Abstract
In humans adeno-associated virus (AAV)-mediated gene transfer is followed by expansion of AAV capsid-specific T cells, evidence of cell damage, and loss of transgene product expression, implicating immunological rejection of vector-transduced cells, which may be prevented by immunosuppressive drugs. We undertook this study to assess the effect of immunosuppression (IS) used for organ transplantation on immune responses to AAV capsid antigens. Recipients of liver or kidney transplants were tested before and 4 weeks after induction of IS in comparison with matched samples from healthy human adults and an additional cohort with comorbid conditions similar to those of the transplant patients. Our data show that transplant patients and comorbid control subjects have markedly higher frequencies of circulating AAV capsid-specific T cells compared with healthy adults. On average, IS resulted in a reduction of AAV-specific CD4+ T cells, whereas numbers of circulating CD8+ effector and central memory T cells tended to increase. Independent of the type of transplant or the IS regimens, the trend of AAV capsid-specific T cell responses after drug treatment varied; in some patients responses were unaffected whereas others showed decreases or even pronounced increases, casting doubt on the usefulness of prophylactic IS for AAV vector recipients.
Parzych and colleagues assess the effect of immunosuppression (IS) when used for organ transplantation on immune responses to adeno-associated virus (AAV) capsid antigens. Transplant patients and comorbid control subjects had markedly higher frequencies of circulating AAV capsid–specific T cells compared with healthy adults. IS treatment resulted in a reduction of AAV-specific CD4+ T cells, whereas numbers of circulating CD8+ effector and central memory T cells tended to increase.
Introduction
Adeno-associated viruses (AAVs) are small single-stranded DNA viruses that belong to the parvovirus family. Their genome contains two open reading frames, rep and cap, which encode regulatory proteins and the virus capsid, respectively. Numerous serotypes that infect primates have been identified, which show variability mainly in Cap and differ in their cell tropism (Gao et al., 2002). AAVs infect humans and other species, although they are not known to cause disease (Weitzman and Linden, 2011). They are dependoviruses and as such coinfect with a helper virus, such as an adenovirus, which provides functions that allow for the replication of AAV (Atchison et al., 1965). AAVs cause persistent infections. In general, they do not integrate but rather remain episomal (Schnepp et al., 2005). Most adult humans are infected with AAVs and more than 60% carry neutralizing antibodies to the more prevalent serotypes (Boutin et al., 2010). Low frequencies of circulating AAV capsid-specific CD8+ and CD4+ T cells have also been reported in humans and other primates (Li et al., 2011).
Recombinant AAVs are used for gene transfer (Mueller and Flotte, 2008; Vandenberghe et al., 2009; Mingozzi and High, 2011). In AAV vectors, the rep and cap genes are deleted and replaced with an expression cassette encoding a gene of interest. In most mammalian species, such as mice, dogs, and nonhuman primates, AAV-mediated gene transfer through the circulation results in sustained expression of the recombinant protein (Nathwani et al., 2007; Wang et al., 2005, 2010). In humans, on the other hand, AAV-mediated gene transfer is followed by an expansion of AAV capsid-specific T cells and a loss of transgene product expression (Manno et al., 2006; Mingozzi et al., 2007, 2009). Although a causative link between AAV capsid-specific T cells and loss of transgene product expression accompanied by evidence of cell damage, such as rises in transaminase levels, on hepatic gene transfer has not yet been proven, immunosuppression (IS) is being used in clinical AAV-mediated gene transfer to prevent T cell-mediated destruction of AAV-transduced cells. In a gene transfer trial in which patients with hemophilia received an AAV vector of serotype 8 encoding factor IX, which was targeted to the liver, an increase in liver transaminases accompanied by a decrease in factor IX expression was successfully treated with a short course of prednisone (Nathwani et al., 2011). AAV-mediated gene transfer combined with transient IS thus appears to prevent immune-mediated destruction of vector-transduced cells.
IS regimens have been optimized to allow for organ transplantation by preventing de novo responses to alloreactive histocompatibility antigens. Their effects on memory T cells induced by persisting viruses are not yet fully understood. Some IS drugs such as rapamycin (sirolimus), which is commonly used to prevent transplant rejection, have been shown to enhance responses to acute viral infections and promote memory formation if used at low doses (Araki et al., 2009). Antimetabolites, which target nucleic acid synthesis and thus cell proliferation, prevent induction of primary responses, which require T cell expansion, but may not be effective against ongoing responses to a persisting virus.
This study was undertaken to assess the effect of IS typically used after organ transplantation on T and B cell responses to AAV capsid antigens. To this end, recipients of liver or kidney transplants were tested just before and 4 weeks after surgery, and induction of IS by a variety of regimens that typically included combination therapies composed of steroids, tacrolimus (Prograf), Simulect (anti-CD25α chain antibody), azathioprine (Aza), mycophenolate mofetil (CellCept or MMF), and/or rapamycin (sirolimus). AAV-specific responses of transplant patients were compared with matched samples from healthy human adults, which were collected twice in the same interval to determine variations in responses without IS. Many of the transplant patients required liver replacement after excessive alcohol consumption or hepatitis virus infections acquired by intravenous drug use. To assess the effect of these comorbid conditions on AAV-specific immune responses, a third cohort of individuals with chronic liver disease that did not yet require transplantation was enrolled and tested as well. These individuals are referred to as comorbid control subjects.
Our data show, as we reported previously (Li et al., 2011), that most individuals have immunological memory to AAV capsid as evidenced by neutralizing antibodies or AAV capsid-specific T cells. T cell responses to AAV capsid were higher in transplant patients and comorbid control subjects than in healthy human adults. Matched samples from healthy human adults showed some variability between the two time points tested, but overall responses were stable. On average, IS of transplant patients resulted in a reduction of AAV-specific CD4+ T cells, while numbers of circulating CD8+ effector and central memory T cells tended to increase. Independent of the type of transplant or the IS regimens, the trend of AAV capsid-specific T cell responses after drug treatment varied; in some patients responses were unaffected whereas others showed decreases or even pronounced increases.
Materials and Methods
Isolation and preservation of lymphocytes
Peripheral blood mononuclear cells (PBMCs) were isolated as described (Li et al., 2011). They were tested immediately after isolation or frozen in 90% fetal bovine serum (FBS) and 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) at −80°C. All samples from a given individual were tested on the same day to minimize assay variability.
