Background: Human pyrin is an important regulator of inflammation and is involved in the pathogenesis of familial Mediterranean fever (FMF).
Results: Ribotoxic stress activates the human pyrin inflammasome.
Conclusion: p38 MAPK signaling is required for assembly of the human pyrin inflammasome.
Significance: Understanding the mechanism of activation of the pyrin inflammasome is crucial for the development of therapeutics to treat FMF.
Keywords: Inflammation, MAP Kinases (MAPKs), p38, Ribosomes, Stress, Pyrin, Caspase-1, Familial Mediterranean Fever, Inflammasome, Ribotoxic Stress
Abstract
Human pyrin with gain-of-function mutations in its B30.2/SPRY domain causes the autoinflammatory disease familial Mediterranean fever by assembling an ASC-dependent inflammasome that activates caspase-1. Wild-type human pyrin can also form an inflammasome complex with ASC after engagement by autoinflammatory PSTPIP1 mutants. How the pyrin inflammasome is activated in the absence of disease-associated mutations is not yet known. We report here that ribotoxic stress triggers the assembly of the human pyrin inflammasome, leading to ASC oligomerization and caspase-1 activation in THP-1 macrophages and in a 293T cell line stably reconstituted with components of the pyrin inflammasome. Knockdown of pyrin and selective inhibition of p38 MAPK greatly attenuated caspase-1 activation by ribotoxic stress, whereas expression of the conditional mutant ΔMEKK3:ER* allowed the activation of caspase-1 without ribotoxic stress. Disruption of microtubules by colchicine also inhibited pyrin inflammasome activation by ribotoxic stress. Together, our results indicate that ribotoxic stress activates the human pyrin inflammasome through a mechanism that requires p38 MAPK signaling and microtubule stability.
Introduction
Pathogens and metabolic danger signals trigger the assembly of cytoplasmic multiprotein complexes termed inflammasomes, which serve as molecular scaffolds for the activation of caspase-1 in innate immune cells (1, 2). Activated caspase-1 processes inactive pro-IL-1β and pro-IL-18 into the active cytokines IL-1β and IL-18, respectively. It is also involved in the unconventional secretion of these cytokines and other proinflammatory mediators such as HMGB1 (high-mobility group box 1) (3). Deregulated inflammasome activity can lead to several autoinflammatory periodic fever syndromes in humans such as the Muckle-Wells, familial Mediterranean fever (FMF),3 and pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA) syndromes (4, 5). This has also been implicated in chronic inflammatory and metabolic diseases such as gout, atherosclerosis, silicosis, and type 2 diabetes (6, 7).
FMF is caused by mutations in the MEFV gene, which encodes pyrin (8, 9), but the molecular mechanism by which these mutations lead to increased production of IL-1β is not yet clear. Cell-based reconstitution studies in our laboratory demonstrated that human pyrin can form an inflammasome complex with ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and procaspase-1, leading to ASC oligomerization, caspase-1 activation, and pro-IL-1β processing (10). Furthermore, our studies showed that binding of autoinflammatory PAPA-associated PSTPIP1 (proline-serine-threonine phosphatase-interacting protein 1) mutants to human pyrin can activate the pyrin inflammasome, leading to increased IL-1β production (5). Based on these studies, it was proposed that wild-type pyrin is a proinflammatory mediator capable of activating caspase-1 and that FMF-associated pyrin mutations may represent gain-of-function mutations that deregulate pyrin inflammasome activation (10). In support of this, recent studies in mice have shown that expression of humanized pyrin proteins carrying FMF-associated mutations results in exaggerated IL-1β cytokine production and severe autoinflammation (11). Despite these recent developments, the role of pyrin in the activation of caspase-1 remains unclear.
The p38 MAPKs play important roles in various intracellular responses, including proliferation, differentiation, apoptosis, tumorigenesis, and inflammation (12). Activation of p38 MAPKs has been shown to increase production of proinflammatory cytokines, and their specific inhibition potently blocks production of these cytokines and reduces chronic inflammation in inflammatory diseases such as rheumatoid arthritis and inflammatory bowel diseases (13). The mechanism by which p38 MAPKs increase production of proinflammatory cytokines, particularly IL-1β and IL-18, is not fully understood. In this study, we investigated whether p38 MAPKs play a role in activation of the pyrin inflammasome. We provide evidence that the activation of p38 MAPK by ribotoxic stress can trigger formation of a pyrin inflammasome, resulting in the oligomerization of ASC and the subsequent activation of caspase-1.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Antibodies against caspase-1, pyrin, IL-1β, and NLRP3 have been described previously (5, 14, 15). Anti-ASC antibody was from Santa Cruz Biotechnology. Anti-phospho-p38 and anti-p38 antibodies were from Cell Signaling. Anti-JNK antibody was from BD Biosciences. Anti-FLAG antibody, anisomycin, deoxynivalenol (DON; vomitoxin), 4-hydroxytamoxifen (4-HT), colchicine, paclitaxel, nigericin, LPS, and cycloheximide were from Sigma. SB203580 and SP600125 were from EMD Biosciences. Anti-phospho-JNK antibody and recombinant human IFNγ was from Invitrogen. The plasmid encoding ΔMEKK3:ER* was a kind gift from S. J. Cook.
