Abstract
A detailed account of the enantioselective total synthesis of (-)-zampanolide, a macrolide marine natural product with high anti-cancer activity is described. For synthesis of the 4-methylene tetrahydropyran unit of (-)-zampanolide, initially, we relied upon an oxidative C-H activation of an alkenyl ether and intramolecular cyclization to provide the substituted tetrahydropyran ring. However, this strategy was unsuccessful. Subsequently, we found that a cinnamyl ether is critical for the successful oxidative intramolecular cyclization reaction. The synthesis also features a cross metathesis reaction to construct a tri-substituted olefin, a ring-closing metathesis to form a highly functionalized macrolactone and a chiral phosphoric acid promoted N-acyl aminal formation to furnish (-)-zampanolide stereoselectively and in good yield. The synthetic (-)-zampanolide had effects on cultured cells and on tubulin assembly consistent with properties reported for the natural product.
Keywords: Natural product, Total Synthesis, C-H bond activation, Metathesis, Stereoselective catalysis
Introduction
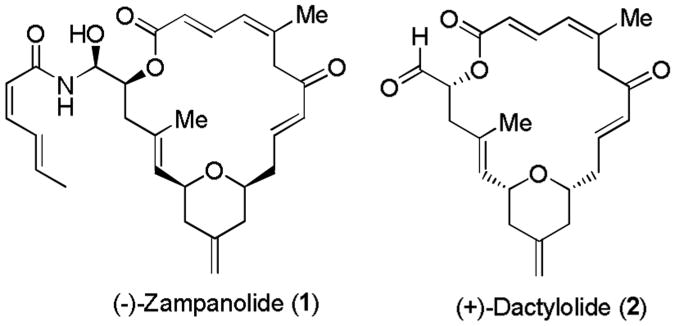
Zampanolide (1, Figure 1), an unsaturated 20-membered macrolide with an N-acyl hemiaminal side chain, was initially isolated by Tanakai and Higa in 1996 from the marine sponge Fasciospongia rimosa in Okinawa, Japan.[1] More recently, (-)-zampanolide was isolated from the Tongan marine sponge, Cacospongia mycofijiensis by Northcote, Miller, and co-workers. [2] Zampanolide exhibited potent cytotoxicity against a variety of cell lines. It has shown IC50 values of 1.1 and 2.9 nM, against SKM-1 and U937 cell lines, respectively. Moreover, zampanolide is very active against HL-60, 1A9, and particularly A2780AD cells, which showed resistance to paclitaxel based on overexpression of P-glycoprotein.[2] Its biological mechanism of action involves the stabilization of microtubules with enhancement of microtubule assembly and blocking cell division in the G2/M phase of the cell cycle.[2, 3] Macrolide (+)-dactylolide (2, Figure 1) was isolated from the sponge Dactylospongia sp.[4] Interestingly, it displayed only modest cytotoxicity. However, it contains opposite configurations of the macrolide core of (-)-zampanolide (1). The biological relevance of the macrolide core of (+)-dactylolide and (-)-zampanolide is not clear.
Figure 1. (-)-Zampanolide 1 and (+)-dactylolide 2.
Both zampanolide and dactylolide have attracted much interest in synthesis and in biological studies of structural variants.[3] Smith and co-workers first reported the total synthesis of (+)-zampanolide, the unnatural antipode, and provided tentative assignment of the absolute configurations of natural (-)-zampanolide.[5] Since then, Hoye et al in 2003[6a] and Uenishi et al in 2009[6b] reported the total synthesis of natural (-)-zampanolide. Recently, we reported an enantioselective synthesis of (-)-zampanolide.[7] Since the isolation of (+)-dactylolide by Riccio and co-workers in 2001,[4] a number of total syntheses and various synthetic approaches to (+)-dactylolide have been reported.[8] The N-acyl side chain of (-)-zampanolide is critical to its potent cytotoxic properties.[1,2] Previous syntheses have furnished zampanolide through direct N-acylaminal formation from unnatural (-)-dactylolide, albeit in only 12% yield. Other major products include zampanolide epimer and bis-acylated products.[6] This direct transformation was achieved through an Al reagent promoted[6a] or a CSA[6b] catalyzed strategy, although in both cases no stereoselectivity was observed. Herein, we report the details of our synthetic efforts that have led to a convergent total synthesis of (-)-zampanolide. The synthesis featured a novel intramolecular oxidative cyclization reaction and an organocatalyic N-acylaminal formation of dactylolide that stereoselectively furnished (-)-zampanolide and no bis-amide byproduct.
Results and Discussion
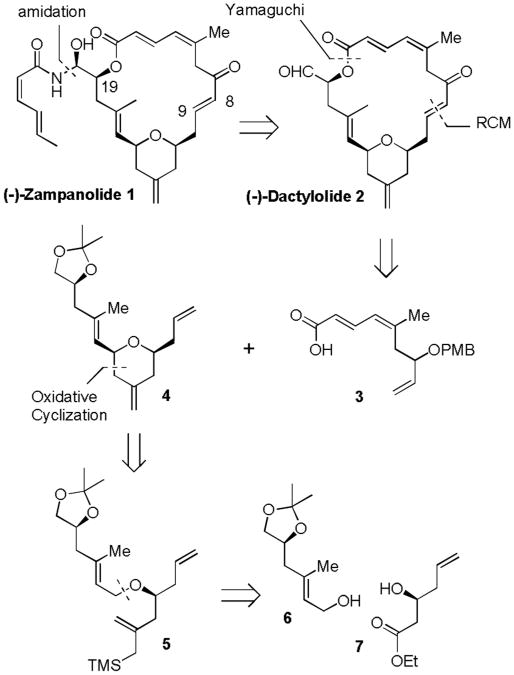
Our initial retrosynthetic analysis of (-)-zampanolide (1) is outlined in Figure 2. Our synthetic strategy was to synthesize (-)-dactylolide (2) and carry out a stereo selective amidation to provide (-)-zampanolide. Strategic disconnection of (-)-dactylolide at the C8-C9 and the C1-C19 ester results in the 4-methylenetetrahydropyran derivative 4 and the unsaturated carboxylic acid. Our plan was to form an ester with the C-19 alcohol and carboxylic acid 3 and then carry out ring closing metathesis to construct the C-20 macrolactone core of (-)-dactylolide. Functionalized 4-methylenetetrahydropyran 4 was planned to be derived from an intramolecular oxidative cyclization of allyl ether 5. This substrate can be synthesized from allylic alcohol 6 and optically active β-hydroxy ester 7.
Figure 2.
First generation retrosynthetic analysis.
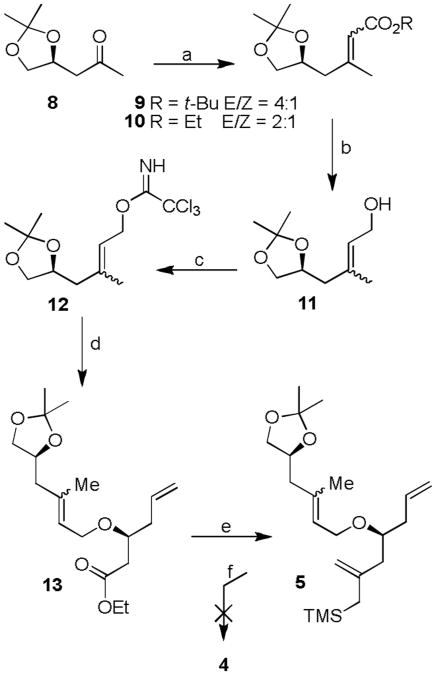
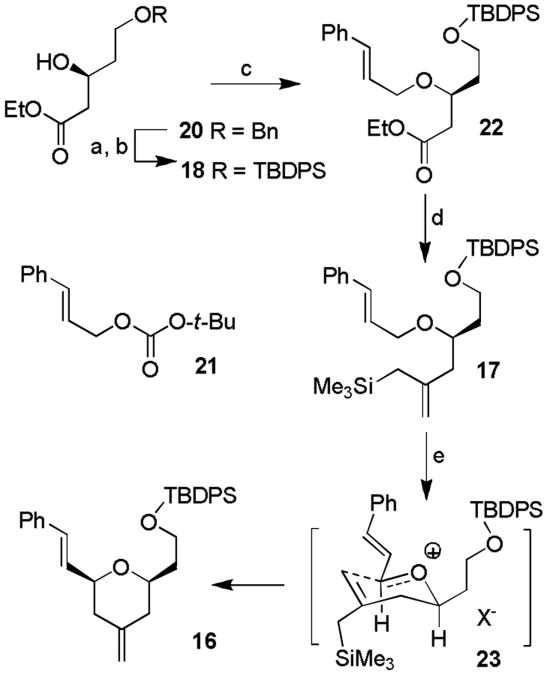
We first examined the feasibility of oxidative cyclization of allylsilane derivative 5 to yield tetrahydropyran 4. The synthesis of 5 is shown in Scheme 1. Optically active methyl ketone 8 was prepared in 5 steps from L-malic acid, as described previously.[9] Horner-Wadsworth-Emmons reaction of ketone 8 with NaH and t-butylphosphonoacetate at 23 °C afforded α,β-unsaturated ester 9 as a mixture (4:1) of E/Z isomers. In contrast, triethyl phosphonoacetate provided 10 with lower E/Z selectivity (2:1). The ester was reduced by exposure to excess DIBAL-H to provide allyl alcohol 11. Treatment of 11 with NaH and trichloroacetonitrile in ether furnished trichloroimidate 12. Initially, we attempted etherification of known alcohol 7[10] with imidate 12 in the presence of a number of acids and Lewis acids such as TfOH, BF3·Et2O, and TMSOTf. However, these conditions resulted in decomposition of starting materials. The use of TBSOTf resulted in a considerable amount of ether 13. The coupling reaction with TIPSOTf proceeded well, providing 13 in 79% yield as a 2:1 mixture of E/Z isomers (by 1H NMR analysis). The ester functionality of 13 was converted to allylsilane derivative 5 using the procedure developed by Benelle and co-workers.[11] With this allylsilane substrate, we have attempted an oxidative cyclization reaction with DDQ under a variety of reaction conditions.[12] However, we could not obtain any desired cyclization. At this point, we speculated that a cinnamyl ether may be more convenient for such an oxidative cyclization reaction. Therefore, we modified our synthetic strategy.