Intracellular cytokine staining
Numbers and functions of AAV capsid-specific CD8+ and CD4+ T cells were assessed by intracellular cytokine staining (ICS) after stimulation with an AAV-2 capsid peptide pool. All peptides were used at a final concentration of 2.5 μg of each peptide per milliliter. Frozen cells were thawed and incubated in RPMI medium overnight at 37°C in 10% CO2. Cells were then washed twice with Hanks' balanced salt solution (HBSS) supplemented with DNase I (2 units/ml), resuspended in RPMI, and stimulated for 6 hr with anti-CD28 (clone CD28.2), anti-CD49d (clone 9F10), in the presence of brefeldin A (Li et al., 2011). Cells were stained with one of two panels for surface markers for 30 min at room temperature in the dark. The first panel consisted of a LIVE/DEAD fixable aqua dead cell staining kit-AmCyan (Invitrogen, Carlsbad, CA), anti-CD14-APC-Cy7 (clone TüK4; Invitrogen), anti-CD19-APC-Cy7 (clone SJ25-C1; Invitrogen), anti-CD8-PE-Texas red (clone SFCI21Thy2D3; Beckman Coulter, Fullerton, CA), anti-CD4-PE-Cy5.5 (clone S3.5; Invitrogen), anti-CD27-QD 655 (clone CLB-27/1; Invitrogen), anti-CD57-PE (clone HCD57; BioLegend, San Diego, CA), and anti-CD45RO-QD 705 (from the laboratory of M. Betts, University of Pennsylvania, Philadelphia, PA). The second panel included a LIVE/DEAD fixable aqua dead cell staining kit-AmCyan (Invitrogen), anti-CD14-APC-Cy7 (clone TüK4; Invitrogen), anti-CD19-APC-Cy7 (clone SJ25-C1; Invitrogen), anti-CD8-PE-Cy5 (clone HIT8a), anti-CD4-PE Cy5.5 (clone S3.5; Invitrogen), anti-CD27-QD 655 (from the laboratory of M. Betts, University of Pennsylvania), anti-CD57-PE (clone HCD57; BioLegend), and anti-CD45RO-PE-Texas red (clone UCHL1; Beckman Coulter). After fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences, San Jose, CA) for 20 min at room temperature, all samples were then stained with anti-interferon (IFN)-γ-APC-Cy5.5 (clone B27), anti-interleukin (IL)-2-APC (clone MQ1-17H12), anti-tumor necrosis factor (TNF)-α-PE-Cy7 (clone MAb11), anti-perforin-Pacific blue (from the laboratory of M. Betts, University of Pennsylvania), and anti-macrophage inflammatory protein (MIP)-1α-FITC (clone 93342; R&D Systems, Minneapolis, MN) for 45 min at room temperature in the dark. Cells were washed twice, fixed with BD stabilizing fixative (BD Biosciences), and then analyzed by fluorescence-activated cell sorting (FACS), using an LSRII flow cytometer (BD Biosciences) and DiVa software. Single-color controls consisted of CompBeads anti-mouse Ig, κ (BD Biosciences). Unless otherwise noted, antibodies were purchased from BD Biosciences. Postacquisition analyses were performed with FlowJo (TreeStar, Ashland, OR). Some of the graphs were generated by SPICE (http://exon.niaid.nih.gov/spice/; Roederer et al., 2011). Cells were gated as follows: single cells were first gated onto lymphocytes and then gated onto the live cells, using a dead cell stain. Cells expressing CD14 or CD19 were excluded in a dump gate. Live cells were then gated onto CD8+ cells or CD4+ cells, which were further gated onto CD45RO and CD27 with CD45ROhiCD27hi-central memory cells, CD45ROhiCD27low effector memory cells, and CD45ROlowCD27low effector cells, which were then gated onto stains for IFN-γ, or IL-2 and TNF-α, or perforin and MIP-1α. Testing for 5 functions allows for a total of 32 different combinations; 31 of those were further analyzed. As perforin by itself tends to be nonspecific, this function was assessed only in combination with any of the other four functions. Responsiveness and the magnitude of the response of any of the subsets were determined by calculating the sum of T cells (normalized to 106 live CD3+ cells) exhibiting any of the 31 different functions on subtraction of normalized background data.
Titration of AAV neutralizing antibodies
AAV-2 neutralizing antibody titers in human were tested as described previously (Li et al., 2011) on HEK293 cells infected with adenovirus vector expressing green fluorescent protein (GFP).
Statistical analyses
Data for T cells, T cell subsets, and AAV-specific antibody titers were compared across the three patient cohorts, using Kruskal–Wallis tests. Then, for markers with significant results, post-hoc Wilcoxon tests were performed to determine between which cohorts the markers were significantly different. To compare the changes of each biomarker before and after transplantation versus that between first and second blood draw of healthy donors, the Wilcoxon rank sum test was used. For all tests results, p<0.05 are considered as significant.
Results
Human cohorts
Blood samples were drawn from a total of 60 patients undergoing transplant surgery. Samples were collected from patients just before surgery and then at 1 month after surgery and initiation of IS. Of the 60 transplant patients 34 were further analyzed; of those, 31 received liver transplants and 3 received kidney transplants from deceased donors for a number of indications such as chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection (41%), alcohol abuse (15%), autoimmune diseases such as primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC), lupus (15%), or others. Fifteen percent were female and 85% were male. The majority of patients were white (71%), 26% were African-Americans, and 1 patient (3%) was Hispanic. Patients were on average 56 years old, ranging from 25 to 75 years of age. At first, all consenting transplant patients were recruited into the study; but then patients who received T cell-depleting drugs such as thymoglobulin were excluded, which had the effect of enriching for liver transplant recipients in the cohort as thymoglobulin is not typically used in these patients at our center (University of Pennsylvania, Philadelphia, PA). Patients received a variety of regimens after surgery, most commonly combinations of tacrolimus (Prograf), azathioprine (Imuran), or mycophenolate derivatives (CellCept), and steroids. More detailed characteristics of the transplant patients and of the drug regimens employed are shown in Supplementary Table S1A (supplementary data are available online at www.liebertonline.com/hum).
Additional blood samples were drawn twice at monthly intervals from normal healthy donors from the Philadelphia area (Supplementary Table S1B). Donors were age-matched but percentages of African-Americans and females were higher than in the transplant patient group.
One blood sample was drawn and tested from 19 adults undergoing treatment for liver disease. This cohort was included to determine the effect of comorbid conditions such as liver disease due to drug or alcohol use and/or viral hepatitis infection as well as hepatic dysfunction on AAV capsid-specific T cell responses. These patients, from here on referred to as comorbid control subjects, were on average 55 years old, ranging from 48 to 72 years of age; 32% were female, 68% were male, 62% were white, 38% were African-American, and one individual was Hispanic. All suffered from liver disease caused most commonly by HCV (58%), PBC or PSC (16%), or alcohol (16%) (Supplementary Table S1C).
Samples that yielded a minimum of ∼107 live peripheral blood mononuclear cells were analyzed further, resulting in a total of 34 matched samples from transplant patients, 16 matched samples from healthy adults, and 19 samples from patients who served as comorbid control subjects.