siRNA Knockdown
Knockdown of pyrin was performed by transfection of siRNA oligonucleotides targeting human pyrin (Qiagen). Scrambled siRNA (control) or pyrin-specific siRNA was transfected into 293T-C1AP or THP-1 cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Cell Treatments and Immunoblot Analysis
293T-C1AP and 293T-C1AN cells, which stably express N-terminally FLAG-tagged procaspase-1, ASC, and pyrin or NLRP3, respectively, were generated by stably transfecting 293T-C1A cells (293T cells expressing procaspase-1 and ASC) with constructs encoding pyrin or NLRP3 as described previously (5, 14, 16). Cells were treated with the following drugs as indicated in each experiment: anisomycin (20 μm), DON (10 μm), cycloheximide (10 μg/ml), puromycin (8 μg/ml), nigericin (10 μm), SB203580 (20 μm), SP600125 (20 μm), IFNγ (20 ng/ml), and LPS (500 ng/ml). Cells were lysed in buffer containing 20 mm HEPES (pH 7.5), 0.5% Nonidet P-40, 50 mm KCl, 150 mm NaCl, 1.5 mm MgCl2, 1 mm EGTA, and protease inhibitors. Cell lysates were fractionated by SDS-PAGE and then transferred to PVDF membranes (Bio-Rad). In some experiments, cell culture supernatants were precipitated by methanol/chloroform as described previously (14) and then immunoblotted with appropriate antibodies.
Chemical Cross-linking
Chemical cross-linking was performed using disuccinimidyl suberate (DSS; Pierce) as described previously (5, 17, 18). Briefly, for analysis of pyrin oligomerization, lysates from IFNγ-primed THP-1 cells treated with or without anisomycin were cross-linked with DSS, immunoprecipitated with anti-pyrin antibody, and immunoblotted with anti-pyrin antibody as described (5). For analysis of ASC oligomerization, THP-1 cells were lysed after stimulation, and the cell lysates were centrifuged at 6000 rpm for 10 min. The resulting pellet fractions were resuspended in PBS, cross-linked with DSS, and analyzed by immunoblotting with anti-ASC antibody as described previously (17, 18).
RESULTS
Ribotoxic Stress Induces Pyrin-dependent Caspase-1 Activation
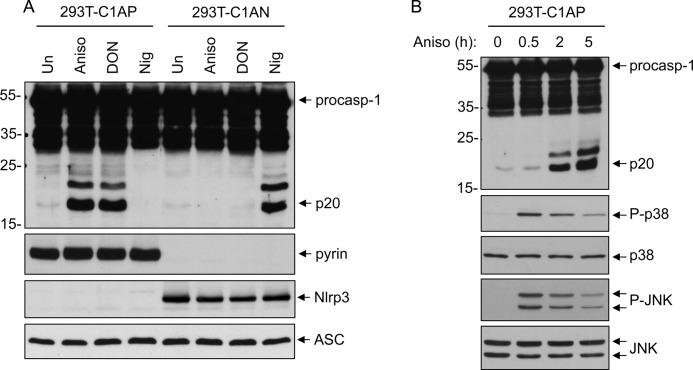
Molecular evidence suggests that human pyrin forms an inflammasome complex with ASC to activate caspase-1 (5, 10). However, the signaling pathway that engages and activates the pyrin inflammasome is currently unknown. Considering that the p38 MAPK stress-signaling pathway is involved in inflammation and possibly in the activation of caspase-1 in response to stress (13, 19), we investigated if activation of the p38 MAPK pathway might activate the pyrin inflammasome. To this end, we used a 293T cell line stably reconstituted with the human pyrin inflammasome components procaspase-1, ASC, and pyrin (293T-C1AP cells) (5). As a control, we used a 293T cell line stably reconstituted with the human NLRP3 inflammasome components procaspase-1, ASC, and NLRP3, (293T-C1AN cells) (5, 14, 16). The two cell lines were treated either with anisomycin, a ribosome inhibitor that potently activates p38 MAPK and JNK by a “ribotoxic stress response” mechanism (20), or with nigericin, a pore-forming toxin that activates the NLRP3 inflammasome. As shown in Fig. 1A, anisomycin treatment induced robust caspase-1 activation only in the pyrin-expressing 293T-C1AP cells but not in the NLRP3-expressing 293T-C1AN cells as evidenced by the processing of procaspase-1 to the p20 subunit. Similar results were obtained with the mycotoxin DON (Fig. 1A, third lane), a potent ribosome inhibitor and ribotoxic stress activator (21). In contrast, the NLRP3 stimulus nigericin was able to activate caspase-1 only in 293T-C1AN cells, but not in 293T-C1AP cells. Consistent with its well documented effects (22), anisomycin induced the activation of both p38 MAPK and JNK in 293T-C1AP cells as determined by the phosphorylation of each kinase (Fig. 1B). These results suggest that ribotoxic stress can specifically activate the pyrin inflammasome, leading to robust caspase-1 activation.
FIGURE 1.