Scheme 1.
Attempted synthesis of dihydropyran 4. Reagents and Conditions: a) dimethyl-t-butyl phosphonoacetate (4.4 equiv.), NaH (3.8 equiv.), THF, 23 °C, 17 h, 74%; b) DIBAL-H (8 equiv.), CH2Cl2, −15 °C, 5 min, 91%; c) NaH (10 mol-%), CCl3CN (1.0 equiv.), ether, 23 °C, 1 h, 98%; d) TIPSOTf (1.1 equiv.), CH2Cl2, 23 °C, 12 h, 79% (brsm); e) CeCl3 (5 equiv.), TMSCH2MgCl (5 equiv.), THF, 23 °C, 12 h, 73%; f) DDQ (2 equiv.), Lewis acid, CH2Cl2, −78 °C to 23 °C, 4 h, decomposition.
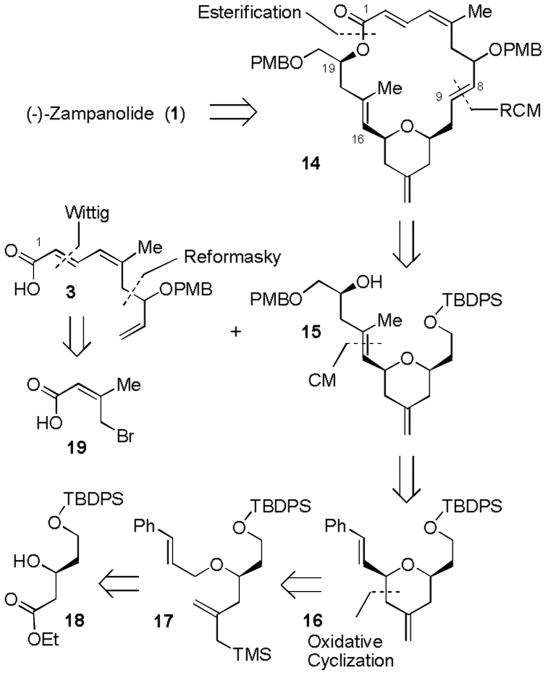
Our alternative retrosynthetic analysis is illustrated in Figure 3. Disconnection of the N-acyl aminal side chain would provide protected macrolactone core 14. Further disassembly of the macrolactone would give rise to tetrahydropyran derivative 15 and trisubstituted olefin 3. Functionalized 4-methylenedihydropyran unit 15 was planned to be synthesized from dihydropyran 16 by a cross metathesis reaction. The dihydropyran 16 with a cinnamyl side chain would be assembled from cinnamyl ether 17 by an oxidative cyclization reaction. We presumed that oxidative activation of a cinnamyl group with DDQ would proceed favorably compared to allylic ether 5. The oxidative cyclization substrate 17 can be derived from β-hydroxy ester 18. This β-hydroxy ester would be prepared in optically active form by an enantioselective hydrogenation process. The polyene unit 3 could be obtained by a Reformatsky reaction of bromo olefin 19 and acrolein, followed by a Wittig olefination.
Figure 3.
Our alternative synthetic plan.
Based upon our revised plan, optically active β-hydroxy ester 20 was synthesized based upon Noyori hydrogenation.[13] This was converted to cinnamyl ether 22 as shown in Scheme 2. Catalytic hydrogenation of 20 over 10% Pd-C, followed by selective protection of the primary alcohol with TBDPSCl and imidazole in THF at 0 °C afforded the corresponding silyl ether. The free secondary hydroxyl was then protected with as a cinnamyl ether using a Tsuji-Trost reaction with a catalytic amount of Pd(PPh3)4 and t-butyl cinnamyl carbonate 21 to provide 22 in 71% yield.[14] The ester group in 22 was converted to allylsilane 17 by treatment with excess trimethylsilylmethylmagnesium bromide in the presence of CeCl3, as described by Bunnelle and co-workers. [11] We then investigated a variety of reaction conditions for effective oxidative cyclization of key substrate 17. The results are shown in Table 1. As can be seen, our initial attempt of using DDQ in the presence of InCl3 as a promoter resulted in the desired dihydropyran cyclization product 16 in 57% yield (entry 1) as a single diastereoisomer (by 1HNMR analysis). The cis stereochemical identity of dihydropyran 16 was established by 1H NMR NOESY experiments. The oxidative cyclization presumably proceeded through the Zimmerman-Traxler transition state shown in stereochemical model 23. The presence of 2,6-dichloropyridine did not improve reaction yields (entry 2). Other Lewis acids did not offer any advantage (entries 3-6).[12] The cyclization reaction in the presence of CSA, however, proved to be the most effective in both CH2Cl2 as well as CH3CN (entries 7 and 8). The catalytic version of this reaction in the presence of PPTS marginally improved the yield to 71% (entry 9). The reaction was then carried out on a 4 gram scale at −38 °C in CH3CN to provide 16 in 81% yield.
Scheme 2.
Synthesis of tetrahydropyran 16. Reagents and conditions: a) Pd/C (3 mol-%), H2, EtOH, 23 °C, 4 h; b) TBDPSCl (1.5 equiv.), imidazole (1.5 equiv.), THF, 0 °C, 1 h, 71% in two steps; c) 21 (2 equiv.), Pd(PPh3)4 (5.5 mol-%), THF, reflux, 36 h, 71%; d) CeCl3 (5 equiv.), TMSCH2MgCl (5 equiv.), THF, −78 °C to 23 °C overnight, 81%; e) see Table 1. TBDPS = tert-butyldiphenylsilyl, TMS = trimethylsilyl, DDQ = 2,3-dicloro-5,6-dicyano-1,4-benzoquinone, PPTS = pyridinium p-toluenesulfonate.
Table 1. Reaction optimization for oxidative cyclization of 17[a].
| Entry | Acid | Solvent | Yield |
|---|---|---|---|
| 1 | InCl3 | CH2Cl2 | 57% |
| 2 | InCl3[b] | CH2Cl2 | 55% |
| 3 | AlCl3 | CH2Cl2 | N.D. |
| 4 | LiClO4 | CH2Cl2 | 41% |
| 5 | LiClO4[b] | CH2Cl2 | 46% |
| 6 | TiCl4 | CH2Cl2 | N.D. |
| 7 | CSA | CH2Cl2 | 65% |
| 8 | CSA | CH3CN | 69% |
| 9 | PPTS | CH3CN | 71% [c] |
| 10 | PPTS | CH3CN | 81% [d] |
Reaction conditions: the reaction was carried out on a 0.5 mmol scale, acid (1.5 equiv.), 4 Å MS, for CH2Cl2 at −78 °C, for CH3CN at −38 °C, 6-8 h;
with 2,6-dichloropyridine (1.5 equiv.);
20 mol-% DDQ and 2 equiv CAN
4 grams scale 17. CAN = ceric ammonimum nitrate.
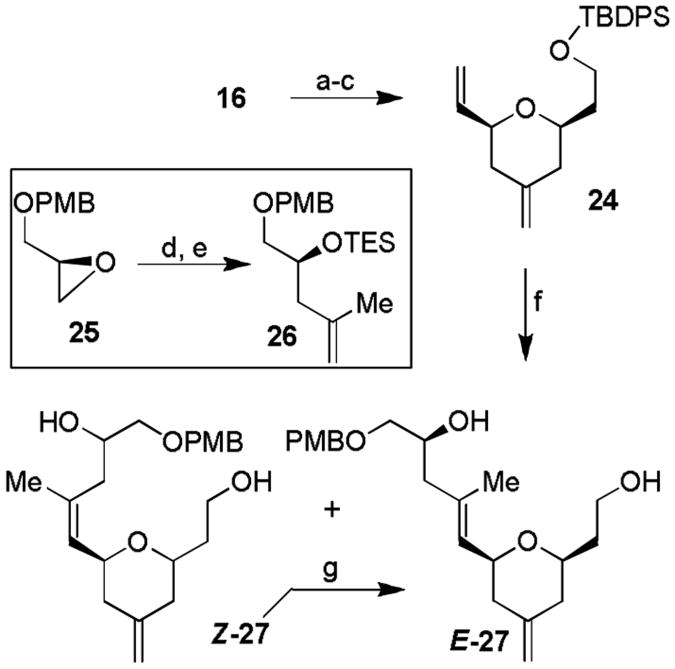
For elaboration of the C17-C20 chain of (-)-zampanolide with an E-trisubstituted olefin, we initially relied upon a cross metathesis of 16 with the corresponding olefin substrate 26. However, our many attempts under a variety of conditions and catalysts resulted in no desired cross metathesis product. This is possibly due to the lack of reactivity of the styrene side chain. Even our attempted cross metathesis with ethylene resulted in no cross metathesis product 24. We therefore devised an alternative strategy to install this side chain. As shown in Scheme 3, we carried out selective cleavage of the styrenyl double bond using dihydroxylation with AD-mix-α, followed by diol cleavage with NaIO4 as described previously.[15] The resulting unstable aldehyde was subjected to Wittig reaction with methylenetriphenylphosphorane to provide alkene 24 in 78% yield for the three steps. For the planned cross metathesis with 24, we prepared 26 by ring opening of a PMB-protected glycidyl derivative 25 with isopropenylmagnesium bromide, followed by protection of the resulting alcohol as a TES ether. Cross metathesis of 24 and 26 was carried out with second generation Grubbs catalyst [16] in CH2Cl2 at reflux for 9 h to furnish trisubstituted olefin as a E/Z mixture of isomers (ratio 1.7:1) in 57% combined yield. The mixture of olefin was treated with HF.Py to remove both silyl groups, and the resulting diols were separated by silica gel chromatography. Olefin Z-27 was subjected to photochemical isomerization to provide the E-27 isomer in 51% yield (brsm 68%).