T cell subsets
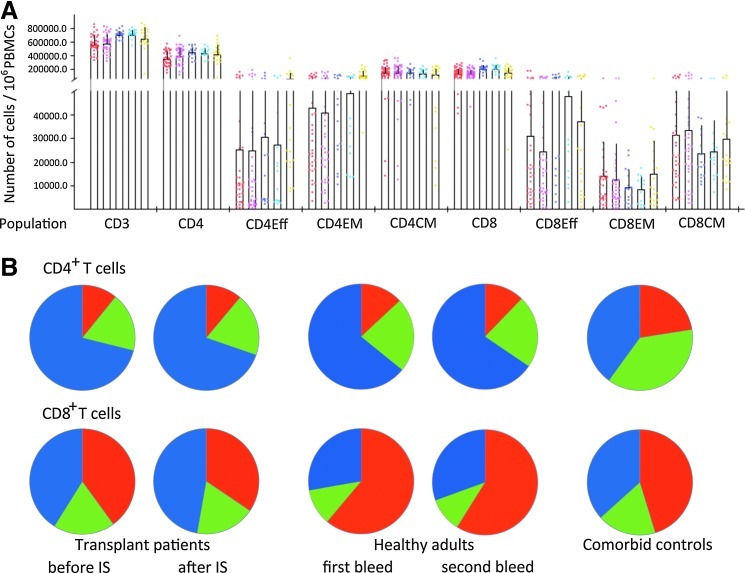
Samples were initially analyzed for numbers of T cells and T cell subsets including CD4+ and CD8+ effector cells, effector memory cells and memory cells. All data were normalized to numbers of cells per 106 live lymphoid PBMCs. As shown in Fig. 1, overall numbers of CD3+ cells were lower in transplant patients than in healthy adults, which was also reflected by lower numbers of CD4+ cells. The distribution of CD4+ T cell subsets was similar to that seen in healthy humans. Both cohorts had mainly central memory CD4+ T cells followed by effector memory CD4+ T cells; effector CD4+ T cells composed less than 10% of the overall CD4+ T cell population. Samples from comorbid control subjects were markedly different; in these patients effector memory CD4+ T cells were as common as central memory CD4+ T cells and numbers of effector CD4+ T cells were markedly higher than in the two other cohorts. CD8+ T cell subset distribution in transplant patients before surgery and IS treatment differed from that of healthy humans and was more comparable to that of the comorbid control subjects. In healthy human adults, effector CD8+ T cells were dominant followed by central memory CD8+ T cells; effector memory CD8+ T cells composed less than 10% of the CD8+ T cell subset. In healthy adults, CD8+ T cell subset distribution was similar at the two tested time points. In contrast, comorbid control subjects and transplant patients before IS had higher proportions of central and effector memory CD8+ T cells and a relative reduction in effector CD8+ T cells. IS did not cause a major reduction of CD3+, CD4+, or CD8+ T cells or a major shift in the dominant T cell subpopulations, although it caused a significant reduction in CD4+ effector T cells and CD8+ effector memory T cells. p Values for the comparisons from Wilcoxon rank sum tests between cohorts are shown in Supplementary Table S2.
FIG. 1.
T cell subsets. (A) Lymphocytes in blood of the three cohorts were analyzed by flow cytometry for expression of CD3, CD4, CD8, and the subset-defining markers CD45RO and CD27 with CD45ROhiCD27hi defining central memory cells, CD45ROlowCD27high defining effector memory T cells, and CD27lowCD45ROlow defining effector cells. Data were normalized to numbers of cells within 106 live PBMCs. Graph shows average frequencies of a given cohort tested at the defined time plus upper whiskers for standard deviations. In addition, data from individuals are shown as dots. Red dots represent transplant patients before immunosuppression (IS), pink dots show the same patients after IS. Purple and blue dots represent the first and second samples from healthy human subjects. Yellow represents results from comorbid control subjects. (B) On the basis of the same data shown in (A), percentages of the various CD4+ and CD8+ T cell subsets were compared in the various cohorts. Central memory cells are shown as blue, effector memory cells as green, and effector cells as red parts of the pie charts.
Magnitude of AAV capsid-specific T cell responses
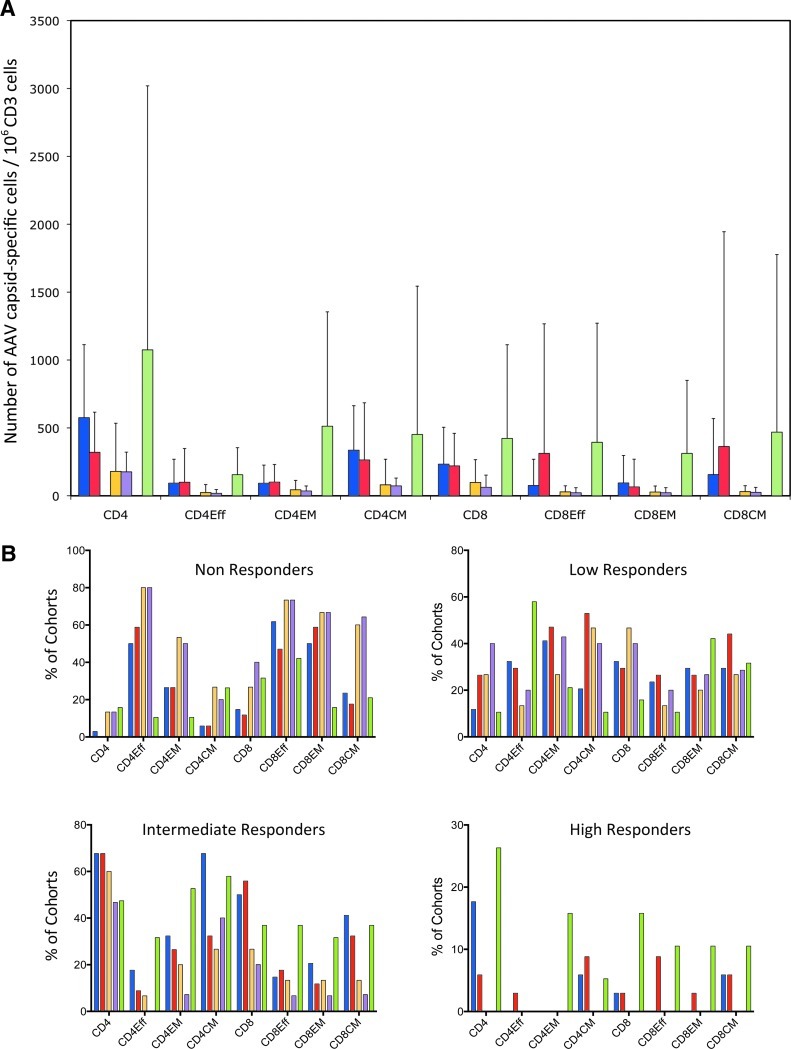
AAV capsid-specific T cell responses were assessed for all CD4+ and CD8+ T cells as well as for the three subsets of effector, effector memory, and central memory T cells. T cells were tested by flow cytometry for the production of IFN-γ, IL-2, TNF-α, MIP-1α, and perforin in response to a peptide pool reflecting the sequence of the AAV capsid. The experiments (Fig. 2A) showed that transplant patients had, across all subsets, higher AAV capsid-specific T cell responses as compared with healthy control subjects. Responses were even higher in the comorbid control subjects. On IS, numbers of AAV capsid-specific CD4+ and CD4+ central memory T cells decreased whereas numbers of effector and central memory CD8+ T cells increased. AAV capsid-specific responses in the various cohorts were further determined by calculating percentages of individuals who failed to carry AAV capsid-specific T cells of a given subset (fewer that 20 AAV capsid-specific cells/106 CD3+ cells after background subtraction), showed a low response (20 to <100 cells), an intermediate response (100 to <1000 cells), or a high response (≥1000 cells) (Fig. 2B). At both blood draws, healthy adults had the highest percentages of non- or low responders across all T cell subsets. Intermediate and high responses were more common in transplant patients, and this pattern was preserved after initiation of IS. Comorbid control subjects again had the highest percentages of intermediate and high responders for most subsets.
FIG. 2.
