Abstract
Sleep apnea is an entity characterized by repetitive upper airway obstruction resulting in nocturnal hypoxia and sleep fragmentation. It is estimated that 2%–4% of the middle-aged population has sleep apnea with a predilection in men relative to women. Risk factors of sleep apnea include obesity, gender, age, menopause, familial factors, craniofacial abnormalities, and alcohol. Sleep apnea has been increasingly recognized as a major health burden associated with hypertension and increased risk of cardiovascular disease and death. Increased airway collapsibility and derangement in ventilatory control responses are the major pathological features of this disorder. Polysomnography (PSG) is the gold-standard method for diagnosis of sleep apnea and assessment of sleep apnea severity; however, portable sleep monitoring has a diagnostic role in the setting of high pretest probability sleep apnea in the absence of significant comorbidity. Positive pressure therapy is the mainstay therapy of sleep apnea. Other treatment modalities, such as upper airway surgery or oral appliances, may be used for the treatment of sleep apnea in select cases. In this review, we focus on describing the sleep apnea definition, risk factor profile, underlying pathophysiologic mechanisms, associated adverse consequences, diagnostic modalities, and treatment strategies.
Keywords: positive airway pressure, obstructive sleep apnea, cardiovascular disease
Introduction
Obstructive sleep apnea (OSA) is a condition characterized by repetitive episodes of complete or partial collapse of the upper airway during sleep resulting in complete cessation (apnea) or reduction (hypopnea) of airflow leading to arousal and hypoxia.1 Apnea is defined as complete cessation of oronasal airflow for at least 10 seconds. Alternatively, the definition of hypopnea requires (1) a drop of ≥ 30% of oronasal airflow from baseline associated with ≥ 4% decrease in oxyhemoglobin saturation, (2) a drop of ≥ 50% of oronasal airflow from baseline and ≥ 3% decrease of oxyhemoglobin saturation, (3) a reduction in airflow as above along with an associated electroencephalographic arousal. In sleep study monitoring, the frequency of apneas and hypopneas per hour of sleep (apnea-hypopnea index [AHI]) is the key measure to define and stratify the severity of OSA, although inherent limitations to this metric include not taking into consideration degree of accompanying hypoxia, length of respiratory events, etc. AHI levels of 5, 15, and 30 have been used as cutpoints to define mild moderate and severe OSA, respectively.1 Apnea can be distinguished as obstructive vs central based upon presence or absence of thoracoabdominal effort.2
Symptoms of OSA may include daytime sleepiness, impaired concentration and mood morning headaches, snoring, and witnessed breathing pauses during sleep observed by the bed partner. There are varying sensitivities and specificities for these specific clinical symptoms, and clinical prediction rules incorporating these symptoms have been shown to be poor predictors in identifying OSA and assessing OSA severity3
Many studies have revealed an association between OSA severity and other common causes of increased mortality such as hypertension,4 stroke,5 coronary artery disease,6 and occupational,7 as well as automobile accidents.8 For this reason, OSA has been increasingly recognized as a major public health issue imposing great economic burden, thereby mandating early recognition and treatment.
Prevalence of OSA
Although OSA was described in the middle of the last century, data describing the prevalence of this disease were not available until 1993 when the results from the Wisconsin Sleep Cohort Study were reported. This study involved 602 participants who were 30–60 years of age and evaluated using overnight polysomnography. The prevalence of OSA (defined as AHI ≥ 5) in this study was 24% in men and 9% in women, and the prevalence of OSA syndrome (OSAS), ie, OSA with associated symptoms (defined as AHI ≥ 5 and daytime sleepiness) was 4% in men and 2% in women.9 The prevalence of OSA was estimated in Southern Pennsylvania households, 1,741 participants between the ages 20 and 100 years were evaluated using overnight PSG. The prevalence of OSA in this cross-sectional study was similar to the Wisconsin Sleep Cohort Study: prevalence of OSA (AHI ≥ 10) was 17% in men and 5% in women and the prevalence of OSAS with concomitant symptoms was 3.3% in men and 1.2% in women.10,11
OSA prevalence studies have been performed in various countries involving individuals of various ethnicities. For example, in an Australian study, the prevalence of OSA was investigated in 485 male participants between the ages 40 and 65 years using a portable, sleep monitoring system. The prevalence of OSA and symptomatic OSAS were 25.9% and 3.1%, respectively12 In Europe, 560 patients were evaluated between the ages 30 and 70 years using overnight PSG. Twenty-six percent of men and 28% of women had OSA (AHI ≥ 5), and 3.4% of men and 3% of women had OSAS (AHI ≥ 5 and daytime sleepiness).13 One of the first Asian epidemiological studies estimated the prevalence of OSA in 259 patients in Hong Kong. The prevalence of OSA (AHI = 5) was 8.8% in men and 3.7% in women, and the prevalence of OSAS (AHI = 5 and daytime sleepiness) was 4.1% in men and 2.1% in women.14,15
It is interesting to note the comparable OSA prevalence estimates in this study involving Asians with a lower body mass index (BMI) compared with the participants of the Wisconsin Study, who have a notably higher BMI suggesting a risk factor profile contributing to OSA in Asians involving factors other than overweight/obesity such as genetic or craniofacial anatomical factors. Epidemiological studies from other Asian countries including Korea and India, have shown similar findings.16–18
In summary, the prevalence of OSA (defined as AHI ≥ 5) were 17%–27% in men and 3%–28% in women. This disparity in the prevalence among these studies, particularly the Spanish study in which 26% of men and 28% of women have OSA,13 may be attributed to methodological differences including varying population age, health status of participants, ethnicity, methods of participant enrollment, use of different definitions of hypopnea, use of different techniques in measuring airflow, and using portable home monitoring such as in the Australian study12
Clinical presentation of OSA
Snoring
Snoring is caused by the vibration of the structures in the oral cavity, and oropharynx is considered one of the most common symptoms for which patients or partners seek medical attention. Habitual snoring is common in the general population; in one report, 40% of women and 60% of men are habitual snorers.9 When considering snoring as a symptom of OSA, approximately 70%–80% of patients who snore have OSA19–21 and 95% of patients who have OSA snore.22
Excessive daytime sleepiness
Daytime sleepiness is the most common daytime symptom in patients with OSA. Because there are many causes of sleepiness, such as insufficient sleep, mood disorders, medication side effects, etc; sleepiness is poorly correlated with the severity of OSA,3 and daytime somnolence is not a specific marker for OSA. Nevertheless, daytime sleepiness is a very useful screening tool to evaluate response to therapy in patients with OSA.23 Various scales used to subjectively assess the degree of sleepiness include Profile of Mood States,24 Stanford Sleepiness Scale,25 and the Epworth Sleepiness Scale.26 Of those scales, the Epworth Sleepiness Scale is widely used because it rates the chance of dozing in different everyday situations within the last month rather than reflecting a momentary mood state. Objective ways of measuring sleepiness include multiple sleep latency testing and the maintenance of wakefulness test.
Other symptoms
Other symptoms of OSA include, but are not restricted to, witnessed apneas, nocturnal choking, unrefreshed sleep, morning headaches, sleep maintenance insomnia, and fatigue. Although clinical symptoms have a poor correlation with the severity of OSA, several prediction models have been developed to provide an OSA screening tool. Most of those models depend on clinical symptoms, anthropometric measurements, and an upper airway anatomy evaluation. Despite the high sensitivity, these prediction rules have minimal clinical utility given the low specificity, and also are of limited use in the pediatric population.27–34
Risk factors of OSA
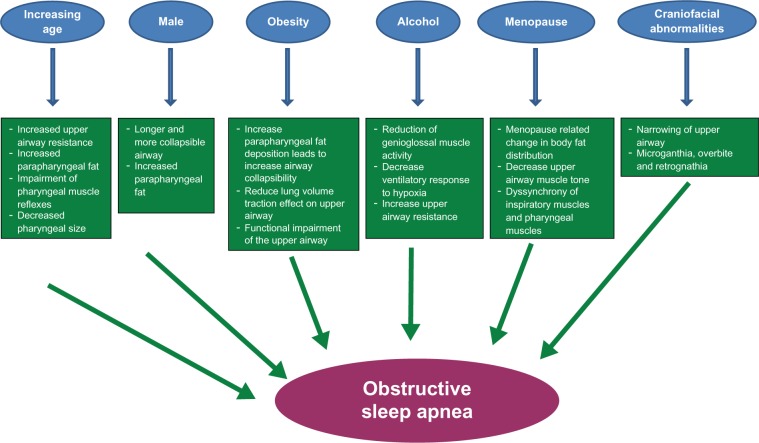
Some of the major risk factors of OSA and their respective pathophysiologic mechanisms are summarized in Figure 1.
Figure 1.
Schematic representation of different risk factors and proposed mechanisms by which they result in obstructive sleep apnea.
Aging
The Sleep Heart Health Study shows a simple, positive linear correlation between age and OSA until the age of approximately 65 years at which point there is a plateau in the prevalence.35 Studies published regarding the prevalence of OSA in the elderly report ranges of 5.6%–70% in referral-based samples and in some population-based studies.36–40 In a review of OSA and the elderly, it has been found that the prevalence of OSA in men is 28%–62% and in women is 19.5%–60%.40 In the elderly, it is thought that OSA may be an entirely different entity altogether and associated with a different constellation of symptoms compared with middle-aged adults. Specifically, the consequences of OSA in the elderly may be more related to behavioral morbidity than cardiovascular outcomes.41 For instance, evidence has shown stronger relationships with cognitive impairments, such as dementia,42 and mood changes, such as depression, and weaker relationships with reporting of snoring in older adults,43 as well as with BMI.44 Also, our analysis of the MrOS Sleep Study, a cohort of elderly men, has identified similar OSA correlates as described in middle-aged adults; however, the strength of the association of risk factors, such as obesity, and symptoms, such as snoring, were comparatively mitigated.45
In an effort to explain the relationship between OSA and age, different hypotheses have been proposed and tested. Function and structure of the upper airway have been a focus of interest in various reports investigating increasing age and upper airway pathophysiology. For example, aging is associated with increased upper airway resistance, increased parapharyngeal fat, decreased pharyngeal size, and impairment of pharyngeal muscle reflexes that are important to maintain upper airway patency, the latter of which is independent of BMI and gender.46–51
Gender
OSA is more common in men, with a male-to-female ratio of 2–4:1 in community-based studies9,10,13,15,16 and approximately 10:1 in sleep clinic referral samples.52 The disparity between the gender-based community and the clinic prevalence of OSA may be explained by fact that women often do not have the classic symptomatology of OSA. Women may be more likely to report morning headaches, difficulty initiating sleep, and fatigue related to OSA compared with reports of restless sleep and witnessed apneas.53 Furthermore, women with OSA are more likely to be treated for depression, to have insomnia, and to have hypothyroidism than men with the same degree of OSA.54
Another factor that may explain this phenomenon is that women are less likely to be referred to sleep centers because of the notion that OSA is a disease of men. Indeed, OSA was first described in men, and earlier studies exclusively enrolled men. Although it is likely that women with OSA are underdiagnosed, there are other gender-based factors at hand. The sex-based disparity of OSA occurrence is likely attributable to a variety of factors, including hormonal influences, and sex-based phenotypes, including physical features such as craniofacial morphology and fat deposition. The structure and the function of the upper airway show gender-based differences, including a shorter and smaller upper airway observed in women compared with men.55,56 Although this upper airway anatomical difference may appear to predispose women rather than men to upper airway collapse, men have been found to have longer airways compared with women, thereby resulting in increased vulnerability to collapse. Data also indicate that women have a more stable upper airway,57 as supported by pharyngeal critical closing pressures that are higher compared with BMI-matched sleep apneic men.58
Another possible explanation of gender-based differences in OSA risk is the variation in the distribution of adipose tissue between men and women. Men tend to have more fat in the upper body including the neck (android), thereby predisposing to upper airway collapse compared with women who tend to have lower body fat (gynoid).59 Consistent with this observation is that the measurement of neck circumference and waist circumference is better correlated with the severity of OSA than BMI.35 An imaging study, magnetic resonance imaging, confirmed that men also have more predominant pharyngeal fat and soft tissue compared with women.60
In summary, it appears that there are important differential structural and functional upper airway differences between men and women, which place men at higher risk for OSA. However, women with OSA often have an atypical symptomatic presentation of OSA symptoms that warrant vigilant screening and recognition.