Scheme 3.
Synthesis of tetrahydropyran E-15. Reagents and conditions: a) AD-mix-α (5 mol-% based on osmium), MeSO2NH2 (1.5 equiv.), t-BuOH/H2O (1:1), 23 °C, 1 h; b) NaIO4 (1.2 equiv.), MeOH/H2O, 23 °C, 2 h; c) Ph3P=CH2 (4.5 equiv.), THF, 40 °C, 16 h. 78% overall yield three steps; d) isopropenylMgBr, (2 equiv.), Li2CuCl4 (5 mol-%), THF, 0 °C, 2 h, 91%; (e) TESOTf (1 equiv.), 2,6-lutidine (1.5 equiv.), CH2Cl2, −30 °C, 1 h, 94%; f) Grubbs II (10 mol-%), 26 (10 equiv.), CH2Cl2, reflux, 9 h, 57%; g) HF/Py, THF, 23 °C, 14 h, E/Z = 1.7:1, overall 81%; h) hv, benzene, 23 °C, 4 h, 51% after two runs.
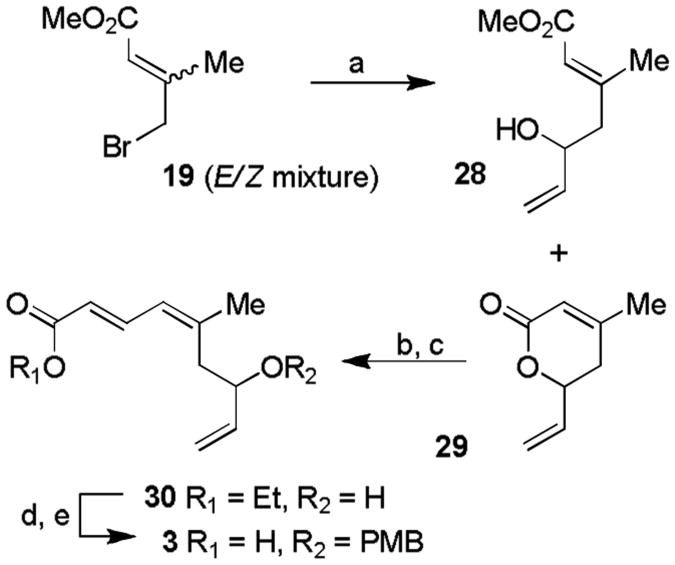
Synthesis of the C1-C9 triene carboxylic acid 3 was carried out using a Reformatsky reaction as the key step. As outlined in Scheme 4, allyl bromide 19 was prepared as an E/Z mixture (1:1) according to the procedure of Fallis and Lei.[17] Treatment of this E/Z mixture with Zn dust, followed by reaction with acrolein, afforded E-alcohol 28 (47%) and α, β-unsaturated δ-lactone 29 (36%) resulting from concomitant lactonization of the Z-alcohol. These products were separated by silica gel chromatography. Treatment of lactone 29 with DIBAL-H, followed by reaction of the resulting lactol with (carbethoxymethylene)-triphenylphosphorane, afforded unsaturated ester 30 in 33% yield for the two steps. The free allylic alcohol was protected as a PMB-ether and saponification of the resulting ester furnished the C1-C9 carboxylic acid fragment 3 in 62% yield in two steps.
Scheme 4.
Synthesis of polyene fragment 3. Reagents and conditions: a) Zn (10 equiv.), acrolein (1.0 equiv.), THF, reflux, 2 h, 84% based on effective starting material; b) DIBAL-H (1.0 equiv.), CH2Cl2, −78 °C, 2 h; c) Ph3P=CHCOOEt (1.3 equiv.), toluene, 100 °C, 16 h, 33% two steps; d) 4-methoxybenzyl 2,2,2-trichloroacetimidate (1.3 equiv.), (+)-CSA (10 mol %), 4 Å MS, CH2Cl2, 0 °C to 23 °C, 24 h, 80%; e) NaOH (3.0 equiv.), EtOH/H2O, 0 °C to 23 °C, 12 h, 77%. DIBAL-H = diisobutyl aluminium hydride, CSA = camphor sulfonic acid.
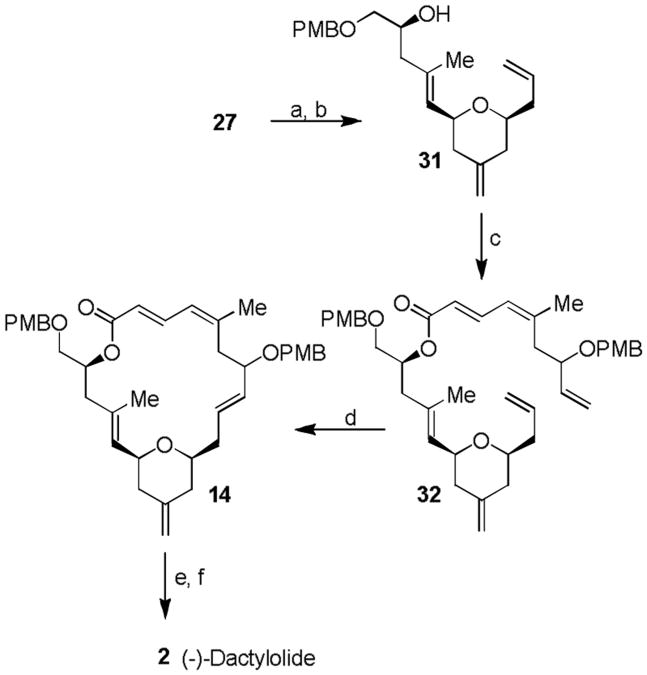
For elaboration of the macrolactone core of (-)-dactylolide or (-)-zampanolide, as shown in Scheme 5, the primary alcohol in 27 was first selectively oxidized with TEMPO and PhI(OAc)2.[18] Wittig olefination of the resulting aldehyde with methylenetriphenyl phosphorane afforded olefin 31 in 60% overall yield. The secondary alcohol in 31 was esterified under Yamaguchi conditions[19] with carboxylic acid 3 using 2,4,6-trichlorobenzoyl chloride in the presence of DMAP to provide a mixture (1:1) of diastereomeric ester 32 in excellent yield. Subjection of this mixture of diastereomers to Grubbs' second generation catalyst (12 mol %) in benzene at 60 °C for 20 h furnished the corresponding 20-member macrolactone. Exposure of the macrolactone mixture to DDQ in aqueous CH2Cl2 removed the PMP group, providing a mixture (1:1 at C7) of alcohols 14 in 65% yield in two steps. Oxidation of 14 with Dess-Martin reagent20 furnished (-)-dactylolide 2 in 80% yield. The spectral data (1H-NMR and 13C-NMR) of (-)-2 is in full agreement with natural (+)-dactylolide except optical rotation of (-)-2 ([α]D24 -148, c 0.09, MeOH).
Scheme 5.
The synthesis of (-)-dactylolide 2. Reagents and conditions: a) PhI(OAc)2 (2.3 equiv.), TEMPO (0.8 equiv.), CH2Cl2, 23 °C, 8.5 h; b) PPh3CH2 (3.5 equiv.), THF, 40 °C, 12 h, 60% in two steps; c) 2,4,6-trichlorobenzoyl chloride (1.5 equiv.), Et3N (2.6 equiv.), DMAP (1 equiv.), toluene, 23 °C, 20 h, 91%; d) Grubbs II (12 mol-%), benzene, 60 °C, 20 h; e) DDQ (2.2 equiv.), CH2Cl2/water, 23 °C, 30 min, 65% in two steps; f) Dess-Martin reagent (6.0 equiv.), NaHCO3 (13.8 equiv.), CH2Cl2, 23 °C, 3.5 h, 80%. DMAP = N, N-dimethyl-4-aminopyridine. TEMPO = 2,2,6,6-Tetramethylpiperidine 1-oxyl.
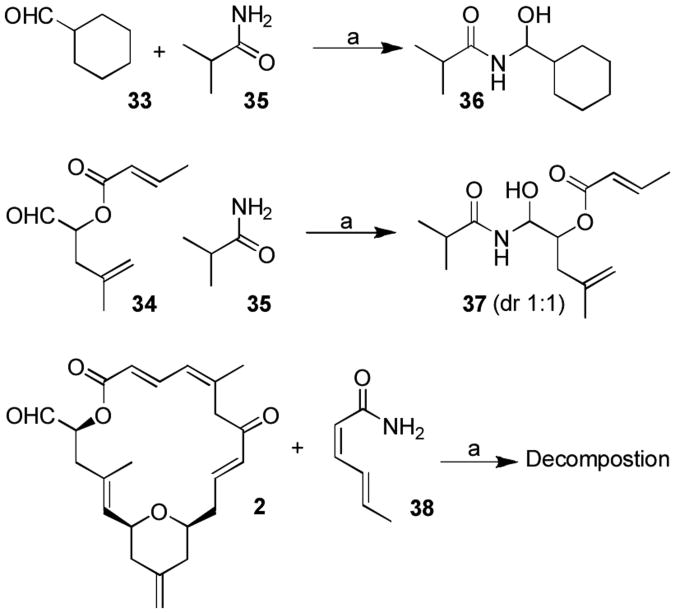
Previous direct N-acylaminal formation proceeded only with limited success, providing (-)-zampanolide (1) along with a mixture of C-20 diastereomers in poor yield (∼12%).[6] To improve this transformation, we first selected cyclohexane carboxaldehyde 33 and aldehyde 34 as model substrates. We investigated N-acylaminal formation using the reaction conditions reported by Williams and co-workers.[21] As shown in Scheme 6, our initial attempts with both aldehyde substrates 33 and 34 and isobutyramide 35 proceeded well, providing N-acylamides 36 (90% yield) and 37 (56% yield) as a 1:1 mixture of diastereomers. However, our attempts to carry out this reaction with (-)-dactylolide 2 and (2Z,4E)-hexa-2,4-dienamide 38 were unsuccessful.
