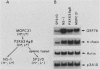
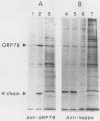
Abstract
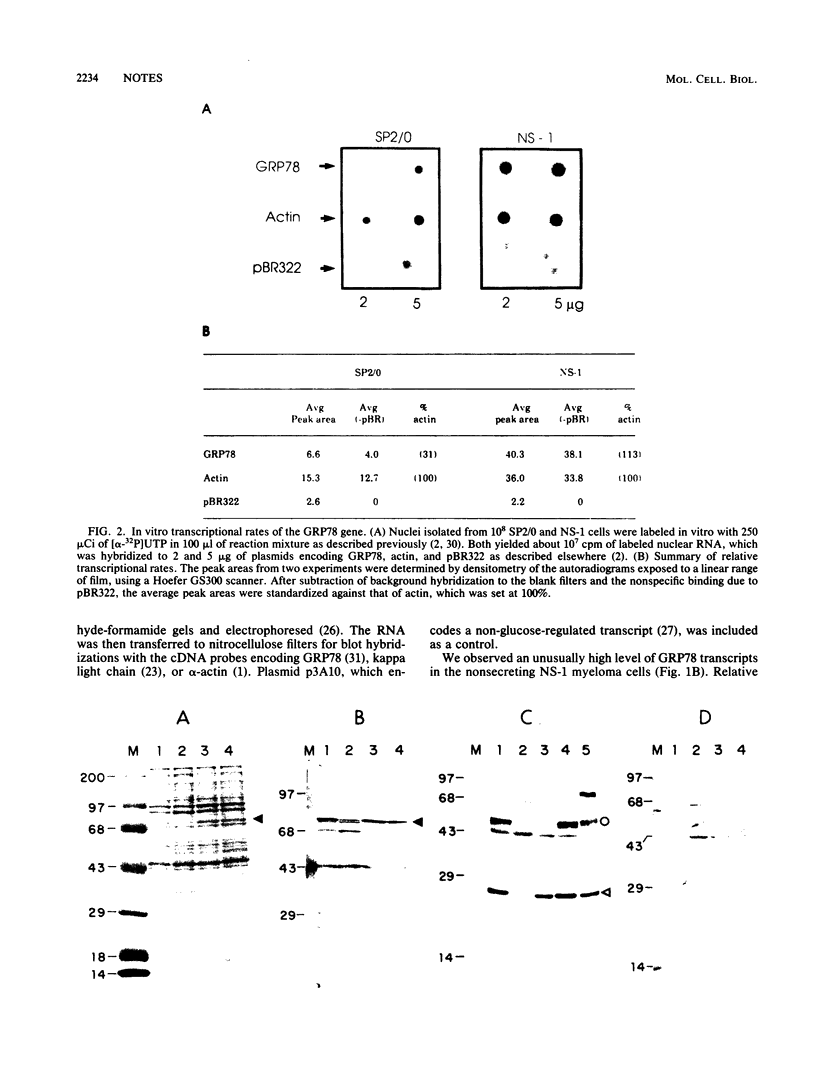
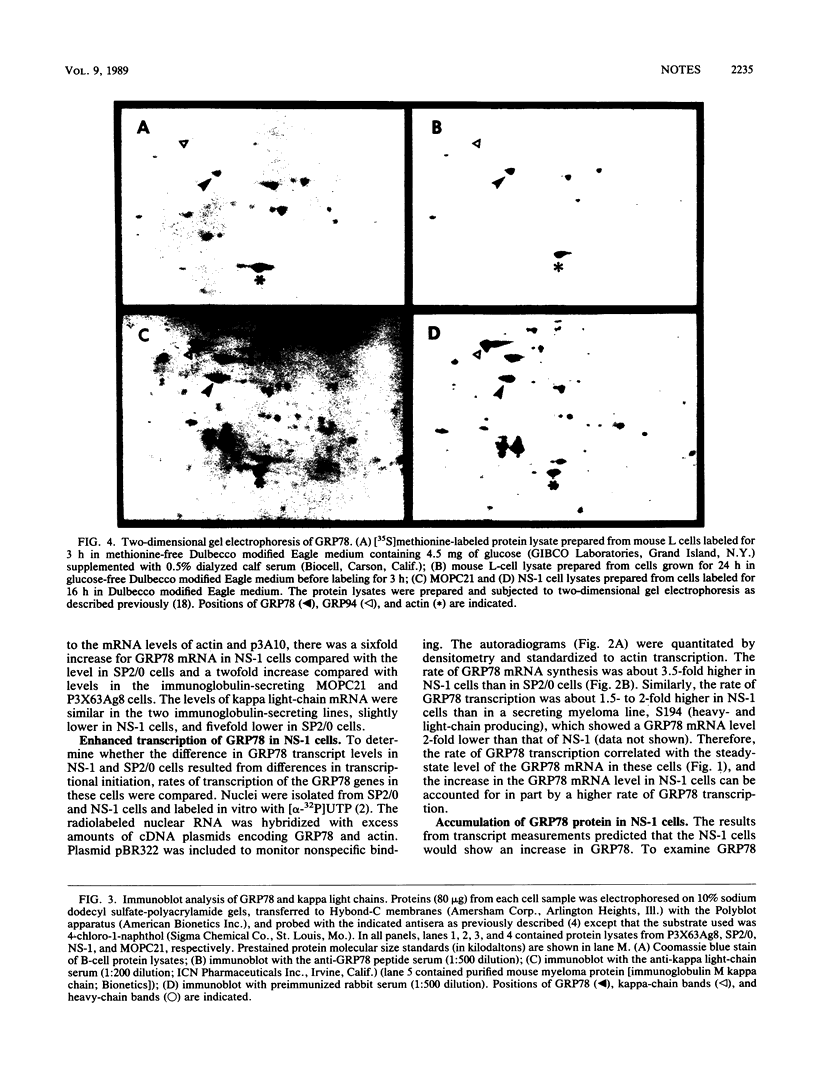
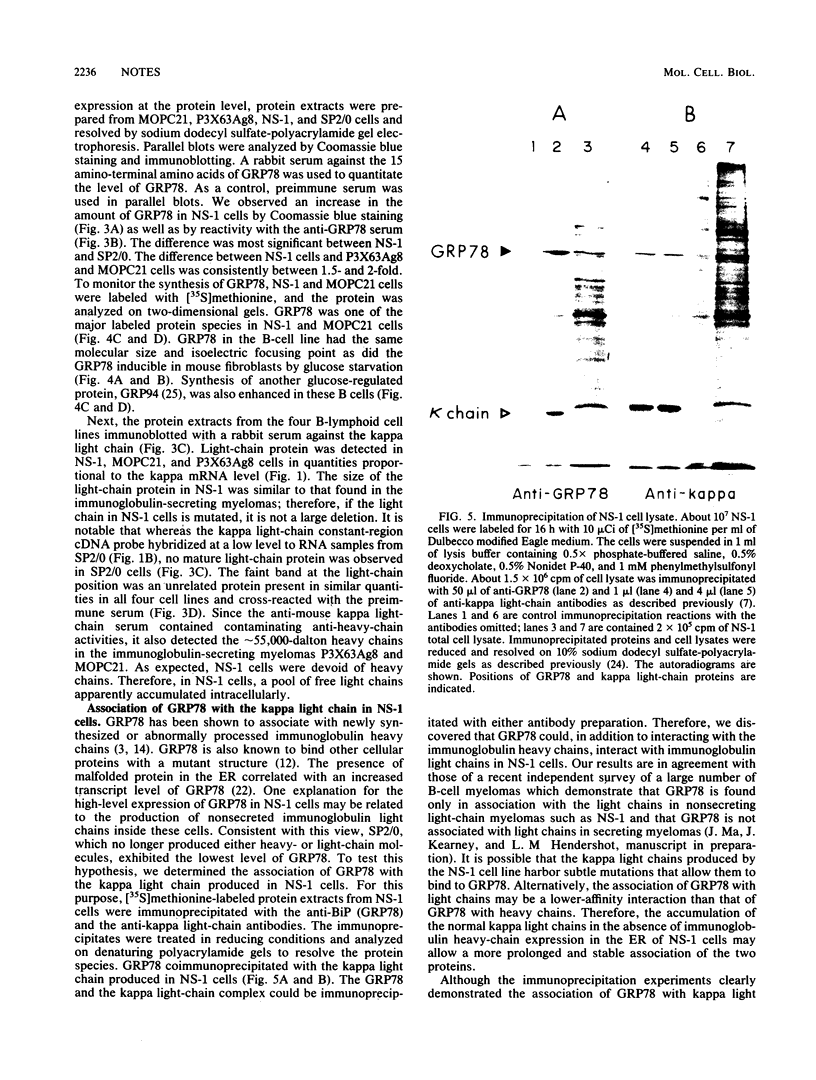
The 78,000-dalton glucose-regulated protein (GRP78) is a stress-inducible protein localized in the endoplasmic reticulum. It has been identified as the immunoglobulin heavy-chain-binding protein. We report here a high level of GRP78 expression in a B-cell myeloma line, NS-1, which produces only kappa light-chain proteins but is unable to secrete them. GRP78 transcription was enhanced in NS-1 cells, resulting in higher levels of GRP78 mRNA and protein than in non-immunoglobulin-producing cells. Furthermore, the nonsecreted light chains in NS-1 cells were found in specific association with GRP78. We hypothesize that in nonsecreting lymphoid cells, the presence of free, unassembled light chains in the endoplasmic reticulum could result in increased transcription of the GRP78 gene and that GRP78 can also bind to immunoglobulin light chains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artishevsky A., Delegeane A. M., Lee A. S. Use of a cell cycle mutant to delineate the critical period for the control of histone mRNA levels in the mammalian cell cycle. Mol Cell Biol. 1984 Nov;4(11):2364–2369. doi: 10.1128/mcb.4.11.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artishevsky A., Wooden S., Sharma A., Resendez E., Jr, Lee A. S. Cell-cycle regulatory sequences in a hamster histone promoter and their interactions with cellular factors. 1987 Aug 27-Sep 2Nature. 328(6133):823–827. doi: 10.1038/328823a0. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos T. J., Bohmann D., Tsuchie H., Tjian R., Vogt P. K. v-jun encodes a nuclear protein with enhancer binding properties of AP-1. Cell. 1988 Mar 11;52(5):705–712. doi: 10.1016/0092-8674(88)90408-4. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Secher D. S., Milstein C. Intracellular immunoglobulin chain synthesis in non-secreting variants of a mouse myeloma: detection of inactive light-chain messenger RNA. J Mol Biol. 1974 Dec 25;90(4):691–701. doi: 10.1016/0022-2836(74)90533-6. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Bole D. G., Kaufman R. J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987 Dec;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige J. J., Keller G. A., Scheffler I. E. Temperature-sensitive Chinese hamster cell mutant with a defect in glycoprotein synthesis: accumulation of the EGF receptor in the endoplasmic reticulum and the role of the glucose-regulated protein GRP78. J Cell Physiol. 1988 Jul;136(1):33–42. doi: 10.1002/jcp.1041360105. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hendershot L. M., Kearney J. F. A role for human heavy chain binding protein in the developmental regulation of immunoglobin transport. Mol Immunol. 1988 Jun;25(6):585–595. doi: 10.1016/0161-5890(88)90081-8. [DOI] [PubMed] [Google Scholar]
- Hendershot L. M., Ting J., Lee A. S. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol Cell Biol. 1988 Oct;8(10):4250–4256. doi: 10.1128/mcb.8.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Kassenbrock C. K., Garcia P. D., Walter P., Kelly R. B. Heavy-chain binding protein recognizes aberrant polypeptides translocated in vitro. Nature. 1988 May 5;333(6168):90–93. doi: 10.1038/333090a0. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Lee A. S. The effect of extracellular Ca2+ and temperature on the induction of the heat-shock and glucose-regulated proteins in hamster fibroblasts. Biochem Biophys Res Commun. 1986 Nov 14;140(3):881–887. doi: 10.1016/0006-291x(86)90717-5. [DOI] [PubMed] [Google Scholar]
- Kim Y. K., Lee A. S. Transcriptional activation of the glucose-regulated protein genes and their heterologous fusion genes by beta-mercaptoethanol. Mol Cell Biol. 1987 Aug;7(8):2974–2976. doi: 10.1128/mcb.7.8.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Phillips M. L., Miller M. M., Teplitz R. L. Monoclonal autoantibody production by hybrid cell lines. Clin Immunol Immunopathol. 