Obesity
OSA is highly prevalent in the obese and overweight population. Several cross-sectional studies revealed a monotonic relationship between OSA and weight, BMI, neck circumference, waist-to-hip ratio, and other measures of body habitus.9–11,14,15,35 Furthermore, fluctuations in weight have been demonstrated to influence severity of OSA. In the Wisconsin Sleep Cohort, a 10% weight gain in subjects with no OSA (AHI < 5) was associated with a 6-fold increase in the odds of developing moderate to severe OSA (AHI ≥ 15) after consideration of relevant confounding factors, including age and gender.61 Also, a 10% weight gain was associated with an approximate 30% worsening in the degree of OSA, and alternatively, a 10% weight loss was associated with an approximate 30% improvement in the degree of OSA. Similar results were shown in the Sleep Heart Health Study, in which an increase in weight by 10 kg was observed to increase the odds of having an AHI (> 15 events per hour) by 5.2-fold in men and 2.5-fold in women over a 5-year period.62
These results indicate that women were less vulnerable to the effect of weight gain-related influence on OSA risk, and this could be explained by gender-dependent differences in fat distribution patterns given men tend to have more truncal obesity, and potentially also due to hormonal factors. The impact of weight on OSA appears to be influenced by age as well. In the Cleveland Family Study, the effect of adiposity measured as BMI on OSA was noted to diminish after the age of 60 years.63 Similar findings have been reported in the Sleep Heart Health Study in which OSA in individuals older than 70 years was weakly related to BMI and other measures of body habitus.35
The effect of weight loss on OSA has been studied in morbidly obese patients with moderate to severe OSA, who underwent bariatric surgery. Dramatic weight loss after surgery was associated with a similar reduction in the degree of OSA and nocturnal hypoxia improvement; however, the majority of these patients still had residual OSA requiring treatment, potentially attributable to persisting excess weight as the average postsurgical BMI was 37.7 kg/m2.64
Genetics
Earlier reports, describing a high prevalence of OSA between family members, suggested that OSA has a familial component apart from the influence of obesity65,66 Several small family-based studies have focused on defining the genetic basis of OSA. In a case series, non-overweight relatives of patients with OSA were found to have more daytime sleepiness, snoring, apneas, and arousals compared with matched controls.67
In the Cleveland Family Study, which is the largest, assembled familial aggregation study investigating OSA, sleep apnea was identified to be more prevalent in relatives of index probands of OSA (21%) compared with neighborhood control subjects (12%).66 In another study, relatives of patients with OSA reported more sleepiness, tiredness, and fatigue.68 Data from twin studies showed that the concordance of snoring, a symptom of OSA, is higher among monozygotic twins than dizygotic.69
The heritability of OSA is partially explained by intermediate, genetically-determined risk factors. Obesity, which is a known risk factor of OSA, is partially under genetic control. A twin study showed that 77% of the variability of the BMI is explained by intrafamilial factors.70 Genome-wide association studies have revealed at least 15 genetic loci linked to obesity-related phenotypes.71 However, there is strong evidence that both OSA and obesity have both shared and unshared genetic determinants as demonstrated in the Cleveland Family study. Different loci linked to apnea have been identified with an intermediate logarithmic odds (LOD) score, a measure of the likelihood of 2 genetic loci being within a measurable distance of each other. After adjustment for BMI, the AHI linkage to one of the loci was markedly attenuated. On the other hand, a locus identified on chromosome 19p demonstrated an LOD of 1.45 after BMI adjustment suggesting that this locus could be of potential interest in complex, multifactorial disease.72
Other risk factors which predispose to OSA that are genetically determined include craniofacial morphologic characteristics and ventilatory control mechanisms. Craniofacial familial features have been identified to be a strong indicator of risk for the development of OSA.68 In addition, craniofacial abnormalities are parts of different genetic disorders in which OSA is highly prevalent, such as Down syndrome and Marfan’s syndrome.73 In animal studies, deficiencies in endothelin 1, retonoic acid receptor aZ, and transforming growth factor X2 have been associated with craniofacial abnormalities. These characteristics were also associated with increased respiratory failure suggesting the possible role of the upper airway and sleep apnea given that phenotypic craniofacial factors are a known risk.
Menopause
Different cross-sectional studies have identified menopause as a risk factor for OSA. A prevalence of OSA in postmenopausal women has been described as 2.7% compared with 0.6% in premenopausal women.10 Consistent with these results, another study estimated the odds of having AHI ≥ 15 in menopausal women by 3.49 compared with 1.07 in premenopausal women after controlling for potential confounders, including age and BMI.77 The effects of hormone replacement therapy in postmenopausal women with OSA in the Sleep Heart Health Study revealed an inverse relationship between OSA severity and hormone replacement therapy was such that in multivariable adjusted models, the odds of having OSA in those taking hormone replacement therapy was ~55% lower than those not taking therapy78
Different mechanisms have been postulated to explain the associative relationship between OSA and the hypoestrogenic state in menopausal women. Menopause-related change in body fat distribution, such as predominant central obesity and increased adiposity around the upper airway, increases the risk for upper airway collapse.59,79 Also, the tone of the muscles such as the geniglossus, a primary pharyngeal dilator, is lower during wakefulness in postmenopausal women compared with premenopausal.80 In addition, estrogen and progestin have a role in regulating the ventilatory drive, which may cause imbalance of forces in favor of increased collapsibility of the upper airway. Finally, low estrogen and progestin levels in menopause may lead to dyssynchrony of the inspiratory muscles and pharyngeal muscles that may result in upper airway obstruction.81
Ethnicity
The current cross-sectional data evaluating the prevalence of OSA in different ethnic groups have shown compatible estimates. Earlier population-based studies that included predominantly Caucasians, however, showed no difference in OSA prevalence between Caucasians and African Americans.35 However, African Americans have been observed to have more severe OSA relative to Caucasians in groups younger than 25 years and older than 65 years.36,82 One study demonstrated that approximately 50% of this OSA risk was attributable to neighborhood disadvantage,83 after adjusting for common OSA risk factors including obesity. The authors postulate that this association between OSA and neighborhood-level conditions may be explained by the environmental and social stressors that are often encountered in low socioeconomic neighborhoods.84 Race-based differences in chemoreceptor and baroreceptor responses also may predispose African Americans to development of OSA.85
Despite similar prevalence rate between different ethnic groups, there were some noteworthy findings; Asian population have lower BMI compared with western populations, yet they have the same prevalence rate of OSA. Furthermore, Asians tend to have more severe OSA than Caucasians after adjustment for other risk factors.86,87 In an elderly US cohort of men, the highest prevalence of OSA was among the Asian-American sample, who, after considering the effects of obesity, had a 2-fold higher OSA prevalence compared with Caucasians.45 As this increased risk in Asians was noted in a US-based Asian sample, these findings support an intrinsic ethnic/genetic susceptibility of OSA risk rather than environmental influence. Data describing OSA in Hispanics are scant. An earlier report from the Sleep Heart Health Study showed that frequent snoring is more common in Hispanics compared with their counterparts after adjusting for BMI.63 Recently, a cross-sectional study conducted in South America in 4 Latin communities, 1.9%–6.4% of the population reported daytime sleepiness, snoring, and witnessed apnea. In a subset of the study population, unattended, simplified respiratory polygraphy was performed demonstrating an OSA prevalence of 10%.88 Variation in OSA ethnicity-based risk may be a result of genetic, environmental, and cultural factors. Also, compared with Caucasians, many minority groups have lower socioeconomic status89 and subsequently higher prevalence of obesity, less health care access, and lower level of health awareness; all of which represent risk factors for OSA. Finally, most epidemiological studies identify ethnicity based on self-report, which is subject to some degree of error, as racial admixture is often not completely taken into account.
Nasal obstruction
Nasal passages represent the gateway of ambient air to the body. Nasal obstruction causes limitation in airflow, an effect that is more pronounced during sleep and that can exacerbate apneas and nocturnal desaturation associated with OSA. Different mechanical factors can cause nasal obstruction including anatomical anomalies, like septal deviation, and inflammatory disease causing mucosal edema, namely rhinitis.
Several reports have examined the relationship between the nose and OSA. Studies with small sample sizes have evaluated the response of intermittent experimental nasal occlusion in healthy subjects. All subjects demonstrated frequent apneas, frequent arousals, and changes in sleep architecture.90–92 Clinical studies investigating the relationship of OSA and nasal obstruction, expressed as nasal resistance measured by rhinometry, have shown inconsistent results. Some studies showed that increased nasal resistance is not a determinant of OSA severity93–95 and treatment of nasal obstruction did not result in substantive changes in severity measures of OSA.96 On the other hand, in a study measuring nasal resistance in more than 500 individuals with symptoms of OSA before performing PSG, daytime nasal obstruction was identified as an independent risk factor for OSA and contributed to 2.3% of the variance.97 In a larger population-based community study, self-reported nasal congestion was identified as an independent risk factor for habitual snoring, including snoring without frank apneas.98
In addition, addressing nasal congestion symptoms is certainly an important component to the approach to enhancing likelihood of positive airway pressure (PAP) adherence. Approaches often involve the use of heated humidification, nasal saline, nasal corticosteroids, and antihistamines. Isolated treatment of increasing nasal passage patency as a treatment for OSA are unlikely to be effective as therapies, such as a nasal strips aimed to increase the caliber of the nasal passages, have failed to demonstrate substantive improvement in OSA degree.99
Craniofacial anatomy
Different craniofacial characteristics have been associated with the development of OSA by causing narrowing of the upper airway and increased upper airway collapsibility including inferiorly positioned hyoid bone, posterior placement of maxilla and mandible, enlarged tongue and soft palate, and smaller velopharyngeal cross-sectional area.100–105 Race-based differences in craniofacial features likely confer varied reasons for increased OSA risk. For example, in the Cleveland Family Study, brachycephaly, decreased middle cranial fossa, and intermaxillary length were associated with increased OSA risk in Caucasians while increased tongue area and soft palate length were more of an OSA risk in African Americans.82 Also, Hispanic patients with OSA have lower maxillary and mandibular positioning.106 Clinical craniofacial morphologic features that are OSA risk factors include micrognathia, retrognathia, or crossbite.
Smoking
Smoking causes difficulty initiating sleep, sleep fragmentation, and daytime sleepiness.107,108 Data are inconsistent as far as identifying smoking as a risk factor for OSA. In the Wisconsin Sleep Cohort Study, a higher risk of snoring and OSA was noted in smokers compared with never-smokers or former smokers with an odds ratio of 2.29 and 4.44, respectively.109 This finding was confirmed by another study reporting that current smokers were 2.5 times more likely to have OSA than former and nonsmokers combined and 2.8 times more likely to have OSA than former smokers alone.110 This effect is noticeable on passive smokers who have a high risk for habitual snoring.111 Several hypotheses have been proposed about the mechanism(s) by which smoking can increase OSA risk. The inflammatory effect of smoking on airway and the changes in lung volumes may predispose to increase upper airway collapsibility, and the effect of nicotine on sleep stability and ventilatory drive may also play a role. Whether smoking is a true risk factor for OSA or a public health problem that is prevalent in a population that is at high risk for OSA remains unclear.
Alcohol
Alcohol causes decreased sleep latency and, in larger quantity, increase the occurence of slow wave sleep.112 Short-term effects of alcohol on OSA were studied in healthy subjects and in subjects with OSA after introducing a certain amount of alcohol or placebo, and the findings of those studies showed that alcohol resulted in increased frequency of apnea, longer apneas, and more frequent hypoxic episodes.113–116 However, 2 other studies failed to replicate those results.117,118 The long-term effects of alcohol on sleep were studied in the Wisconsin Sleep Cohort Study that showed an increase in usual alcohol consumption in men which was associated with increased risk of mild or worse OSA.119 The results of this study were consistent with other cross-sectional studies in different populations.17,43,120
Alcohol likely exacerbates OSA via different mechanisms including selective reduction of genioglossal muscle activity,121 decreased ventilatory responses to hypercapnia and hypoxia,122,123 increased upper airway resistance, and increased tendency of an unstable upper airway to collapse.115,124
Pathophysiology of OSA
Breathing is a function of centrally located breathing centers that control respiratory muscles to allow airflow through the airways to ensure gas exchange in the lungs. Any dysfunction at the level of the respiratory centers (unstable ventilation), upper airway (obstruction), or combination of both can lead to abnormal breathing patterns more prominent during sleep, causing derangements in gas exchange and frequent arousals.
Upper airway patency
The upper airway in humans is a collapsible tube with a predominance of soft tissue and little bony or rigid support. In the normal human, the upper airway is patent during wakefulness and sleep as the net forces tend to keep the upper airway open and requires at least −5 cm H2O to collapse under passive conditions.125 However, this is not the case in obese subjects in which case during sleep, the airway pressure required to collapse the airway, ie, critical closing pressure,126,127 is close to atmospheric pressure and even positive.125 The airway is most at risk for complete collapse at the end of expiration where tissue pressure is higher than intraluminal pressure.125,128 Anatomical factors also increase OSA risk as occurs in obese individuals who have increased parapharyngeal fat.129,130 Certain craniofacial features such as retrognathia may be associated with increased risk of OSA due to a smaller caliber and more crowded upper airway.131
Individual posture may influence upper airway size as the supine position is associated with prolapse of the tongue and palatal structure posteriorly, and that explains the reason why OSA is typically worse in the supine position.132,133 Other forces counteract factors that tend to collapse upper airway including activation of the pharyngeal muscles. More than 20 pharyngeal muscles act in a complex and coordinated matter to maintain upper airway patency. The most extensively studied is the genioglossus muscle that has 3 main neuronal controls: (1) a reflex-mediated activation of the genioglossus through laryngeal mechanoreceptors in response to negative luminal pressure,134–136 (2) respiratory neurons in the medulla, which activate the genioglossus muscle 50–100 ms earlier than the diaphragm to maintain a patent airway just before inspiration,137 and (3) the hypoglossal motor neuron that has a constant excitatory input during a wake state from upper airway serotonergic and adrenergic neurons.138,139
Finally, lung volume affects upper airway patency, ie, as the lungs are connected to the upper airway, it applies caudal traction that stiffens pharyngeal walls and makes it less likely for the upper airway to collapse.140–142 Any decrease in lung volumes such as in obesity and change in posture from upright to supine result in less tension on the pharyngeal walls and a more collapsible airway.143
Ventilatory control
Central respiratory centers in the brain stem tightly regulate oxygen and carbon dioxide levels in the blood via many feedback loops that involve different chemoreceptors and mechanoreceptors, resulting in changes in the pattern and depth of ventilation to maintain blood gases within narrow limits. Such a complex system can become unstable. Instability of the system is best explained by the loop gain principal,144–146 which is an engineering concept. Loop gain is the ratio of a corrective response (ventilation) to the disturbance itself. A high-gain system responds quickly and vigorously to a disturbance, whereas a low-gain system responds slowly and weakly.147 The 2 primary variables influencing loop gain are known as controller gain and plant gain, and both are important in ventilatory stability. The controller gain represents chemoresponsiveness or the hypoxic and hypercapnic ventilatory responses. A high controller gain is, therefore, generally due to rapid hypercapnic responsiveness.148 Plant gain reflects the effectiveness of a given level of ventilation to eliminate CO2. High loop gain destabilizes ventilation in wake and sleep states, although it is less obvious during awake because breathing patterns during wakefulness are highly influenced by behaviors, such as talking and eating. It is believed that high loop gain plays a role in the pathophysiology of OSA in which breathing centers respond quickly and vigorously (high controller) to minor changes in CO2, which results in a drop in CO2 below the apneic threshold, resulting in pauses in breathing leading to CO2 retention and so forth.149–152
Pathogenesis of OSA
Patients with OSA have smaller and more collapsible airways than normal. In the awake state, the airway is patent because of pharyngeal dilator muscle activation. However, in the sleep state, the activity of those muscles is markedly diminished153 and lung volume decreases in the supine position causing substantial restriction in airflow resulting in hypopnea or apnea.143 This mechanical load and CO2 retention activate the pharyngeal muscles in order to restore upper airway patency.154,155 Recruitment of pharyngeal dilator muscles can be achieved and upper airway patency is restored without a cortical arousal in some individuals, whereas in others, the arousal is required for effective muscle recruitment; this phenomenon has been described as compensatory effectiveness.156
Ventilatory instability plays a role in OSA as supported by work describing that loop gain is higher in patient with severe OSA during non-REM sleep than those with mild OSA.144,157 Indeed, after each apnea, patients with OSA hyperventilate, CO2 drops to the apneic threshold, expiratory time becomes prolonged, and the upper airway may collapse again.