Scheme 6.
Model N-acylaminal reaction. Reagents and conditions: a) amide (10 equiv.), Cy2BCl (9 equiv.), Et3N (9 equiv.), Et2O, 0 °C, 1 h.
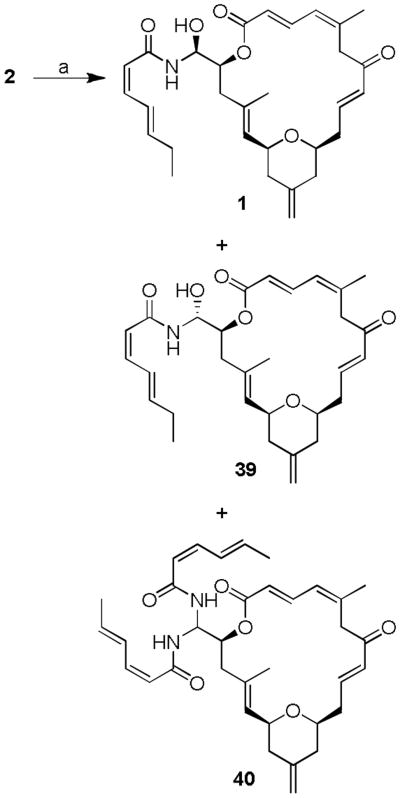
We subsequently planned to optimize direct amidation conditions reported by Uenishi and co-workers.[6b] Reaction of (-)-dactylolide and amide 38 with the p-TsOH was reported to provide (-)-zampanolide 1 (12%), C-20 epimeric product (12%) and the corresponding bis-amide (23%).[6b] In this transformation, the lack of stereoselectivity and the formation of bis-amide limited the yield of desired (-)-zampanolide. In an effort to improve the reaction yield and minimize conversion to the bis-amide byproduct, we investigated reaction of (-)-2 and amide 38 in the presence of Brφnsted acid diphenylphosphoric acid (10 mol-%) in CH2Cl2 at 23 °C for 14 h, as depicted in Scheme 7 As indicated by HPLC analysis, the above reaction condition provided (-)-zampanolide 1, its epimer 39, and bis-amide 40 in better ratio than reported for the p-TsOH catalyzed reaction.[6b]
Scheme 7.
Brφnsted acid catalyzed direct amidation of 2 and 38. a) diphenylphosphoric acid (10 mol-%), CH2Cl2, 23 °C, 14 h. Product distribution (1 : 39 : 40 = 2.7 : 2.5 : 1) was measured by HPLC.
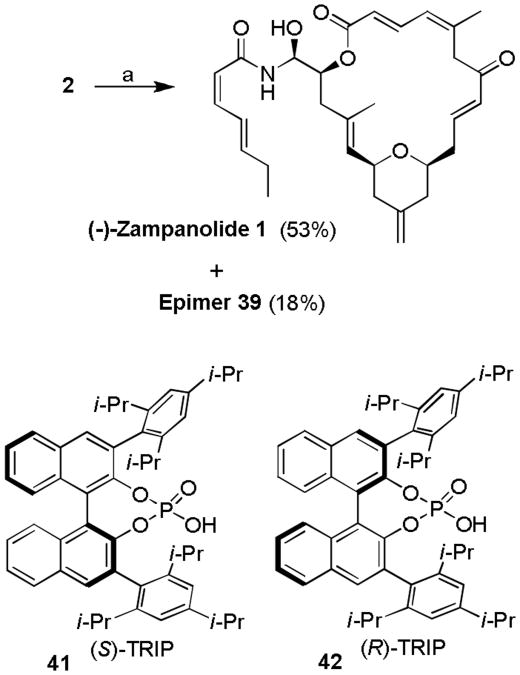
Encouraged by this result, we investigated the effect of chiral phosphoric acid TRIP 41[22] in the N-acyl aminal formation of (-)-2 and amide 38. As shown in Scheme 8, reaction of (-)-2 in the presence of (S)-TRIP (20 mol %) at 23 °C for 12 h furnished N-acyl aminal derivatives diastereoselecivity in good yields and, most significantly, the formation of the byproduct, bis-amide 40 was not observed. The reaction provided (-)-zampanolide (1) in 53% yield and epi-zampanolide 39 in 18% yield after separation by HPLC. In contrast, the corresponding reaction with 38 and (-)-2 in the presence of mismatched (R)-TRIP 42 resulted in (-)-1 and 39 as a 1:1 mixture of diastereomers. Interestingly, the formation of bis-amide byproduct 40 was not observed. The spectral data (1H NMR and 13C NMR) of synthetic (-)-zampanolide (1, [α]D - 94, c 0.08, CH2Cl2) is in full agreement with that of natural zampanolide (Lit.1 [α]D - 101, c 0.12, CH2Cl2).
Scheme 8.
Synthesis of (-)-zampanolide using (S)-TRIP catalyzed amidation: a) amide 38 (3 equiv.), (S)-TRIP (20 mol-%), CH2Cl2, 23 °C, 14 h.
Biological Activities
The biological properties of compounds 1, 39 and 40 were evaluated with purified tubulin combined with heat-treated microtubule-associated proteins, and cytotoxic effects were examined with several cell lines. Paclitaxel was used as a control in all experiments. With tubulin, 1 strongly induced assembly, as was reported previously,[2] while compounds 39 and 40 had only slight activity (data not shown). The cytotoxic activities of the three agents against three human cancer cell lines are summarized in Table 2. We used MCF-7 breast cancer cells and two ovarian lines, OVCAR 8 and its isogenic derivative NCI/ADR-RES, which, like A2780AD,[2] overexpresses P-glycoprotein, an important cellular drug efflux pump. Overexpression of P-glycoprotein produces the major multidrug resistance phenotype. P-glycoprotein overexpressing cell lines are of particular interest in evaluating agents with a paclitaxel-like mechanism of action, since such cell lines are highly resistant to paclitaxel, and multidrug resistance may cause clinical loss of sensitivity to paclitaxel.
Table 2.
The cytotoxic activity of compounds against three human cancer cell lines.a
| IC50 (nM) ± Standard deviation | |||
|---|---|---|---|
|
| |||
| Compounds | MCF-7 | OVCAR 8 | NCI/ADR-RES |
| 1 | 4.0 ± 0.5 | 20 ± 0 | 25 ± 7 |
| 39 | 200 ± 0 | 250 ± 70 | 750 ± 200 |
| 40 | 430 ± 200 | 300 ± 0 | 750 ± 400 |
| Paclitaxel | 2.0 ± 0 | 7.5 ± 2 | >5,000 |
A standard deviation of 0 indicates the same value was obtained in all experiments (usually two independent determinations)
Compound 1 was similar in its cytotoxic activity to paclitaxel in both the MCF-7 and OVCAR 8 cell lines, but it was much more active than paclitaxel in the multidrug resistant NCI/ADR-RES cell line. Compounds 39 and 40 were substantially less active than 1 in all cell lines examined. However, 39 and 40 were more active than paclitaxel in the NCI/ADR-RES cells, indicating that they, too, are not good substrates for P-glycoprotein.
We also did a limited study with 1, 39 and 40 in human CA46 Burkitt lymphoma cells, since this cell line yields a high proportion of cells arrested in the G2/M phase of the cell cycle when treated with antitubulin agents at higher concentrations, such as 10-fold higher than the IC50. With such high concentrations, all three compounds and paclitaxel caused over 90% G2/M arrest in the Burkitt cells. With compound 1 and paclitaxel, the agents were used at 100 nM, while with 39 and 40, the concentration used was 5 μM (data not presented).
Conclusions
In summary, a detailed account of the enantioselective total synthesis of (-)-zampanolide is described. Our initial synthesis route to the zampanolide tetrahydropyran core involved oxidative C-H activation of an allylic ether derivative. However, this strategy did not provide the functionalized tetrahydropyran core for zampanolide. We subsequently devised an alternative oxidative C-H activation strategy where a cinnamyl ether was used in place of the allyl ether. This cyclization method provided the 4-methylene tetrahydropyran derivative in a highly stereoselective manner. The C1-C9 triene carboxylic acid unit 3 was synthesized using a Reformatsky reaction as the key step. Yamaguchi esterification and ring closing olefin metathesis provided the zampanolide core. An organocatalytic N-acyl aminal formation with a chiral phosphoric acid proceeded stereoselectively and in good yield without the formation of the bis-amide byproduct. The current synthesis is amenable to a variety of structural analogs of zampanolide. The synthetic zampanolide had biological properties equivalent to those previously described for the natural product.[1,2]
Experimental Section
General Methods
All moisture sensitive reactions were carried out in an oven dried flask under an argon atmosphere. Anhydrous solvents were obtained as follows: THF, diethyl ether and benzene, distilled from sodium and benzophenone; dichloromethane, pyridine, triethylamine, and diisopropylethylamine, distilled from CaH2. All other solvents were HPLC grade. 1H NMR and 13C NMR spectra were recorded on Varian INOVA300-1 and Bruker Avance ARX-400 spectrometers. NMR data were resolved with Mestrec software. IR spectra were recorded on a Perkin Elmer spectrometer. Optical rotations were recorded on a Perkin Elmer 341 polarimeter. Mass spectra were obtained at the Purdue University Campus-wide Mass Spectrometry Center. Column chromatography was peRformed with Whatman 240-400 mesh silica gel under a low pressure of 3-5 psi. TLC was carried out with E. Merck silica gel 60-F-254 plates. HPLC was peRformed on an Agilent 1100 instrument.