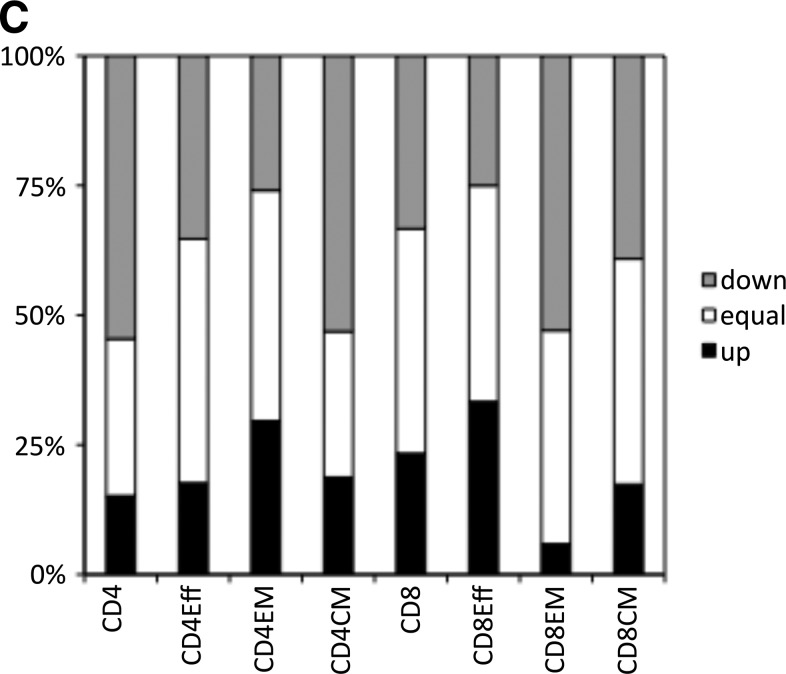
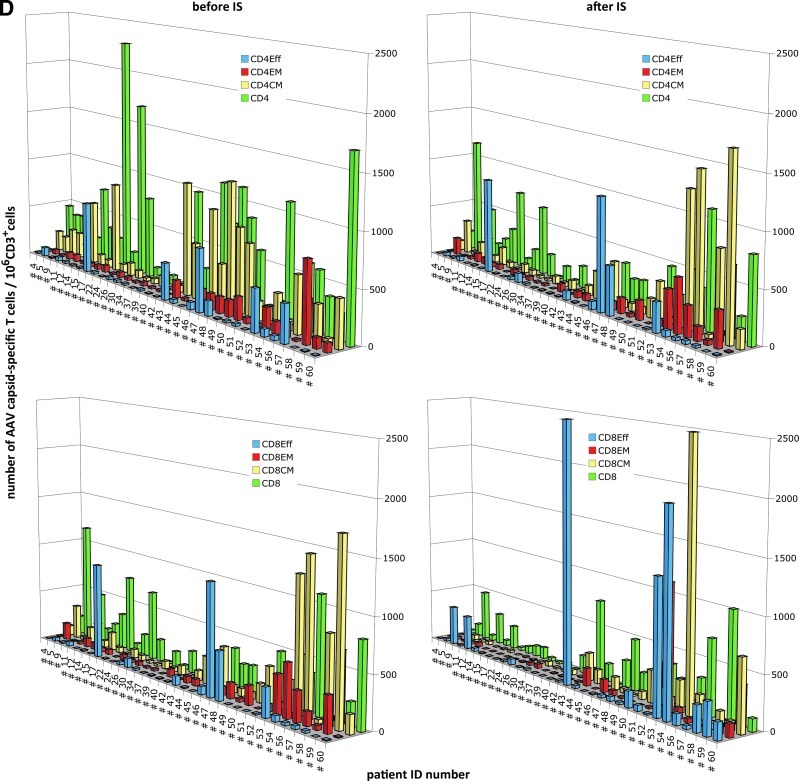
Magnitude of AAV capsid-specific T cell responses. (A) Average numbers of AAV capsid-specific T cells over 106 live CD3 cells for all cohorts. As indicated in the legend, IS patients before and after IS treatment initiation are shown in blue and red, respectively, the two consecutively harvested samples from healthy humans are shown in yellow and purple, respectively, and samples from comorbid control subjects are shown in light green. (B) Percentages of individuals who failed to respond (red; fewer than 20 cells/106 live CD3+ cells of a given subset), showed low responsiveness (yellow; 20 to <100 cells/106 live CD3+ cells), intermediate responsiveness (green; 100 to <1000 cells/106 live CD3+ cells), and high responsiveness (blue; ≤1000 cells/106 live CD3+ cells). Normalized background values were subtracted before the analysis. (C) Percentages of transplant patients who, after IS, either maintained stable numbers of AAV capsid-specific T cells (less than 2-fold changes, white part of columns), showed more that 2-fold decreases (gray part of columns), or increases (black part of columns). (D) AAV capsid-specific CD4+ cells (top) or CD8+ cells (bottom) and their subsets of individual patients before (left) and after (right) IS. Overall responses are shown in green, effector cells in blue, effector memory cells in red, and central memory cells in yellow.
IS, in some patients, changed the overall magnitude of responses by either increasing or decreasing their numbers. In about 40% of patients, the magnitude of AAV capsid-specific responses of most T cell subsets remained unchanged after IS (less than 2-fold differences before and after IS). In more than 50% of patients, IS effected a more than 2-fold decrease in CD4+ T cells, CD4+ central memory T cells, or CD8+ effector memory T cells. Decreases in the other subsets were less common and were observed in 25–40% of patients (Fig. 2C and D). Increases were also observed and more than 25% of patients showed increases in CD4+ central memory and/or CD8+ effector T cells. Some patients showed increases in some subsets that were accompanied by decreases in others. Many of the more dramatic changes were driven by low numbers of AAV capsid-specific T cells and were therefore unlikely to be of biological relevance. Nevertheless, marked changes were also seen in patients who were responders to AAV capsid. Focusing on the latter subset of patients, we assessed whether decreases or increases in responses related to age, sex, race, etiology of disease, or type of IS regimen used at the time of transplant surgery. Excluding patients who were nonresponders before and after IS showed that patients requiring liver transplants due to virus infection or alcohol abuse had a 2-fold higher likelihood to develop increased AAV capsid-specific CD8+ or CD8+ effector T cells after IS. None of the other parameters such as age, sex, race, or drug regimen at the onset of treatment were related to other changes in AAV capsid-specific T cell responses on IS. Patient distribution related to clinical factors and change status is shown in Supplementary Table S3A for CD4+ T cells and in Supplementary Table S3B for CD8+ T cells.
CD4+ T cell functions
Functions of AAV capsid-specific T cells were measured by determining their dominant cytokine/perforin production profiles. Specifically, we analyzed T cells for production of MIP-1α, an inflammation-promoting chemokine, and TNF-α and IFN-γ, which are in general produced by more activated T cells and that can both upregulate MHC molecules and thereby promote T cell-mediated target cell lysis. We also tested for perforin, one of the lytic enzymes that enables T cells to lyse their targets, and IL-2, which is indicative for a more resting stage of T cells. In healthy individuals, 50 versus 60% of AAV capsid-specific CD4+ T cells exhibited single functions at the two time points. The second most common population produced MIP-1α alone or with other functions (36 vs. 44% at first and second bleed) (Supplementary Fig. S1A). The cytokine/perforin production profile was similar in transplant patients before IS although relative portions of CD4+ T cells producing TNF-α or IL-2 alone or in combination with other factors was higher at 32 and 44% respectively, whereas cells producing MIP-1α or in combination with other factors were less common (26%). This pattern shifted after IS, with cells producing MIP-1α becoming more abundant (56%) at the expense of T cells producing TNF-α (32% before IS and 11% after IS). CD4+ T cells from comorbid control subjects differed with more than 50% of AAV capsid-specific CD4+ T cells producing IFN-γ only.