Moreover, several reports have described decreased responsiveness of the pharyngeal muscles to negative upper airway pressure due to damage to the sensory nerves in the upper airway or to the muscle itself. This damage may be due to inflammation caused by vibration, snoring, or trauma.158–161 Data also support the notion of ventilatory variability at sleep–wake transitions as a predictor of OSA severity.162 In addition, humoral and inflammatory mediators released from the visceral adipose tissue may play a role in ventilatory regulation. The most studied is leptin, which binds to its receptors in the hypothalamus, causing reduced satiety and increased ventilation.163 Leptin stimulates respiratory drive, as shown in leptin-deficient or resistant mice that exhibited features of central hypoventilation and obesity.164
Overall, there are a variety of ventilatory control mechanisms that may predispose to an unstable upper airway, including alterations in loop gain, impairments in upper airway motor and neural control, and responsiveness, as well as humoral mechanisms.
OSA and adverse clinical outcomes
OSA, likely via mechanisms of intermittent hypoxia, sympathetic nervous system activation, and alterations in intrathoracic pressures, has been associated with metabolic dysregulation and cardioavascular sequelae, such as diabetes mellitus, hypertension, congestive heart failure, and stroke.
Inflammation, endothelial dysfunction and atherosclerosis
Repetitive episodes of oxygen desaturation and reoxygenation, which is a hallmark feature of OSA, may represent a risk for increased oxidative stress.165 Increase in oxygen free radical production166 results in lipid peroxidation,167 protein oxidation, DNA damage, and alteration in cellular hemostasis, leading to upregulation of certain proinflammatory genes controlled by nuclear factor κB.168 Indeed, compared to healthy control, patients with OSA have elevated tumor necrosis factor α,169,170 interleukin 6,171,172 interleukin 8,173,174 monocyte chemoattractant protein 1,175 and cellular adhesion molecules including intercellular adhesion molecule 1,175 L-selectin,174 and vascular adhesion molecule 1.174,176 This combination of an enhanced systemic inflammatory state along with increased oxidative stress and elevated sympathetic nervous system activation may result in endothelial dysfunction and increased arterial stiffness,177,178 factors that play a central role in the initiation and acceleration of atherosclerosis.178 Atherosclerosis has been identified by recent research as a consequence of an augmented inflammatory state causing endothelial injury that progresses to atheroma formation.179 Furthermore, treatment of OSA with PAP is associated with a reduction in inflammatory markers levels171,180 and improvement in endothelial function measured by pulse wave velocity (ascertained by arterial applanation tonometry) and endothelial-dependent vasodilation.177,181
Hypertension
The primary mechanism considered to link OSA to the development of hypertension is sympathetic nervous system overactivity. It has been postulated that intermittent hypoxia and negative intrathoracic pressure lead to chemoreceptor activation and increased sympathetic outflow, and subsequently endothelial dysfunction that predisposes to increased arterial stiffness and this in turn leads to development of hypertension. This theory was examined in animals when intermittent hypoxia caused by repetitive airway obstruction caused transient elevation blood pressure, and this effect lasted after exposure.182,183 Those findings were substantiated by studies conducted on humans. Using plasma and urinary catecholamines as a method of measuring sympathetic activity, it was shown that urinary catecholamine in morbidly obese hypertensive patients with OSA was higher compared with normotensive, obese individuals without OSA.184 Other studies have reported consistent findings such that catecholamine levels were higher in the mornings and at baseline compared with controls. Moreover, decreased level of catecholamines after successful termination of apnea by tracheostomy or PAP has been described.185–189
Cross-sectional data from the Pennsylvania cohort and the Sleep Heart Health Study showed a high prevalence of hypertension in participants with OSA.190,191 A causal association of OSA and hypertension was supported by longitudinal data from the Wisconsin Cohort Study demonstrating a dose-response relationship between the severity of OSA measured by AHI and incident hypertension with a 2.89 increased odds of developing hypertension in subjects with AHI ≥ 15 during a 4-year follow-up period.192 Recent findings from the Sleep Heart Health Study also demonstrated a significant longitudinal relationship between OSA and the development of hypertension among individuals who were normotensive at baseline examination; however, these relationships were attenuated to borderline significance after taking into account obesity193
There are several factors that may account for the difference between the results of the Wisconsin Cohort Study and the Sleep Heart Health Study, including the different definitions used in the assessment of the AHI as a marker of OSA, differences in the ascertainment of blood pressure measurement (ie, measuring blood pressure at home vs clinic), and using different cutpoints in defining hypertension (140/90 vs 135/85). The estimated effect in the Sleep Heart Health Study, however, was similar to the cross-sectional findings, and the reduction in power due to restricting the sample to those who were normotensive at baseline may have accounted for these marginal findings. The strength of the OSA-hypertension association has also been noted to decreases with age, which may be secondary to competing risk factors or survivorship bias.190,194
Several interventional trials have investigated the relationship between hypertension and elimination of apneas by PAP. Several of these studies have demonstrated that PAP has a blood pressure lowering effect,195–200 whereas others did not.201,202 Inconsistency in the results of those trials can be explained by different methodology, enrolling individuals who are normotesive at baseline, treatment for short duration, or the differential interindividual responsiveness to treatment. Of note, OSA has been clearly recognized as a secondary cause of hypertension in the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.203
Coronary artery disease
It has been shown that OSA is associated with activation of thrombotic pathways and leads to a state of enhanced inflammation that serves as intermediate mechanisms leading to cardiovascular disease.204 Furthermore, repetitive episodes of hypoxia, intrathoracic pressure swings, and increased afterload create a state of demand-supply mismatch causing cardiac ischemia as demonstrated electrocardiographically by ST depression, accompanying episodes of hypoxia and apnea.205–207 There is a linear relationship between AHI and coronary artery calcification score after taking into account potential confounding cardiovascular factors.208 A positive association between AHI and coronary atherosclerotic plaque volume measured by intravascular ultrasound has also been noted.209 These findings are further supported by several small case-control studies, which showed increased OSA in individuals with documented coronary artery disease.5,210–213 The prevalence of OSA in the setting of acute coronary syndrome has been reported to be quite high. Specifically, of 104 patients admitted with acute coronary syndrome, 66.4% had mild to moderate OSA (AHI ≥ 10) and 26.0% had moderate to severe OSA (AHI ≥ 30), with the predominant apnea pattern being obstructive: 72.1%.214
Furthermore, elimination of apnea and hypoxia with PAP is associated with reduction in all-cause mortality and cardiovascular-specific mortality.211,215–218 It has also been reported that patients with untreated severe OSA had a higher incidence of fatal cardiovascular events (1.06 per 100 person-years) and nonfatal cardiovascular events (2.13 per 100 person-years) than that of patients treated with continuous positive airway pressure (CPAP) (0.35, P = 0.0008 and 0.64, P = 0.0001).215
Stroke
Several case–control studies have demonstrated an increased prevalence of OSA in individuals with cerebrovascular disease.219–223 OSA as a preexisting condition or a risk factor for stroke has been investigated in several longitudinal studies. In a population-based study, 1,475 subjects with OSA followed for an average of 4 years, an increased odds of developing stroke in subjects with AHI (odds = 20) compared with controls (OR = 4.48; P = 0.02) was reported.224 In a cohort derived from a sleep referral center, a significant increased risk of stroke, transient ischemic attack, or death from any cause in OSA compared with controls (Hazard ratio = 1.97; P = 0.01) after adjusting for confounding factors including atrial fibrillation was noted. There is also a trend of increased risk of cerebrovascular disease and severity of OSA.225
These results are consistent with findings of OSA and stroke in other observational studies.5,226,227 Studies assessing the effect of OSA treatment on the incidence of stroke are limited. Less vascular problems were observed, including stroke, in subjects with OSA treated by weight loss or tracheostomy compared with no treatment.228,229 If left untreated, OSA was associated with poor outcomes in stroke patients.230,231 Worse functional impairments and a longer period of hospitalization and rehabilitation have been observed in stroke patients with OSA.230 Moreover, poststroke patients with AHI of 15 or greater have been shown to have a higher risk of death.231 Recent findings from The Sleep Heart Health Study cohort have shown an increased risk of stroke in men with mild to moderate OSA, i.e. 6% increased stroke risk for each unit increase in AHI (95% CI: 0.02–0.10).232
Congestive heart failure
There is likely a bidirectional relationship between congestive heart failure and OSA. Heart failure may contribute to OSA development by accumulation of fluid or edema of the upper airway, thereby enhancing collapsibility. Sleep-related reductions in pulmonary function residual capacity may contribute to periodic breathing by reducing oxygen stores.146,233,234 Obstructive apneas and hypopneas can occur during nadirs of neuromuscular output when the relative innervations of the upper airway muscles are insufficient to maintain airway patency.
Conversely OSA may adversely affect cardiac function through mechanisms, including adverse effects of chronic hypertension, nondipping blood pressure, atherosclerosis and ischemic injury, hypoxemia-related and catecholamine-related myocyte injury, and cardiac remodeling. Of 450 patients with a mean left ventricular ejection fraction of 27%, 38% were observed to have OSA and 72% central sleep apnea,235 which is much higher than the reported 5%–10% prevalence of OSA in the healthy middle-aged population.9 Another study of 81 patients with systolic dysfunction found a high prevalence of central sleep apnea, occurring in 40% of the sample with another 11% with OSA.236 Cross-sectional results from the Sleep Heart Health Study demonstrated that modest levels of OSA (ie, AHI ≥ 11) were associated with a 2.38 higher odds of self-reported heart failure, independent of other risk factors.5 Prospective data from the Sleep Heart Health Study have reported that middle-aged men with an AHI ≥ 30 had an approximately 60% increased 8-year incidence of heart failure compared with men with an AHI <5 (Gottlieb, unpublished data).
