Abstract
In Saccharomyces cerevisiae, the class C vacuole protein sorting (Vps) proteins, together with Vam2p/Vps41p and Vam6p/Vps39p, form a complex that interacts with soluble N-ethylmaleimide-sensitive factor attachment protein receptor and Rab proteins to “tether” vacuolar membranes before fusion. To determine a role for the corresponding mammalian orthologues, we examined the function, localization, and protein interactions of endogenous mVps11, mVps16, mVps18, mVam2p, and mVam6. We found a significant proportion of these proteins localized to early endosome antigen-1 and transferrin receptor-positive early endosomes in Vero, normal rat kidney, and Chinese hamster ovary cells. Immunoprecipitation experiments showed that mVps18 not only interacted with Syntaxin (Syn)7, vesicle-associated membrane protein 8, and Vti1-b but also with Syn13, Syn6, and the Sec1/Munc18 protein mVps45, which catalyze early endosomal fusion events. Moreover, anti-mVps18 antibodies inhibited early endosome fusion in vitro. Mammalian mVps18 also associated with mVam2 and mVam6 as well as with the microtubule-associated Hook1 protein, an orthologue of the Drosophila Hook protein involved in endosome biogenesis. Using in vitro binding and immunofluorescence experiments, we found that mVam2 and mVam6 also associated with microtubules, whereas mVps18, mVps16, and mVps11 associated with actin filaments. These data indicate that the late Vps proteins function during multiple soluble N-ethylmaleimide-sensitive factor attachment protein receptor-mediated fusion events throughout the endocytic pathway and that their activity may be coordinated with cytoskeletal function.
INTRODUCTION
Soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) proteins are central to membrane fusion, forming tight 4 helix bundles that pull opposing membranes together (Hay, 2001). Although much is known about how SNARE complexes form, less is known about the upstream processes that regulate SNARE assembly. Some specificity is contributed by the SNARE proteins themselves, which catalyze fusion only in particular combinations due to the conformation of both the SNARE domain and N-terminal regulatory domain (Sollner, 2003). However, this intrinsic specificity is unlikely to suffice in vivo, and many other SNARE-interacting proteins have been proposed to contribute to the specificity of membrane fusion (Wickner and Haas, 2000; Chen and Scheller, 2001; Hey, 2001; Wendler and Tooze, 2001; Sollner, 2003).
One important function that controls fusion specificity is the aggregation of compartments before the formation of trans-SNARE pairs or SNARE-pins. So-called “tethering” factors provide this function and are typically comprised of oligomeric complexes that associate directly or indirectly with SNARE proteins, Rab proteins, and Sec1/Munc-like (S/M) proteins (Waters and Pfeffer, 1999; Whyte and Munro, 2002). Only limited sequence or mechanistic conservation has been observed between tethering complexes, implying that each complex is tailored to provide and integrate a number of events specific to each fusion step (Waters and Pfeffer, 1999; Waters and Hughson, 2000).
Some of the yeast late vacuole protein sorting (Vps) proteins act as membrane tethers during fusion to yeast endosomes and vacuoles (Peterson and Emr, 2001; Price et al., 2000; Sato et al., 2000; Srivastava et al., 2000). These proteins form a hetero-oligomeric complex comprised of the class C Vps proteins (Vps18p, Vps16p, Vps11p, and the S/M protein Vps33p; Rieder and Emr, 1997; Seals et al., 2000) as well as a subcomplex consisting of the class B Vps proteins Vam6p and Vam2p (Price et al., 2000). The resulting homotypic vacuolar protein sorting (HOPS) complex localizes to subdomains of the vacuole where fusion occurs (Price et al., 2000; Seals et al., 2000; Wurmser et al., 2000; Eitzen et al., 2002; Wang et al., 2003). The HOPS complex also interacts with both SNAREs (such as Vam3p, Nyv1p, and Vti1p) and the Rab GTPase Ypt7p (Price et al., 2000; Sato et al., 2000; Seals et al., 2000; Wurmser et al., 2000; Eitzen et al., 2002; Wang et al., 2003). Mammalian orthologues of the HOPS proteins have been described and seem to also function in late endosomal fusion events. Overexpression of components such as mVps18 or mVam6 clusters late endocytic compartments and immunoprecipitation experiments show that Syntaxin (Syn)7, which catalyzes late endosomal fusion events, associates with the mammalian class C Vps proteins (Mullock et al., 2000; Caplan et al., 2001; Kim et al., 2001; Poupon et al., 2003).
The Drosophila orthologue of Vps18 (Deep Orange, DOR) also interacts with the Hook protein, and mammalian isoforms of Hook associate with the cytoskeleton (Lloyd et al., 1998; Kramer and Phistry, 1999; Sevrioukov et al., 1999; Sunio et al., 1999; Narayanan et al., 2000; Walenta et al., 2001). This latter association is interesting because fusion events within the endocytic pathway are also controlled spatially by the cytoskeleton, which serves to transport and anchor compartments at the right place to undergo fusion (Qualmann et al., 2000). Moreover, other putative tethering complexes have been found to associate with the cytoskeleton, suggesting that cytoskeletal functions are coordinated with the SNARE-based fusion apparatus.
This study expands the role of the mammalian HOPS proteins in the endocytic system. We find that the endogenous HOPS proteins significantly colocalize with early endosomal compartments. We also find that mVps18 associates not only with late but also early endosomal SNAREs such as Syn13 and Syn6 as well as the S/M protein mVps45. Additionally, antibodies specific to mVps18 inhibit the fusion of early endosomes in vitro. Interestingly, the mammalian HOPS proteins differentially interact with the actin and microtubule cytoskeleton, suggesting they may coordinate cytoskeletal attachment and movement of endosomes with their SNARE-dependent fusion.
MATERIALS AND METHODS
Unless otherwise stated all reagents were from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich (St. Louis, MO).
Cloning of cDNAs
Using standard BLAST algorithms, mouse cDNAs encoding mouse (m)Vps18, mVps16, and mVps11, and mVam2 and mVam6 were identified by searching the mouse expressed sequence tag (EST) database for contiguous sequences that showed significant homology to known yeast open reading frames. PCR primers designed to amplify a ∼300-base pair fragment of each coding region were used to screen several mouse cDNA libraries arrayed in microtiter plates (Origene, Rockville, MD). Sequencing cDNAs encoding the full-length protein showed complete identity with recently described sequences in GenBank. The sequences we used were mVps18 (BC039176), mVps16 (AB056721) and mVps11 (AK090030), mVam2 (XM_127209), and mVam6 (XM_031720). Mouse homologues of Drosophila Hook were identified by BLAST searching the mouse EST database. A C-terminal fragment of one contiguous sequence was amplified by PCR and sequenced. This fragment corresponds to the mouse homologue of the human Hook1 isoform (GenBank NM_015888). The eGFP-Rab5 expressing plasmid was a kind gift from Prof. Phillip Stahl (Washington University, St. Louis, MO) (Roberts et al., 1999).
Antibodies
PCR fragments encoding regions of mVps11, mVps16, mVps18, mVam2, mVam6, and Hook1 (Figure 1, A and B) were cloned into pGEX (Amersham Biosciences, Piscataway, NJ) or pMAL (New England Biolabs, Beverly, MA) vectors to produce glutathione S-transferase (GST) or maltose binding protein (MBP) fusion proteins (Table 1) according to manufacturers' instructions. When truncated proteins were produced, BL21RIL codon plus cells were used (Strategene, La Jolla, CA) (Shin et al., 1997) and led to the production of full-length soluble proteins in every instance.
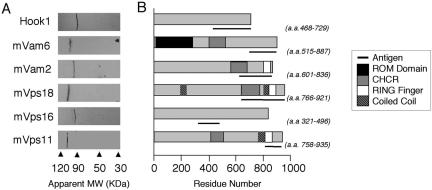
Figure 1.
Antibody detection and domain structure of mammalian late Vps proteins. (A) Affinity-purified polyclonal antibodies to the following proteins were used to immunoblot 50 μg of postnuclear supernatant from B16 melanoma cells: Hook1 (∼85 kDa), mVam6 (∼101 kDa), mVam2 (∼98.5 kDa), mVps18 (∼110 kDa), mVps16 (∼95 kDa), and mVps11(∼108 kDa). (B) Schematic of each protein's predicted domain structure and the region to which antibodies were generated (-).
Table 1.