Specific activation of the pyrin inflammasome by ribotoxic stress inducers. A, immunoblots of caspase-1, pyrin, NLRP3, and ASC in lysates of 293T-C1AP or 293T-C1AN cells stimulated with anisomycin (Aniso), DON, or nigericin (Nig) for 5 h as indicated. Un, untreated. B, immunoblots of caspase-1, phospho-p38 (P-p38), p38, phospho-JNK (P-JNK), and JNK in lysates of 293T-C1AP treated with anisomycin for the indicated times. procasp-1, procaspase-1.
Ribotoxic Stress-induced Caspase-1 Activation Is Dependent on p38 MAPK
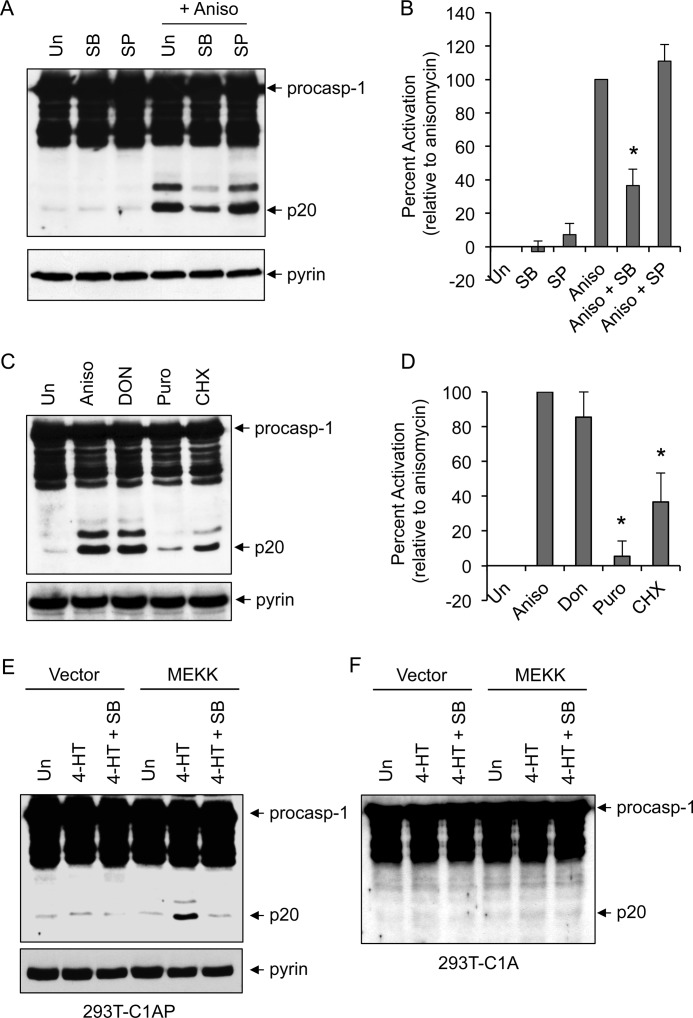
Because anisomycin induced the activation of both p38 MAPK and JNK in 293T-C1AP cells (Fig. 1B), we next examined the effect of selective p38 MAPK inhibition on anisomycin-induced caspase-1 activation. Pretreatment with SB203580, which is known to selectively inhibit p38α and p38β MAPKs (13), significantly reduced caspase-1 activation by anisomycin in 293T-C1AP cells, whereas the selective JNK inhibitor SP600125 showed little effect (Fig. 2, A and B). These results indicate that p38 MAPK activity is required for the activation of caspase-1 by anisomycin in 293T-C1AP cells. Consistent with these results, ribosome inhibitors that weakly activate p38 (cycloheximide) or do not activate p38 (puromycin) (23) induced significantly less or very little caspase-1 activation, respectively (Fig. 2, C and D).
FIGURE 2.
p38 MAPK-dependent activation of caspase-1. A and C, immunoblots of caspase-1 in lysates of 293T-C1AP cells stimulated with anisomycin (Aniso) for 5 h in the absence (untreated (Un)) or presence of SB203580 (SB) or SP600125 (SP) (A) or with the indicated drugs for 5 h (C). procasp-1, procaspase-1; Puro, puromycin; CHX, cycloheximide. B and D, quantification of caspase-1 activation in multiple independent experiments with 293T-C1AP cells treated as described for A and C as determined by densitometry of the caspase-1 p20 band produced by the different treatments. Percent activation represents the percentages of the densitometric values of the caspase-1 p20 band produced by the different treatments relative to the densitometric value of the caspase-1 p20 produced by anisomycin treatment alone (n = 3). *, p < 0.001 (Student's t test). E and F, immunoblots of caspase-1 in lysates of 293T-C1AP (E) or 293T-C1A (F) cells transfected with empty vector (first to third lanes) or the ΔMEKK3:ER* plasmid (fourth to sixth lanes) for 18 h and then treated with 4-HT (1 μm) for an additional 6 h in the presence or absence of SB203580 as indicated. The lower panels in A, C, and E show pyrin immunoblots.