Frequencies of CD4+ effector T cells were low in healthy adults and were mainly monofunctional, producing IFN-γ, TNF-α, or IL-2 (64 vs. 70%, first vs. second bleed). CD4+ effector T cells from transplant patients were more polyfunctional, although those producing only TNF-α (23%) predominated before IS. Frequencies of CD4+ effector T cells with most functions declined after onset of IS, with the exception of those producing IFN-γ or MIP-1α alone or with perforin, which increased (5–27% for IFN-γ; 34–43% for MIP-1α with perforin). CD4+ effector cells of comorbid control subjects produced mainly IFN-γ (17%) or MIP-1α (Supplementary Fig. S1B).
CD4+ effector memory T cells were rare in healthy adults; those that were detectable produced mainly MIP-1α (17%) or TNF-α or IFN-γ alone (17%) or in combination with other factors (17%). In transplant patients CD4+ effector memory T cells producing IL-2 alone or in combination with other factors were more common before IS (31%); after initiation of IS, CD4+ effector memory T cells producing TNF-α only became dominant (51%). CD4+ effector memory T cells from comorbid control subjects again were clearly distinct with a large fraction being single positive for IFN-γ (27%), MIP-1α (25%), or TNF-α (18%) (Supplementary Fig. S1C).
Numbers of CD4+ central memory T cells were low in healthy individuals and produced at roughly equal proportions MIP-1α, TNF-α, or IFN-γ alone or in combination. Transplant patients showed higher numbers of CD4+ central memory T cell responses with a similar cytokine profile, which for the more common functions remained stable after IS. Central memory CD4+ T cells from comorbid control subjects were dominated by cells producing only IFN-γ (Supplementary Fig. S1D).
Statistical analyses of AAV capsid-specific CD4+ T cell responses
We used Wilcoxon rank sum tests to compare the responses of transplant patients at baseline (before IS) with those of healthy donors (first blood draw). The following differences in AAV-specific CD4+ T cell responses were found to be significant: Transplant patients had higher CD4+ T cell responses and this was combined with significant differences in cells producing all five factors or IFN-γ in combination with perforin. At baseline, transplant patients had higher numbers of overall CD4+ effector T cells and, within this subset, higher numbers of cells producing IL-2 together with TNF-α. Transplant patients also had higher numbers of effector memory CD4+ T cells although no single function was significantly different from those of healthy donors. Central memory CD4+ T cells were also more frequent in transplant patients, and the difference in such cells producing IL-2 with MIP-1α reached significance (Supplementary Table S4A). Kruskal–Wallis tests comparing AAV capsid-specific CD4+ T cells across transplant patients at baseline, healthy human donors (first blood draw), and comorbid control subjects showed significant differences in the sum of AAV capsid-specific CD4+ cells and all three CD4+ T cell subsets. None of the functions of the overall CD4+ T cell response were found to be different. For CD4+ T effector cells, those that produced (1) IL-2, MIP-1α, and perforin with or without TNF-α or (2) IL-2 only were different. Differences were more pronounced for effector memory CD4+ T cells, which showed significance for subsets producing (1) IFN-γ and MIP-1α with or without TNF-α, (2) IL-2, MIP-1α, and perforin with or without TNF-α, (3) IL-2 and MIP-1α, (4) IL-2 and TNF-α, or (5) MIP-1α with TNF-α. p Values from Wilcoxon rank sum tests between cohorts are shown in Supplementary Table S4B–D for variables with p<0.05 from the Kruskal–Wallis tests.
By Wilcoxon rank sum tests, we next compared changes in AAV capsid-specific CD4+ T cell numbers and functions after IS with changes in healthy donors between the first and second bleed to determine whether any of the changes in transplant patients were likely to have been caused by IS rather than by natural variations in CD4+ T cell responses. For the overall CD4+ T cell response, only the sum was significantly different. For CD4+ effector T cells, the sum of the response was not significantly different although changes in subsets producing IFN-γ with IL-2 and MIP-1α or TNF-α only were different. No significant difference was observed for effector memory CD4+ T cells. Central memory CD4+ T cells, although they failed to show significant IS-induced changes in the magnitude, had differences in functions. Specifically they showed differences in portions of cells producing IFN-γ, IL-2, MIP-1α and cell producing MIP-1α, perforin, IFN-γ, IL-2, and TNF-α (Supplementary Table S4E).
CD8+ T cell functions
CD8+ T cells are assumed to play a dominant role in rejecting AAV-transduced cells after gene transfer. Only a fraction of healthy human adults had circulating AAV capsid-specific CD8+ T cells. Transplant patients and comorbid control subjects had markedly higher numbers of AAV capsid-specific CD8+ T cells. In transplant patients before IS, these cells produced IFN-γ, MIP-1α, TNF-α, and IL-2 at approximately equal ratios. After IS CD8+ T cells producing only MIP-1α became dominant (55%), whereas those producing IFN-γ, IL-2, and especially TNF-α declined, presumably due to Prograf, which inhibits the production of some cytokines. The profile of AAV capsid-specific CD8+ T cells of comorbid control subjects resembled that of transplant patients after IS (Supplementary Fig. S2).
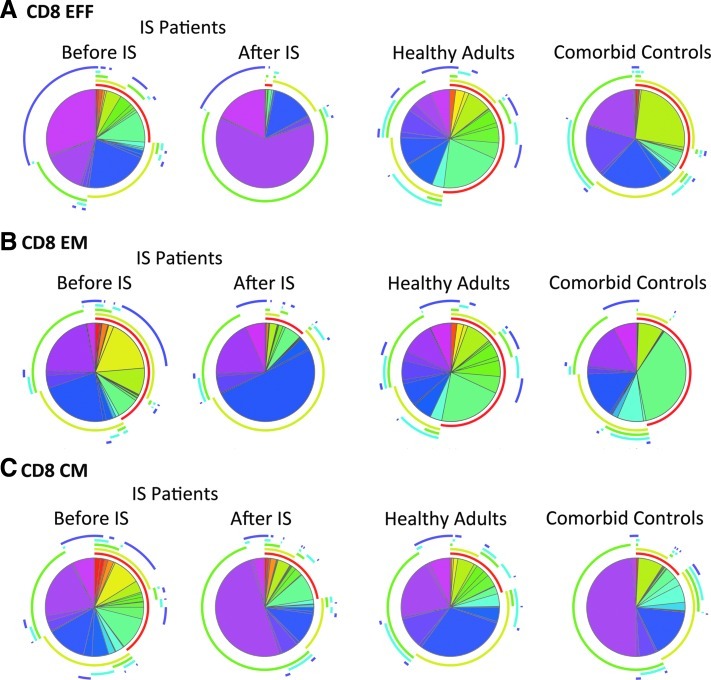
Only a few healthy adults carried AAV-specific CD8+ effector T cells. In transplant patients, who more commonly had circulating CD8+ effector T cells, such cells were mainly monofunctional, producing TNF-α (30%), IL-2 (21%), IFN-γ (10%), or MIP-1α (14%). Perforin-producing cells were rare. After IS, average numbers of CD8+ effector T cells increased and produced mainly MIP-1α (62%). Comorbid control subjects again were unique in carrying large portions of CD8+ effector T cells that produced IFN-γ together with IL-2 (25%) or MIP-1α together with perforin (Fig. 3A).