Compound 9
To a suspension of NaH (1.35 g, 60% on silcon oil, 33.7 mmol) on THF (40 mL) at 0 °C was added a solution of t-butyl 2-(dimethoxyphosphoryl) acetate (8.7 g, 38.8 mmol) in THF (10 mL). The mixture was stirred at 0 °C for 30 min and at room temperature for 30 min. After cooling to 0 °C, to the solution was added ketone 8 (1.69 g, 10.7 mmol) in THF (10 mL). After stirring at 0 °C for 10 min and at room temperature overnight, the reaction was quenched with saturated NH4Cl. The aqueous layer was extracted with ether twice. The combined organic layer was washed with brine, dried (Na2SO4) and concentrated. The residue was purified by silica gel chromatography with hexanes/ethyl acetate = 4:1 to give a mixture of E/Z isomers, and pure ethyl acetate was used to recover excess t-butyl 2-(dimethoxyphosphoryl) acetate. The mixture was bound to a silica gel column and eluted with hexanes/ethyl acetate = 9:1 to yield the Z-isomer (0.4 g) and E-isomer (1.7 g), overall yield 77%. Z-isomer: 1H NMR (400 MHz, CDCl3) δ 5.69 (s, 1H),4.36-4.27 (m, 1H), 4.07 (dd, J = 6.0, 8.1 Hz, 1H), 3.62 (dd, J = 7.1, 8.0 Hz, 1H), 3.07 (dd, J = 4.7, 12.7 Hz, 1H), 2.68 (dd, J = 7.8, 12.7 Hz, 1H), 1.95 (d, J = 1.3 Hz, 3H), 1.46 (s, 9H), 1.42 (s, 3H), 1.35 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.6, 154.5, 119.5, 108.8, 79.6, 75.3, 69.1, 36.7, 28.1, 28.0, 26.8, 26.3, 25.6. IR (thin film, cm−1) 2981, 1706, 1646, 1500, 1368, 1254, 1221, 1072, 968, 860, 844. [δ]D23 = −43° (c 2.3, ethyl acetate). Rf = 0.29, hexanes/ethyl acetate = 90:10, KMnO4 stain. MS (ESI) m/z 279 (M+Na +). E-isomer: 1H NMR (400 MHz, CDCl3) δ 5.63 (d, J = 1.2 Hz, 1H), 4.32-4.24 (m, 1H), 4.06 (dd, J = 5.9, 8.1 Hz, 1H), 3.57 (dd, J = 6.8, 8.1 Hz, 1H), 2.46 (dd, J = 6.9, 13.4 Hz, 1H), 2.27 (dd, J = 7.0, 14.1 Hz, 1H), 2.17 (d, J = 1.3 Hz, 3H), 1.48 (s, 9H), 1.42 (s, 3H), 1.36 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 165.9, 153.4, 119.5, 109.1, 79.7, 73.8, 69.1, 44.8, 28.1, 26.9, 25.5, 18.6. IR (thin film, cm−1) 2982, 1714, 1651, 1455, 1380, 1184, 1110, 1073, 862, 829. [δ]D23 = −11.7° (c 2.2, ethyl acetate). Rf = 0.28, hexanes/ethyl acetate = 90:10, KMnO4 stain. MS (ESI) m/z 279 (M+Na +).
Compound 11
To a solution of the E-isomer of 9 (0.85 g, 3.3 mmol) in CH2Cl2 (20 mL) at −15 °C was added DIBAL-H (1.18 g, 1.47 mL, 8.3 mmol). The mixture was stirred at −15 °C for 5 min and quenched with saturated NH4Cl. After stirring at room temperature for 5 min, the resulting gel was diluted with CH2Cl2 and filtered through celite. The aqueous layer was extracted with CH2Cl2 once. The combined organic layer was dried (Na2SO4) and concentrated, followed by silica gel chromatography with hexanes/ether (2:3) as eluent to yield 11 as a colorless oil (0.56 g, 91%). 1H NMR (400 MHz, CDCl3) δ 5.63 (t, J = 6.8 Hz, 1H), 4.27-4.18 (m, 1H), 4.12 (d, J = 6.6 Hz, 2H), 4.01 (dd, J = 6.0, 8.0 Hz, 1H), 3.53 (t, J = 7.5 Hz, 1H), 2.34 (dd, J = 7.0, 13.9 Hz, 1H), 2.17 (dd, J = 6.2, 14.0 Hz, 1H), 1.98 (brs, 1H), 1.69 (s, 3H), 1.39 (s, 3H), 1.32 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 135.0, 126.1, 108.9, 76.7, 69.1, 58.9, 43.5, 26.8, 25.5, 16.7. IR (thin film, cm−1) 3413, 2985, 2935, 1668, 1455, 1371, 1216, 1157, 1062, 999, 831, 790. [δ]D23 = +1.5° (c 2.6, ethyl acetate). Rf = 0.27, hexanes/ethyl ether (2:3), KMnO4 stain. MS (ESI) m/z 209 (M+Na +).
Compound 12
To a suspension of NaH (60% in silicon oil, 10 mol-%, 8.6 mg, 0.22 mmol) in diethyl ether (1.5 mL) at 0 °C was added a solution of 11 (400 mg, 2.15 mmol) in ether (1 mL) dropwise. Then the solution was stirred at room temperature for 20 min and cooled to −40 °C. Next, CCl3CN (215 μL, 2.15 mmol) was added. Then the mixture was stirred sequentially at −40 °C for 1 h and room temperature for 1 h, followed by addition of MeOH (9 μL). The solution was concentrated and suspended in hexanes. The suspension was filtered through celite. The filtrate was concentrated and checked by 1H NMR. The resulting yellow oil 12 (697 mg, 98%) was pure enough and used in the next step without further purification. 1H NMR (400 MHz, CDCl3) δ 8.27 (s, 1H), 5.54 (t, J = 6.8 Hz, 1H), 4.82 (d, J = 6.8 Hz, 2H), 4.30-4.21 (m, 1H), 4.02 (dd, J = 5.9, 8.1 Hz, 1H), 3.57 (t, J = 7.5 Hz, 1H), 2.44 (dd, J = 6.7, 13.4 Hz, 1H), 2.25 (dd, J = 6.9, 14.0 Hz, 1H), 1.79 (s, 3H), 1.42 (s, 3H), 1.36 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 162.6, 139.1, 120.3, 108.9, 74.2, 69.1, 65.8, 43.5, 26.9, 25.6, 17.2. IR (thin film, cm−1) 3470, 3344, 2986, 1738, 1662, 1455, 1370, 1291, 1229, 1157, 1068, 984, 827, 799, 650. [δ]D23 = −1.9° (c 2.0, ethyl acetate). Rf = 0.28, hexanes/ethyl acetate = 90:10, KMnO4 stain. MS (ESI) m/z 352 (M+Na +).
Compound 13
To a suspension of 7 (40 mg, 0.25 mmol), 12 (90 mg, 0.27 mmol) and 4 Å MS (40 mg) in CH2Cl2 (1 mL) was added TIPSOTf (25 μL,) at −78 °C and, the mixture was stirred at room temperature overnight. The reddish suspension was quenched with Et3N (0.1 mL) and concentrated. The residue was purified by silica gel chromatography with hexanes/ethyl ether = 4:1 as eluent to give the target product 13 as a colorless oil (32 mg, E/Z = 2:1) containing residual 7 (20 mg). The yield was 79% brsm. 1H NMR (300 MHz, CDCl3) δ 5.87-5.73 (m, 2H), 5.57-5.35 (m, 2H), 5.12-5.06 (m, 4H), 4.27-3.95 (m, 12H), 3.88-3.79 (m, 2H), 3.57-3.43 (m, 2H), 2.55-2.15 (m, 12H), 1.78 (s, 1.7H), 1.69 (s, 4.3H), 1.41 (s, 1.7H), 1.40 (s, 4.3H), 1.34 (s, 6H), 1.25 (t, J = 7.2 Hz, 6H). Rf = 0.40 (hexanes/ethyl acetate = 4:1, KMnO4 stain).