Plasmids used to isolate antibodies
| Gene | Immunized vector | Plasmid | Purification vector | Plasmid |
|---|---|---|---|---|
| mVPS18 | pGEX | pPL1180 | pMAL | pPL1407 |
| mVPS16 | pMAL | pPL1244 | pMAL | pPL1244 |
| mVPS11 | pMAL | pPL1241 | pMAL | pPL1699 |
| mVam2 | pGEX | pPL1091 | pMAL | pPL1479 |
| mVam6 | pMAL | pPL1654 | pMAL | pPL1654 |
| Hook1 | pMAL | pPL1553 | pMAL | pPL1553 |
Serum collected from immunized rabbits was exhaustively subtracted by passage over a column made by conjugating a soluble protein fraction derived from bacteria expressing both GST and MBP to Affi-gel10 and Affi-gel 15 Sepharose (Bio-Rad, Hercules CA). Subtracted serum was then passed over a column containing covalently bound recombinant antigen (Table 1), eluted with 100 mM glycine pH 2.5, and then exhaustively dialyzed against phosphate-buffered saline (PBS). Antibodies specific for mVps11, mVps16, and mVam2 were further immunodepleted using saponin-extracted, aldehyde-fixed nuclei.
Antibody specificity was characterized using a series of immunoblotting and immunofluorescent experiments against cultured cell types and tissues. Figure 1 shows the results of immunoblotting mouse B16F10 postnuclear supernatant with each antibody.
Previous studies have defined two splice variants of mVam2, one of which lacks the C-terminal Zn finger domain (McVey Ward et al., 2001). This variant occurs by insertion of an extra exon near the C terminus that introduces a premature stop codon. Although the intron/exon structures of the mouse and human genes are similar, the extra human exon is not conserved in the mouse genome. We did not observe any splice variants (in this region) in the mouse EST database, consistent with previous studies in human cells, showing this is not an abundant transcript (McVey Ward et al., 2001). Our anti-mVam2 antibodies recognized a single band corresponding to full-length mVam2 (Figure 1A). In agreement with Kramer and colleagues (Walenta et al., 2001) our antibodies to mouse Hook1 recognized a single band of ∼85 kDa. No cross reactivity was observed with the other smaller Hook isoforms, Hook2 and Hook3 (Walenta et al., 2001).
All other antibodies were characterized previously or were from commercial sources (Table 2) except rabbit polyclonal antibodies to mouse Vti1-b (amino acids 1–207), Vamp7 (amino acids 1–180), which were raised against GST fusion proteins and affinity purified essentially as described for antibodies to mouse Vps proteins above. Plasmids encoding mouse Vti1-b were a kind gift from Dr. Gabrielle Fischer Von-Mollard (University of Oregon, Eugene, OR). Antibodies to Syn7 (rabbit and goat) and mVps45 were described previously (Tellam et al., 1997, Mullock et al., 2000).
Table 2.
Antibodies used
| Antigen | Clone/Catalog no. | Antibody | Source |
|---|---|---|---|
| EEA1 | (E41120) | MAb | Transduction Laboratories (Lexington, KY) |
| Syn6 | (S5542) | MAb | Transduction Laboratories |
| hTfR | (H68.4) | MAb | Zymed Laboratories (South San Francisco, CA) |
| CD63 | (RFAC4) | MAb | Biodesign (Saco, ME) |
| Actin | (JLA20) | MAb | DSHBa |
| β-Tubulin | (E7) | MAb | DSHBa |
| LAMP1 | (H4A3) | MAb | DSHBa |
| LAMP2 | (H4B4) | Mab | DSHBa |
| Lactate dehydroginase | (HH17) | Mab | Sigma-Aldrich |
| Syn13 | (110 132) | PAb (rabbit) | Synaptic Systems (Goettingen, Germany) |
| Texas Red anti-rabbit | (T-2767) | PAb (goat) | Molecular Probes (Eugene, OR) |
| Texas Red anti-mouse | (T-862) | PAb (goat) | Molecular Probes |
| Alexa 488 anti-rabbit | (A11008) | PAb (goat) | Molecular Probes |
| Alexa 488 anti-mouse | (A11001) | PAb (goat) | Molecular Probes |
| Cy5 anti-rabbit | (611 110122) | PAb (goat) | Rockland (Gilbertsville, PA) |
| Cy5.5 anti-mouse | (610 713124) | PAb (goat) | Rockland |
DSHB, Developmental Studies Hybridoma Bank, (University of Iowa, Iowa City, IA)
Cell culture Vero, CHO and NRK cells (ATCC number CCl-81, CCL-61 and CRL-6509) were cultured using standard conditions. For transient transfections Vero cells were seeded at 5 × 105 cells/well into 6 well plates (PGC Scientifics, Frederick, MD) containing a sterile glass coverslip and allowed to grow for a further 24 h before transfection. Transfection was performed in 1 ml of serum-free media containing 5 μg of plasmid DNA/well as detailed (in Poupon et al., 2003) and fixed 24 h after transfection.
Immunofluorescence Localization
Cells grown on coverslips were fixed using immersion in methanol (-20°C for 5 min) or 2% (wt/wt) paraformaldehyde (20 min at 25°C). Aldehyde-fixed cells were quenched and permeabilized (0.2% [vol/vol] Triton X-100 and 50 mM glycine in PBS pH 7.2 [5 min at 25°C]). Saponin extraction before fixation was performed as follows. After washing three times in PBS, cells were immersed in 0.05% (wt/vol) saponin, 80 mM PIPES pH 6.5, 5 mM EGTA, and 1 mM MgCl2 (60 s at 25°C), washed once quickly with PBS, and fixed. Cells were then incubated in 2% (vol/vol) goat serum in PBS (60 min at 25°C) and then with primary and secondary antibodies.
Quantitation
Quantitation was performed using the program “CoolLocalizer” (version 1.0.7; CytoLite, Stockholm, Sweden). Tagged image files depicting confocal micrographs were analyzed by setting the region of interest to include whole, saponin-extracted, individual cells immunostained with the stated markers. Cells that met the above criteria were then selected for analysis at random. The colocalization of markers (percentage) was defined by multiplying the Pearson's coefficient by 100. During each analysis n = 5 Vero cells.
Cell Fractions
Preconfluent (95%) cultures in 150-mm2 dishes were washed three times with PBS and scraped into HES buffer (20 mM HEPES pH 7.2, 1 mM EDTA, 250 mM sucrose, 100 mM NaCl containing 1 mM phenylmethylsulfonyl fluoride and 5× Complete EDTA free protease inhibitors cocktail; Roche Diagnostics, Mannheim, Germany) prechilled to 4°C. Postnuclear supernatant (PNS) was made by centrifuging cell lysates (4000 × g for 5 min) prepared by passage (10×) through a 21-gauge needle. The supernatant was used to make crude cell membrane and crude cytosolic fractions by centrifuging the PNS at 100,000 × g (60 min at 4°C). Detergent-solubilized membranes were made by resuspending crude membrane fractions in HES containing 1% 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate (CHAPS) and 100 mM NaCl (5 min at 25°C) followed by incubation for (30 min 4°C) before centrifugation at 17,000 × g (10 min at 4°C) (Wade et al., 2001). Nuclei isolated from the initial low-speed pellet were layered on top of a 2.2 M sucrose cushion and subjected to 30,000 rpm in an SW60 rotor for 60 min at 4°C. The pellet was saponin extracted, paraformaldehyde fixed, and blocked in serum as described previously. Bovine brain cytosol was prepared as described previously (Wattenberg and Rothman, 1986).
Sedimentation Experiments
Microtubules were assembled using the CytoDYNAMIX kit according to manufacturer's instructions (Cytoskeleton, Denver, CO). Bovine brain cytosol (2 mg) was incubated with 5 μg of taxol-stabilized microtubules (30 min at 37°C). The microtubule fraction was then collected after centrifugation through a sucrose cushion as described previously (Walenta et al., 2001) and subjected to immunoblot analysis. Actin polymerization was performed using 2 mg of bovine brain cytosol, 2 mM ATP, 20 nM phalloidin, and 100 μM nocodozole (all from Sigma-Aldrich) as described previously (Fucini et al., 2000). Polymerized actin was isolated and washed after centrifugation as described previously (Fucini et al., 2000) and subjected to immunoblot analysis.