FIG. 3.
CD8+ T cell response to AAV capsid. The pie charts show the distribution of AAV capsid-specific CD8+ T cell functions. Contributions of individual functions are highlighted by the pie arcs, which show IFN-γ in red, IL-2 in light green, MIP-1α in bright green, perforin in blue, and TNF-α in purple. (A) CD8+ effector T cells; (B) CD8+ effector memory T cells; (C) CD8+ central memory T cells.
AAV capsid-specific CD8+ effector memory T cells were relatively rare in all cohorts, especially in healthy adults. Transplant patients before IS showed increased numbers of cells producing IL-2 (22%) or TNF-α in combination with IL-2 and IFN-γ (28%). After IS the response became dominated by cells producing only IL-2 (50%). Responding comorbid control subjects carried mainly IFN-γ only-producing CD8+ effector memory T cells (Fig. 3B).
AAV capsid-specific CD8+ central memory T cells were again rare in healthy human adults. Several of the transplant patients had high numbers of central memory CD8+ T cells that, before IS, exhibited a multitude of functions. After IS, average numbers of AAV capsid-specific CD8+ central memory T cells tended to increase in most of the functionally distinct classes, and this was especially pronounced for T cells producing TNF-α (66%). IL-2-producing AAV capsid-specific CD8+ central memory T cells decreased after IS. Central memory CD8+ T cells from comorbid control subjects were dominated by those that produced only MIP-1α (Fig. 3C).
In addition, we compared in transplant patients numbers of global and AAV capsid-specific CD8+ T cells or subsets thereof expressing CD57, a marker for terminal T cell differentiation (Mollet et al., 1998). By Student t test, there was a significant difference in CD57 expression only on effector memory CD8+ T cells; numbers of those expressing CD57 decreased significantly on IS (p=0.003). For CD57 expression on AAV capsid-specific CD8+ T cells we analyzed those producing MIP-1α, the factor that was most commonly produced by such cells in response to their cognate antigen, and found no significant changes on IS (data not shown).
Statistical analyses of AAV capsid-specific CD8+ T cell responses
Comparing transplant patients at baseline with healthy human adults showed differences in the magnitude of the overall AAV capsid-specific CD8+ T cell response, with significant differences in cells producing MIP-1α and perforin with or without MIP-1α. Effector, effector memory, and central memory CD8+ T cells did not differ in magnitude of the response but did differ in functionalities. Specifically, effector CD8+ T cells showed differences in subsets producing (1) IFN-γ, IL-2, and perforin, (2) IL-2 alone, and (3) MIP-1α with perforin. Effector memory CD8+ T cells differed in subsets producing IFN-γ combined with perforin or MIP-1α. Central memory CD8+ T cells differed only in those producing IFN-γ together with IL-2 (Supplementary Table S5A). Comparisons of the three cohorts showed differences in the magnitude of the effector and central memory CD8+ T cell response to AAV capsid. Regarding individual functions, differences were seen for the overall CD8+ T cell response for cells producing (1) IFN-γ, IL-2, and MIP-1α with or without perforin, (2) IFN-γ, MIP-1α, perforin, and TNF-α, (3) IL-2, MIP-1α, and perforin with/without TNF-α and IL-2, and (4) MIP-1α, IL-2, and perforin. CD8+ effector T cells differed in cells producing (1) all five functions and cells producing (2) IFN-γ, MIP-1α, and perforin, (3) IL-2, MIP-1α, and perforin with/without TNF-α, (4) MIP-1α and perforin, or (5) IL-2 only. CD8+ effector memory cells differed in those producing (1) IFN-γ with perforin, (2) IL-2, MIP-1α, and perforin with/without TNF-α, or (3) MIP-1α only. CD8+ central memory cells differed in those that produced (1) IL-2, MIP-1α, and perforin with/without TNF-α and (2) MIP-1α and TNF-α with or without perforin. p Values for the Kruskal-Wallis tests are shown in Supplementary Table S5B–E for samples with p<0.05.
The analysis of changes induced by IS compared with changes in healthy adults during the 4-week sampling interval showed no significant differences in the overall CD8+ T cell response; significant changes in the magnitude of AAV capsid-specific effector CD8+ T cells and within this subset of cells producing IL-2 only; no changes in the sum of the effector memory CD8+ T cell response but differences in the subset producing IFN-γ, IL-2, MIP-1α, and perforin; and no changes in the CD8+ central memory response, although within this subset T cells producing MIP-1α, perforin, and TNF-α differed (Supplementary Table S5A–E).
AAV-specific antibody titers
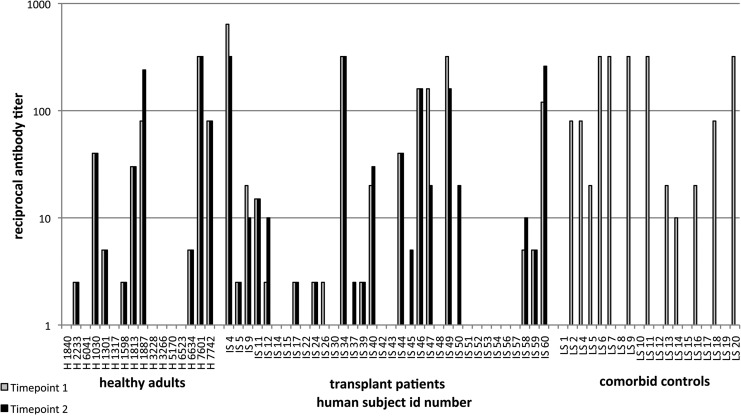
Sera from individuals of the three cohorts were tested for neutralizing antibodies to AAV-2 (Fig. 4). In most healthy adults as well as in transplant patients, titers remained stable between sample collections or at most varied by one dilution factor, which is within the error of the assay. Only one subject in the healthy adult cohort (subject 1887) showed a marked increase in titer between the first and second samples, whereas one of the transplant patients (patient 47) showed a pronounced decrease. The healthy subject had low numbers of AAV capsid-specific T cells in the blood, which failed to increase in the second sample. Patient 47 was an intermediate to high responder. His CD4+ T cells decreased after IS whereas his number of AAV capsid-specific CD8+ T cells increased. Otherwise there were no significant differences in average antibody titer between the three cohorts or between samples collected at different times.
FIG. 4.
AAV-specific neutralizing antibody titers. The graph shows titers of neutralizing antibodies to AAV-2. Lightly shaded columns, titers measured in the first samples; solid columns, titers measured in the second samples taken 4 weeks later. ID numbers: H, healthy adults; IS, transplant patients; CC, comorbid control subjects.
Discussion
AAV-mediated gene transfer vehicles are unique among vectors that are being extensively used in clinical investigation, in that they are completely gutted and thereby unable to synthesize their own antigens. Immune responses to viral antigens, which are thought to eliminate AAV-transduced cells and prevent sustained expression of therapeutic transgene products, is thus limited to a short period of time before the AAV gene transfer particles have been degraded and the resulting peptides have been eliminated. This process, as clinical experience with immune responses to capsid antigens of various types of AAV gene transfer vehicles has shown, is expected to take 4–8 weeks (Mingozzi et al., 2007; Nathwani et al., 2011). One would assume that AAV-mediated gene transfer combined with a short course of IS would prevent immune responses to the structural antigens of AAV vectors and thereby improve their therapeutic benefits.