Cardiac arrhythmias
There is biologic plausibility that OSA-associated hypoxia, fluctuating intrathoracic pressure, and imbalance in autonomic nervous system function may predispose to cardiac arrhythmogenesis.237–239 In the Sleep Heart Health Study cohort, an increased prevalence of complex ventricular ectopy and atrial fibrillation in patients with an AHI ≥ 30 was observed.240 A causal association between sleep apnea and arrhythmias is supported by the findings from a case-cross over study performed in the Sleep Heart Health Study, which examined the temporal distributions of arrhythmias in association with the occurrence of apneas and hypopneas on PSG, demonstrating that the occurrence of discrete paroxysmal atrial fibrillation or nonsustained ventricular tachycardia was 17-fold higher in 90 seconds immediately following an apnea or hypopnea compared with a time period following nonobstructed breathing.241 In a multicenter cohort of older community-dwelling males, there was a dose-response relationship between the severity of sleep apnea and the prevalence of arrhythmias in elderly men. In this study, complex ventricular ectopy was associated most strongly with OSA and hypoxia, whereas atrial fibrillation was most strongly associated with central sleep apnea.242
Untreated sleep apnea has been identified as a risk factor of recurrent atrial fibrillation after treatment by cardioversion.243 Furthermore, the future development of atrial fibrillation in individuals with sleep apnea was retrospectively investigated; nocturnal hypoxia was identified as a future predictor of arrhythmia development.244
Metabolic consequences
Several plausible biological mechanisms have been investigated linking OSA and insulin resistance/diabetes mellitus. Intermittent hypoxia associated with increased sympathetic activity directly can alter glucose metabolism 245,246 or indirectly increase other counter-regulatory hormones like cortisol and, possibly, growth hormone that increases insulin resistance.247,248 Moreover, increased oxidative stress associated with intermittent hypoxia generates oxygen free radicals 166,249 and produces a state of inflammatory response that has been shown to increase insulin resistance.250 Both OSA and insulin resistance are in large part a product of the worldwide epidemic of obesity.251 However, it is unclear whether increased insulin resistance in patients with OSA occurs through pathways of oxidative stress or, alternatively, through a state of increased systemic inflammatory response.252 A number of reports have investigated the complex relationship between OSA and insulin resistance. Using various methods to measure insulin resistance, several population-based, cross-sectional studies reported an independent association between insulin resistance, glucose intolerance, and diabetes with markers of OSA severity, namely AHI and hypoxia.253–258 These findings were supported by several smaller clinic-based studies.259–264
However, longitudinal data confirming this relationship is sparse. Using snoring as surrogate for OSA, habitual snoring was identified as an independent risk factor for the incidence of self-reported diabetes.265 Similarly, a 2-fold increased risk of laboratory-confirmed diabetes was noted in relation to regular snoring.266
When assessing OSA via PSG, disparate results have been described. Longitudinally, moderate to severe OSA was a significant independent risk factor for incident diabetes in an Australian cohort (fully adjusted OR = 13.45; 95% confidence interval [CI] = 1.59–114.11) with CIs that were notably wide, suggesting lack of measurement precision. Alternatively, an association between OSA and future development of diabetes after a 4-year follow-up period was not found in the Wisconsin Cohort Study. Specifically, the odds ratio for developing diabetes mellitus within 4 years in those with an AHI of 15 or more compared with those with an AHI < 5 was not statistically significant when adjusting for age, sex, and body habitus.267 The discrepancy in these results may be explained by short follow-up periods, small sample sizes, or inconsistencies in measuring markers of insulin resistance, obesity, and OSA. In one study, improvement of insulin sensitivity in patients with OSA after 2 days of treatment remained stable for 3 months, an effect that was more pronounced in individuals with a BMI less than 30 kg/m2.268 These findings were countered by one randomized study. After 3 months of treatment, no difference was noted in insulin sensitivity between intervention and placebo group.269
A series of other studies with a host of limitations, including small sample sizes, etc, have also not identified an improvement in insulin resistance with OSA treatment.270–274 Results from a randomized, crossover, controlled trial did not provide evidence, that impaired glucose tolerance normalizes after 2 months of therapeutic CPAP treatment.275 However, insulin resistance was measured by the oral glucose tolerance test, and insulin glucose product improved in those with severe sleep apnea, suggesting beneficial metabolic effects of CPAP in those with severe disease.276
Another metabolic derangement associated with OSA is dyslipidemia. Mice models of chronic intermittent hypoxia, a hallmark feature of OSA, showed increase in triglyceride and cholesterol biosynthesis and secretion through upregulation of certain liver enzymes that increase availability of fatty acids and lipoprotein secretion.277,278 Similar results were seen in patients with OSA with increased plasma triglycerides and low-density lipoprotein noted to correlate with nocturnal hypoxia.279 Furthermore, treatment of OSA led to a reduction in total cholesterol in adults280,281 and pediatric282 populations. In one study, however, abnormalities in the lipid profile were noted to be attributable to obesity more than OSA, and treatment of OSA by PAP had no effect on total cholesterol and triglyceride levels.283
Cognitive function and motor vehicle accidents
Patients with OSA are often unaware of their nocturnal symptoms. However, daytime symptoms have a great impact on cognitive function and quality of life. Cognitive function is commonly classified in clinical and research studies into three broad categories: attention, memory and learning, and executive performance.284 Patients with OSA commonly experience difficulties in working, memory problems, and concentration, and these symptoms have been best correlated with a degree of hypoxia at night.285 On the other hand, treatment of OSA is linearly associated with improvement of cognitive dysfunction, and patients with severe disease tend to benefit the most.286–288
As high vigilance is required for safe driving, it is no surprise that patients with OSA are at higher risk for motor vehicle accidents. Indeed, patients with AHI > 15 were significantly more likely to have multiple accidents in 5 years (OR = 7.3).289 Improvement in cognitive and motor function with treatment of OSA is associated with a reduction in collision rate.8,290,291
Quality of life
Patients with OSA often report poor quality of life292,293 and poor general health status.294 Different factors can contribute to this perception: excessive daytime sleepiness,295 insomnia, social dysfunction, impaired work performance,296 and higher prevalence of psychiatric problems such as depression.297
To evaluate the degree of impairment in quality of life, different methods have been used in clinical practice and research, which are divided into generic instruments that allow comparison between populations or studies, eg, the Medical Outcomes Study Short Form-36,298 and alternatively, specific instruments that are designed to detect changes among patients with specific disorders, eg, Calgary sleep apnea quality of life index.299 Both of these methods have been used to quantify the degree of impairment and the improvement of quality of life after treatment of OSA.300 Several clinical trials showed improvement of quality of life with consistent usage of PAP after variable periods of follow-up from 2 weeks to 4 months. Those studies showed that the degree of improvement depends mainly on the degree of impairment at baseline.286,301–306
Diagnosis of OSA
The diagnosis of OSA is based upon clinical presentation and physical findings suggestive of the disease in conjunction with objective data obtained from sleep study monitoring. OSA is a highly prevalent disease and, if left untreated, may result in considerable social, economic, and health sequelae. Bearing this in mind, early recognition by the health care provider is of paramount importance.
Polysomnography
PSG is the gold-standard method to diagnose OSA and provides a method to determine the PAP level required for treatment. During PSG, detailed information is obtained by using electroencephalogram, electromyogram, electro-oculogram, electrocardiogram, snore microphone, body position and leg movement, oronasal airflow, chest wall effort, and oxyhemoglobin saturation, as well as video recording.307 Full sleep study monitoring is performed during usual sleep hours with 6 hours of recording optimally needed to establish the diagnosis. Usually, the patient returns to the laboratory for a follow-up sleep study to establish adequate pressure required to eliminate respiratory events. In order to enhance efficiency, split night studies may be performed, which involves an initial portion of sleep recording required to establish the diagnosis followed by PAP titration. Split night studies have inherent drawbacks due to short recording time for the diagnostic portion, representing a less than ideal diagnostic method for those patients with a low pretest probability (as longer sleep time are often needed to establish the diagnosis), and given that the therapeutic part of the split night study may not provide sufficient time to establish the optimal therapeutic PAP setting.
Many factors can affect the accuracy of PSG in ascertaining the diagnosis of OSA. PSG is a highly specific and sensitive test for patients with a high pretest probability. However, this is not the case for patients with low-test probability of OSA. Considering an AHI cutoff of 15 results in a false negative rate of approximately 20%.308–311 Factors that may result in night-to-night variability are body position and use of drugs that affect the percentage of REM sleep (ie, apnea tend to occur in supine position and in REM sleep).312,313
Home portable monitoring
With an increasing number of patients who are referred for the evaluation for OSA and by following current guidelines for the diagnosis of OSA, it has been estimated that 600 sleep studies per 100,000 individuals per year would be required.314 This has placed great pressure on the limited number of sleep laboratories in the country and may contribute to delay in OSA diagnosis and treatment. For this reason, portable home sleep monitoring emerged as a potential alternative for the diagnosis of OSA.
Per the American Academy of Sleep Medicine guidelines, there are different levels of sleep monitoring with levels II–IV categorized as unattended monitoring involving a minimum of 7 channels (Level II), 4 channels (Level III), or 1 channel (Level IV).315 Although portable sleep monitoring is less costly and more convenient for the patient, there are several disadvantages. Absence of direct supervision by a sleep technician increases the likelihood of data loss or obtaining poor quality or uninterruptable sleep studies with some reports of equipment failure or data loss between 8%–16%.316,317
In the Sleep Heart Health Study, ~60 participants underwent both unattended portable monitoring and in-laboratory monitoring within 2 weeks of one another. The intraclass correlation coefficients of the respiratory variables demonstrated good reliability with values of ~0.7–0.8.316 In another study, portable sleep monitoring and PSG were noted to have the best performance in patients with AHI < 5 or AHI > 30.317 Data from a randomized controlled trial comparing PSG and portable sleep monitoring showed that outcomes of quality of life and sleepiness were similar in the 2 groups.318
On the basis of current literature, the American Academy of Sleep Medicine released clinical guidelines regarding the use of portable sleep monitoring. In laboratory, PSG remains the gold-standard method for OSA diagnosis; however, portable sleep monitoring is a diagnostic option in patients with high pretest probability of moderate to severe OSA without significant comorbidity or concern for concomitant sleep disorders. Portable sleep monitoring should not be used for OSA screening, diagnosing other sleep disorders, or in the absence of a comprehensive sleep evaluation by a board certified or board eligible sleep professional.319
Treatment of OSA
Treatment of OSA is focused on reversal of underlying pathology. PAP has been used to act as pneumatic splint in order to keep pharyngeal pressures above pharyngeal critical pressure and, hence, prevent recurrent airway obstruction. However, certain subgroups of patients with craniofacial abnormalities as a primary OSA risk factor may benefit from upper airway surgical intervention or oral appliance to restore normal upper airway anatomy. Furthermore, patient counseling about sleep posture (ie, discouraging sleep in the supine position), sleep hygiene (maintaining regular sleep–wake times and discouraging the use of sedatives, hypnotics, and alcohol particularly given their ability to reduce upper airway muscle tone and worsen OSA), and weight loss is an integral part of the management of OSA.
Positive airway pressure
PAP was first introduced as a treatment of OSA in 1983.320 Since then, and with advances in technology, different ways in delivering PAP have evolved with a large number of clinical trials investigating the efficacy in improving different clinical outcomes. Continuous PAP, known as CPAP, is commonly used to treat OSA by delivering a constant pressure throughout inspiration and expiration to maintain upper airway patency during sleep. It consists of a flow generator that delivers airflow at a constant pressure to the patient through a mask via a tubing system. CPAP was evaluated in clinical trials and was compared to sham CPAP, pills, or no treatment, using parallel or crossover designs. The results were in favor of CPAP comparatively reducing AHI and improving daytime sleepiness assessed subjectively by Epworth sleeping scale196,286,305,321–331 and objectively by using multiple sleep latency testing.286,305,322–324,326,328,332 Although the effect of CPAP on other outcomes is less established, several reports noted improvement in sleep quality (increased stages 3 and 4)333 and quality of life.328,332,334,335
The effect of treatment of OSA on cardiovascular disease, metabolic disorders, cognitive function, and road traffic accidents has already been discussed earlier in this review. However, most of these studies enrolled patients with moderate to severe OSA; therefore, whether these outcomes are improved in milder degrees of OSA is not as substantiated. In one study, a beneficial effect of CPAP over conservative therapy in individuals with mild OSA in terms of mood, energy level, and general health, as assessed by the Medical Outcome Survey Short Form-36, was observed, and it was most evident among individuals with hypertension or diabetes.336 In another study, those with an AHI in the range 5–10 noted an improvement in cognitive function, psychological well-being, and quality of life with CPAP use.286
Another mode of PAP is bi-level PAP. Bi-level PAP delivers 2 different set pressures: one during inspiration and a different lower pressure during expiration, called inspiratory PAP (IPAP) and expiratory PAP (EPAP), respectively. It has the same indication as CPAP; however, bi-level PAP allows exhalation at a lower pressure, which theoretically makes it more tolerable than CPAP, especially if pressure needs are high.337 Another advantage of bi-level PAP is the pressure differential increase in ventilation via pressure support, which may be important in patients with concomitant hypoventilation, lung restriction, or increased dead space.
Auto-PAP allows unattended in-home titration of PAP by adjusting the pressure to the minimum required. Compared to CPAP, auto-PAP is similar in ability to eliminate respiratory events and to improve subjective sleepiness.338 Adaptive servoventilation (ASV) is one of the new versions of PAP that may be helpful in the treatment of complex sleep apnea (treatment-emergent central sleep apnea) and periodic breathing.339,340 It essentially delivers a constant EPAP, but the inspiratory pressure is autoadjustable to meet a certain percentage of the recent average ventilation or flow depending upon the machine type. The difference between the IPAP and EPAP, in essence, fluctuates to create a tidal volume that is 90% of the average sensed ventilation. ASV has a backup rate that is activated by central apnea. PAP is a chronic treatment for a long-lasting disease. Several factors play a role in the patient’s compliance that is conventionally defined as regular use of the device for more than 70% of nights for more than 4 hours each night.341,342 Several observational studies have estimated the PAP discontinuation rate between 5% and 50%, and most of these cases occurred during the first 3 months of use.342 Factors that predict long-term adherence to therapy are early adherence to therapy, increased OSA severity, and excessive daytime sleepiness.331,343,344 The majority of patients discontinue treatment due to side effects that commonly relate to mask interface problems, such as air leak, skin breakdown, claustrophobia, and mouth dryness. These side effects can be addressed by changing the mask type accordingly (nasal mask vs nasal pillows vs full face-mask) and by ensuring proper mask fit. Other complications are related to nasal symptoms, such as congestion, dryness, and epistaxis. Symptomatic relief can be achieved by using heated humidification and nasal saline solution.345 Pressure intolerance can also sometimes negatively impact patient compliance with CPAP. In these situations, the ramp feature can be used, which gradually increases the pressure from a lower setting to the goal pressure over a specified period of time to allow acclimatization. Other methods to address pressure intolerance include use of expiratory pressure relief or bi-level PAP.