Immunoprecipitation
Polyclonal antibody-saturated protein A agarose (Invitrogen, Carlsbad, CA) was cross-linked using dimethyl pimelimidate (Accoceberry et al., 2001). Beads (50 μl) were added to detergent-solubilized (CHAPS) membrane fractions (1.2 mg) containing 100 mM NaCl in a volume of 400 μl. After incubation at 4°C for 3 h on a shaking platform, beads were collected by centrifugation (21,000 × g for 2 min at 25°C) and washed three times in 0.5× HES (containing 100 mM NaCl) and 0.5× PBS. Beads were eluted twice (sequentially) with 25 μl of 100 mM glycine pH 2.5. Eluate was combined with Laemmli sample buffer, the pH neutralized (10 μl of 1 M Tris pH 7.0), and analyzed by immunoblotting. Where appropriate, 2-mercaptoethanol (10% vol/vol) was used. Human IgG (Sigma-Aldrich) was added to the blocking reagent (5% [wt/vol] nonfat dried milk in PBS containing 0.01% [vol/vol]) Tween 20) at a final concentration of 1 mg/ml for immunoblotting.
Endosome Fusion Assay
Two 150-mm2 dishes of CHO cells (80% confluent) were washed with PBS (containing 1 mM CaCl2, 1 mM MgCl2, and 10 mM glucose) and were allowed to endocytose either biotinylated rabbit IgG or avidin-conjugated alkaline phosphatase (Av-ALP) for 5 min at 37°C. Cells were washed twice in cold PBS pH 5.0 and then three times in cold PBS. Cell were lysed (as described above) in 250 mM sucrose, 3 mM imidazole pH 7.0, 5× Complete EDTA free protease inhibitors cocktail (Roche Diagnostics). PNS preparations were then incubated on ice in the presence or absence of antibodies for 20 min. Reactions were then initiated by mixing both treated PNS fractions with bovine brain cytosol and an ATP-regenerating system (10 mM ATP, 1 M creatine phosphate, and 3.7 mg/ml creatine phosphokinase, 20 mM PIPES pH 6.8) in triplicate. Fusion reactions also contained 60 μg/ml biocytinin to prevent nonspecific formation of IgG–enzyme complexes. Fusion was allowed to proceed at 37°C and then stopped with the addition of 0.1 volume 10% (vol/vol) Triton X-100 in PBS. Immune complexes were captured on 50 μl of protein A agarose (Invitrogen, Carlsbad, CA). Enzyme activity was measured kinetically by conversion of paranitrophenol at 405 nm (Gorvel et al., 1991).
RESULTS
Endogenous mVps18 Localizes to Early Endosomes
Polyclonal antibodies raised to recombinant protein fragments of mouse isoforms of mammalian (m)Vps18, mVps16, mVps11, mVam2, mVam6, and Hook1 (Figure 1A) were used in a series of immunolocalization experiments (Figures 2, 3, 4, 5, 6). The yeast class C Vps proteins Vps18/Pep3p and Vps11/Pep5p localize and function on the vacuolar membrane (Rieder and Emr, 1997; Wang et al., 2003). This suggests that the mammalian counterparts might localize to and function in late-endocytic compartments. Previous studies indicate that mVps18 colocalizes with several endocytic markers (Kim et al., 2001). Surprisingly, we found that anti-mVps18 clearly labeled compartments positive for the early sorting endosomal marker early endosome antigen-1 (EEA1) in Vero cells (Gaullier et al., 1999) (Figure 2, A and B). Localization of mVps18 to EEA1-positive compartments was observed in cells fixed with either aldehyde or methanol (Figures 2, A and B, and 3F, respectively), and anti-mVps18 antibody labeling was abolished when the recombinant antigen, but not GST or MBP alone, was included during the labeling procedure (our unpublished data). Morphometric analysis of several micrographs (n = 5) showed that 87 ± 4% of pixels positive for mVps18 were also positive for EEA1. Interestingly, there was a subset of EEA-positive compartments that did not have appreciable mVps18 labeling. There was some colocalization with transferrin receptor (TfR) that labeled a broader pattern of endosomal compartments (Figure 2C). However, there was little immunolocalization of mVps18 upon late endocytic structures (Figure 2, D and E) positive for LAMP1, LAMP2 (Gough et al., 1999), or CD63 (Metzelaar et al., 1991) (our unpublished data). We found 32 ± 12% were positive for TfR labeling, whereas only 6 ± 2.9% of pixels positive for mVps18 were also positive for CD63.
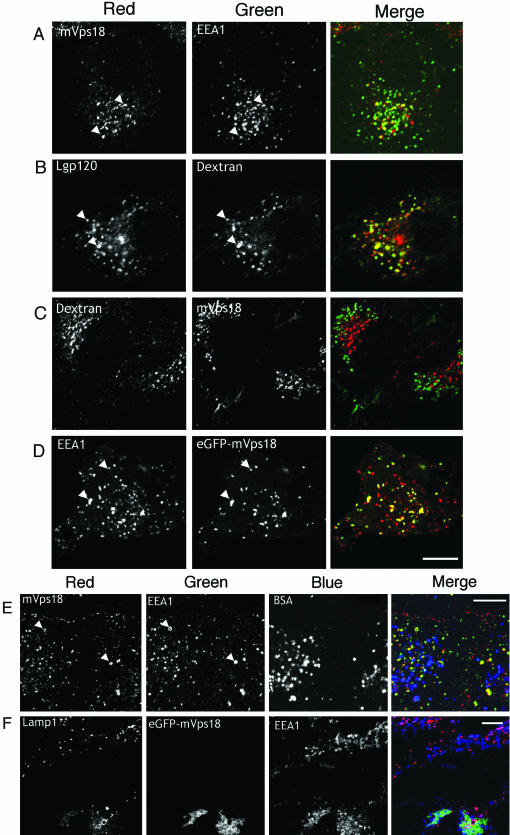
Figure 2.
Immunolocalization of mVps18 in Vero cells. Vero cells were fixed, permeabilized, and labeled with polyclonal anti-mVps18 antibodies together with Texas Red-conjugated goat anti-rabbit (labeling anti-mVps18) secondary and double labeled with monoclonal antibodies to EEA1 (A and B), TfR (C), LAMP1 (D), or LAMP2 (E) together with Alexa488-conjugated secondary antibody and visualized by confocal microscopy. Cells labeled for EEA1 and TfR (A–C) were fixed in aldehyde, whereas cells labeled for LAMPs were fixed with methanol (D and E). Arrowheads denote examples of colocalization between vesicles positive for mVps18 and either EEA1 or TfR (B and C). Bar, 5 μm (top); 10 μm (other).
Figure 3.
Localization of mVps18 to early endosomes in NRK and Vero cells. NRK cells were aldehyde fixed and immunolabeled for mVps18 (red) and EEA1 (green) together with anti-rabbit Texas Red- and Alexa488-conjugated anti-mouse. Arrowheads highlight examples of EEA1-mVps18 colocalization (A). To label late endosomal/lysosomal compartments, NRK cells were allowed to endocytose TexasFigure 3 (cont). Red-conjugated dextran for 60 min followed by a 4-h chase in the presence of Leupeptin (200 μm) before methanol fixation and labeling with anti-Lgp120 (B) or anti-Vps18 (C). NRK cells expressing eGFP-mVps18 at 24 h posttransfection were counterimmunolabeled with anti-EEA1 (D) monoclonal antibodies together with Texas Red-conjugated anti-mouse secondary antibodies. Bar, 10 μm. (E) Vero cells were allowed to endocytose Cy5-conjugated BSA for 60 min followed by a 4-h chase in the presence of leupeptin (200 μm) (E). Cells were aldehyde fixed and immunolabeled with anti-mVps18 and Texas Red-conjugated anti-rabbit antibodies and with anti-EEA1 and Alexa488-conjugated antimouse secondary antibodies. The merged image shows lysosomal BSA in blue, EEA1 in green, and mVps18 in red. Yellow areas depict colocalization between mVps18 and EEA1 (examples are also highlighted with arrowheads). Bar, 10 μm. (F) Vero cells were transiently transfected with an eGFP-mVps18 expressing plasmid and methanol-fixed and labeled with monoclonal anti-LAMP1 (Texas Red) and polyclonal anti-EEA1 (Cy5). Bar, 10 μm.
Figure 4.