IS has been optimized to allow for organ allotransplantation. In general, IS regimens consist of combinations of drugs with different modes of action to prevent induction of immune responses against alloantigens of the transplant. Such drugs include mycophenolate mofetil (MMF), which on being metabolized into mycophenolic acid inhibits inosine monophosphate dehydrogenase, the enzyme that controls synthesis of purine, which is essential for proliferation of B and T lymphocytes (Gummert et al., 1999; Ritter and Pirofski, 2009). Azathioprine, on conversion into thioinosinic acid, is another purine synthesis inhibitor (Gummert et al., 1999). Tacrolimus prevents dephosphorylation of NF-AT, a family of transcription factors that control production of cytokines by T cells (Kiani et al., 2000). Sirolimus (also known as rapamycin) inhibits the mammalian target of rapamycin (mTOR) pathway by directly binding the mTOR complex 1, which is essential to increase protein synthesis and metabolism on T cell activation. The exact mode of action of steroids in unknown; they have antiinflammatory activity and inhibit protein synthesis (Sehgal and Sirolimus, 2003).
Although IS is known to lessen graft rejection by inhibiting de novo T cell responses to alloantigens, less is known about its effects on memory T cell responses to common human pathogens or ongoing immune responses to persisting viruses such as AAVs. Increased susceptibility of patients on IS to pathogens or reactivation of persisting viruses suggests that IS can reduce recall immune responses (Scroggs et al., 1987; Glenn, 1981; Lucas et al., 1997). Previous studies with kidney transplant recipients on IS maintenance treatment showed selective effects on various T cell subsets. Specifically, IS caused a long-term reduction in naive CD4+ T cells and effector CD8+ T cells with relative increases in effector memory CD4+ T cells and effector and central memory CD8+ T cells (Grigoryev et al., 2010). IS was also shown to lack efficacy in reducing allergic diseases (Shroff et al., 2012), suggesting that its effects are selective and not necessarily predictable in the context of existing immune responses to viral antigens.
The observation that a 4- to 8-week course of prednisolone can dramatically reduce circulating levels of AAV capsid-specific T cells and prevent rejection of AAV-transduced hepatocytes has enabled long-term expression of factor IX from an AAV vector in men with severe hemophilia B (Nathwani et al., 2011). We conducted this study to assess the effect of IS regimens used for liver or kidney transplantation on AAV capsid-specific immune responses to determine whether such regimens could be used transiently to reduce T cell responses and thus likely rejection of AAV-transduced cells after gene transfer. T and B cell responses were analyzed before and early, at 4 weeks, after surgery to determine the effect of the initial induction therapy. Responses of transplant patients were compared with those of age-matched healthy adults who were also analyzed at a 4-week interval to distinguish variations of immune responses to a persisting and presumably periodically reactivating virus from changes induced by IS. From this comparison it became obvious that transplant candidates before surgery had markedly higher T cell responses to AAV capsid antigens compared with healthy adults, which may reflect more frequent viral infections or reactivations due to lower immunological resistance under conditions of chronic disease. We therefore also included a cohort of individuals who had similar underlying diseases as the transplant patients, such as chronic hepatitis B or C virus infections or liver destruction due to alcohol abuse or autoimmunity. T cell responses of these comorbid control subjects were in some aspects similar to those of transplant patients, although in other aspects they were unique.
At baseline, transplant patients had higher numbers of circulating AAV capsid-specific CD4+ T cells of all three subsets compared with healthy adults. Comorbid control subjects also had higher AAV capsid-specific responses of the more activated effector and effector memory CD4+ T cells, whereas there was no difference in numbers of central memory CD4+ T cells compared with healthy adults. Numbers of the two former subsets were even higher in comorbid control subjects compared with transplant patients. Differences were not as pronounced for AAV capsid-specific CD8+ T cells, for which numbers diverged only for effector and central memory CD8+ T cells between the three cohorts. Comparing numbers of functionally distinct T cell subsets, differences were seen between transplant patients, healthy adults, and comorbid control subjects mainly for CD4+ effector T cells producing IL-2 in combination with other factors and CD4+ effector memory T cells producing MIP-1α in combination with other factors. Differences in CD8+ T cell responses between comorbid control subjects and the other two cohorts mainly reflected cells producing MIP-1α and/or perforin across all three subsets. The finding that transplant patients at baseline, as well as comorbid control subjects, had higher numbers of AAV capsid-specific T cells was unexpected and suggests either more frequent infections with distinct AAV serotypes, which share conserved T cell epitopes (Chen et al., 2006), or more common reactivations of persisting virus. We view the former as unlikely, as antibody responses to AAV-2 revealed approximately equal numbers of seronegative individuals within the three cohorts. Reactivation of endogenous AAV would require helper functions, which during primary infection are provided by adenoviruses. Genotoxic stress has been shown to allow for replication of AAV in the absence of adenovirus at least in vitro (Yalkinoglu et al., 1988), and it is feasible that diseases that cause extensive kidney or liver cell damage promote replication of AAV. The higher frequencies of AAV capsid-specific T cells in comorbid control subjects as compared with transplant candidates may reflect extensive cell damage in the former cohort, thus reducing numbers of cells that may allow for AAV replication.
Our main question was whether IS changes numbers of AAV capsid-specific T cells beyond those seen over time in healthy adults. Our results show that IS caused a significant reduction in AAV capsid-specific CD4+ T cells, which could be attributed to a reduction in those belonging to the central memory subset mainly producing MIP-1α and/or TNF-α in combination with other factors. IS had no effect on total numbers of AAV capsid-specific CD8+ T cells; it reduced numbers of CD8+ effector memory T cells producing MIP-1α alone or in combination with other factors but increased numbers of AAV capsid-specific central memory CD8+ T cells, especially those producing perforin in combination with MIP-1α and other factors. Average numbers of effector CD8+ T cells increased, again affecting mainly cells producing perforin in combination with other factors, although this increase did not reach statistical significance.
Numbers of AAV capsid-specific T cells in individual transplant patients either remained stable, decreased, or even increased after initiation of IS. Decreases could be a reflection of direct IS-induced T cell death or they could reflect changes in T cell trafficking. Increases could also reflect trafficking or homeostatic proliferation of T cells after IS-induced T cell depletion (Hickman and Turka, 2005), or they could reflect antigen-driven expansion of T cells due to reactivation of AAV. Changes in AAV capsid-specific T cells (i.e., decreases in overall CD4+ T cells, central memory CD4+ T cells, and effector memory CD8+ T cells with increases in central memory CD8+ T cells) did not faithfully track changes in overall T cell subset distributions after IS, thus making changes due to homeostatic proliferation unlikely. Expansion of T cells or changes in migration due to AAV reactivation can be envisioned. Reactivation of persisting adenoviruses is a well-documented complication of IS after organ transplantation (Ardehali et al., 2001) and in turn may support AAV replication. AAV-specific antibody titers failed to increase after IS. This suggests that AAVs were most likely not reactivated after IS, although we cannot rule out some limited replication that failed to reach the threshold for activation of B cells.