Oral appliances
As PAP has emerged as an effective treatment of OSA, oral appliances have been identified as an alternative treatment modality. Per the American Academy of Sleep Medicine guidelines, oral appliances are indicated for treatment of mild and/or positional OSA. Oral appliances are broadly divided into devices that protrude the mandible forward known as mandibular advancement devices and tongue-retaining devices. The goal of these devices is to maintain upper airway patency during sleep by changing upper airway geometry and by increasing the pharyngeal occlusion pressure.346,347 Most clinical trials have used mandibular advancement devices as a prototype of oral appliances. Relative contraindications for the use of oral appliances include temporomandibular joint disease. Potential side effects include myofascial pain, excessive salivation, temporomandibular joint pain, tooth pain, dry mouth, and gum irritation.348
Studies comparing PAP to oral appliances showed significant differences in favor of PAP. PAP was more efficacious in improving AHI compared to oral appliances. However, the difference in symptomatic improvement was less clear.324,326,349–351
Surgery
Surgical treatment of OSA aims to increase cross-sectional area of the upper airway by removal of redundant tissue, and hence eliminating the obstructive episodes during sleep. Different procedures have been described including uvulopalatopharyngoplasty (UPPP), which was first introduced in 1981,352 and consists of removal of the tonsils, uvula, and part of the soft palate. Overall, UPPP effectively addresses OSA in only 50% of cases treated with this modality.353 Potential complications of this procedure include velopharyngeal insufficiency and postoperative pain. With advances in technology, various other techniques have been used, such as multilevel, temperature-controlled, radiofrequency tissue ablation, laser-assisted uvulopalatoplasty, and maxillomandibular osteotomy. Compared with no treatment, no difference was noted in daytime sleepiness and quality of life in patients who underwent laser-assisted uvulopalatoplasty.354,355 Similar results were reported using temperature-controlled, radiofrequency tissue ablation or radiofrequency surgery of soft palate.356–359 Nevertheless, certain patients with OSA might benefit from surgery. Using anatomical staging system to stratify patients with OSA was more effective in predicting outcome than did the severity-based staging.360
Conclusion
For the past 3–4 decades, OSA has been increasingly recognized as a highly prevalent disorder and source of adverse health consequences, resulting in major population health burden. In the United States, approximately 12 million people, 30–60 years of age, have OSA,9 and 38,000 die each year from cardiovascular disease attributed to OSA.361 OSA is associated with a host of signs and symptoms adversely affecting quality of life, including snoring, daytime somnolence, neurocognitive deficits, and irritability. Substantial morbidity and economic costs are associated with untreated OSA, including those related to daytime sleepiness and hypertension, and cardiovascular comorbidity. Despite the notion that OSA is a prevalent and treatable condition associated with negative health consequences, at least 75% of severe OSA cases are undiagnosed.362,363 Therefore, there is a crucial need to increase recognition of this disorder and address the public health impact of this common condition, including the extent to which treatment of OSA may modify the course of other chronic health conditions, such as insulin resistance, cardiac arrhythmogenesis, heart failure, and cardiovascular disease.
Acknowledgments
Supported by NIH (National Heart Lung Blood Institute) and K23 HL079114, NIH M01 RR00080, American Heart Association National Scientist Development Award 0530188N, Central Society of Clinical Research Award, and NCI 1U54CA116867. The project described was also supported by UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999 Aug 1;22(5):667–689. [PubMed] [Google Scholar]
- 2.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159(2):502–507. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30(9):1121–1127. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindberg E, Carter N, Gislason T, Janson C. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med. 2001;164(11):2031–2035. doi: 10.1164/ajrccm.164.11.2102028. [DOI] [PubMed] [Google Scholar]
- 8.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340(11):847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 9.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 10.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 11.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 12.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med. 1995;151(5):1459–1465. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 13.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apneahypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 14.Ip MS, Lam B, Tang LC, Lauder IJ, Ip TY, Lam WK. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest. 2004;125(1):127–134. doi: 10.1378/chest.125.1.127. [DOI] [PubMed] [Google Scholar]
- 15.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119(1):62–69. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170(10):1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 17.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169(2):168–173. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 18.Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest. 2006;130(1):149–156. doi: 10.1378/chest.130.1.149. [DOI] [PubMed] [Google Scholar]
- 19.Puvanendran K, Goh KL. From snoring to sleep apnea in a Singapore population. Sleep Res Online. 1999;2(1):11–14. [PubMed] [Google Scholar]
- 20.Tami TA, Duncan HJ, Pfleger M. Identification of obstructive sleep apnea in patients who snore. Laryngoscope. 1998;108(4 Pt 1):508–513. doi: 10.1097/00005537-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Vaidya AM, Petruzzelli GJ, Walker RP, McGee D, Gopalsami C. Identifying obstructive sleep apnea in patients presenting for laser-assisted uvulopalatoplasty. Laryngoscope. 1996;106(4):431–437. doi: 10.1097/00005537-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb DJ, Yao Q, Redline S, Ali T, Mahowald MW. Does snoring predict sleepiness independently of apnea and hypopnea frequency? Am J Respir Crit Care Med. 2000;162(4 Pt 1):1512–1517. doi: 10.1164/ajrccm.162.4.9911073. [DOI] [PubMed] [Google Scholar]
- 23.Douglas NJ. Systematic review of the efficacy of nasal CPAP. Thorax. 1998;53(5):414–415. doi: 10.1136/thx.53.5.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beutler LE, Ware JC, Karacan I, Thornby JI. Differentiating psychological characteristics of patients with sleep apnea and narcolepsy. Sleep. 1981;4(1):39–47. doi: 10.1093/sleep/4.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(5):1162–1168. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 26.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103(1):30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 27.Viner S, Szalai JP, Hoffstein V. Are history and physical examination a good screening test for sleep apnea? Ann Intern Med. 1991;115(5):356–359. doi: 10.7326/0003-4819-115-5-356. [DOI] [PubMed] [Google Scholar]
- 28.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18(3):158–166. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 29.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 30.Kirby SD, Eng P, Danter W, et al. Neural network prediction of obstructive sleep apnea from clinical criteria. Chest. 1999;116(2):409–415. doi: 10.1378/chest.116.2.409. [DOI] [PubMed] [Google Scholar]
- 31.Dixon JB, Schachter LM, O’Brien PE. Predicting sleep apnea and excessive day sleepiness in the severely obese: indicators for polysomnography. Chest. 2003;123(4):1134–1141. doi: 10.1378/chest.123.4.1134. [DOI] [PubMed] [Google Scholar]
- 32.Kushida CA, Efron B, Guilleminault C. A predictive morphometric model for the obstructive sleep apnea syndrome. Ann Intern Med. 1997;127(8 Pt 1):581–587. doi: 10.7326/0003-4819-127-8_part_1-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 33.Tsai WH, Remmers JE, Brant R, Flemons WW, Davies J, Macarthur C. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167(10):1427–1432. doi: 10.1164/rccm.200112-110OC. [DOI] [PubMed] [Google Scholar]
- 34.Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23(7):929–938. doi: 10.1093/sleep/23.7.929. [DOI] [PubMed] [Google Scholar]
- 35.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 36.Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1946–1949. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 37.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Morbidity, mortality and sleep-disordered breathing in community dwelling elderly. Sleep. 1996;19(4):277–282. doi: 10.1093/sleep/19.4.277. [DOI] [PubMed] [Google Scholar]
- 38.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Natural history of sleep disordered breathing in community dwelling elderly. Sleep. 1993;16(Suppl 8):S25–S29. doi: 10.1093/sleep/16.suppl_8.s25. [DOI] [PubMed] [Google Scholar]
- 40.Ancoli-Israel S. Epidemiology of sleep disorders. Philadelphia, PA: WB. Saunders; 1989. [PubMed] [Google Scholar]
- 41.Young T. Sleep-disordered breathing in older adults: is it a condition distinct from that in middle-aged adults? Sleep. 1996;19(7):529–530. doi: 10.1093/sleep/19.7.529. [DOI] [PubMed] [Google Scholar]
- 42.Mant A, Saunders NA, Eyland AE, Pond CD, Chancellor AH, Webster IW. Sleep-related respiratory disturbance and dementia in elderly females. J Gerontol. 1988;43(5):M140–M144. doi: 10.1093/geronj/43.5.m140. [DOI] [PubMed] [Google Scholar]
- 43.Enright PL, Newman AB, Wahl PW, Manolio TA, Haponik EF, Boyle PJ. Prevalence and correlates of snoring and observed apneas in 5,201 older adults. Sleep. 1996;19(7):531–538. doi: 10.1093/sleep/19.7.531. [DOI] [PubMed] [Google Scholar]
- 44.Ancoli-Israel S, Gehrman P, Kripke DF, et al. Long-term follow-up of sleep disordered breathing in older adults. Sleep Med. 2001;2(6):511–516. doi: 10.1016/s1389-9457(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 45.Mehra R, Stone KL, Blackwell T, et al. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55(9):1356–1364. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eikermann M, Jordan AS, Chamberlin NL, et al. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131(6):1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med. 2006;119(1):72, e9–e14. doi: 10.1016/j.amjmed.2005.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shochat T, Loredo J, Ancoli-Israel S. Sleep Disorders in the Elderly. Curr Treat Options Neurol. 2001;3(1):19–36. doi: 10.1007/s11940-001-0021-x. [DOI] [PubMed] [Google Scholar]
- 49.Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J Sleep Res. 2000;9(4):389–393. doi: 10.1046/j.1365-2869.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 50.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10(9):2087–2090. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 51.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102(2):547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 52.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 1: clinical features. Sleep. 2002;1525(4):412–419. [PubMed] [Google Scholar]
- 53.Ambrogetti A, Olson LG, Saunders NA. Differences in the symptoms of men and women with obstructive sleep apnoea. Aust N Z J Med. 1991;21(6):863–866. doi: 10.1111/j.1445-5994.1991.tb01408.x. [DOI] [PubMed] [Google Scholar]
- 54.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28(3):309–314. [PubMed] [Google Scholar]
- 55.Mohsenin V. Gender differences in the expression of sleep-disordered breathing: role of upper airway dimensions. Chest. 2001;120(5):1442–1447. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 56.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166(10):1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 57.Mohsenin V. Effects of gender on upper airway collapsibility and severity of obstructive sleep apnea. Sleep Med. 2003;4(6):523–529. doi: 10.1016/s1389-9457(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 58.Jordan AS, McEvoy RD, Edwards JK, et al. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol. 2004;558(Pt 3):993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Millman RP, Carlisle CC, McGarvey ST, Eveloff SE, Levinson PD. Body fat distribution and sleep apnea severity in women. Chest. 1995;107(2):362–366. doi: 10.1378/chest.107.2.362. [DOI] [PubMed] [Google Scholar]
- 60.Whittle AT, Marshall I, Mortimore IL, Wraith PK, Sellar RJ, Douglas NJ. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54(4):323–328. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 62.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165(20):2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 63.O’Connor GT, Lind BK, Lee ET, et al. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health Study. Sleep. 2003;26(1):74–79. [PubMed] [Google Scholar]
- 64.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122(6):535–542. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 65.Strohl KP, Saunders NA, Feldman NT, Hallett M. Obstructive sleep apnea in family members. N Engl J Med. 1978;299(18):969–973. doi: 10.1056/NEJM197811022991801. [DOI] [PubMed] [Google Scholar]
- 66.Redline S, Tishler PV, Tosteson TD, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151(3 Pt 1):682–687. doi: 10.1164/ajrccm/151.3_Pt_1.682. [DOI] [PubMed] [Google Scholar]
- 67.Mathur R, Douglas NJ. Family studies in patients with the sleep apnea-hypopnea syndrome. Ann Intern Med. 1995;122(3):174–178. doi: 10.7326/0003-4819-122-3-199502010-00003. [DOI] [PubMed] [Google Scholar]
- 68.Guilleminault C, Partinen M, Hollman K, Powell N, Stoohs R. Familial aggregates in obstructive sleep apnea syndrome. Chest. 1995;107(6):1545–1551. doi: 10.1378/chest.107.6.1545. [DOI] [PubMed] [Google Scholar]
- 69.Ferini-Strambi L, Calori G, Oldani A, et al. Snoring in twins. Respir Med. 1995;89(5):337–340. doi: 10.1016/0954-6111(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 70.Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. JAMA. 1986;256(1):51–54. [PubMed] [Google Scholar]
- 71.Loos RJ. Recent progress in the genetics of common obesity. Br J Clin Pharmacol. 2009;68(6):811–829. doi: 10.1111/j.1365-2125.2009.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palmer LJ, Buxbaum SG, Larkin EK, et al. Whole genome scan for obstructive sleep apnea and obesity in African-American families. Am J Respir Crit Care Med. 2004;169(12):1314–1321. doi: 10.1164/rccm.200304-493OC. [DOI] [PubMed] [Google Scholar]
- 73.Hollister DW, Godfrey M, Sakai LY, Pyeritz RE. Immunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndrome. N Engl J Med. 1990;323(3):152–159. doi: 10.1056/NEJM199007193230303. [DOI] [PubMed] [Google Scholar]
- 74.Kurihara Y, Kurihara H, Suzuki H, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368(6473):703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- 75.Lohnes D, Mark M, Mendelsohn C, et al. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120(10):2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 76.Sanford LP, Ormsby I, Gittenberger-de Groot AC, et al. TGF-beta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124(13):2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 78.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167(9):1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 79.Deegan PC, McNicholas WT. Predictive value of clinical features for the obstructive sleep apnoea syndrome. Eur Respir J. 1996;9(1):117–124. doi: 10.1183/09031936.96.09010117. [DOI] [PubMed] [Google Scholar]