To provide further evidence that p38 MAPK is involved in activation of the pyrin inflammasome, we transfected the 293T-C1AP cells with a construct encoding the conditional protein kinase ΔMEKK3:ER (24). The ΔMEKK3:ER* protein consists of the kinase domain of MEKK3 fused in-frame to a modified form of the hormone-binding domain of the estrogen receptor. The ΔMEKK3:ER* protein remains inactive until cells are treated with 4-HT, allowing activation of the MAPK and SAPK kinases without cellular stress or damage (24). Similar to the anisomycin effect, treatment of the ΔMEKK3:ER*-expressing 293T-C1AP cells with 4-HT resulted in the activation of caspase-1 (Fig. 2E). Furthermore, selective inhibition of p38 MAPK by SB203580 reduced MEKK3-mediated caspase-1 activation in these cells. No caspase-1 activation was observed after 4-HT stimulation of ΔMEKK3:ER*-expressing 293T-C1A cells (Fig. 2F), which lack pyrin, indicating that pyrin is required for the activation of caspase-1 in response to activation of the ΔMEKK3:ER* protein kinase. Taken together, these observations indicate that p38 MAPK is a critical signaling molecule in the pyrin inflammasome activation pathway.
Knockdown of Pyrin Reduces Caspase-1 Activation by Anisomycin
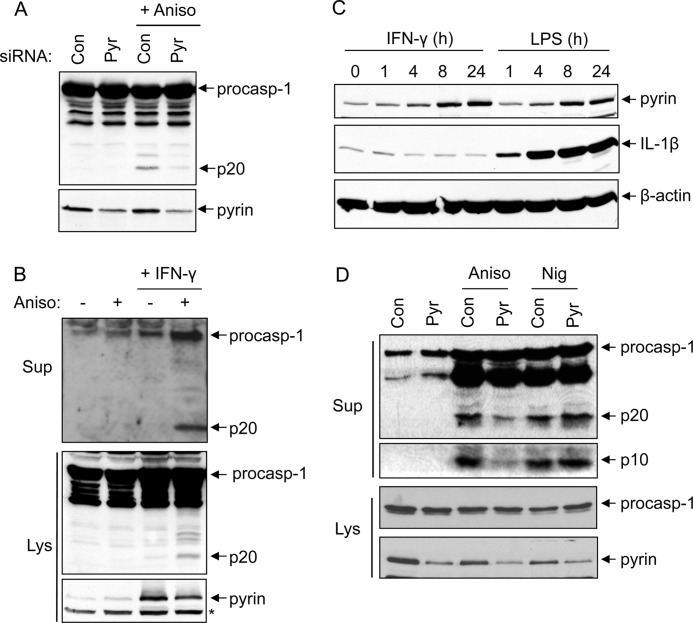
To further validate the critical role of pyrin in caspase-1 activation by activated p38 MAPK, we knocked down pyrin expression in 293T-C1AP cells by siRNA (Fig. 3A, lower panel). As expected, caspase-1 activation by anisomycin was notably reduced in cells transfected with pyrin-specific siRNA, but not in cells transfected with scrambled siRNA (Fig. 3A, upper panel), indicating that pyrin is indeed required for caspase-1 activation by anisomycin in these cells.
FIGURE 3.
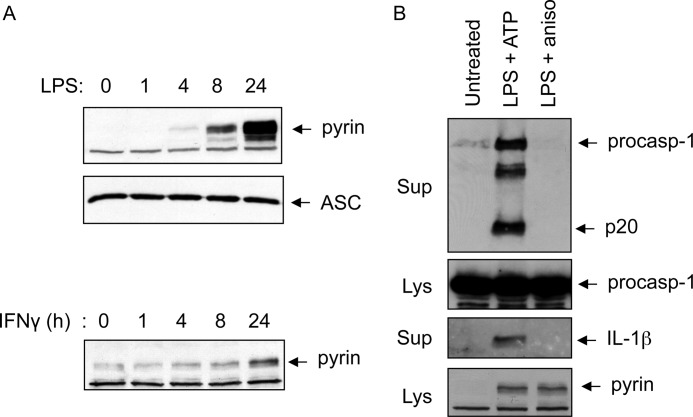
Knockdown of pyrin reduces caspase-1 activation in response to anisomycin. A, immunoblots of caspase-1 and pyrin in cell lysates of 293T-C1AP cells transfected with non-silencing siRNA (control (Con)) or siRNA specific to pyrin (Pyr) for 48 h and then treated with anisomycin (Aniso) for 5 h as indicated. B, immunoblots of caspase-1 in culture supernatants (Sup; upper panel) or cell lysates (Lys; middle panel) of PMA-differentiated THP-1 cells treated with (+) or without (−) anisomycin (5 h) following priming with or without IFNγ for 18 h as indicated. The lower panel shows an immunoblot of pyrin in the same samples. The asterisk indicates a nonspecific band. C, immunoblots of pyrin, IL-1β, and β-actin in PMA-differentiated THP-1 cells stimulated with IFNγ or LPS for the indicated times. D, immunoblots of caspase-1 in culture supernatants as determined with anti-caspase-1 p20 antibody (first panel) or anti-caspase-1 p10 antibody (second panel) of PMA-differentiated THP-1 cells transfected with non-silencing or pyrin-specific siRNA for 48 h and then primed with IFNγ for an additional 18 h, followed by treatment with anisomycin or nigericin (Nig) for 5 h as indicated. The third and fourth panels show immunoblots of procaspase-1 (procasp-1) and pyrin in the lysates of the same samples. The results are representative of at least three independent experiments.
