1(dSBE2)-Luc(Del-2), pNKX2-1(dSBEctr)-Luc(Del-ctr), on day 1 (all with pGV). Cells were harvested after 2 days of treatment and luciferase activity was analyzed. (*: P<0.01 vs. WT; #: P<0.01 vs. Mut-1; Δ: P<0.05 vs. Mut-2; □: P<0.05 vs. Del-ctr). (E) chromatin immunoprecipitation (ChIP) assay for SMAD binding to the NKX2-1 distal promoter. Differentiated hESC-H9 cells were cultured in M, 10 ng/mL Activin-A, 100 ng/mL Activin-A, or 100 ng/mL Activin-A+10 μM SB431542 for another 2 days. Cells were harvested and ChIP performed with an isogenic or anti-SMAD2 antibodies. Enrichment for the NKX2-1 promoter in the precipitated DNA was analyzed by qPCR using primers that flank the distal promoter region. Results from triplicate experiments are shown as fold change of DNA enrichment. *P<0.05.
Discussion
High concentrations of Activin-A (50–100 ng/mL) or GDF11 (250 ng/mL) are necessary and sufficient to commit hESC-derived definitive endoderm committed progeny to an NKX2-1 positive respiratory epithelial fate. Activin-A induces NKX2-1 expression by binding to the ALK4 receptor, which phosphorylates SMAD2 that then binds to SBE domains in the NKX2-1 promoter and induces its expression.
Previous studies in mouse models have revealed that active beta-catenin acts dominantly to specify NKX2-1 lung endoderm progenitors in the anterior foregut [7,8]. We, therefore, tested if Wnt3a, known to signal via the canonical signaling pathway, affects respiratory specification from hESC-committed DE. Wnt3a alone did not induce expression of NKX2-1, and when combined with Activin-A, also did not synergistically induce NKX2-1 expression.
Another growth factor that induces NKX2-1 positive respiratory endoderm in vivo is FGF2 as high concentrations of FGF2 fates anterior foregut endoderm to respiratory endoderm in e8.5–9 murine embryo explant cultures [9]. Several studies also demonstrated that m/hESC-derived DE is specified to respiratory endoderm by FGF2 [10,11,15,16]. We were unable to demonstrate that FGF2 alone induces NKX2-1 expression in vitro.
As high concentrations of Activin-A (50–100 ng/mL) committed DE to NKX2-1 expressing cells, we evaluated the effect of other members of the TGFβ superfamily, including TGFβ1 and GDF11, which like Activin-A, signal via SMAD2/3 and BMP4, which signals via SMAD1/5/8. GDF11 could be used instead of Activin-A to specify DE to NKX2-1 positive cells, whereas TGFβ1 could not. However, TGFβ1 also did not preclude the specification to NKX2-1 expressing cells induced by Activin-A. Similar results were observed for BMP4. These results differ from recent studies wherein respiratory endoderm was generated by multiple steps with different cytokines or growth factors combination, including culturing with Activin A for 4 days [11,15,16]. We induced DE commitment in 2D culture, in the presence of Activin-A, and subsequently committed a fraction of these cells to FOXA2, GATA6, PAX9, and NKX2-1 positive cells, consistent with respiratory endoderm, by continued exposure to 50–100 ng/mL Activin-A alone for another 4 days. Moreover, we demonstrate that addition of the ALK4/5/7 inhibitor, SB431542, alone or together with Activin-A prevented induction of NKX2-1 expression. Although NKX2.1 expression (Delta CT=9) is induced by Acitivin A, no SPC expression is found in our culture system. There are 2 possible reasons. First, additional signaling pathways are needed to implement the remainder of the respiratory program (8); second, the expression level of NKX2.1 is still not enough to induce SPC expression.
Both high concentrations of Activin-A and GDF11 resulted in SMAD2 phosphorylation, which did not occur when low doses of Activin-A were used or after addition of TGFβ1. Activin-A binds chiefly to ALK4, GDF11 binds both ALK4 and ALK5, whereas TGFβ1 only binds ALK5. This specificity for Activin-A/GDF11 is different from the dependency of undifferentiated hESC on SMAD2 mediated signaling from either TGFβ1 or Activin-A for selfrenewal [34]. ChIP using SMAD2 antibodies in hESC progeny treated with Activin-A, demonstrated significant enrichment for the NKX2-1 promoter. A luciferase-NKX2-1 promoter reporter assay demonstrated an increase in activity when conditions wherein SMAD2 becomes activated were used. When the putative SBEs were mutated or eliminated, significantly lower levels of luciferase activity were detected.