- 80.Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84(3):1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- 81.Hudgel DWSP. The Human Airway During Sleep. In: Saunders NASC, editor. Sleep and Breathing. New York: Marcel Dekker; 1994. pp. 191–208. [Google Scholar]
- 82.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155(1):186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 83.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149(3):342–347. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 84.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: how and why do these relationships change with age? Psychol Bull. 2002;128(2):295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 85.Crisostomo I, Zayyad A, Carley DW, et al. Chemo- and baroresponses differ in African-Americans and Caucasians in sleep. J Appl Physiol. 1998;85(4):1413–1420. doi: 10.1152/jappl.1998.85.4.1413. [DOI] [PubMed] [Google Scholar]
- 86.Ong KC, Clerk AA. Comparison of the severity of sleep-disordered breathing in Asian and Caucasian patients seen at a sleep disorders center. Respir Med. 1998;92(6):843–848. doi: 10.1016/s0954-6111(98)90386-9. [DOI] [PubMed] [Google Scholar]
- 87.Li KK, Kushida C, Powell NB, Riley RW, Guilleminault C. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. 2000;110(10 Pt 1):1689–1693. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 88.Bouscoulet LT, Vazquez-Garcia JC, Muino A, et al. Prevalence of sleep related symptoms in four Latin American cities. J Clin Sleep Med. 2008;154(6):579–585. [PMC free article] [PubMed] [Google Scholar]
- 89.Tarasiuk A, Greenberg-Dotan S, Simon T, Tal A, Oksenberg A, Reuveni H. Low socioeconomic status is a risk factor for cardiovascular disease among adult obstructive sleep apnea syndrome patients requiring treatment. Chest. 2006;130(3):766–773. doi: 10.1378/chest.130.3.766. [DOI] [PubMed] [Google Scholar]
- 90.Olsen KD, Kern EB, Westbrook PR. Sleep and breathing disturbance secondary to nasal obstruction. Otolaryngol Head Neck Surg. 1981;89(5):804–810. doi: 10.1177/019459988108900522. [DOI] [PubMed] [Google Scholar]
- 91.Lavie P, Fischel N, Zomer J, Eliaschar I. The effects of partial and complete mechanical occlusion of the nasal passages on sleep structure and breathing in sleep. Acta Otolaryngol. 1983;95(1–2):161–166. doi: 10.3109/00016488309130930. [DOI] [PubMed] [Google Scholar]
- 92.Zwillich CW, Pickett C, Hanson FN, Weil JV. Disturbed sleep and prolonged apnea during nasal obstruction in normal men. Am Rev Respir Dis. 1981;124(2):158–160. doi: 10.1164/arrd.1981.124.2.158. [DOI] [PubMed] [Google Scholar]
- 93.Blakley BW, Mahowald MW. Nasal resistance and sleep apnea. Laryngoscope. 1987;97(6):752–754. doi: 10.1288/00005537-198706000-00023. [DOI] [PubMed] [Google Scholar]
- 94.Atkins M, Taskar V, Clayton N, Stone P, Woodcock A. Nasal resistance in obstructive sleep apnea. Chest. 1994;105(4):1133–1135. doi: 10.1378/chest.105.4.1133. [DOI] [PubMed] [Google Scholar]
- 95.Miljeteig H, Hoffstein V, Cole P. The effect of unilateral and bilateral nasal obstruction on snoring and sleep apnea. Laryngoscope. 1992;102(10):1150–1152. doi: 10.1288/00005537-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 96.Kerr P, Millar T, Buckle P, Kryger M. The importance of nasal resistance in obstructive sleep apnea syndrome. J Otolaryngol. 1992;21(3):189–195. [PubMed] [Google Scholar]
- 97.Lofaso F, Coste A, d’Ortho MP, et al. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J. 2000;16(4):639–643. doi: 10.1034/j.1399-3003.2000.16d12.x. [DOI] [PubMed] [Google Scholar]
- 98.Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med. 2001;25161(12):1514–1519. doi: 10.1001/archinte.161.12.1514. [DOI] [PubMed] [Google Scholar]
- 99.Wenzel M, Schonhofer B, Siemon K, Kohler D. Nasal strips without effect on obstructive sleep apnea and snoring. Pneumologie. 1997;51(12):1108–1110. [PubMed] [Google Scholar]
- 100.Jamieson A, Guilleminault C, Partinen M, Quera-Salva MA. Obstructive sleep apneic patients have craniomandibular abnormalities. Sleep. 1986;9(4):469–477. doi: 10.1093/sleep/9.4.469. [DOI] [PubMed] [Google Scholar]
- 101.Lowe AA, Santamaria JD, Fleetham JA, Price C. Facial morphology and obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1986;90(6):484–491. doi: 10.1016/0889-5406(86)90108-3. [DOI] [PubMed] [Google Scholar]
- 102.Lowe AA, Ono T, Ferguson KA, Pae EK, Ryan CF, Fleetham JA. Cephalometric comparisons of craniofacial and upper airway structure by skeletal subtype and gender in patients with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1996;110(6):653–664. doi: 10.1016/s0889-5406(96)80043-6. [DOI] [PubMed] [Google Scholar]
- 103.Maltais F, Carrier G, Cormier Y, Series F. Cephalometric measurements in snorers, non-snorers, and patients with sleep apnoea. Thorax. 1991;46(6):419–423. doi: 10.1136/thx.46.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zucconi M, Ferini-Strambi L, Palazzi S, Orena C, Zonta S, Smirne S. Habitual snoring with and without obstructive sleep apnoea: the importance of cephalometric variables. Thorax. 1992;47(3):157–161. doi: 10.1136/thx.47.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwab RJ. Upper airway imaging. Clin Chest Med. 1998;19(1):33–54. doi: 10.1016/s0272-5231(05)70430-5. [DOI] [PubMed] [Google Scholar]
- 106.Will MJ, Ester MS, Ramirez SG, Tiner BD, McAnear JT, Epstein L. Comparison of cephalometric analysis with ethnicity in obstructive sleep apnea syndrome. Sleep. 1995;18(10):873–875. doi: 10.1093/sleep/18.10.873. [DOI] [PubMed] [Google Scholar]
- 107.Wetter DW, Young TB. The relation between cigarette smoking and sleep disturbance. Prev Med. 1994;23(3):328–334. doi: 10.1006/pmed.1994.1046. [DOI] [PubMed] [Google Scholar]
- 108.Phillips BA, Danner FJ. Cigarette smoking and sleep disturbance. Arch Intern Med. 1995;155(7):734–737. [PubMed] [Google Scholar]
- 109.Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154(19):2219–2224. [PubMed] [Google Scholar]
- 110.Kashyap R, Hock LM, Bowman TJ. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath. 2001;5(4):167–172. doi: 10.1007/s11325-001-0167-5. [DOI] [PubMed] [Google Scholar]
- 111.Franklin KA, Gislason T, Omenaas E, et al. The influence of active and passive smoking on habitual snoring. Am J Respir Crit Care Med. 2004;170(7):799–803. doi: 10.1164/rccm.200404-474OC. [DOI] [PubMed] [Google Scholar]
- 112.Yules RB, Lippman ME, Freedman DX. Alcohol administration prior to sleep. The effect on EEG sleep stages. Arch Gen Psychiatry. 1967;16(1):94–97. doi: 10.1001/archpsyc.1967.01730190096012. [DOI] [PubMed] [Google Scholar]
- 113.Scanlan MF, Roebuck T, Little PJ, Redman JR, Naughton MT. Effect of moderate alcohol upon obstructive sleep apnoea. Eur Respir J. 2000;16(5):909–913. doi: 10.1183/09031936.00.16590900. [DOI] [PubMed] [Google Scholar]
- 114.Taasan VC, Block AJ, Boysen PG, Wynne JW. Alcohol increases sleep apnea and oxygen desaturation in asymptomatic men. Am J Med. 1981;71(2):240–245. doi: 10.1016/0002-9343(81)90124-8. [DOI] [PubMed] [Google Scholar]
- 115.Dawson A, Bigby BG, Poceta JS, Mitler MM. Effect of bedtime alcohol on inspiratory resistance and respiratory drive in snoring and nonsnoring men. Alcohol Clin Exp Res. 1997;21(2):183–190. [PubMed] [Google Scholar]
- 116.Tsutsumi W, Miyazaki S, Itasaka Y, Togawa K. Influence of alcohol on respiratory disturbance during sleep. Psychiatry Clin Neurosci. 2000;54(3):332–333. doi: 10.1046/j.1440-1819.2000.00701.x. [DOI] [PubMed] [Google Scholar]
- 117.Berry RB, Desa MM, Light RW. Effect of ethanol on the efficacy of nasal continuous positive airway pressure as a treatment for obstructive sleep apnea. Chest. 1991;99(2):339–343. doi: 10.1378/chest.99.2.339. [DOI] [PubMed] [Google Scholar]
- 118.Scrima L, Hartman PG, Hiller FC. Effect of three alcohol doses on breathing during sleep in 30–49 year old nonobese snorers and nonsnorers. Alcohol Clin Exp Res. 1989;13(3):420–427. doi: 10.1111/j.1530-0277.1989.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 119.Peppard PE, Austin D, Brown RL. Association of alcohol consumption and sleep disordered breathing in men and women. J Clin Sleep Med. 2007;3(3):265–270. [PMC free article] [PubMed] [Google Scholar]
- 120.Tanigawa T, Tachibana N, Yamagishi K, et al. Usual alcohol consumption and arterial oxygen desaturation during sleep. JAMA. 2004;292(8):923–925. doi: 10.1001/jama.292.8.923-b. [DOI] [PubMed] [Google Scholar]
- 121.Krol RC, Knuth SL, Bartlett D., Jr Selective reduction of genioglossal muscle activity by alcohol in normal human subjects. Am Rev Respir Dis. 1984;129(2):247–250. [PubMed] [Google Scholar]
- 122.Johnstone RE, Reier CE. Acute respiratory effects of ethanol in man. Clin Pharmacol Ther. 1973;14(4):501–508. doi: 10.1002/cpt1973144part1501. [DOI] [PubMed] [Google Scholar]
- 123.Sahn SA, Lakshminarayan S, Pierson DJ, Weil JV. Effect of ethanol on the ventilatory responses to oxygen and carbon dioxide in man. Clin Sci Mol Med. 1975;49(1):33–38. doi: 10.1042/cs0490033. [DOI] [PubMed] [Google Scholar]
- 124.Mitler MM, Dawson A, Henriksen SJ, Sobers M, Bloom FE. Bedtime ethanol increases resistance of upper airways and produces sleep apneas in asymptomatic snorers. Alcohol Clin Exp Res. 1988;12(6):801–805. doi: 10.1111/j.1530-0277.1988.tb01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82(4):1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 126.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144(3 Pt 1):494–498. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 127.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64(2):535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 128.Schwab RJ. Pro: sleep apnea is an anatomic disorder. Am J Respir Crit Care Med. 2003;168(3):270–271. 273. doi: 10.1164/rccm.2305014. discussion. [DOI] [PubMed] [Google Scholar]
- 129.Katz I, Stradling J, Slutsky AS, Zamel N, Hoffstein V. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990;141(5 Pt 1):1228–1231. doi: 10.1164/ajrccm/141.5_Pt_1.1228. [DOI] [PubMed] [Google Scholar]
- 130.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990;3(5):509–514. [PubMed] [Google Scholar]
- 131.Lyberg T, Krogstad O, Djupesland G. Cephalometric analysis in patients with obstructive sleep apnoea syndrome. I. Skeletal morphology. J Laryngol Otol. 1989;103(3):287–292. doi: 10.1017/s0022215100108734. [DOI] [PubMed] [Google Scholar]
- 132.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143(6):1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 133.Gold AR, Marcus CL, Dipalo F, Gold MS. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002;121(5):1531–1540. doi: 10.1378/chest.121.5.1531. [DOI] [PubMed] [Google Scholar]
- 134.Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle responses to upper airway pressure changes: afferent pathways. J Appl Physiol. 1982;52(2):445–450. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- 135.Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol. 1982;52(2):438–444. doi: 10.1152/jappl.1982.52.2.438. [DOI] [PubMed] [Google Scholar]
- 136.Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol. 1991;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Lunteren E. Muscles of the pharynx: structural and contractile properties. Ear Nose Throat J. 1993;72(1):27–29. 33. [PubMed] [Google Scholar]
- 138.Fogel RB, Trinder J, Malhotra A, et al. Within-breath control of genioglossal muscle activation in humans: effect of sleep-wake state. J Physiol. 2003;550(Pt 3):899–910. doi: 10.1113/jphysiol.2003.038810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532(Pt 2):467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65(5):2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 141.Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. J Appl Physiol. 1991;70(3):1328–1336. doi: 10.1152/jappl.1991.70.3.1328. [DOI] [PubMed] [Google Scholar]
- 142.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26(7):851–856. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 143.Ballard RD, Irvin CG, Martin RJ, Pak J, Pandey R, White DP. Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol. 1990;68(5):2034–2041. doi: 10.1152/jappl.1990.68.5.2034. [DOI] [PubMed] [Google Scholar]
- 144.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163(5):1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 145.Hudgel DW, Gordon EA, Thanakitcharu S, Bruce EN. Instability of ventilatory control in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158(4):1142–1149. doi: 10.1164/ajrccm.158.4.9712105. [DOI] [PubMed] [Google Scholar]
- 146.Cherniack NS. Respiratory dysrhythmias during sleep. N Engl J Med. 1981;305(6):325–330. doi: 10.1056/NEJM198108063050606. [DOI] [PubMed] [Google Scholar]
- 147.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172(11):1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 148.Asyali MH, Berry RB, Khoo MC. Assessment of closed-loop ventilatory stability in obstructive sleep apnea. IEEE Trans Biomed Eng. 2002;49(3):206–216. doi: 10.1109/10.983454. [DOI] [PubMed] [Google Scholar]
- 149.Longobardo GS, Gothe B, Goldman MD, Cherniack NS. Sleep apnea considered as a control system instability. Respir Physiol. 1982;50(3):311–333. doi: 10.1016/0034-5687(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 150.Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol. 1986;61(4):1438–1443. doi: 10.1152/jappl.1986.61.4.1438. [DOI] [PubMed] [Google Scholar]
- 151.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(11):1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jordan AS, Wellman A, Edwards JK, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol. 2005;99(5):2020–2027. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wheatley JR, White DP. The influence of sleep on pharyngeal reflexes. Sleep. 1993;16(Suppl 8):S87–S89. doi: 10.1093/sleep/16.suppl_8.s87. [DOI] [PubMed] [Google Scholar]
- 154.Stanchina ML, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165(7):945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 155.Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27(6):1105–1112. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168(6):645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 157.Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. J Appl Physiol. 2005;99(6):2440–2450. doi: 10.1152/japplphysiol.00772.2005. [DOI] [PubMed] [Google Scholar]
- 158.Kimoff RJ, Sforza E, Champagne V, Ofara L, Gendron D. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164(2):250–255. doi: 10.1164/ajrccm.164.2.2010012. [DOI] [PubMed] [Google Scholar]
- 159.Svanborg E. Upper airway nerve lesions in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164(2):187–189. doi: 10.1164/ajrccm.164.2.2105010c. [DOI] [PubMed] [Google Scholar]
- 160.Friberg D, Gazelius B, Hokfelt T, Nordlander B. Abnormal afferent nerve endings in the soft palatal mucosa of sleep apnoics and habitual snorers. Regul Pept. 1997;71(1):29–36. doi: 10.1016/s0167-0115(97)01016-1. [DOI] [PubMed] [Google Scholar]
- 161.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(5):541–546. doi: 10.1164/rccm.200308-1100OC. [DOI] [PubMed] [Google Scholar]
- 162.Ibrahim LH, Patel SR, Modarres M, et al. A measure of ventilatory variability at wake-sleep transition predicts sleep apnea severity. Chest. 2008;134(1):73–78. doi: 10.1378/chest.07-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 164.O’Donnell CP, Schaub CD, Haines AS, et al. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1477–1484. doi: 10.1164/ajrccm.159.5.9809025. [DOI] [PubMed] [Google Scholar]