Immunolocalization of mammalian Vps11 and Vps16 in permeabilized cells. Vero cells were permeabilized with saponin and fixed before immunolabeling with antibodies to mVps18 (A), mVps16 (B), or mVps11 (C) together with Texas Red-conjugated goat anti-rabbit secondary antibodies. Cells were colabeled with antibodies to EEA1 (A-C), LAMP1 (D), and CD63 (E), as indicated together with Alexa488-conjugated goat antimouse secondary antibodies. Filled arrowheads (A–C) denote examples of colocalization between mVps18, mVps16 mVps11, and EEA1. Some of the filamentous pattern observed for the class C Vps proteins is indicated by open arrowheads (A and B). Bar, 10 μm.
Figure 5.
Immunolocalization of mVam2. Saponin-permeabilized Vero cells were methanol fixed and immunolabeled with anti-mVam2 (A and B) and anti-LAMP1 (A) or anti-EEA1 (B) antibodies, together with Texas Red-conjugated anti-rabbit and Alexa488 anti-mouse secondary antibodies. Both the red and green channels from the inset designated in the merged image are shown on the far right. Note the filamentous pattern of mVam2 labeling present in aldehyde-fixed cells (B) but less prominent in methanol-fixed cells (A). Rab5 colocalization with both mVam2 (C) and mVps18 (D) was done by transiently expressing eGFP-Rab5. Bar, 10 μm (except for micrographs from inset).
Figure 6.
Cytoskeletal localization of the mammalian late Vps proteins. Vero cells were methanol fixed and labeled with anti-mVps18. A field at low magnification (A) and a field at high magnification (B), designated by the box is shown. The fluorescence signal was intensified in the high-power field to visualize the filamentous pattern that could be discerned in addition to the vesicular-like pattern (open arrowheads). Bar, 10 μm (top); 10 μm (bottom). Saponin-permeabilized Vero cells were also aldehyde fixed and labeled with polyclonal antibodies to mVps16 (C and D), or mVam2 (E and F). Cells were counterlabeled for actin filaments with Alexa488-conjugated phalloidin (C and E) or microtubules (D and F) by using monoclonal anti-tubulin antibodies together with Alexa488-conjugated goat anti-mouse secondary antibodies. Anti-mVps16 colocalized to actin filaments, whereas mVam2 colocalized with microtubules. Bar, 5 μm. Micrographs were taken of cellular subregions where the filamentous pattern could be clearly discerned. (G) Bovine brain cytosol (2 mg) was mixed with taxol-stabilized microtubules (Taxol) or unpolymerized tubulin (ø). Samples were incubated at 37°C for 30 min. Pellets obtained after centrifugation at 100,000 × g were immunoblotted along with an 20% equivalent sample of the total input protein before centrifugation (input). (H) Bovine brain cytosol (2 mg) was incubated in the absence (ø) or presence (phalloidin) of 20 μM phalloidin and 100 μM nocodazole for 30 min at 37°C. Pellets obtained after centrifugation at 100,000 × g were immunoblotted along with an equivalent sample of the total input protein before centrifugation (input). Lactate dehydrogenase was used as a negative control for cytoskeletal association.
We performed additional experiments on normal rat kidney (NRK) cells and again found colocalization between mVps18 and EEA1 (Figure 3A). MVps18 also localized diffusely through the cell and likely reflects the larger cytosolic pool of protein in this cell type (∼60% total by fractionation; our unpublished data). We also found that mVps18 showed little colocalization with endocytosed dextran (1-h pulse/4-h chase) that was used to label Lgp120-positive late endosomal structures (Figure 3, B and C). To better assess the relative distribution of mVps18 between early and late endosomes, we performed triple labeling experiments using anti-EEA1 and anti-mVps18 antibodies in Vero cells that had internalized Texas Red-conjugated bovine serum albumin (BSA) to late endosomes (1-h pulse/4-h chase) (Figure 3E). Antibodies to mVps18 showed high levels of colocalization with EEA1 but not with late endosomal BSA.
Although we used polyclonal antibodies raised to a large region of mVps18, we were not certain whether these antibodies recognized a small subpopulation of mVps18 proteins by immunofluorescence. Therefore, we analyzed the distribution of a functional eGFP-mVps18 fusion protein characterized previously (Poupon et al., 2003) (Figure 3F). In transient transfection experiments, eGFP-mVps18 reaches very high levels after 48 h whereupon significant clustering of late endosomal compartments is observed (Poupon et al., 2003). After 24 h (posttransfection) before major clustering is observed, we found eGFP-mVps18 on EEA1-positive vesicles in NRK cells. In previous studies, the ability of mVps18 or mVam6 overexpression to cause clustering of late endosomal compartments supported the idea that these proteins function in part to tether late endosomal compartments together (Caplan et al., 2001; Poupon et al., 2003). Although those studies observed minor clustering of early endosomal compartments in NRK cells, we found that overexpression of eGFP-mVps18 in Vero cells did result in clusters of endosomes that contained both EEA1-positive and LAMP1-positive compartments (Figure 3F). Interestingly, the structures that contained each of these markers were still discrete but clustered within the same region of the cell, suggesting that under these conditions, mVps18 overexpression could cluster both early and late endosomes (Figure 3F).
Endogenous mVps11 and mVps16 Localize to Early Endosomes in Permeabilized Cells
The data mentioned above indicate that mVps18 may control aspects of early endosomal traffic and are consistent with recent genetic studies in yeast indicating that Vps18p, Vps11p, and Vps16p may have additional roles earlier in the endocytic pathway besides fusion to the vacuole (Srivastava et al., 2000; Peterson and Emr, 2001). Furthermore, fractionation studies in yeast indicate that the class C proteins fractionate more like the endosomal-localized Pep12 marker protein rather than completely as a vacuolar protein (Rieder and Emr, 1997). To further test this hypothesis, we next used antibodies to mVps16 and mVps11 to determine whether these proteins also localized to early endosomes. Preliminary experiments showed the localization of these proteins to EEA1-positive compartments; however, a large cytosolic pool of these proteins obscured visualization of the membrane associated pool. Therefore, saponin permeabilization before fixation was used to deplete the cytosolic pool of each protein. In saponin-permeabilized cells, mVps18 was still localized to EEA1-positive compartments (Figure 4A) and verified that cell morphology as well as the localization of mVps18 was not altered by saponin treatment. Using this method of fixation, we found that both mVps16 (Figure 4B) and mVps11 (Figure 4C) also localized predominantly to EEA1-positive early endosomal structures that were distinct from late endosomal and lysosomal compartments positive for LAMP1 or CD63 (Figure 4, D and E). We also observed a nonvesicular, filamentous pattern for mVps18, mVps16, and mVps11 antibodies toward the periphery of the cell (Figure 4, A and B, open arrows), which was further analyzed in subsequent experiments (see below).
Immunolocalization of mVam2
Having localized the mammalian class C Vps proteins to early endocytic structures, we were interested in the intracellular distribution of mVam2, because its yeast counterpart, Vam2p/Vps41p, physically interacts with the yeast class C Vps proteins (Price et al., 2000; Seals et al., 2000).
Figure 5 shows saponin-permeabilized cells labeled with antibodies specific to mVam2 and counterlabeled with monoclonal antibodies specific for LAMP1 (Figure 5A) and EEA1 (Figure 5B). Here again, although we found only low levels of mVam2 localizing with late endosomal markers, we found clear instances of localization with EEA1. Additionally, we found that mVam6 antibodies also labeled numerous filamentous structures (Figure 5, A and B). These were much more prominent when we used aldehyde fixation (e.g., EEA1; Figure 5B) than when we used methonal fixation (e.g., labeling with LAMP1). Because the predominantly vesicular pattern of mVam2 immunolabeling in methanol-fixed cells could not be compared with that of aldehyde-fixed cells, we performed additional localization experiments between mVam2 and GFP-Rab5 introduced by transient transfection (Figure 5, C and D). As expected, both mVam2 (Figure 5C) and mVps18 (Figure 5D) colocalized with GFP-Rab5. Although, a significant amount of the GFP-Rab5 did not localize with mVam2- or mVps18-positive compartments, the level we observed is consistent with the localization of the latter proteins with EEA1, which also localizes with only a portion of Rab5-positive structures (Roberts et al., 1999). Together, these results indicate that at least a portion of mVam2 also localizes with early endosomal compartments.