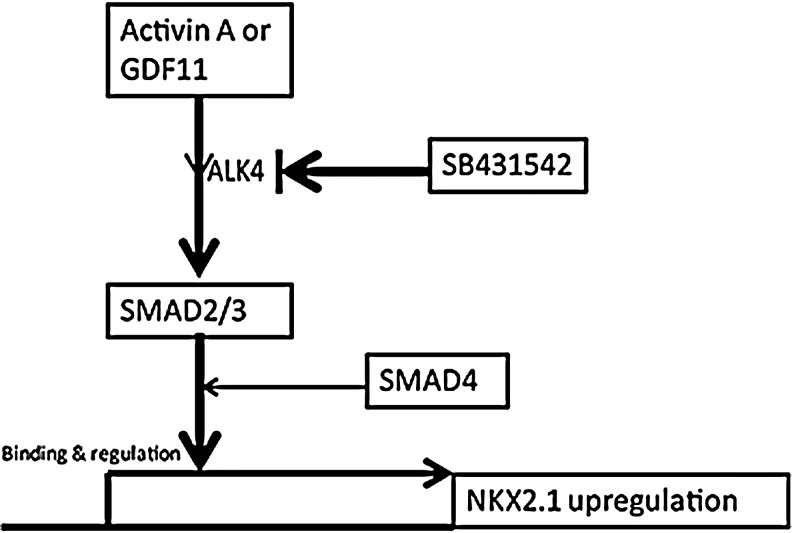
In conclusion, our study demonstrates that binding of Activin-A to ALK4 activates SMAD2, which then induces NKX2-1 expression after definitive endoderm formation (Fig. 5).
FIG. 5.
Model of SMAD Regulation of NKX2-1 transcription in Human pluripotent stem cells. Arrows represent induction, and hammer-ended lines represent inhibition.
Supplementary Material
Acknowledgments
The authors thank Wendy Vandendries for technical help, Antonio LoNigro and Dr Laura Ordovas for their help with molecular cloning and Dr Yemiao Chen for his help with text and figure editing. The work was supported in part by grants from K.U.Leuven (EIW-B4855-EF/05/11, ETH-C1900-PF, EME-C2161-GOA/11/012) and FWO Odysseus funding to CMV.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kimura S. Hara Y. Pineau T. Fernandez-Salguero P. Fox CH. Ward JM. Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;1:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 2.Bohinski RJ. Di Lauro R. Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol. 1994;14:5671–5681. doi: 10.1128/mcb.14.9.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikeda K. Clark JC. Shaw-White JR. Stahlman MT. Boutell CJ. Whitsett JA. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem. 1995;270:8108–8114. doi: 10.1074/jbc.270.14.8108. [DOI] [PubMed] [Google Scholar]
- 4.Lazzaro D. Price M. de Felice M. Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 5.Liu D. Yi M. Smith M. Mendelson CR. TTF-1 response element is critical for temporal and spatial regulation and necessary for hormonal regulation of human surfactant protein-A2 promoter activity. Am J Physiol Lung Cell Mol Physiol. 2008;2:L264–L271. doi: 10.1152/ajplung.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minoo P. Su G. Drum H. Bringas P. Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(-/-) mouse embryos. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- 7.Goss AM. Tian Y. Tsukiyama T. Cohen ED. Zhou D. Lu MM. Yamaguchi TP. Morrisey EE. Wnt2/2b and β-Catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris-Johnson KS. Domyan ET. Vezina CM. Sun X. Beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serls AE. Doherty S. Parvatiyar P. Wells JM. Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- 10.Ameri J. Ståhlberg A. Pedersen J. Johansson JK. Johannesson MM I Artner and Semb. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells. 2010;28:45–56. doi: 10.1002/stem.249. [DOI] [PubMed] [Google Scholar]
- 11.Green MD. Chen A. Nostro MC. d'Souza SL. Schaniel C. Lemischka IR. Gouon-Evans V. Keller G. Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotech. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rippon HJ. Polak JM. Qin M. Bishop AE. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells. 2006;24:1389–1398. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- 13.Winkler ME. Mauritz C. Groos S. Kispert A. Menke S. Hoffmann A. Gruh I. Schwanke K. Haverich A, et al. Serum-free differentiation of murine embryonic stem cells into alveolar type II epithelial cells. Cloning Stem Cells. 2008;10:49–64A–C. doi: 10.1089/clo.2007.0075. [DOI] [PubMed] [Google Scholar]
- 14.Van Haute L. De Block G. Liebaers I. Sermon K. De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longmire TA. Ikonomou L. Hawkins F. Christodoulou C. Cao Y. Jean JC. Kwok LW. Mou H. Rajagopal J, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mou H. Zhao R. Sherwood R. Ahfeldt T. Lapey A. Wain J. Sicilian L. Izvolsky K. Musunuru K, et al. Generation of mutipotent lung and airway progenitors from mouse ESCs and patient-soecufuc cystic fibrosis iPSCS. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rankin SA. Gallas AL. Neto A. Gómez-Skarmeta JL. Zorn AM. Suppression of Bmp4 signaling by the zinc-finger repressors Osr1 and Osr2 is required for Wnt/beta-catenin-mediated lung specification in Xenopus. Development. 