- 165.Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath. 2003;7(3):105–110. doi: 10.1007/s11325-003-0105-9. [DOI] [PubMed] [Google Scholar]
- 166.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162(2 Pt 1):566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 167.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27(1):123–128. [PubMed] [Google Scholar]
- 168.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112(17):2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 169.Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126(5):1473–1479. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 170.Riha RL, Brander P, Vennelle M, et al. Tumour necrosis factor-alpha (-308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J. 2005;26(4):673–678. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- 171.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107(8):1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 172.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28(2):87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 173.Alzoghaibi MA, Bahammam AS. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: a pilot study. Sleep Breath. 2005;9(3):119–126. doi: 10.1007/s11325-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 174.Ohga E, Nagase T, Tomita T, et al. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol. 1999;87(1):10–14. doi: 10.1152/jappl.1999.87.1.10. [DOI] [PubMed] [Google Scholar]
- 175.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol. 2003;94(1):179–184. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 176.Ursavas A, Karadag M, Rodoplu E, Yilmaztepe A, Oral HB, Gozu RO. Circulating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndrome. Respiration. 2007;74(5):525–532. doi: 10.1159/000097770. [DOI] [PubMed] [Google Scholar]
- 177.Kohler M, Craig S, Nicoll D, Leeson P, Davies RJ, Stradling JR. Endothelial function and arterial stiffness in minimally symptomatic obstructive sleep apnea. Am J Respir Crit Care Med. 2008;178(9):984–988. doi: 10.1164/rccm.200805-717OC. [DOI] [PubMed] [Google Scholar]
- 178.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(5):613–618. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 179.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 180.Kobayashi K, Nishimura Y, Shimada T, et al. Effect of continuous positive airway pressure on soluble CD40 ligand in patients with obstructive sleep apnea syndrome. Chest. 2006;129(3):632–637. doi: 10.1378/chest.129.3.632. [DOI] [PubMed] [Google Scholar]
- 181.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169(3):348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 182.Fletcher EC, Lesske J, Qian W, Miller CC, III, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19(6 Pt 1):555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 183.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99(1):106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fletcher EC, Miller J, Schaaf JW, Fletcher JG. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep. 1987;10(1):35–44. doi: 10.1093/sleep/10.1.35. [DOI] [PubMed] [Google Scholar]
- 185.Jennum P, Wildschiodtz G, Christensen NJ, Schwartz T. Blood pressure, catecholamines, and pancreatic polypeptide in obstructive sleep apnea with and without nasal Continuous Positive Airway Pressure (nCPAP) treatment. Am J Hypertens. 1989;2(11 Pt 1):847–852. doi: 10.1093/ajh/2.11.847. [DOI] [PubMed] [Google Scholar]
- 186.Lapinski M, Przybylowski T, Lewandowski J, et al. Diurnal blood pressure rhythm and urinary catecholamine excretion in obstructive sleep apnoea and essential hypertension. J Hypertens Suppl. 1993;11(5):S292–S293. [PubMed] [Google Scholar]
- 187.Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest. 1993;103(3):722–727. doi: 10.1378/chest.103.3.722. [DOI] [PubMed] [Google Scholar]
- 188.Dimsdale JE, Coy T, Ziegler MG, Ancoli-Israel S, Clausen J. The effect of sleep apnea on plasma and urinary catecholamines. Sleep. 1995 Jun;18(5):377–381. [PubMed] [Google Scholar]
- 189.Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest. 2001;120(3):887–893. doi: 10.1378/chest.120.3.887. [DOI] [PubMed] [Google Scholar]
- 190.Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160(15):2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 191.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 192.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 193.O’Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159–1164. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111(5):614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 195.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107(1):68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 196.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163(2):344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 197.Heitmann J, Ehlenz K, Penzel T, et al. Sympathetic activity is reduced by nCPAP in hypertensive obstructive sleep apnoea patients. Eur Respir J. 2004;23(2):255–262. doi: 10.1183/09031936.04.00015604. [DOI] [PubMed] [Google Scholar]
- 198.Martinez-Garcia MA, Gomez-Aldaravi R, Soler-Cataluna JJ, Martinez TG, Bernacer-Alpera B, Roman-Sanchez P. Positive effect of CPAP treatment on the control of difficult-to-treat hypertension. Eur Respir J. 2007;29(5):951–957. doi: 10.1183/09031936.00048606. [DOI] [PubMed] [Google Scholar]
- 199.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47(5):840–845. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 200.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359(9302):204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 201.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27(6):1229–1235. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 202.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129(6):1459–1467. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 203.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 204.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 205.Hanly P, Sasson Z, Zuberi N, Lunn K. ST-segment depression during sleep in obstructive sleep apnea. Am J Cardiol. 1993;71(15):1341–1345. doi: 10.1016/0002-9149(93)90552-n. [DOI] [PubMed] [Google Scholar]
- 206.Franklin KA, Nilsson JB, Sahlin C, Naslund U. Sleep apnoea and nocturnal angina. Lancet. 1995;345(8957):1085–1087. doi: 10.1016/s0140-6736(95)90820-x. [DOI] [PubMed] [Google Scholar]
- 207.Alonso-Fernandez A, Garcia-Rio F, Racionero MA, et al. Cardiac rhythm disturbances and ST-segment depression episodes in patients with obstructive sleep apnea-hypopnea syndrome and its mechanisms. Chest. 2005;127(1):15–22. doi: 10.1378/chest.127.1.15. [DOI] [PubMed] [Google Scholar]
- 208.Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia-Touchard A, Lopez-Jimenez F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133(4):927–933. doi: 10.1378/chest.07-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Turmel J, Series F, Boulet LP, et al. Relationship between atherosclerosis and the sleep apnea syndrome: an intravascular ultrasound study. Int J Cardiol. 2009;132(2):203–209. doi: 10.1016/j.ijcard.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 210.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest. 1996;109(3):659–663. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- 211.Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1910–1913. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 212.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28(3):596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 213.Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99(1):26–30. doi: 10.1016/j.amjcard.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 214.Mehra R, Principe-Rodriguez K, Kirchner HL, Strohl KP. Sleep apnea in acute coronary syndrome: high prevalence but low impact on 6-month outcome. Sleep Med. 2006;7(6):521–528. doi: 10.1016/j.sleep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 215.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 216.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 217.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 218.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22(2):217–223. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 220.Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27(3):401–407. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 221.Mohsenin V, Valor R. Sleep apnea in patients with hemispheric stroke. Arch Phys Med Rehabil. 1995;76(1):71–76. doi: 10.1016/s0003-9993(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 222.Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161(2 Pt 1):375–380. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 223.Wessendorf TE, Teschler H, Wang YM, Konietzko N, Thilmann AF. Sleep-disordered breathing among patients with first-ever stroke. J Neurol. 2000;247(1):41–47. doi: 10.1007/pl00007787. [DOI] [PubMed] [Google Scholar]
- 224.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 226.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118(9):955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 227.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37(9):2317–2321. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 228.Partinen M, Jamieson A, Guilleminault C. Long-term outcome for obstructive sleep apnea syndrome patients. Mortality. Chest. 1988;94(6):1200–1204. doi: 10.1378/chest.94.6.1200. [DOI] [PubMed] [Google Scholar]
- 229.Partinen M, Guilleminault C. Daytime sleepiness and vascular morbidity at seven-year follow-up in obstructive sleep apnea patients. Chest. 1990;97(1):27–32. doi: 10.1378/chest.97.1.27. [DOI] [PubMed] [Google Scholar]
- 230.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26(3):293–297. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 231.Sahlin C, Sandberg O, Gustafson Y, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. 2008;168(3):297–301. doi: 10.1001/archinternmed.2007.70. [DOI] [PubMed] [Google Scholar]
- 232.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea hypopnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cherniack NS, Longobardo GS. Cheyne-Stokes breathing. An instability in physiologic control. N Engl J Med. 1973;288(18):952–957. doi: 10.1056/NEJM197305032881810. [DOI] [PubMed] [Google Scholar]
- 234.Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol. 1982;53(3):644–659. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- 235.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 236.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97(21):2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 237.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99(9):1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 238.Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension. 2000;35(1 Pt 1):144–147. doi: 10.1161/01.hyp.35.1.144. [DOI] [PubMed] [Google Scholar]
- 239.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98(8):772–776. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 240.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009;54(19):1797–1804. doi: 10.1016/j.jacc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169(12):1147–1155. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 244.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 245.Hoffman RP, Sinkey CA, Dopp JM, Phillips BG. Systemic and local adrenergic regulation of muscle glucose utilization during hypoglycemia in healthy subjects. Diabetes. 2002;51(3):734–742. doi: 10.2337/diabetes.51.3.734. [DOI] [PubMed] [Google Scholar]
- 246.Porte D, Jr, Williams RH. Inhibition of insulin release by norepinephrine in man. Science. 1966;152(726):1248–1250. doi: 10.1126/science.152.3726.1248. [DOI] [PubMed] [Google Scholar]
- 247.Bratel T, Wennlund A, Carlstrom K. Pituitary reactivity, androgens and catecholamines in obstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP) Respir Med. 1999;93(1):1–7. doi: 10.1016/s0954-6111(99)90068-9. [DOI] [PubMed] [Google Scholar]
- 248.Grunstein RR, Handelsman DJ, Lawrence SJ, Blackwell C, Caterson ID, Sullivan CE. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapy. J Clin Endocrinol Metab. 1989;68(2):352–358. doi: 10.1210/jcem-68-2-352. [DOI] [PubMed] [Google Scholar]
- 249.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(5):625–630. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 250.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293(5535):1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 251.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 252.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24(5):816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 253.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165(5):677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 254.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 255.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 256.Lam JC, Lam B, Lam CL, et al. Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med. 2006;100(6):980–987. doi: 10.1016/j.rmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 257.Okada M, Takamizawa A, Tsushima K, Urushihata K, Fujimoto K, Kubo K. Relationship between sleep-disordered breathing and lifestyle-related illnesses in subjects who have undergone health-screening. Intern Med. 2006;45(15):891–896. doi: 10.2169/internalmedicine.45.1592. [DOI] [PubMed] [Google Scholar]
- 258.Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006;29(6):777–783. doi: 10.1093/sleep/29.6.777. [DOI] [PubMed] [Google Scholar]
- 259.Peltier AC, Consens FB, Sheikh K, Wang L, Song Y, Russell JW. Autonomic dysfunction in obstructive sleep apnea is associated with impaired glucose regulation. Sleep Med. 2007;8(2):149–155. doi: 10.1016/j.sleep.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 260.Kono M, Tatsumi K, Saibara T, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131(5):1387–1392. doi: 10.1378/chest.06-1807. [DOI] [PubMed] [Google Scholar]
- 261.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25(9):735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 262.Strohl KP, Novak RD, Singer W, et al. Insulin levels, blood pressure and sleep apnea. Sleep. 1994;17(7):614–618. doi: 10.1093/sleep/17.7.614. [DOI] [PubMed] [Google Scholar]
- 263.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 264.Makino S, Handa H, Suzukawa K, et al. Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clin Endocrinol (Oxf) 2006;64(1):12–19. doi: 10.1111/j.1365-2265.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 265.Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med. 2000;248(1):13–20. doi: 10.1046/j.1365-2796.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 266.Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol. 2002;155(5):387–393. doi: 10.1093/aje/155.5.387. [DOI] [PubMed] [Google Scholar]
- 267.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169(2):156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 269.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62(11):969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Brooks B, Cistulli PA, Borkman M, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79(6):1681–1685. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 271.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29(4):720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 272.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165(4):447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 273.Smurra M, Philip P, Taillard J, Guilleminault C, Bioulac B, Gin H. CPAP treatment does not affect glucose-insulin metabolism in sleep apneic patients. Sleep Med. 2001;2(3):207–213. doi: 10.1016/s1389-9457(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 274.Saarelainen S, Lahtela J, Kallonen E. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res. 1997;6(2):146–147. doi: 10.1046/j.1365-2869.1997.00034.x. [DOI] [PubMed] [Google Scholar]