We attempted to correlate changes in AAV capsid-specific T cell responses on IS that went beyond natural variability in healthy human subjects to patients' characteristics, their underlying diseases, or specifics of the drug regimen. Subtle correlations were most likely undetectable because of the modest number of patients and differences in diseases and treatment. Our statistical analyses did show that patients requiring transplants due to alcohol abuse or viral infections had a ≥2-fold higher likelihood of developing increased overall CD8+ T cell responses and CD8+ effector T cell responses to AAV capsid as compared with other patients.
Our study does not support the notion that transplant IS regimens given at the time of gene transfer are likely to reduce AAV capsid-specific T cell responses as detectable in blood. Instead, in some patients, IS may cause increased responses, especially of perforin-expressing CD8+ T cells, which are the likely culprits of immunological destruction of AAV-transduced cells. One caveat must be stressed: Our study was conducted with transplant patients who, before IS, already had markedly higher numbers of circulating AAV capsid-specific CD4+ and CD8+ T cells compared with healthy adults. This most likely was linked to the underlying tissue damage, as comorbid control subjects with similar diseases also had elevated responses. Additional studies are needed to determine the magnitude of the AAV capsid-specific T cell response in patients with genetic defects that might be amenable to gene transfer, such as that in patients with hemophilia B, which may also be elevated due to chronic tissue damage after bleeds.
In summary, IS regimens used for organ transplantation may not effectively reduce AAV-specific T cells. Recipients of AAV gene transfer vectors should be monitored carefully for several weeks to assess, if possible, fluctuations in amounts of transgene product, levels of enzymes indicative of damage to the injected tissue, and circulating AAV capsid- and transgene product-specific T cells to promptly identify upcoming immune responses that may eliminate AAV-transduced cells. In recipients of AAV-8 vectors expressing factor IX a transient treatment with steroids at the time of rising transaminases successfully abrogated further liver damage and markedly reduced numbers of circulating AAV capsid-specific T cells (Nathwani et al., 2011). For the time being, careful monitoring and, if needed, a short course of steroids should remain the protocol of choice to address periods of immune rejection after AAV-mediated gene transfer.
Supplementary Material
Acknowledgments
This work was supported by a grant from the NIH/NHLBI (grant 5 P01 HL078810) and by funds from the State of Pennsylvania. Dr. High is an Investigator of the Howard Hughes Medical Institute, which supported this work. Dr. Levine's contribution was supported in part by NIDDK grant 1K08DK092282-01. The authors acknowledge the contributions of Mary Shaw, Debra McCorriston, and Brian Conboy, who facilitated the clinical coordination of patient enrollment into this study.
Author Disclosure Statement
KH has patents on the use of AAV for gene transfer.
References
- Araki K. Turner A.P. Shaffer V.O., et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardehali H. Volmar K. Roberts C., et al. Fatal disseminated adenoviral infection in a renal transplant patient. Transplantation. 2001;71:998–999. doi: 10.1097/00007890-200104150-00029. [DOI] [PubMed] [Google Scholar]
- Atchison R.W. Casto B.C. Hammon W.M. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S. Monteilhet V. Veron P., et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Chen J. Wu Q. Yang P., et al. Determination of specific CD4 and CD8 T cell epitopes after AAV2- and AAV8-hF.IX gene therapy. Mol. Ther. 2006;13:260–269. doi: 10.1016/j.ymthe.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Glenn J. Cytomegalovirus infections following renal transplantation. Rev. Infect Dis. 1981;3:1151–1178. doi: 10.1093/clinids/3.6.1151. [DOI] [PubMed] [Google Scholar]
- Grigoryev Y.A. Kurian S.M. Avnur Z., et al. Deconvoluting post-transplant immunity: Cell subset-specific mapping reveals pathways for activation and expansion of memory T, monocytes and B cells. PLoS One. 2010;5:e13358. doi: 10.1371/journal.pone.0013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummert J.F. Ikonen T. Morris R.E. Newer immunosuppressive drugs: A review. J. Am. Soc. Nephrol. 1999;10:1366–1380. doi: 10.1681/ASN.V1061366. [DOI] [PubMed] [Google Scholar]
- Hickman S.P. Turka L.A. Homeostatic T cell proliferation as a barrier to T cell tolerance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1713–1721. doi: 10.1098/rstb.2005.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani A. Rao A. Aramburu J. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity. 2000;12:359–372. doi: 10.1016/s1074-7613(00)80188-0. [DOI] [PubMed] [Google Scholar]
- Li H. Lasaro M.O. Jia B., et al. Capsid-specific T-cell responses to natural infections with adeno-associated viruses in humans differ from those of nonhuman primates. Mol. Ther. 2011;19:2021–30. doi: 10.1038/mt.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas K.G. Pollok K.E. Emanuel D.J. Post-transplant EBV induced lymphoproliferative disorders. Leuk. Lymphoma. 1997;25:1–8. doi: 10.3109/10428199709042491. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. Maus M.V. Hui D.J., et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. Meulenberg J.J. Hui D.J., et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F. High K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nat. Rev. Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- Mollet L. Sadat-Sowti B. Duntze J, et al. CD8hi+CD57+ T lymphocytes are enriched in antigen-specific T cells capable of down-modulating cytotoxic activity. Int. Immunol. 1998;10:311–323. doi: 10.1093/intimm/10.3.311. [DOI] [PubMed] [Google Scholar]
- Mueller C. Flotte T.R. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- Nathwani A.C. Gray J.T. McIntosh J., et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani A.C. Tuddenham E.G. Rangarajan S., et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter M.L. Pirofski L. Mycophenolate mofetil: Effects on cellular immune subsets, infectious complications, and antimicrobial activity. Transpl. Infect. Dis. 2009;11:290–297. doi: 10.1111/j.1399-3062.2009.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M. Nozzi J.L. Nason M.C. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79A:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepp B.C. Jensen R.L. Chen C.L., et al. Characterization of adeno-associated virus genomes isolated from human tissues. J. Virol. 2005;79:14793–14803. doi: 10.1128/JVI.79.23.14793-14803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggs M.W. Wolfe J.A. Bollinger R.R. Sanfilippo F. Causes of death in renal transplant recipients. A review of autopsy findings from 1966 through 1985. Arch. Pathol. Lab. Med. 1987;111:983–987. [PubMed] [Google Scholar]
- Sehgal S.N. Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant. Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- Shroff P. Mehta R.S. Chinen J., et al. Presentation of atopic disease in a large cohort of pediatric liver transplant recipients. Pediatr. Transplant. 2012;16:379–384. doi: 10.1111/j.1399-3046.2012.01684.x. [DOI] [PubMed] [Google Scholar]
- Vandenberghe L.H. Wilson J.M. Gao G. Tailoring the AAV vector capsid for gene therapy. Gene Ther. 2009;16:311–319. doi: 10.1038/gt.2008.170. [DOI] [PubMed] [Google Scholar]
- Wang L. Calcedo R. Nichols T.C., et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- Wang L. Wang H. Bell P., et al. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol. Ther. 2010;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman M.D. Linden R.M. Adeno-associated virus biology. Methods Mol. Biol. 2011;807:1–23. doi: 10.1007/978-1-61779-370-7_1. [DOI] [PubMed] [Google Scholar]
- Yalkinoglu A.O. Heilbronn R. Burkle A., et al. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 1988;48:3123–3129. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.