Mammalian HOPS Proteins Associate with the Cytoskeleton
Although the filamentous structures for mVam2 observed after aldehyde fixation were prominent, they were also observed in methanol-fixed cells. In addition, inspection of either intact or saponin-permeabilized cells showed filamentous patterns for mVps18, mVps16, and mVps11 labeling, especially toward the basal surface of the cells (Figure 4, A and B, open arrows; our unpublished data), and could clearly be seen when fluorescence signals from areas relatively devoid of vesicular structures were amplified (Figure 6, A and B). To determine whether these filamentous structures corresponded to particular cytoskeletal elements, we performed a series of double labeling experiments by using anti-mVps18, anti-mVps16, anti-Vps11, and anti-mVam2 antibodies in concert with the fungal toxin phalloidin (conjugated with Alexa Fluor448) to label actin filaments, or with a monoclonal anti-α tubulin antibody to label microtubules (Figure 6, C–F). We then focused on cellular regions where the filamentous pattern was more discernible. These data showed that apart from their vesicular localization, the class C Vps protein mVps16 (Figure 6C) (and mVps18 and mVps11; our unpublished data) colocalized with actin filaments. No colocalization was observed between mVps16 (as well as mVps18 and mVps11; our unpublished data) and microtubules (Figure 6D). In contrast, mVam2 did not colocalize with phalloidin labeled actin filaments (Figure 6E). Rather, mVam2 was localized to microtubules (Figure 6F).
We then used an in vitro binding assay to verify that the class C Vps proteins (mVps16 and mVps18) associated specifically with phalloidin polymerized actin filaments (Figure 6H), whereas mVam2 and mVam6 associated with taxol-stabilized microtubules (Figure 6G). Microtubules were polymerized in vitro, incubated in bovine brain cytosol, and collected by centrifugation before analyzing protein recruitment from cytosol by immunoblotting. Actin filaments were generated by adding phalloidin and ATP to bovine brain cytosol and collected by centrifugation. Consistent with previous results, we found that mammalian Hook1 also associated with microtubules and interestingly also associated with actin filaments. The cytosolic lactate dehydrogenase enzyme was included to show that the association was specific and not due to contamination by cytosolic proteins. Thus, by both immunolocalization experiments and in vitro binding experiments we find that components of the mammalian HOPS complex differentially associated with both the actin and microtubule cytoskeleton.
Proteins Interacting with mVps18
Previous studies have established that mVps18 can be coimmunoprecipitated in a complex with mVps16 and mVps11 (Kim et al., 2001). To confirm this interaction and to investigate further protein interactions, antibodies specific to either mVps18, Hook1, or GST were used to perform immunoprecipitation experiments from a rat liver membrane fraction solubilized in 1% CHAPS. From this fraction, we were able to isolate between 20 and 30% of the total mVps18 in this fraction, which was sufficient for us to detect at least some of the potential interactions of mVps18. Consistent with previous results (Kim et al., 2001) mVps16 coimmunoprecipitated with mVps18 as did Syn7 (Figure 7) and mVps11 (our unpublished data). We also found that similar to the yeast HOPS proteins (Price et al., 2000), mVps18 also associated with mVam2 and mVam6. This is consistent with previous results showing that over expressed eGFP-mVps18 also associated with mVam6 and mVps11 (Poupon et al., 2003).
Figure 7.
Coimmunoprecipitation of proteins associated with mVps18. A detergent-soluble crude membrane fraction was subjected to immunoprecipitation with antibodies to mVps18, GST or Hook1 as indicated. Immunoprecipitated complexes were analyzed by immunoblotting with the antibodies to the indicated proteins. For comparison a 10% equivalent of input protein is shown except in the case of mVps16, Syn13, Vam2m, and mVam6 where a 20% equivalent is shown.
Based on previous studies showing the interaction of Drosophila Vps18 (Deep Orange) with the protein Hook (Kramer and Phistry, 1999; Sevrioukov et al., 1999; Narayanan et al., 2000), we also examined whether mVps18 could associate with the mammalian Hook1 isoform, which has a similar localization pattern as mVam2. Figure 7 shows that Hook1 was specifically coimmunoprecipitated with anti-mVps18 antibodies and that mVps18 was coprecipitated with anti-Hook1 antibodies.
We also found that aside from its interaction with Syn7, mVps18 also interacted with Vti1-b, another t-SNARE light chain that functions with Syn7 to effect late endosomal fusion (Mullock et al., 2000; Wade et al., 2001; Antonin et al., 2002). In addition, we found that mVps18 also associated with Syn13, a heavy chain t-SNARE, and the t-SNARE light chain Syn6, both of which are involved in early endosome fusion (Mills et al., 2001; Zerial and McBride, 2001). Separate blotting experiments were performed to show that the Syn13 antibodies did not cross react with Syn7, which migrated slightly faster during SDS-PAGE (our unpublished data). These results are consistent with the localization of mVps18 to EEA1/Rab5-positive endosomes. Interestingly, mVps18 did not coimmunoprecipitate with EEA1, another putative tethering factor that associates with Syn13 and Rab5 (Christoforidis et al., 1999). We also found that mVps18 associated with the S/M protein mVps45. Thus, in addition to its association with another S/M protein mVps33a (Kim et al., 2001), mVps18 seems to be able to form a complex with more than one S/M protein. The interaction of mVps18 with mVps45 is consistent with a function in early endosomes because mVps45 binds to Syn6, Syn13, and Rabenosyn 5, all effectors of early endosome fusion (Tellam et al., 1997; Nielsen et al., 2000).
We also found that mVps18 also associated with Vamp8, a v-SNARE that participates in both early endosomal and late endosomal homotypic fusion events (Prekeris et al., 1999; Wade et al., 2001; Antonin et al., 2002). Interestingly, Vamp7 did not coimmunoprecipitate with mVps18, indicating that mVps18 may not participate directly in Vamp7-dependent fusion events (McVey Ward et al., 2000; Bogdanovic et al., 2002). These combined data suggest that an mVps18-containing complex of proteins can bind to multiple SNAREs, most likely a cis-SNARE complex, which stands in contrast to studies in yeast that indicate Vps18 binds exclusively to uncomplexed Syntaxin (Vam3p) (Sato et al., 2000). However, yeast Vam2 and Vam6 have been found to associate with SNARE complexes, indicating that mVps18 might associate with SNAREs in the context of its larger HOPS complex (Price et al., 2000).
A Function for mVps18 in Early Endosomal Fusion
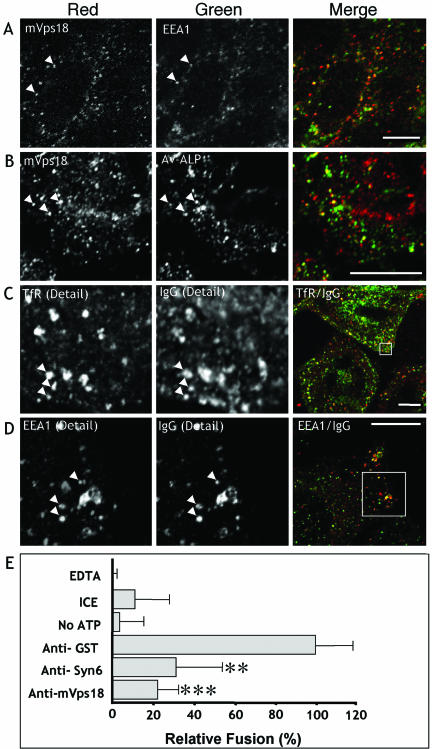
Having immunolocalized mVps11, mVps16, and mVps11 to early endosomes and coimmunoprecipitated mVps18 with early endosomal fusion machinery, we assessed whether mVps18 could play a role in early endosome fusion by using an in vitro assay (Gorvel et al., 1991). We loaded endosomes from two populations of CHO cells with a 5-min pulse of either IgG-Biotin or Av-ALP. We then assayed the effects of specific antibodies upon content mixing that was allowed to proceed for 30 min at 37°C in vitro in the presence of cytosol and ATP before isolation and quantitation of IgG–ALP complexes. We confirmed that mVps18 localized to EEA1-positive compartments in this cells type (Figure 8A). Immunolocalization experiments showed that Av-ALP complexes were resident on mVps18-positive compartments after a 5-min chase (Figure 8B). Figure 8, C and D, also confirmed that a 5-min pulse of endocytosed biotinylated IgG colocalized with EEA1 and TfR (closed arrows).
Figure 8.