Compound 5
Finely powdered CeCl3·7H2O (0.228 g, 0.61 mmol) was heated at 160 °C under high vacuum for 2.5 h and cooled to room temperature under argon. The white powder was suspended in 1 mL of THF and stirred at room temperature for 2 h. Then the white suspension was cooled to −78 °C and to it was added TMSCH2MgCl (0.615 mL, 1 M in diethyl ether, 0.615 mmol). The yellow suspension was stirred at −78 °C for 1 h, and to it was added 13 (40 mg, 0.12 mmol) in THF (0.4 mL). Then the mixture was stirred sequentially at −78 °C for 2 h and room temperature overnight. The white suspension was cooled to 0 °C and diluted with anhydrous ether (5 mL), followed by addition of HCl (1 mL, 2 M). Then the reaction mixture was diluted with water/ether. The aqueous layer was extracted with ether twice. The combined organic layer was washed with brine, dried (Na2SO4) and concentrated. The residue was purified by silca gel chromatography with hexanes/ethyl acetate (93:7) as eluent to give the product as a colorless oil, the E/Z mixture (ca. 2:1) of 5 (32 mg, 73%). 1H NMR (400 MHz, CDCl3) δ 5.90-5.80 (m, 2H), 5.48 (t, J = 6.6 Hz, 0.66H), 5.40 (t, J = 6.6 Hz, 1.38H), 5.13-5.07 (m, 4H), 5.10-5.04 (m, 4H), 4.65 (s, 2H), 4.59 (s, 2H), 4.27-4.14 (m, 2H), 4.05-3.98 (m, 6H), 3.57-3.46 (m, 4H), 2.46-2.37 (m, 2H), 2.30-2.17 (m, 8H), 2.08 (dd, J = 6.3, 14.1 Hz, 2H), 1.78 (s, 2H), 1.70 (s, 4H), 1.58 (s, 1.4H), 1.55 (s, 2.6H), 1.42 (s, 2H), 1.41 (4H), 1.35 (s, 6H), 0.02 (s, 18H). 13C NMR (100 MHz, CDCl3) δ 144.5, 135.6, 135.2, 135.1, 124.8, 124.1, 116.8, 109.7, 109.6, 108.9, 108.8, 108.7, 74.6, 74.5, 69.3, 65.6, 65.4, 65.3, 43.7, 42.9, 38.5, 36.5, 27.1, 27.0, 26.9, 25.7, 25.6, 24.1, 23.2, 17.0, -1.3. IR (thin film, cm−1) 3075, 2953, 1633, 1435, 1370, 1248, 1157, 1068, 857, 695. Rf = 0.41 (hexanes/ethyl acetate =4:1, PMA stain). MS (ESI) m/z 389 (M+Na +).
Optimization of oxidative cyclization
To a reaction vial charged with 17 (56 mg, 0.1 mmol) and 4 Å MS (0.1 g) was added solvent (4 mL) at −38 °C under argon and DDQ (34 mg, 0.15 mmol) and acid (1.5 equiv.). Then the mixture was stirred until full conversion as monitored by TLC (hexanes/ethyl acetate = 98:2, developed four times, ca. 6-8 h to reach completion). Then the reaction was quenched with Et3N (0.2 mL) and filtered under pressure through a silica gel pad. The concentrated residue was purified by silica gel chromatography with hexanes/ethyl acetate (95:5) as eluent to give 16 as a colorless oil.
Compound 16
Cyclization reaction with stiochiometric amount of DDQ (Table 1, entry 10): To a flask charged with DDQ (1.56 g, 6.87 mmol), PPTS (1.78 g, 6.87 mmol) and 4 Å MS (9.0 g) under argon at −38 °C was added anhydrous acetonitrile (100 mL). A solution of (R)-tert-butyl(3-(cinnamyloxy)-5-((trimethylsilyl)methyl)hex-5-enyloxy)diphenylsilane 17 (2.63 g, 4.72 mmol, 97% ee) in acetonitrile (31 mL) was added dropwise for 5 min. The mixture was stirred at −38 °C for 3 h. The reaction was quenched with triethylamine (3 mL) and filtered through a pad of Celite. The filtrate was concentrated and then dissolved in ethyl acetate, washed with brine, and dried (Na2SO4). Evaporation of the solvent gave a residue which was purified by silica gel chromatography with hexanes/ethyl acetate (98:2) to provide compound 16 as a pale yellow oil (1.81g, 81%). 1H-NMR (300 MHz, CDCl3) δ 7.69-7.66 (m, 4H), 7.45-7.21 (m, 11H), 6.59 (d, J = 16.0 Hz, 1H), 6.24 (dd, J = 16.0, 5.7 Hz, 1H), 4.78 (s, 2H), 3.99-3.86 (m, 2H), 3.81-3.74 (m, 1H), 3.70-3.62 (m, 1H), 2.36 (d, J = 14.8 Hz, 1H), 2.28 (d, J = 14.4, 1H), 1.92-1.76 (m, 2H), 1.05 (s, 9H). 13C-NMR (75 MHz, CDCl3) δ 144.3, 136.8, 135.5, 133.9, 133.8, 130.1, 129.5, 128.4, 127.6, 126.4, 108.9, 78.5, 75.1, 60.1, 41.0, 40.7, 39.0, 26.8, 19.2. IR (thin film, cm−1) 3070, 2857, 1959, 1823, 1651, 1590, 1427, 1359, 1110, 966, 892, 741, 703, 614. Rf = 0.36 (hexanes/ethyl acetate = 95:5, UV) The Rf is almost the same as that of the starting material, but development with hexanes/ethyl acetate = 99:1 three times gave a slightly higher Rf than that of the starting material. [α]d20 = +3.5 (c 1.2, ethyl acetate). MS (ESI) m/z 505 (M+Na+). HRMS (ESI) calculated for (C32H38O2SiNa) 505.2539, found 505.2536.
Determination of ee
To a solution of 16 (30 mg, 60.4 μmol) in THF (1 mL) was added TBAF (0.4 mL, 1 M in THF, 0.4 mmol). Then the solution was stirred at room temperature for 4 h and concentrated. The residue was purified by silica gel chromatography with hexanes/ethyl aceate (v/v = 3:2) as eluent to give the free alcohol as a colorless oil (8.0 mg, 60%), 97% ee. 1H NMR (400 MHz, CDCl3) δ 7.39-7.23 (m, 5H), 6.23 (dd, J = 6.1, 16.0 Hz, 1H), 6.58 (d, J = 16.0 Hz, 1H), 4.79 (s, 2H), 4.03-3.99 (m, 1H), 3.86-3.82 (m, 2H), 3.68-3.62 (m, 1H), 2.64 (s, 1H), 2.36 (d, J = 13.3 Hz, 1H), 2.23 (d, J = 13.3 Hz, 1H), 2.20-2.08 (m, 2H), 1.94-1.84 (m, 1H), 1.83-1.76 (m, 1H). 13C NMR (100 MHz, CDCl3) δ143.4, 130.6, 129.5, 128.4, 127.6, 126.4, 109.3, 79.0, 78.7, 61.2, 40.7, 40.4, 37.9. IR (thin film, cm−1) 3401, 2942, 2900, 1719, 1650, 1450, 1421, 1315, 1062, 966, 746, 693. Rf = 0.35, hexanes/ethyl acetate = 7:3 UV. [α]d20 = +6.8 (c 0.73, ethyl acetate). HPLC, Daicel IC column, hexanes/IPA = 9:1, 0.5 mL/min, 254 nm, t1 = 14.1 min, t2 = 14.9 min. Peak at t2 is the major enantiomer.
Compound 16
Catalytic version (Table 1, entry 9): To a suspension of DDQ (0.147 g, 20 mol-%), PPTS (1.63 g, 6.48 mmol), CAN (3.51 g, 6.48 mmol), and 4 Å MS (3.6 g) on CH3CN (120 mL) was added a solution of 17 (1.80 g, 3.24 mmol) in CH3CN (10 mL). The suspension was stirred at -38 °C for 16 h and quenched with Et3N (1 mL). The mixture was filtered through a silica gel column with hexanes/ethyl acetate (v/v =95:5) as eluent. The concentrated product was purified by silica gel chromatography with hexanes/ethyl acetate (v/v = 95:5) as eluent to give methylenetetrahydropyran 16 as a reddish oil (1.17 g, 71%). The analytical data comply with the reported value.
Compound 36
To a reaction vial charged with with isobutyramide (20.9 mg, 0.24 mmol) in diethyl ether (1.2 mL) was added Et3N (56 μL, 0.4 mmol) and Cy2BCl (0.24 mL, 1 M in hexanes, 0.24 mmol) in sequence to yield a white suspension. The mixture was stirred at 0 °C for 15 min, and to it was added cyclohexanecarbaldehyde (24 μL, 0.2 mmol). After being stirred at 0 °C for 1 h, the reaction mixture was loaded onto a silica gel column with 0.5 mL of CH2Cl2 to dissolve the white precipitate. The column was eluted with hexanes/ethyl acetate = 3:2 to give the product 36 as a white solid (40 mg, 99%). 1H NMR (400 MHz, CDCl3) δ 6.12 (d, J = 7.1 Hz, 1H), 5.08-5.04 (m, 1H), 3.95 (d, J = 3.7 Hz, 1H), 2.39-2.32 (m, 1H), 1.78-1.65 (m, 4H), 1.54-1.44 (m, 1H), 1.21-0.99 (m, 12H). 13C NMR (100 MHz, CDCl3) δ 178.2, 77.6, 42.1, 35.6, 28.13, 27.9, 26.2, 25.7, 25.6, 19.5, 19.2. IR (thin film, cm−1) 1693, 1639, 1512, 1366, 1249, 1171, 778. Rf = 0.20 (hexanes/ethyl acetate = 3:2, PMA stain). MS (ESI) m/z 222 (M+Na +).
Compound 34
To a solution of TBS glycidol (2 g, 10.6 mmol) in THF (20 mL) was added Li2CuCl4 (5.3 mL, 0.1 M in THF) and isoprenyl magnesium bromide (0.5 M in THF, 26 mL, 13 mmol) at −78 °C. After stirring at −78 °C for 15 min and at room temperature for 2 h, crotonyl chloride (1.22 mL, 12.8 mmol) was added, and the reaction mixture was stirred at 0 °C for 5 min. Then the reaction was quenched with water and extracted with ethyl acetate twice. The combined organic layer was washed with sat. NaHCO3 and brine, dried (Na2SO4) and concentrated. The residue was purified by flash chromatography with hexanes/ethyl acetate = 97:3 as eluent to give TBS ether as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 6.98-6.91 (m, 1H), 5.81 (dd, J = 1.6, 15.5 Hz, 1H), 5.23-5.16 (m, 1H), 4.77 (s, 1H), 4.71 (s, 1H), 3.66 (d, J = 5.2 Hz, 2H), 2.36 (dd, J = 7.3, 13.9 Hz, 1H), 2.24 (dd, J = 6.0, 13.8 Hz, 1H), 1.86 (dd, J = 1.5, 6.9 Hz, 3H), 1.75 (s, 3H), 0.88 (s, 9H), 0.02 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 166.0, 144.4, 141.5, 122.8, 113.1, 72.2, 64.0, 38.9, 25.7, 22.5, 18.1, 17.9, -5.4. IR (thin film, cm−1) 2930, 1722, 1657, 1258, 1182, 1101, 836, 777. Rf = 0.31 (hexanes/ethyl acetate = 97:3, PMA stain). MS (ESI) m/z 321 (M+Na +).