To provide more evidence supporting the role of pyrin in caspase-1 activation by activated p38 MAPK, we treated phorbol 12-myristate 13-acetate (PMA)-differentiated THP-1 cells with anisomycin. Unlike 293T-C1AP cells, anisomycin treatment did not trigger caspase-1 activation in PMA-differentiated THP-1 cells (Fig. 3B, second lane). We reasoned that this unresponsiveness to anisomycin might be due to the low expression level of pyrin in the PMA-differentiated THP-1 cells (Fig. 3B, lower panel, first and second lanes). To up-regulate pyrin expression, we primed THP-1 cells with IFNγ, which was shown previously to induce pyrin expression (5). Treatment of THP-1 cells for 8–24 h with IFNγ or LPS induced notable pyrin up-regulation (Fig. 3C). Stimulation of the IFNγ-primed THP-1 cells with anisomycin caused caspase-1 activation as observed in 293T-C1AP cells (Fig. 3B, fourth lane). Knocking down pyrin expression by siRNA in the IFNγ-primed THP-1 cells attenuated caspase-1 activation in response to anisomycin, but not to nigericin (Fig. 3D). Collectively, these results demonstrate that the pyrin inflammasome is important for the activation of caspase-1 in response to ribotoxic stress in both the pyrin inflammasome-reconstituted 293T-C1AP cells and the human THP-1 macrophage cell line.
Anisomycin Facilitates ASC Oligomerization
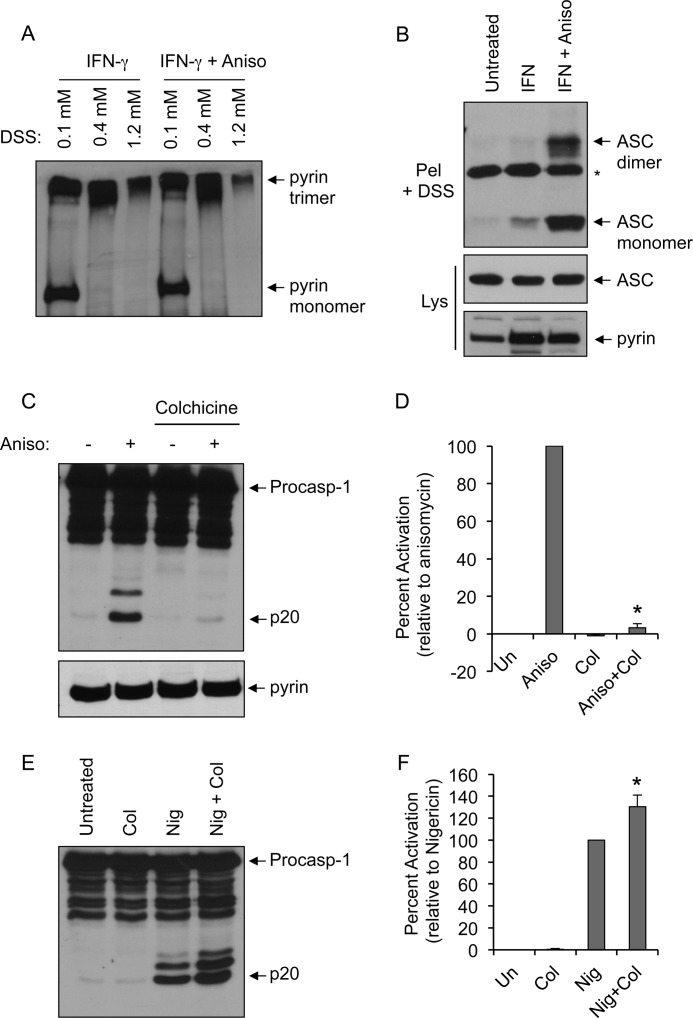
To understand how p38 MAPK activation by anisomycin triggers the pyrin inflammasome assembly, we first determined whether anisomycin could affect the oligomerization status of pyrin. Consistent with our previously published results (5), pyrin was present as a homotrimer in IFNγ-primed THP-1 cells (Fig. 4A). However, anisomycin treatment did not change the oligomerization status of pyrin, suggesting that anisomycin-induced p38 MAPK signaling acts at the level of ASC recruitment and oligomerization (17). Indeed, by using the ASC oligomerization assay, which measures the amount of oligomeric ASC in the Nonidet P-40-insoluble low-speed pellets of stimulated cells (17, 18), we found a high amount of oligomeric ASC in the pellet fraction of IFNγ/anisomycin-treated THP-1 cells (Fig. 4B, third lane), but not in that of untreated or IFNγ-treated cells (first and second lanes, respectively). These results suggest that anisomycin-induced p38 MAPK signaling promotes the recruitment of ASC to the pyrin homotrimer, which facilitates ASC oligomerization.
FIGURE 4.