Antibodies to mVps18 inhibit a Syn6-dependent early endosome in vitro fusion assay. CHO cells were aldehyde fixed and labeled for mVps18 and EEA1 (A) as indicated and closed arrows denote colocalization (A). CHO cells allowed to endocytose avidin-conjugated alkaline phosphatase (B) for 5 min before fixation and immunolabeling for mVps18 and Av-ALP by using biotinylated Alexa488-conjugated antibodies. Bar, 5 μm. CHO cells allowed to endocytose biotin conjugated rabbit IgG (C and D) for 5 min before fixation and immunolabeling with monoclonal anti-TfR (C) or anti-EEA1 (D) in combination with biotinylated antibody conjugated to avidin. Images at right show low power merged images. The designated inset is shown in detail at left. Bar, 5 μm. (E) Membranes from CHO cells allowed to endocytose either Biotin-IgG or Av-ALP for 5 min were incubated in the presence or absence of the indicated antibodies for 20 min on ice and then mixed with bovine brain cytosol and incubated at 37°C for 30 min. Immunocomplexes formed upon fusion were isolated on protein A agarose beads and quantitated by alkaline phosphatase activity (mean ± SD). Omission of the ATP-regenerating system, inclusion of EDTA (to chelate Mg2+ ions), or reactions kept on ice showed significantly less IgG-Biotin-Av-ALP activity, and the latter condition was used as the background. Fusion reactions containing anti-GST antibodies (50 μg/ml) were taken as 100% relative fusion, which represented ∼30% of the total enzyme activity within the PNS fractions. Significant inhibition was observed when anti-mVps18 polyclonal antibodies were included (150 μg/ml; ***p = 0.0012) and when anti-Syn6 polyclonal antibodies were included (225 μg/ml; **p = 0.0072).
After the fusion reaction was complete, ∼30% of the total ALP in endosomes was found within the IgG–ALP complex indicating that ∼30% of the endosomes had fused. The reaction was temperature and ATP dependent because reactions maintained at 0°C, or that contained EDTA or that did not contain the ATP-regenerating system had little content mixing (Figure 8E). Antibodies specific to mVps18 caused a significant inhibition of content mixing (Figure 8E), whereas the addition of anti-GST antibodies, or nonspecific antibodies to 600 μg/ml, had no effect on the fusion assay. Inclusion of polyclonal antibodies to Syn6 also gave significant inhibition, consistent with previous studies showing a role for the t-SNARE complex of Syn13 and Syn6 in catalyzing early endosomal fusion events in vitro (McBride et al., 1999, Mills et al., 2001). These same anti-Syn6 antibodies did not inhibit a late endosome heterotypic fusion assay with lysosomes (B. Mullock, P. Pryor, and J.P. Luzio, unpublished data), further indicating that the assay we used was specific for early endosome fusion. Separate experiments demonstrated that this early endosome fusion assay was resistant to a batch of polyclonal anti-Syn7 antibodies previously shown to inhibit a late endosome/lysosome fusion assay (Mullock at el., 2000; our unpublished data). These data show that antibodies specific to mVps18 specifically inhibit content mixing between early endosomes in a Syn6-dependent and Syn7-independent insensitive in vitro fusion assay.
DISCUSSION
In addition to its proposed role during late endosomal fusion (Poupon et al., 2003), our data indicate a role for mVps18 in early endosomal biogenesis. We found that a significant proportion of the class C Vps proteins, as well as mVam2, localize to EEA1- and Rab5-positive endosomes under a variety of fixation conditions and within a variety of cell types. This localization need not exclude a direct role for these factors in late endosome fusion. Indeed, the localization of these proteins to earlier compartments in the endosomal system could indicate where these factors are initially loaded onto membranes. These proteins would then program regions of the early endosome for fusion with late endosomes after the separation of this subdomain by endosome maturation or the formation of endosomal carrier vesicles (Gruenberg et al., 1989; Stoorvogel et al., 1993). The SNARE requirements for this fusion event are not yet defined but would be predicted to include many of those that we found associated with mVps18.
The model that the HOPS proteins are involved in early endosome fusion events is not only supported by their localization but also the association of mVps18 with known effectors of early endosome fusion that include Syn6 and Syn13 (Prekeris et al., 1998; Simonsen et al., 1999). Furthermore, mVps18 also associated with the S/M protein mVps45, which has also been implicated in control of early endosome fusion (Tellam et al., 1997; Nielsen et al., 2000) and represents the second S/M protein (besides Vps33a) to which mVps18 can associate (Kim et al., 2001). In addition, we find that under certain conditions, overexpression of eGFP-mVps18 induces clustering of not only late endosomal compartments but also early endosomes. Previous studies have implicated a role for mVps18 in late endosome fusion based on its ability to tightly cluster late endosomes (Poupon et al., 2003). Although those studies showed only low levels of clustering EEA1-positive compartments, we found significant clustering of early endosomal compartments (Figure 3F), which is likely explained by our use of different cell types. Finally, we observe that mVps18 specific antibodies inhibit early endosome fusion in vitro. Like other early endosome fusion assays (Mills et al., 2001), the assay we used was also sensitive to antibodies to Syn6 but not Syn7 demonstrating its specificity. Further studies are required to establish whether the HOPS proteins are involved in the same rab5-mediated homotypic fusion reaction that has previously been characterized (Gorvel et al., 1991), or whether this represents a different type of early endosomal fusion with different requirements. Interestingly, although we find that mVps18 does localize to Rab5-positive compartments, mVps18 is concentrated differently than Rab5, indicating that each of these proteins might occupy different endosomal subdomains (Figure 5D).
The possibility that the class C Vps proteins mediate some type of Syn6-/Syn13–dependent early endosome fusion event in vivo is also consistent with recent studies in yeast. The severe phenotype of vps11, vps16, vps18 and vps33 mutants cannot be explained by loss of vacuole fusion events alone, implying that fusion events earlier in the endocytic pathway also rely on these proteins (Srivastava et al., 2000; Peterson and Emr, 2001). Indeed, the class C Vps phenotype is mimicked when fusion to the vacuole and endosomal compartments are both blocked by mutation of VAM3 and PEP12 SNARE genes (Peterson and Emr, 2001). The localization of the yeast class C Vps proteins is also consistent with a broader function in the endosomal system. Although GFP fusion proteins to the various HOPS complex components do localize to the vacuole, fractionation experiments show that Vps18, Vps11, and Vps16 fractionates with Pep12p, a SNARE localized to endosomal compartments (Rieder and Emr, 1997). Additionally, the class C complex interacts genetically and physically with Vps8p, which is involved in transport steps before vacuole fusion (Horazdovsky et al., 1996; Woolford et al., 1998) and, in turn, interacts with Vps21p, the yeast orthologue of Rab5 (Horazdovsky et al., 1996; Woolford et al., 1998). Finally, both yeast Vps11p and Vps18p interact with Vac1p/Pep7p (Peterson et al., 1999), which is also involved in fusion to the yeast endosome (Peterson and Emr, 1999; Srivastava et al., 2000). The closest mammalian Vac1p homologue is Rabenosyn-5, which binds Rab5 and Rab4 (de Renzis et al., 2002) and interacts with the S/M protein mVps45 (Nielsen et al., 2000). Like the proposed dual role for the mammalian class C Vps complex, Rabenosyn-5 is not only required for early endosome fusion but also its overexpression slows the delivery of cathepsin D to late endosomes (Nielsen et al., 2000). Together, these observations support the idea that the HOPS proteins in both yeast and animal cells participate in more than one fusion event.
Much more work is required to decipher the array of protein–protein interactions among the mammalian HOPS proteins that occur while they execute their function. Our coimmunoprecipitation experiments confirm that mVps18 interacts with Syntaxin heavy chains, yet we also coimmunoprecipitated a number of other t-SNAREs, including Syn6 and Vti1-b as well as the v-SNARE Vamp8. These results contrast with previous data in yeast where Vps18p associated exclusively with the uncomplexed Syntaxin Vam3p (Sato et al., 2000). Instead, our results indicate that mVps18 associates with cis-SNARE complexes. These data are, however, consistent with other studies in yeast showing that HOPS components (Vam6p-Vam2p) associate with cis-SNARE complexes (Price et al., 2000). It is likely that variation in the methods used between these different studies in yeast account for their discrepancy. However, further experimentation with recombinant proteins from both yeast and animal systems should better resolve this issue.