To a solution of TBS ether prepared as described above (50 mg, 0.168 mmol) in MeOH/CH2Cl2 (v/v 4:1, 5 mL) was added HCl (170 μL, 2 M) and water (170 μL). Then the reaction was quenched with sat. NaHCO3 and diluted with water. The aqueous layer was extracted with ethyl acetate three times. The combined organic layer was washed with brine, dried (Na2SO4) and concentrated. The residue was purified by silica gel chromatography with hexanes/ethyl acetate (7:3) as eluent to give the alcohol as a colorless oil (25 mg, 70%). 1H NMR (400 MHz, CDCl3) δ 7.05-6.93 (m, 1H), 5.85 (dd, J = 1.7, 15.5 Hz, 1H), 5.15-5.10 (m, 1H), 4.81 (s, 1H), 4.76 (s, 1H), 3.78-3.72 (m, 1H), 3.68-3.62 (m, 1H), 2.37 (dd, J = 7.5, 14.1 Hz, 1H), 2.30 (dd, J = 6.0, 14.2 Hz, 1H), 2.06 (t, J = 6.0 Hz, 1H), 1.88 (d, J = 5.9 Hz, 3H), 1.77 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 166.5, 145.3, 141.0, 122.4, 113.5, 73.1, 64.5, 39.0, 22.4, 17.9. IR (thin film, cm−1) 3441, 2918, 1719, 1655, 1445, 1310, 1293, 1184, 1103, 1048, 969, 894, 838. Rf = 0.28 (hexanes/ethyl acetate = 7:3, I2 or KMnO4 stain). MS (ESI) m/z 207 (M+Na +).
To a suspension of the above alcohol (25 mg, 0.136 mmol) and NaHCO3 (0.1 g, 1.2 mmol) in CH2Cl2 (5 mL) was added Dess-Martin reagent (0.68 mL, 0.3 M in CH2Cl2, 0.204 mmol). The mixture was vigorously stirred for 2 h and quenched with a Na2S2O3/NaHCO3 solution. The aqueous layer was extracted with CH2Cl2 twice. The combined organic layer was dried (Na2SO4) and concentrated. The residue was filtered through a silica gel plug with hexanes/ethyl acetate (7:3) as eluent to give 34 as a colorless oil (21 mg, 83%). The compound was unstable and used immediately in the next step. 1H NMR (300 MHz, CDCl3) δ 9.56 (s, 1H), 7.13-7.01 (m, 1H), 5.92 (dd, J = 1.7, 15.6 Hz, 1H), 5.20 (dd, J = 5.7, 9.0 Hz, 1H), 4.85 (s, 1H), 4.78 (s, 1H), 2.56 (dd, J = 4.6, 14.9 Hz, 1H), 2.45 (dd, J = 8.9, 15.8 Hz, 1H), 1.91 (dd, J = 1.6, 6.9 Hz, 3H), 1.76 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 198.3, 165.6, 146.6, 139.4, 121.4, 114.3, 76.1, 36.9, 22.3, 18.0. IR (thin film, cm−1) 2943, 2851, 1722, 1656, 1444, 1377, 1294, 1263, 1178, 1105, 969, 898, 837, 688. Rf = 0.52 (hexanes/ethyl acetate = 7:3, I2 stain or KMnO4). MS (ESI) m/z 205 (M+Na +).
Compound 37
To a solution of amide 35 (10.4 mg, 0.12 mmol) and Et3N (28μL), 0.2 mmol) in diethyl ether (0.4 mL) was added Cy2BCl solution (0.20 mL, 0.6 M in hexanes, 0.12 mmol). The resulting white suspension was stirred at 0 °C for 15 min, and to it was added aldehyde 34 (28.4 mg, 0.1 mmol) in diethyl ether (0.2 mL). After being stirred at 0 °C for 1 h, the mixture was quenched with THF/phosphate buffer (pH = 7.4)/30% H2O2 (v/v/v = 1:1:1, 2 mL). Then the aqueous layer was extracted with ethyl acetate three times. The combined organic layer was washed with brine, dried (Na2SO4), and concentrated. The residue was purified by silica gel chromatography with hexanes/ethyl acetate (v/v = 1:1) as eluent to yield 37 as a mixture of diastereomixtures in the ratio of 1:1, (15 mg, 56%). 1H NMR (300 MHz, CDCl3) δ 10.2 (s, 1H), 9.9 (s, 1H), 7.06-6.93 (m, 2H), 6.70 (d, J = 7.6 Hz, 1H), 6.60 (d, J = 8.3 Hz, 1H), 5.86-5.84 (m, 1H), 5.81-5.79 (m, 1H), 5.16-5.07 (m, 2H), 4.82-8.81 (m, 2H), 4.75-4.74 (m, 2H), 2.48-2.34 (m, 4H), 2.15-2.02 (m, 2H), 1.90-1.87 (m, 6H), 1.75 (s, 3H), 1.73 (s, 3H). 1.20-1.16 (m, 12H). IR (thin film, cm-1) 3339, 1711, 1659, 1531, 1444, 1293, 1183, 1103, 1045, 969, 893. Rf = 0.35 (hexanes/ethyl acetate = 1:1, KMnO4). MS (ESI) m/z 292 (M+Na +).
Compounds 1, 39 and 40
Amidation using diphenyl phosphate as catalyst. The reaction mixture containing dactylolide 2 (0.5 mg, 1.31 μmol), amide 38 (0.30 mg, 2.76 μmol) and diphenyl phosphate (0.03 mg, 0.13 μmol) in CH2Cl2 (30 μL) was stirred at 23 °C for 14 h. The mixture was loaded onto a silica gel column with hexanes/ethyl acetate (1:1) as eluent to get a mixture of 1, 38, 39 and 40 that was further separated on HPLC (IC, hexanes/i-PrOH = 1:1, 0.5 mL/min, 254 nM). The ratio of 1 to 39 was close to 1:1.
Bisamide 40
1H-NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 12.1, 14.7 Hz, 1H), 7.48-7.41 (m, 2H), 6.85-6.80 (m, 1H), 6.45-6.39 (m, 3H), 6.33 (d, J = 7.3 Hz, 1H), 6.10 (d, J = 11.6 Hz, 1H), 6.06-5.98 (m, 2H), 5.93 (d, J = 14.7 Hz, 1H), 5.92 (d, J = 16.2 Hz, 1H), 5.68-5.61 (m, 2H), 5.41 (d, J = 11.0 Hz, 1H), 5.40 (d, J = 11.0 Hz, 1H), 5.16 (d, J = 8.0 Hz, 1H), 4.72 (s, 2H), 4.17 (d, J = 13.6 Hz, 1H), 3.97-3.93 (m, 1H), 3.26 (t, J = 10.4 Hz, 1H), 3.00 (d, J = 13.4 Hz, 1H), 2.39-2.33 (m, 2H), 2.24-2.19 (m, 2H), 2.13 (d, J = 12.7 Hz, 1H), 2.06 (d, J = 13.6 Hz, 1H), 1.93 (d, J = 12.4 Hz, 2H), 1.86 (d, J = 3.9 Hz, 6H), 1.79 (s, 3H), 1.71 (s, 3H). MS (ESI) m/z 611 (M+Na+).
(-)-Zampanolide 1 and 39
To a flask containing (-)-2 (7 mg, 18.3 μmol), and (2Z, 4E)-hexa-2,4-dienamide 38 (6.1 mg, 55 μmol), (S)-TRIP 41 (2.7 mg, 20 mol-%) in CH2Cl2 (0.7 mL) was added. The resulting mixture was stirred at 23 °C for 12 h. After this period, the reaction was loaded on a short silica gel column and eluted with hexanes/ethyl acetate (3:2) to provide a crude mixture of (S)-TRIP 41, 1 and 39. The mixture was separated in HPLC to give (-)-zampanolide 1 (4.6 mg, 51%) and 39 (1.6 mg, 18%). HPLC condition: hexanes/i-PrOH = 1:1, 0.5 mL/min, 254 nm, t(S)-TRIP = 7.4 min, tamide38 = 12.5 min, tzampanolide 1= 15.3 min, t39 = 23.3 min.
Zampanolide 1
Rf = 0.35 (hexanes/ethyl acetate = 1:1, UV, PMA). [α]D20 = -94 (c 0.08, CH2Cl2). 1H-NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 11.6, 14.9 Hz, 1H), 7.45-7.40 (m, 1H), 6.85-6.79 (m, 1H), 6.46 (t, J = 11.3 Hz, 1H), 6.31 (d, J = 7.8 Hz, 1H), 6.11 (d, J = 11.9 Hz, 1H), 6.08-6.02 (m, 1H), 5.95 (d, J = 15.0 Hz, 1H), 5.94 (d, J = 16.4 Hz, 1H), 5.46-5.43 (m, 2H), 5.31-5.27 (m, 1H), 5.20 (d, J = 7.9 Hz, 1H), 4.73 (s, 2H), 4.13 (d, J = 13.0 Hz, 1H), 3.98-3.94 (m, 1H), 3.66 (brs, 1H), 3.29 (t, J = 10.5 Hz, 1H), 3.04 (d, J = 13.6 Hz, 1H), 2.41 (d, J = 13.6 Hz, 1H), 2.38-2.29 (m, 1H), 2.29-2.20 (m, 2H), 2.14 (d, J = 13.4 Hz, 1H), 2.09 (d, J = 13.4 Hz, 1H), 1,97-1.91 (m, 2H), 1.87 (d, J = 7.0 Hz, 3H), 1.81 (s, 3H), 1.72 (s, 3H). 13C-NMR (125 MHz, CDCl3) δ197.9, 167.6, 166.8, 146.3, 143.8, 143.6, 143.5, 140.2, 140.1, 132.0, 131.4, 129.9, 128.1, 125.2, 120.2, 116.8, 109.1, 76.5, 75.8, 75.3, 71.4, 45.1, 41.8, 40.9, 40.6, 40.1, 23.6, 18.6, 16.6. IR (thin film, cm−1) 3368, 2924, 2854, 2360, 1636, 1539, 1456, 1357, 1281, 1260, 1210, 1149, 1086, 979, 889, 803, 666. MS (ESI) m/z 518 (M+Na+). HRMS(ESI) calculated for (C29H37NO6Na) 518.2519, found 518.2520.