Role of ASC and microtubules in anisomycin-induced caspase-1 activation. A, PMA-differentiated THP-1 cells were primed with IFNγ for 16 h and then treated with or without anisomycin (Aniso) for 3 h as indicated. Cell lysates were cross-linked with increasing concentrations of DSS and immunoprecipitated with anti-pyrin antibody, followed by immunoblotting with anti-pyrin antibody. B, immunoblots of oligomeric ASC pyroptosomes in DSS-cross-linked cell pellets (Pel + DSS) of PMA-differentiated THP-1 cells treated with or without anisomycin after IFNγ priming as indicated (upper panel). The middle and lower panels show ASC and pyrin in the cell lysates (Lys) of the same samples. The asterisk indicates a nonspecific band. C and E, immunoblots of caspase-1 in 293T-C1AP cells treated with (+) or without (−) anisomycin following pretreatment with colchicine (5 μm) as indicated (C) or in 293T-C1AN cells treated with or without nigericin (Nig) following pretreatment with colchicine (Col; 5 μm) as indicated (E). procasp-1, procaspase-1. D and F, quantification of caspase-1 activation in multiple independent experiments with 293T-C1AP (D) or 293T-C1AN (F) cells treated as described for C and E as determined by densitometry of the caspase-1 p20 band produced by the different treatments. Percent activation represents the percentages of the densitometric values of the caspase-1 p20 band produced by the different treatments relative to the densitometric value of the caspase-1 p20 produced by anisomycin (D) or nigericin (F) treatment alone (n = 3). *, p < 0.001 (Student's t test). Un, untreated.
Destabilization of Microtubules Inhibits Anisomycin-induced Caspase-1 Activation
Colchicine, a microtubule-destabilizing drug, has been clinically used for the treatment of FMF (25). Although the precise molecular mechanism by which colchicine reduces inflammation in FMF patients remains largely unknown, we have previously demonstrated that both colchicine and nocodazole blocked pyrin inflammasome-mediated caspase-1 activation by autoinflammatory PAPA-associated PSTPIP1 mutants (5). To assess the importance of microtubule polymerization in anisomycin-induced caspase-1 activation, we stimulated 293T-C1AP cells with anisomycin in the presence or absence of colchicine. As shown in Fig. 4 (C and D), colchicine almost completely abrogated anisomycin-induced caspase-1 activation. In contrast, colchicine treatment significantly increased nigericin-induced caspase-1 activation in 293T-C1AN cells (Fig. 4, E and F). These results indicate that microtubule stability is important for pyrin inflammasome activation, but not for activation of the NLRP3 inflammasome.
DISCUSSION
FMF is an inherited autoinflammatory disease caused by missense mutations in human pyrin (8, 9). The exact role of pyrin in inflammation and the signaling pathway(s) that regulate its activity are not fully understood. Earlier studies proposed that pyrin functions as an anti-inflammatory molecule by inhibiting the activity or activation of caspase-1 (26). However, subsequent studies showed that human pyrin can assemble an inflammasome complex with ASC to activate caspase-1 when coexpressed in HEK-293T cells or when engaged by PAPA-associated PSTPIP1 mutants in HEK-293T and human THP-1 macrophages (5, 10). This indicates that pyrin functions as a mediator rather than as an inhibitor of caspase-1 activation. This role has been supported by additional studies in macrophages (27) and underscored by knock-in studies in mice expressing humanized pyrin proteins carrying FMF-associated mutations (11). Using a HEK-293T cell line stably reconstituted with components of the human pyrin inflammasome and THP-1 macrophages with pyrin knockdown, our work further supports the role of pyrin as a mediator of caspase-1 activation by demonstrating that ribotoxic stress can activate the human pyrin inflammasome. Several ribosome inhibitors that damage the 28 S ribosomal RNA and/or interfere with its functioning can induce a response called the ribotoxic stress response, which stimulates MAPK signaling (20, 23). Among these, anisomycin and the trichothecene mycotoxin DON are two of the most potent ribotoxic stress inducers and activators of the p38 MAPK pathway (23). Our results demonstrate that these agents, through their ability to activate p38 MAPK, can activate caspase-1 by a pyrin-dependent mechanism, indicating that p38 MAPK is a critical mediator of pyrin inflammasome activation. The key role of p38 in activation of the pyrin inflammasome was supported by experiments showing that expression of the conditional protein kinase ΔMEKK3:ER* (24), which allows the activation of p38 MAPK with 4-HT and without cellular stress or damage, can induce the activation of caspase-1 in 293T-C1AP cells. Furthermore, SB203580, a small molecule selective inhibitor of p38 MAPK, can effectively suppress caspase-1 activation by anisomycin and ΔMEKK3:ER*. The regulation of pyrin activity by p38 MAPK suggests that p38 MAPK inhibitors represent potential therapeutics, alone or in combination with colchicine, for the treatment of FMF.