We also find that mVam2 and mVam6 interact with mVps18 and also show significant localization to EEA1- and Rab5-positive endosomes. Yeast Vam6p and Vam2p are thought to form a subcomplex, and Vam6p has been proposed to act as a GTP exchange factor for the Ypt7p Rab protein (Wurmser et al., 2000). Although Ypt7p is homologous both in sequence and proposed function to Rab7, we have not yet been able to see interaction of Rab7 with the mammalian HOPS components (our unpublished data). Furthermore, we were unable to find significant colocalization of mVps18 with either eGFP-Rab7 or eGFP fusions with activated forms of Rab7 (Q67L) (our unpublished data). Interestingly, the lysosomal clustering evoked by overexpression of mVam6 that has been documented previously is independent of Rab7 (Caplan et al., 2001). Thus, it remains to be determined whether the mVam2-mVam6 subcomplex provides a GTP exchange function in animal cells and which GTPase(s) it controls.
All of the HOPS proteins we analyzed associated with the cytoskeleton. Furthermore, we were also able to demonstrate interaction between mVps 18 and the microtubule-associated Hook1 protein that is also implicated in endosomal biogenesis by virtue of its homology with Drosophila Hook protein (Lloyd et al., 1998; Sevrioukov et al., 1999). We believe that the cytoskeletal association of these components likely represents an important aspect of their function. Interestingly, the clustering of endosomal compartments induced by overexpression of eGFP-mVps18 is also accompanied by dramatic changes in the organization of actin that forms a network of actin polymers that surround the clusters of endosomes (Poupon et al., 2003). Biochemical analysis revealed that the class C Vps proteins associated with actin filaments, whereas mVam6 and mVam2 associated with microtubules. Despite the ability of the mVam6–mVam2 complex to associate with class C mVps18 protein, our cytoskeletal fractionation experiments showed that only Hook1 was capable of associating with both filaments and microtubules generated in vitro. These data imply that the association of the class C Vps proteins with the mVam6–mVam2 proteins is precluded by association with the cytoskeleton.
Further analysis will be devoted toward determining the function of the association between the mHOPS proteins and the cytoskeleton. Microtubules and actin filaments provide a network important for the movement and positioning of both early and late endosomes as well as lysosomes (Rogers and Gelfand, 2000; Cordonnier et al., 2001). Membrane traffic to and through late endosomes is not only sensitive to microtubule and actin filament depolymerization but is controlled by many proteins that interact with actin and microtubules such as Rho family GTPases, myosins, kinesins, and the dynein/dynactin complex (Allen, 1995; Murphy et al., 1996; Chimini and Chavrier, 2000; Ellis and Mellor, 2000; Rogers and Gelfand, 2000; Apodacia, 2001; Muller et al., 2001; Eitzen, 2003). If vesicle movement as well as immobilization is an important factor in determining the efficiency of cargo transport (Kelly, 1990; Qualmann et al., 2000), then there should exist mechanisms to integrate cytoskeletal interactions with downstream fusion events. Perhaps, the members of the mammalian HOPS complex could function at this interface by orchestrating a switch between vesicle movement and vesicle fusion. Thus, the HOPS proteins would join a growing collection of proteins that interact with both the cytoskeleton and effectors of fusion such as RILP, Rab6, Rab27a, BLOC-1, and the Exocyst complex (Rogers and Gelfand, 2000; Schroer, 2000; Falcon-Perez et al., 2002; Langford, 2002; Lipschutz and Mostov, 2002; Short et al., 2002; Yi et al., 2002). Alternatively, the cytoskeleton and particularly actin could play a more direct role in the fusion process, a function distinct from its role in vesicle movement or positioning. Two different in vitro late endosome fusion assays show dependence on actin polymerization under situations where the spatial distribution of compartments might not be a critical factor (Jahraus et al., 2001; Eitzen et al., 2002). The yeast vacuolar homotypic fusion assay not only requires proper actin polymerization and depolymerization but also the activity of Cdc42p (Eitzen et al., 2001; Muller et al., 2001). Kinetic analysis of vacuole fusion shows that Cdc42p and actin are required at the Ypt7p-dependent step of vesicle tethering (Eitzen et al., 2001). Actin itself is also coconcentrated with HOPS components along vertices of tethered vacuoles where fusion occurs (Eitzen et al., 2002; Wang et al., 2003). Thus, actin could be an integral part of the glue that tethers the fusion apparatus of opposing membranes together.
Acknowledgments
This work was supported by a grant from the Human Frontiers Science Program to R.C.P., J.P.L., and David E. James and by a grant to R.C.P. from the AHA (9730275N). S.C.W.R. was supported by AHA postdoctoral fellowship grants 0120475Z and 0325605Z. V.P. was supported by a Wellcome Trust traveling fellowship awarded to J.P.L.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–06–0358. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-06-0358.
Abbreviations used: Av-ALP, avidin-alkaline phosphatase conjugate; EEA1, early endosome antigen-1; GST, glutathione S-transferase; HOPS, homotypic protein sorting and vacuolar fusion complex; MBP, maltose binding protein; PNS, postnuclear supernatant; S/M, Sec1/Munc like protein; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor(s); TfR, transferrin receptor; Vps, vacuole protein sorting.
References
- Accoceberry, I., Thellier, M., Datry, A., Desportes-Livage, I., Biligui, S., Danis, M., and Santaelli, X. (2001). One-step purification of Enterocytozoon bieneusi from human stools by immunoaffinity expanded bed adsorption. J. Clin. Microbiol. 39, 1947-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, V. (1995). Membrane traffic motors. FEBS Lett. 369, 101-106. [DOI] [PubMed] [Google Scholar]
- Antonin, W., Dulubova, I., Arac, D., Pabst, S., Plitzner, J., Rizo, J., and Jahn, R. (2002). The N-terminal domains of Syn7 and Vti1b form three-helix bundles that differ in their ability to regulate SNARE complex assembly. J. Biol. Chem. 277, 36449-36456. [DOI] [PubMed] [Google Scholar]
- Apodacia, G. (2001). Endocytic traffic in polarized epithelial cells: role of the actin and Microtubule cytoskeleton. Traffic 2, 149-159. [DOI] [PubMed] [Google Scholar]
- Bogdanovic, A., Bennett, N., Kieffer, S., Louwagie, M., Morio, T., Garin, J., Satre, M., and Bruckert, F. (2002). Syn7, Syn 8, Vti1 and Vamp7 (vesicle-associated membrane protein 7) form an active S.N.A.R.E. complex for early macropinocytic compartment fusion in Dictyostelium discoideum. Biochem. J. 368, 29-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan, S., Hartnell, L.M., Aguilar, R.C., Naslavsky, N., and Bonifacino, J. (2001). Human Vam6p promotes lysosomal clustering and fusion in vivo. J. Cell Biol. 154, 109-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.A., and Scheller, R.H. (2001). SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2, 98-106. [DOI] [PubMed] [Google Scholar]
- Chimini, G., and Chavrier, P. (2000). Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2, E191-E196. [DOI] [PubMed] [Google Scholar]
- Christoforidis, S., Mcbride, H., Burgoyne, R.D., and Zerial, M. 1999. The Rab5 effectors EEA1 is a core component of endosome docking. Nature 397, 621-625. [DOI] [PubMed] [Google Scholar]
- Cordonnier, M.-N., Dauzonne, D., Louvard, D., and Coudrier, E. (2001). Actin filaments and myosin I alpha cooperate with microtubules for the movement of lysosomes. Mol. Biol. Cell 12, 4013-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Renzis, S., Sonnichsen, B., and Zerial, M. (2002). Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat. Cell Biol. 4, 124-133. [DOI] [PubMed] [Google Scholar]