Zampanolide diastereomer 39
Rf = 0.35 (hexanes/ethyl acetate = 1:1, UV, PMA). 1H-NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 11.6, 15.0 Hz, 1H), 7.46-7.39 (m, 1H), 6.86-6.80 (m, 1H), 6.46 (t, J = 11.3 Hz, 1H), 6.35 (d, J = 7.5 Hz, 1H), 6.12 (d, J = 11.6 Hz, 1H), 6.06 (dd, J = 6.9, 15.0 Hz, 1H), 6.00 (d, J = 15.0 Hz, 1H), 5.94 (d, J = 16.3 Hz, 1H), 5.54-5.51(m, 1H), 5.45 (d, J = 11.2 Hz, 1H), 5.37-5.31 (m, 1H), 5.24 (d, J = 8.6 Hz, 1H), 4.73 (brs, 2H), 4.11 (d, J = 13.7 Hz, 1H), 3.98-3.94 (m, 1H), 3.60 (br, 1H), 3.34-3.27 (m, 1H), 3.05 (d, J = 13.7 Hz, 1H), 2.51 (dd, J = 11.0, 13.8 Hz, 1H), 2.39-2.23 (m, 3H), 2.15 (d, J = 13.7 Hz, 1H), 2.10 (d, J = 13.1 Hz, 1H), 2.00-1.93 (m, 2H), 1.87 (d, J = 6.6 Hz, 3H), 1.82 (s, 3H), 1.71 (s, 3H).
Biological Methods
Tubulin and heat-treated microtubule-associated proteins were prepared as described previously.[23] Evaluation of compound-induced microtubule assembly by turbidimetry at 350 nm was performed as described previously.[24] Evaluation of cytotoxicity of agents against the MCF-7, OVCAR-8, and NCI/ADR-RES human cancer cell lines, generously provided by the National Cancer Institute drug screening group, was performed by the rhodamine B method,[25] in which cellular protein is the parameter quantitated. Paclitaxel was generously provided by the Drug Chemistry and Synthesis Branch of the National Cancer Institute.
Supplementary Material
Acknowledgments
Financial support for this work was provided in part by the National Institutes of Health and Purdue University.
Footnotes
Supplementary Materials: 1H NMR and 13C NMR spectra for all new compounds and HPLC traces for compounds 1, 16, 39 associated with this article can be found in the online version.
References
- 1.Tanaka J, Higa T. Tetrahedron Lett. 1996;37:5535–5538. [Google Scholar]
- 2.Field JJ, Singh AJ, Kanakkanthara A, Halafihi T, Northcote PT, Miller JH. J Med Chem. 2009;52:7328–7332. doi: 10.1021/jm901249g. [DOI] [PubMed] [Google Scholar]
- 3.Qi YK, Ma ST. ChemMedChem. 2011;6:399–409. doi: 10.1002/cmdc.201000534. [DOI] [PubMed] [Google Scholar]
- 4.Cutignano A, Bruno I, Bifulco G, Casapullo A, Debitus C, Gomez-Paloma L, Riccio R. Eur J Org Chem. 2001:775–778. doi: 10.1021/np010053+. [DOI] [PubMed] [Google Scholar]
- 5.a) Smith AB, III, Safonov IG, Corbett RM. J Am Chem Soc. 2001;123:12426–12427. doi: 10.1021/ja012220y. [DOI] [PubMed] [Google Scholar]; b) Smith AB, III, Safonov IG, Corbett RM. J Am Chem Soc. 2002;124:11102–11113. doi: 10.1021/ja020635t. [DOI] [PubMed] [Google Scholar]
- 6.a) Hoye TR, Hu M. J Am Chem Soc. 2003;125:9576–9577. doi: 10.1021/ja035579q. [DOI] [PubMed] [Google Scholar]; b) Uenishi J, Iwamoto T, Tanaka J. Org Lett. 2009;11:3262–3265. doi: 10.1021/ol901167g. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh AK, Cheng X. Org Lett. 2011;13:4108–4111. doi: 10.1021/ol201626h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Smith AB, III, Safonov IG. Org Lett. 2002;4:635–637. doi: 10.1021/ol017271e. [DOI] [PubMed] [Google Scholar]; b) Ding F, Jennings MP. Org Lett. 2005;7:2321–2324. doi: 10.1021/ol0504897. [DOI] [PubMed] [Google Scholar]; c) Aubele DL, Wan S, Floreancig PE. Angew Chem. 2005;117:3551–3554. doi: 10.1002/anie.200500564. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:3485–3488. doi: 10.1002/anie.200500564. [DOI] [PubMed] [Google Scholar]; d) Sanchez CC, Keck GE. Org Lett. 2005;7:3053–3056. doi: 10.1021/ol051040g. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Louis I, HungeRford NL, Humphries EJ, McLeod MD. Org Lett. 2006;8:1117–1120. doi: 10.1021/ol053092b. [DOI] [PubMed] [Google Scholar]; f) Zurwerra D, Gertsch J, Altmann KH. Org Lett. 2010;12:2302–2305. doi: 10.1021/ol100665m. [DOI] [PubMed] [Google Scholar]; g) Yun SY, Hansen EC, Volchkov I, Cho EJ, Lo WY, Lee D. Angew Chem. 2010;122:4357–4359. doi: 10.1002/anie.201001681. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:4261–4263. doi: 10.1002/anie.201001681. [DOI] [PubMed] [Google Scholar]
- 9.Bode ML, Gates PJ, Gebretnsae SY, Vleggaar R. Tetrahedron. 2010;66:2026–2036. [Google Scholar]
- 10.Doroh B, Sulikowski GA. Org Lett. 2006;8:903–906. doi: 10.1021/ol0530225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayanan BA, Bunnelle WH. Tetrahedron Lett. 1987;28:6261–6264. [Google Scholar]
- 12.For related DDQ cyclizations, see references: Hayashi Y, Mukaiyama T. Chem Lett. 1987;9:1811–1814.Ying BP, Trogden BG, Kohlman DT, Liang SX, Xu YC. Org Lett. 2004;6:1523–1526. doi: 10.1021/ol036314j.Zhang Y, Li CJ. Angew Chem. 2006;118:1983–1986.Angew Chem Int Ed. 2006;45:1949–1952. doi: 10.1002/anie.200503255.Tu W, Liu L, Floreancig PE. Angew Chem. 2008;120:4252–4255.Angew Chem Int Ed. 2008;47:4184–4187. doi: 10.1002/anie.200706002.Tu W, Floreancig PE. Angew Chem. 2009;121:4637–4641.Angew Chem Int Ed. 2009;48:4567–4571. doi: 10.1002/anie.200901489.Yu B, Jiang T, Li J, Su Y, Pan X, She X. Org Lett. 2009;11:3442–3445. doi: 10.1021/ol901291w.
- 13.Claffey MM, Hayes CJ, Heathcock CH. J Org Chem. 1999;64:8267–8274. doi: 10.1021/jo9910987. [DOI] [PubMed] [Google Scholar]
- 14.Chrobok A, Gossinger E, Grunberger K, Kahlig H, White MJ, Wuggenig F. Tetrahedron. 2007;63:8336–8350. [Google Scholar]
- 15.Zhang W, Carter RG. Org Lett. 2005;7:4209–4212. doi: 10.1021/ol051544e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 17.Lei B, Fallis AG. Can J Chem. 1991;69:1450–1456. [Google Scholar]
- 18.Jauch J. Angew Chem Int Ed. 2000;39:2764–2765. doi: 10.1002/1521-3773(20000804)39:15<2764::aid-anie2764>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull Chem Soc Jpn. 1979;52:1989–1993. [Google Scholar]
- 20.Dess DB, Martin JC. J Org Chem. 1983;4:4155. [Google Scholar]
- 21.Kiren S, Shangguan N, Williams LJ. Tetrahedron Lett. 2007;48:7456–7459. [Google Scholar]
- 22.For a review of TRIP in the organocatalysis, see: Akiyama T. Chem Rev. 2007;107:5744–5758. doi: 10.1021/cr068374j. for application examples, see: Hoffmann S, Seayad AM, List B. Angew Chem. 2005;117:7590–7593. doi: 10.1002/anie.200503062.Angew Chem Int Ed. 2005;44:7424–7427. doi: 10.1002/anie.200503062.
- 23.Hamel E, Lin CM. Biochemistry. 1984;23:4173–4184. doi: 10.1021/bi00313a026. [DOI] [PubMed] [Google Scholar]
- 24.Pryor DE, O'Brate A, Bilcer G, Díaz JF, Wang Y, Wang Y, Kabaki M, Jung MK, Andreu JM, Ghosh AK, Giannakakou P, Hamel E. Biochemistry. 2002;41:9109–9115. doi: 10.1021/bi020211b. [DOI] [PubMed] [Google Scholar]
- 25.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M. J Natl Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.