The microtubule-disrupting drug colchicine is very effective in the treatment of FMF, and the colchicine responsiveness is an important diagnostic tool for FMF (25, 28). Our results suggest that this therapeutic benefit of colchicine is likely due to its ability to inhibit the activity of the pyrin inflammasome. Treatment of 293T-C1AP cells with colchicine inhibited pyrin-mediated caspase-1 activation in response to anisomycin. In contrast, colchicine treatment had no inhibitory effect on NLRP3-mediated caspase-1 activation in response to nigericin in 293T-C1AN cells. These results indicate that the colchicine responsiveness is highly specific to the pyrin inflammasome pathway and could explain the therapeutic benefit of colchicine in the treatment of FMF. It is not yet clear how colchicine can inhibit the activity of the pyrin inflammasome. However, because pyrin is associated with microtubules (29), we can speculate that the disruption of microtubules by colchicine might lead to dissociation of pyrin from microtubules. This could prevent pyrin from assembling with ASC and procaspase-1 to form a functional inflammasome. Although colchicine is also effective in the treatment of gouty arthritis, which is mediated by the NLRP3 inflammasome, colchicine does not inhibit the activity of the NLRP3 inflammasome, but it appears to interfere with the uptake of urate crystals by macrophages (30).
One of the reasons for the controversy regarding pyrin function is the lack of mouse genetic evidence to support a role for mouse pyrin in caspase-1 activation. Mouse pyrin lacks the C-terminal B30.2/SPRY domain, which harbors most of the FMF mutations, and therefore may not have a similar function and/or regulation as the human protein. Supporting this, mice with a targeted disruption of the pyrin gene do not show any obvious defects in inflammation (26), whereas mice with knock-in of humanized pyrin transgenes carrying FMF-associated mutations in the B30.2/SPRY domain show constitutive caspase-1 activation in macrophages and severe inflammation similar to FMF (11). Interestingly, knock-in of a humanized pyrin transgene without FMF-associated mutations leads to embryonic lethality in mice (11). These observations suggest that the B30.2/SPRY domain regulates the inflammasome activity of human pyrin. The absence of this domain in mouse pyrin might lead to an altered or even loss of pyrin inflammasome activity. Consistent with this, our results shows that the expression of mouse pyrin could be up-regulated by LPS or IFNγ treatment in peritoneal macrophages (Fig. 5A). However, treatment of these macrophages with anisomycin did not induce caspase-1 activation or result in IL-1β release, whereas treatment with the NLRP3 inflammasome activator ATP did both (Fig. 5B). Unlike human pyrin, mouse pyrin did not interact with PSTPIP1,4 which could explain the recent observations that knock-in of the disease-associated PSTPIP1 A230T mutant in mice does not lead to development of an autoinflammatory disease (31). Together, these observations further support the idea that mouse pyrin is not activated by the same signaling pathway(s) that activate human pyrin.
FIGURE 5.
Anisomycin does not activate caspase-1 in mouse peritoneal macrophages. A, immunoblots of pyrin and ASC in mouse peritoneal macrophages treated with LPS (upper and middle panels) or IFNγ (lower panel) for the indicated times. B, immunoblots of caspase-1 or IL-1β in culture supernatants (Sup) of mouse peritoneal macrophages treated with LPS plus ATP or LPS plus anisomycin (aniso) as indicated. Immunoblots of caspase-1 and pyrin in the cell lysates (Lys) are indicated. procasp-1, procaspase-1.
In conclusion, we have identified the ribotoxic stress pathway as an important mediator of pyrin inflammasome activation. We propose that the activation of p38 MAPK signaling by ribotoxic stress stimulates the recruitment of ASC to the pyrin homotrimer, which induces its oligomerization. It remains to be determined whether p38 MAPK directly phosphorylates the pyrin homotrimer to induce the assembly of the pyrin inflammasome.
This work was supported, in whole or in part, by National Institutes of Health Grants AG14357 and AR055398 (to E. S. A).