2012;139:3010–3020. doi: 10.1242/dev.078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das A. Acharya S. Gottipati KR. McKnight JB. Chandru H. Alcorn JL. Boggaram V. Thyroid transcription factor-1 (TTF-1) gene: identification of ZBP-89, Sp1, and TTF-1 sites in the promoter and regulation by TNF-α in lung epithelial cells. AJP: lung cellular and molecular physiology. 2011;301:L427–L440. doi: 10.1152/ajplung.00090.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C. Ling X. Yuan B. Minoo P. A novel DNA element mediates transcription of Nkx2.1 by Sp1 and Sp3 in pulmonary epithelial cells. Biochim Biophys Acta. 2000;1490:213–224. doi: 10.1016/s0167-4781(99)00183-9. [DOI] [PubMed] [Google Scholar]
- 20.Minoo P. Hu L. Xing Y. Zhu NL. Chen H. Li M. Borok Z. Li C. Physical and functional interactions between homeodomain NKX2.1 and winged helix/forkhead FOXA1 in lung epithelial cells. Mol Cell Biol. 2007;27:2155–2165. doi: 10.1128/MCB.01133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H. Lu MM. Zhang L. Whitsett JA. Morrisey EE. GATA6 regulates differentiation of distal lung epithelium. Development. 2002;129:2233–2246. doi: 10.1242/dev.129.9.2233. [DOI] [PubMed] [Google Scholar]
- 22.Zhou B. Zhong Q. Minoo P. Li C. Ann DK. Frenkel B. Morrisey EE. Crandall ED. Borok Z. Foxp2 inhibits Nkx2.1-mediated transcription of SP-C via interactions with the Nkx2.1 homeodomain. Am J Respir Cell Mol Biol. 2008;38:750–758. doi: 10.1165/rcmb.2007-0350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Hamdan H. Liu H. Li C. Jones C. Lee M. deLemos R. Minoo P. Structure of the human Nkx2.1 gene. Biochim Biophys Acta. 1998;1396:336–348. doi: 10.1016/s0167-4781(97)00210-8. [DOI] [PubMed] [Google Scholar]
- 23.Kubo A. Shinozaki K. Shannon JM. Kouskoff V. Kennedy M. Woo S. Fehling HJ. Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 24.Roelandt P. Pauwelyn KA. Sancho-Bru P. Subramanian K. Bose B. Ordovas L. Vanuytsel K. Geraerts M. Firpo M, et al. Human embryonic and rat adult stem cells with primitive endoderm-like phenotype can be fated to definitive endoderm, and finally hepatocyte-like cells. PLoS One. 2010;5:e12101. doi: 10.1371/journal.pone.0012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLin VA. Rankin SA. Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- 26.Hsu DS. Acharya CR. Balakumaran BS. Riedel RF. Kim MK. Stevenson M. Tuchman S. Mukherjee S. Barry W, et al. Characterizing the developmental pathways TTF-1, NKX2–8, and PAX9 in lung cancer. PNAS. 2009;106:5312–5317. doi: 10.1073/pnas.0900827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y. Rath N. Hannenhalli S. Wang Z. Cupolas T. Kimura S. Atochina-Vasserman E. Lu MM. Beers MF, et al. GATA and Nkx factors synergistically regulate tissue-specific gene expression and development in vivo. Development. 2007;134:189–198. doi: 10.1242/dev.02720. [DOI] [PubMed] [Google Scholar]
- 28.Maeda Y. Davé V. Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 29.Antonica F. Kasprzyk DF. Opitz R. Iacovino M. Liao XH. Dumitrescu AM. Refetoff S. Peremans K. Manto M, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71. doi: 10.1038/nature11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litingtung Y. Lei L. Westphal H. Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 31.Derynck R. Zhang Y. Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 32.Lee KL. Lim SK. Orlov YL. Yit le Y. Yang H. Ang LT. Poellinger L. Lim B. Graded nodal/activin signaling titrates conversion of quantitative phospho-Smad2 levels into qualitative embryonic stem cell fate decisions. PLoS Genet. 2011;7:e1002130. doi: 10.1371/journal.pgen.1002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massagué J. Seoane J. Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 34.James D. Levine AJ. Besser D. Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.