- 275.Redline S, Mehra R, Wang X, Babineau D, Aylor J, Ismail-Beigi F. A double-blind cross-over trial of CPAP vs sham-CPAP on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Am J Respir Crit Care Med. 2010;181:A2539. [Google Scholar]
- 276.Redline S, Mehra RWX, Babineau D, Aylor J, Ismail-Beigi F. A double-blind cross-over trial of CPAP vs sham-CPAP on metabolic control in individuals with impaired glucose tolerance and sleep apnea. American Thoracic Society Abstracts. 2010 [Google Scholar]
- 277.Savransky V, Nanayakkara A, Li J, et al. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175(12):1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Li J, Savransky V, Nanayakkara A, Smith PL, O’Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol. 2007;102(2):557–563. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 279.Savransky V, Jun J, Li J, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103(10):1173–1180. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Steiropoulos P, Tsara V, Nena E, et al. Effect of continuous positive airway pressure treatment on serum cardiovascular risk factors in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2007;132(3):843–851. doi: 10.1378/chest.07-0074. [DOI] [PubMed] [Google Scholar]
- 281.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax. 2004;59(9):777–782. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177(10):1142–1149. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Barcelo A, Barbe F, Llompart E, et al. Effects of obesity on C-reactive protein level and metabolic disturbances in male patients with obstructive sleep apnea. Am J Med. 2004;117(2):118–121. doi: 10.1016/j.amjmed.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 284.Engleman HM, Douglas NJ. Sleep 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(7):618–622. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7(6):498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 286.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(2):461–467. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 287.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163(5):565–571. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 289.Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep. 1997;20(8):608–613. doi: 10.1093/sleep/20.8.608. [DOI] [PubMed] [Google Scholar]
- 290.Hack M, Davies RJ, Mullins R, et al. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea. Thorax. 2000;55(3):224–231. doi: 10.1136/thorax.55.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ellen RL, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Son-Hing M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2(2):193–200. [PubMed] [Google Scholar]
- 292.Yang EH, Hla KM, McHorney CA, Havighurst T, Badr MS, Weber S. Sleep apnea and quality of life. Sleep. 2000;23(4):535–541. [PubMed] [Google Scholar]
- 293.Baldwin CM, Griffith KA, Nieto FJ, O’Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24(1):96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 294.Briones B, Adams N, Strauss M, et al. Relationship between sleepiness and general health status. Sleep. 1996;19(7):583–588. doi: 10.1093/sleep/19.7.583. [DOI] [PubMed] [Google Scholar]
- 295.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health Study. Sleep. 1998;21(1):27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 296.Grunstein RR, Stenlof K, Hedner JA, Sjostrom L. Impact of self-reported sleep-breathing disturbances on psychosocial performance in the Swedish Obese Subjects (SOS) Study. Sleep. 1995;18(8):635–643. doi: 10.1093/sleep/18.8.635. [DOI] [PubMed] [Google Scholar]
- 297.Millman RP, Fogel BS, McNamara ME, Carlisle CC. Depression as a manifestation of obstructive sleep apnea: reversal with nasal continuous positive airway pressure. J Clin Psychiatry. 1989;50(9):348–351. [PubMed] [Google Scholar]
- 298.Ware J. The SF-36 health survey. In: Spilker B, editor. Quality of Life in Pharmacoeconomic in Clinical Trials. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1996. pp. 337–345. [Google Scholar]
- 299.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med. 1998;158(2):494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 300.Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med. 2001;2(6):477–491. doi: 10.1016/s1389-9457(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 301.Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6(3):199–204. doi: 10.1046/j.1365-2869.1997.00043.x. [DOI] [PubMed] [Google Scholar]
- 302.D’Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure – a prospective study. Chest. 1999;115(1):123–129. doi: 10.1378/chest.115.1.123. [DOI] [PubMed] [Google Scholar]
- 303.Bennett LS, Barbour C, Langford B, Stradling JR, Davies RJ. Health status in obstructive sleep apnea: relationship with sleep fragmentation and daytime sleepiness, and effects of continuous positive airway pressure treatment. Am J Respir Crit Care Med. 1999;159(6):1884–1890. doi: 10.1164/ajrccm.159.6.9808107. [DOI] [PubMed] [Google Scholar]
- 304.Piccirillo JF, Gates GA, White DL, Schectman KB. Obstructive sleep apnea treatment outcomes pilot study. Otolaryngol Head Neck Surg. 1998;118(6):833–844. doi: 10.1016/S0194-5998(98)70277-3. [DOI] [PubMed] [Google Scholar]
- 305.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115(3):771–781. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 306.Bolitschek J, Schmeiser-Rieder A, Schobersberger R, Rosenberger A, Kunze M, Aigner K. Impact of nasal continuous positive airway pressure treatment on quality of life in patients with obstructive sleep apnoea. Eur Respir J. 1998;11(4):890–894. doi: 10.1183/09031936.98.11040890. [DOI] [PubMed] [Google Scholar]
- 307.Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20(6):406–422. [PubMed] [Google Scholar]
- 308.Chediak AD, Acevedo-Crespo JC, Seiden DJ, Kim HH, Kiel MH. Nightly variability in the indices of sleep-disordered breathing in men being evaluated for impotence with consecutive night polysomnograms. Sleep. 1996;19(7):589–592. doi: 10.1093/sleep/19.7.589. [DOI] [PubMed] [Google Scholar]
- 309.Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Variability in the apnea-hypopnea index and its consequences for diagnosis and therapy evaluation. Respiration. 2009;77(1):32–37. doi: 10.1159/000167790. [DOI] [PubMed] [Google Scholar]
- 310.Bittencourt LR, Suchecki D, Tufik S, et al. The variability of the apnoea-hypopnoea index. J Sleep Res. 2001;10(3):245–251. doi: 10.1046/j.1365-2869.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 311.Ahmadi N, Shapiro GK, Chung SA, Shapiro CM. Clinical diagnosis of sleep apnea based on single night of polysomnography vs two nights of polysomnography. Sleep Breath. 2009;13(3):221–226. doi: 10.1007/s11325-008-0234-2. [DOI] [PubMed] [Google Scholar]
- 312.Richard W, Kox D, den Herder C, Laman M, van Tinteren H, de Vries N. The role of sleep position in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2006;263(10):946–950. doi: 10.1007/s00405-006-0090-2. [DOI] [PubMed] [Google Scholar]
- 313.Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol. 1991;71(2):488–497. doi: 10.1152/jappl.1991.71.2.488. [DOI] [PubMed] [Google Scholar]
- 314.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169(6):668–672. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 315.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 316.Iber C, Redline S, Kaplan Gilpin AM, et al. Polysomnography performed in the unattended home versus the attended laboratory setting – Sleep Heart Health Study methodology. Sleep. 2004;27(3):536–540. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 317.Tonelli de Oliveira AC, Martinez D, Vasconcelos LF, et al. Diagnosis of obstructive sleep apnea syndrome and its outcomes with home portable monitoring. Chest. 2009;135(2):330–336. doi: 10.1378/chest.08-1859. [DOI] [PubMed] [Google Scholar]
- 318.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146(3):157–166. doi: 10.7326/0003-4819-146-3-200702060-00004. [DOI] [PubMed] [Google Scholar]
- 319.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 320.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 321.Ballester E, Badia JR, Hernandez L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(2):495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- 322.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness: a randomized, controlled trial. Ann Intern Med. 2001;134(11):1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 323.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(6):773–780. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 324.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(6):656–664. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 325.Chakravorty I, Cayton RM, Szczepura A. Health utilities in evaluating intervention in the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2002;20(5):1233–1238. doi: 10.1183/09031936.00.00014401. [DOI] [PubMed] [Google Scholar]
- 326.Engleman HM, McDonald JP, Graham D, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166(6):855–859. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- 327.Henke KG, Grady JJ, Kuna ST. Effect of nasal continuous positive a irway pressure on neuropsychological function in sleep apnea-hypopnea syndrome. A randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2001;163(4):911–917. doi: 10.1164/ajrccm.163.4.9910025. [DOI] [PubMed] [Google Scholar]
- 328.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353(9170):2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 329.Mansfeld DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169(3):361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 330.Marshall NS, Neill AM, Campbell AJ, Sheppard DS. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax. 2005;60(5):427–432. doi: 10.1136/thx.2004.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 332.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572–575. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 333.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1459–1463. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 334.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164(4):608–613. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 335.Sin DD, Mayers I, Man GC, Ghahary A, Pawluk L. Can continuous positive airway pressure therapy improve the general health status of patients with obstructive sleep apnea? a clinical effectiveness study. Chest. 2002;122(5):1679–1685. doi: 10.1378/chest.122.5.1679. [DOI] [PubMed] [Google Scholar]
- 336.Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3 Pt 1):858–865. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]
- 337.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 338.Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep. 2004;27(2):249–253. doi: 10.1093/sleep/27.2.249. [DOI] [PubMed] [Google Scholar]
- 339.Kasai T, Usui Y, Yoshioka T, et al. Effect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circ Heart Fail. 2010;3(1):140–148. doi: 10.1161/CIRCHEARTFAILURE.109.868786. [DOI] [PubMed] [Google Scholar]
- 340.Philippe C, Stoica-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337–342. doi: 10.1136/hrt.2005.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Pepin JL, Krieger J, Rodenstein D, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999;160(4):1124–1129. doi: 10.1164/ajrccm.160.4.9802027. [DOI] [PubMed] [Google Scholar]
- 342.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 343.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7(1):81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 344.Engleman HM, Asgari-Jirhandeh N, McLeod AL, Ramsay CF, Deary IJ, Douglas NJ. Self-reported use of CPAP and benefits of CPAP therapy: a patient survey. Chest. 1996;109(6):1470–1476. doi: 10.1378/chest.109.6.1470. [DOI] [PubMed] [Google Scholar]
- 345.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007;132(3):1057–1072. doi: 10.1378/chest.06-2432. [DOI] [PubMed] [Google Scholar]
- 346.Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake calibre of the velopharynx. Thorax. 1999;54(11):972–977. doi: 10.1136/thx.54.11.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Ng AT, Gotsopoulos H, Qian J, Cistulli PA. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;168(2):238–241. doi: 10.1164/rccm.200211-1275OC. [DOI] [PubMed] [Google Scholar]
- 348.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244–262. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 349.Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996;109(5):1269–1275. doi: 10.1378/chest.109.5.1269. [DOI] [PubMed] [Google Scholar]
- 350.Ferguson KA, Ono T, Lowe AA, al-Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax. 1997;52(4):362–368. doi: 10.1136/thx.52.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tan YK, L’Estrange PR, Luo YM, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur J Orthod. 2002;24(3):239–249. doi: 10.1093/ejo/24.3.239. [DOI] [PubMed] [Google Scholar]
- 352.Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic azbnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 1981;89(6):923–934. doi: 10.1177/019459988108900609. [DOI] [PubMed] [Google Scholar]
- 353.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19(2):156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 354.Ferguson KA, Heighway K, Ruby RR. A randomized trial of laserassisted uvulopalatoplasty in the treatment of mild obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167(1):15–19. doi: 10.1164/rccm.2108050. [DOI] [PubMed] [Google Scholar]
- 355.Larrosa F, Hernandez L, Morello A, Ballester E, Quinto L, Montserrat JM. Laser-assisted uvulopalatoplasty for snoring: does it meet the expectations? Eur Respir J. 2004;24(1):66–70. doi: 10.1183/09031936.04.00082903. [DOI] [PubMed] [Google Scholar]
- 356.Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2003;128(6):848–861. doi: 10.1016/S0194-59980300461-3. [DOI] [PubMed] [Google Scholar]
- 357.Stuck BA, Sauter A, Hormann K, Verse T, Maurer JT. Radiofrequency surgery of the soft palate in the treatment of snoring. A placebo-controlled trial. Sleep. 2005;28(7):847–850. doi: 10.1093/sleep/28.7.847. [DOI] [PubMed] [Google Scholar]
- 358.Ceylan K, Emir H, Kizilkaya Z, et al. First-choice treatment in mild to moderate obstructive sleep apnea: single-stage, multilevel, temperature-controlled radiofrequency tissue volume reduction or nasal continuous positive airway pressure. Arch Otolaryngol Head Neck Surg. 2009;135(9):915–919. doi: 10.1001/archoto.2009.117. [DOI] [PubMed] [Google Scholar]
- 359.Back LJ, Liukko T, Rantanen I, et al. Radiofrequency surgery of the soft palate in the treatment of mild obstructive sleep apnea is not effective as a single-stage procedure: a randomized single-blinded placebo-controlled trial. Laryngoscope. 2009;119(8):1621–1627. doi: 10.1002/lary.20562. [DOI] [PubMed] [Google Scholar]
- 360.Li HY, Wang PC, Lee LA, Chen NH, Fang TJ. Prediction of uvulopalatopharyngoplasty outcome: anatomy-based staging system versus severity-based staging system. Sleep. 2006;29(12):1537–1541. doi: 10.1093/sleep/29.12.1537. [DOI] [PubMed] [Google Scholar]
- 361.National Commission on Sleep Disorders Research. Wake up America: a national sleep alert. Bethesda. MD. 1995:2. [Google Scholar]
- 362.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]