L. Solorzano, P. Datta, T. Fernandes-Alnemri, and E. S. Alnemri, unpublished data.
- FMF
- familial Mediterranean fever
- PAPA
- pyogenic arthritis, pyoderma gangrenosum, and acne
- DON
- deoxynivalenol
- 4-HT
- 4-hydroxytamoxifen
- DSS
- disuccinimidyl suberate
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1. Schroder K., Tschopp J. (2010) The inflammasomes. Cell 140, 821–832 [DOI] [PubMed] [Google Scholar]
- 2. Franchi L., Muñoz-Planillo R., Núñez G. (2012) Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 13, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lamkanfi M. (2011) Emerging inflammasome effector mechanisms. Nat. Rev. Immunol. 11, 213–220 [DOI] [PubMed] [Google Scholar]
- 4. Park H., Bourla A. B., Kastner D. L., Colbert R. A., Siegel R. M. (2012) Lighting the fires within: the cell biology of autoinflammatory diseases. Nat. Rev. Immunol. 12, 570–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu J. W., Fernandes-Alnemri T., Datta P., Wu J., Juliana C., Solorzano L., McCormick M., Zhang Z., Alnemri E. S. (2007) Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol. Cell 28, 214–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strowig T., Henao-Mejia J., Elinav E., Flavell R. (2012) Inflammasomes in health and disease. Nature 481, 278–286 [DOI] [PubMed] [Google Scholar]
- 7. Wen H., Ting J. P., O'Neill L. A. (2012) A role for the NLRP3 inflammasome in metabolic diseases–did Warburg miss inflammation? Nat. Immunol. 13, 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. International FMF Consortium (1997) Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell 90, 797–807 [DOI] [PubMed] [Google Scholar]
- 9. French FMF Consortium (1997) A candidate gene for familial Mediterranean fever. Nat, Genet. 17, 25–31 [DOI] [PubMed] [Google Scholar]
- 10. Yu J. W., Wu J., Zhang Z., Datta P., Ibrahimi I., Taniguchi S., Sagara J., Fernandes-Alnemri T., Alnemri E. S. (2006) Cryopyrin and pyrin activate caspase-1, but not NF-κB, via ASC oligomerization. Cell Death Differ. 13, 236–249 [DOI] [PubMed] [Google Scholar]
- 11. Chae J. J., Cho Y. H., Lee G. S., Cheng J., Liu P. P., Feigenbaum L., Katz S. I., Kastner D. L. (2011) Gain-of-function pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice. Immunity 34, 755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coulthard L. R., White D. E., Jones D. L., McDermott M. F., Burchill S. A. (2009) p38MAPK: stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 15, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ashwell J. D. (2006) The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 6, 532–540 [DOI] [PubMed] [Google Scholar]
- 14. Fernandes-Alnemri T., Yu J. W., Datta P., Wu J., Alnemri E. S. (2009) AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernandes-Alnemri T., Yu J. W., Juliana C., Solorzano L., Kang S., Wu J., Datta P., McCormick M., Huang L., McDermott E., Eisenlohr L., Landel C. P., Alnemri E. S. (2010) The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Juliana C., Fernandes-Alnemri T., Kang S., Farias A., Qin F., Alnemri E. S. (2012) Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandes-Alnemri T., Wu J., Yu J. W., Datta P., Miller B., Jankowski W., Rosenberg S., Zhang J., Alnemri E. S. (2007) The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fernandes-Alnemri T., Alnemri E. S. (2008) Assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol. 442, 251–270 [DOI] [PubMed] [Google Scholar]
- 19. Johansen C., Moeller K., Kragballe K., Iversen L. (2007) The activity of caspase-1 is increased in lesional psoriatic epidermis. J. Invest. Dermatol. 127, 2857–2864 [DOI] [PubMed] [Google Scholar]
- 20. Laskin J. D., Heck D. E., Laskin D. L. (2002) The ribotoxic stress response as a potential mechanism for MAP kinase activation in xenobiotic toxicity. Toxicol. Sci. 69, 289–291 [DOI] [PubMed] [Google Scholar]
- 21. Shifrin V. I., Anderson P. (1999) Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J. Biol. Chem. 274, 13985–13992 [DOI] [PubMed] [Google Scholar]
- 22. Iordanov M. S., Pribnow D., Magun J. L., Dinh T. H., Pearson J. A., Chen S. L., Magun B. E. (1997) Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the α-sarcin/ricin loop in the 28S rRNA. Mol. Cell. Biol. 17, 3373–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kataoka T. (2012) Translation inhibitors and their unique biological properties. Eur. J. Pharmacol. 676, 1–5 [DOI] [PubMed] [Google Scholar]
- 24. Garner A. P., Weston C. R., Todd D. E., Balmanno K., Cook S. J. (2002) ΔMEKK3:ER* activation induces a p38α/β2-dependent cell cycle arrest at the G2 checkpoint. Oncogene 21, 8089–8104 [DOI] [PubMed] [Google Scholar]
- 25. Stojanov S., Kastner D. L. (2005) Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr. Opin. Rheumatol. 17, 586–599 [DOI] [PubMed] [Google Scholar]
- 26. Chae J. J., Komarow H. D., Cheng J., Wood G., Raben N., Liu P. P., Kastner D. L. (2003) Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol. Cell 11, 591–604 [DOI] [PubMed] [Google Scholar]
- 27. Seshadri S., Duncan M. D., Hart J. M., Gavrilin M. A., Wewers M. D. (2007) Pyrin levels in human monocytes and monocyte-derived macrophages regulate IL-1β processing and release. J. Immunol. 179, 1274–1281 [DOI] [PubMed] [Google Scholar]
- 28. Ozturk M. A., Kanbay M., Kasapoglu B., Onat A. M., Guz G., Furst D. E., Ben-Chetrit E. (2011) Therapeutic approach to familial Mediterranean fever: a review update. Clin. Exp. Rheumatol. 29, S77–S86 [PubMed] [Google Scholar]
- 29. Mansfield E., Chae J. J., Komarow H. D., Brotz T. M., Frucht D. M., Aksentijevich I., Kastner D. L. (2001) The familial Mediterranean fever protein, pyrin, associates with microtubules and colocalizes with actin filaments. Blood 98, 851–859 [DOI] [PubMed] [Google Scholar]
- 30. Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 [DOI] [PubMed] [Google Scholar]
- 31. Wang D., Höing S., Patterson H. C., Ahmad U. M., Rathinam V. A., Rajewsky K., Fitzgerald K. A., Golenbock D. T. (2013) Inflammation in mice ectopically expressing human pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA) syndrome-associated PSTPIP1 A230T mutant proteins. J. Biol. Chem. 288, 4594–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]