1981 Mar;18(3):368–374. doi: 10.1016/0090-1229(81)90130-6. [DOI] [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988 Mar 31;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Kwan S. P., Rudikoff S., Seidman J. G., Leder P., Scharff M. D. Nucleic acid and protein sequences of phosphocholine-binding light chains. J Exp Med. 1981 May 1;153(5):1366–1370. doi: 10.1084/jem.153.5.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S., Delegeane A. M., Baker V., Chow P. C. Transcriptional regulation of two genes specifically induced by glucose starvation in a hamster mutant fibroblast cell line. J Biol Chem. 1983 Jan 10;258(1):597–603. [PubMed] [Google Scholar]
- Lee A. S. The accumulation of three specific proteins related to glucose-regulated proteins in a temperature-sensitive hamster mutant cell line K12. J Cell Physiol. 1981 Jan;106(1):119–125. doi: 10.1002/jcp.1041060113. [DOI] [PubMed] [Google Scholar]
- Lin A. Y., Lee A. S. Induction of two genes by glucose starvation in hamster fibroblasts. Proc Natl Acad Sci U S A. 1984 Feb;81(4):988–992. doi: 10.1073/pnas.81.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarella R. A., Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94). J Biol Chem. 1987 Jun 25;262(18):8875–8883. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Resendez E., Jr, Attenello J. W., Grafsky A., Chang C. S., Lee A. S. Calcium ionophore A23187 induces expression of glucose-regulated genes and their heterologous fusion genes. Mol Cell Biol. 1985 Jun;5(6):1212–1219. doi: 10.1128/mcb.5.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J., Wooden S. K., Kriz R., Kelleher K., Kaufman R. J., Lee A. S. The nucleotide sequence encoding the hamster 78-kDa glucose-regulated protein (GRP78) and its conservation between hamster and rat. Gene. 1987;55(1):147–152. doi: 10.1016/0378-1119(87)90258-7. [DOI] [PubMed] [Google Scholar]
- Wooden S. K., Kapur R. P., Lee A. S. The organization of the rat GRP78 gene and A23187-induced expression of fusion gene products targeted intracellularly. Exp Cell Res. 1988 Sep;178(1):84–92. doi: 10.1016/0014-4827(88)90380-1. [DOI] [PubMed] [Google Scholar]