- Eitzen, G., Thorngren, N., and Wickner, W. (2001). Rho1p and CDC42p act after Ypt7p to regulate vacuole docking. EMBO J. 20, 5650-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen, G., Wang, L., Thorngren, N., and Wickner, W. (2002). Remodeling of organelle-bound actin is required for yeast vacuole fusion. J. Cell Biol. 158, 669-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen, G. (2003). Actin remodeling to facilitate membrane fusion. Biochim. Biophys. Acta 1641, 175-181. [DOI] [PubMed] [Google Scholar]
- Ellis, S., and Mellor, H. (2000). Regulation of endocytic traffic by rho family GTPases. Trends Cell Biol. 10, 85-88. [DOI] [PubMed] [Google Scholar]
- Falcon-Perez, J.M., Starcevic, M., Gautam, R., and Dell'Angelica, E.C. (2002). BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet-dense granules. J. Biol. Chem. 277, 28191-28199. [DOI] [PubMed] [Google Scholar]
- Fucini, R.V., Navarrete, A., Vadakkan, C., Lacomis, L., Erdjument-Bromage, H., Tempst, P., and Stamnes, M. (2000). Activated ADP-ribosylation factor assembles distinct pools of actin on Golgi membranes. J. Biol. Chem. 275, 18824-18829. [DOI] [PubMed] [Google Scholar]
- Gaullier, J.M., Simonsen, A., D'Arrigo, A., Bremnes, B., and Stenmark, H. (1999). FYVE finger protein as effectors of phosphatidylinositol 3-phosphate. Chem. Physiol. Lipids 98, 87-94. [DOI] [PubMed] [Google Scholar]
- Gorvel, J.-P., Chavrier, P., Zerial, M., and Gruenberg, J. (1991). Rab5 controls early endosome fusion in vitro. Cell 64, 915-925. [DOI] [PubMed] [Google Scholar]
- Gough, N.R., Zweifel, M.E., Martinez-Augustin, O., Aguilar, R.C., Bonifacino, J.S., and Fambourgh, D.M. (1999). Utilization of the indirect lysosomal targeting pathway by lysosome-associated membrane proteins (LAMPs) is influenced largely by the C-terminal residue of their GYXXf targeting signals. J. Cell Sci. 112, 4257-4269. [DOI] [PubMed] [Google Scholar]
- Gruenberg, J., Griffiths, G., Howell, K.E. (1989). Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J. Cell Biol. 108, 1301-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, J.C. (2001). SNARE complex structure and function. Exp. Cell Res. 271, 10-21. [DOI] [PubMed] [Google Scholar]
- Horazdovsky, B.F., Cowles, C.R., Mustol, P., Holmes, M., and Emr, S.D. (1996). A novel RING finger protein, Vps8p, functionally interacts with the small GTPase, Vps21p, to facilitate soluble vacuolar protein localization. J. Biol. Chem. 271, 33607-33615. [DOI] [PubMed] [Google Scholar]
- Jahraus, A., Egeberg, M., Hinner, B., Habermann, A., Sackman, E., Pralle, A., Faulstich, H., Rybin, V., Defacque, H., and Griffiths, G. (2001). ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol. Biol. Cell 12, 155-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, R.B. (1990). Associations between microtubules and intracellular organelles. Curr. Opin. Cell Biol. 2, 105-108. [DOI] [PubMed] [Google Scholar]
- Kim, B.Y., Kramer, H., Yamamoto, A., and Kominami, E. (2001). Molecular characterization of mammalian homologues of class C Vps proteins that interact with Syn-7. J. Biol. Chem. 276, 29393-29402. [DOI] [PubMed] [Google Scholar]
- Kramer, H., and Phistry, M. (1999). Genetic Analysis of hook, a gene required for endocytic trafficking in Drosophila. Genetics 151, 675-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford, G.M. (2002). Myosin-V, a versatile motor for short-range vesicle transport. Traffic 3, 859-865. [DOI] [PubMed] [Google Scholar]
- Yi, Z., Yokota, H., Torii, S., Aoki, T., Hosaka, M., Zhao, S., Takata, K., Takeuchi, T., and Izumi, T. (2002). The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol. Cell Biol. 22, 1858-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipschutz, J.H., and Mostov, K.E. (2002). Exocytosis: the many masters of the exocyst. Curr. Biol. 12, R212-R214. [DOI] [PubMed] [Google Scholar]
- Lloyd, V., Ramaswami, M., and Kramer, H. (1998). Not just pretty eyes: Drosophila eye-color mutations and lysosomal delivery. Trends Cell Biol. 8, 257-259. [DOI] [PubMed] [Google Scholar]
- McBride, H.M., Rybin, V., Murphy, C., Giner, A., Teasdale, R., and Zerial, M. (1999). Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and Syn 13. Cell 98, 377-386. [DOI] [PubMed] [Google Scholar]
- McVey Ward, D., Pevsner, J., Scullion, M. A., Vaughn, M. and Kaplan. J. (2000). Syn7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages. Mol. Biol. Cell 11, 2327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey Ward, D., Radisky, D., Scullion, M.A., Tuttle, M.S., Vaughn, M., and Kaplan, J. (2001). hVPS41 is expressed in multiple isoforms and can associate with vesicles through a RING-H2 finger motif. Exp. Cell Res. 267, 126-134. [DOI] [PubMed] [Google Scholar]
- Metzelaar, M.J., Wijngaard, P.L., Peters, P.J., Sixma, J.J., Nieuwenhuis, H.K., and Clevers, H.C. (1991). CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J. Biol. Chem. 266, 3239-3245. [PubMed] [Google Scholar]
- Mills, I.G., Urbe, S., and Clague, M.J. (2001). Relationships between EEA1 binding partners and their role in endosome fusion. J. Cell Sci. 114, 1959-1965. [DOI] [PubMed] [Google Scholar]
- Muller, O., Johnson, D.I., and Mayer, A. (2001). Cdc42p functions at the docking stage of yeast vacuole membrane fusion. EMBO J. 20, 5657-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock, B.M., Smith, C.W., Bright, N.A., Ihrke, G., Lindsay, M., Parton, R.G., James, D.E., Luzio, J.P., and Piper, R.C. (2000). Syn 7 is localized to late endosomes compartments, associated with Vamp 8, and is required for endosome, lysosome fusion. Mol. Biol. Cell 11, 3137-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, C., Saffrich, R., Grummt, M., Gournier, H., Rybin, V., Rubino, M., Auvinen, P., Lütcke, A., Parton, R.G., and Zerial, M. (1996). Endosome dynamics regulated by a Rho protein. Nature 384, 427-432. [DOI] [PubMed] [Google Scholar]
- Narayanan, R., Kramer, H., and Ramaswami, M. (2000). Drosophila endosomal proteins Hook and Deep Orange regulate synapse size but not synaptic vesicle recycling. J. Neurobiol. 45, 105-119. [DOI] [PubMed] [Google Scholar]
- Nielsen, E., Christoforidis, S., Uttenweiler-Joseph, S., Miaczynska, M., Dewitte, F., Wilm, M., Hoflack, B., and Zerial, M. (2000). Rabenosyn-5, a novel Rab5 effecter, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J. Cell Biol. 151, 601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris, R., Klumperman, J., Chen, Y.A., and Scheller, R.H. (1998). Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J. Cell Biol. 143, 957-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris, R., Yang, B., Oorschot, V., Klumperman, J., and Scheller, R.H. (1999). Differential roles of Syntaxin7 and Syntaxin8 during endosomal trafficking. Mol. Biol. Cell 10, 3891-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, M.R., Burd, C.G., and Emr, S.D. (1999). Vac1p coordinates Vps21 Rab GTPase and Vps34 PtdIns 3-kinase signaling, essential for Pep12- and Vps45p-dependent vesicle docking/fusion at the endosome. Curr. Biol. 9, 159-162. [DOI] [PubMed] [Google Scholar]
- Peterson, M.R., and Emr, S.D. (2001). The class C Vps complex functions at multiple stages of the vacuolar transport pathway. Traffic 2, 476-486. [DOI] [PubMed] [Google Scholar]
- Poupon, V., Stewart, A., Gray, S.R., Piper, R.C., and Luzio, J.P. (2003). The role of mVps18 in clustering, fusion, and intracellular localization of late endocytic organelles. Mol. Biol. Cell 14, 4015-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, A., Seals, D., Wickner, W., and Ungerman, C. (2000). The docking stages of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J. Cell Biol. 148, 1231-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann, B., Kessels, M.M., and Kelly, R. (2000). Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol. 150, F111-F116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, S.E., and Emr, S.D. (1997). A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol. Biol. Cell 8, 2307-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R.L., Barbieri, K.M., Pryse, K.M., Chua, M., Morisaki, J.H., and Stahl, P.D. (1999). Endosome fusion in living cells overexpressing GFP-rab5. J. Cell Sci. 112, 3667-3675. [DOI] [PubMed] [Google Scholar]
- Rogers, S.L., and Gelfand, V.I. (2000). Membrane trafficking, organelle transport and the cytoskeleton. Curr. Opin. Cell Biol. 12, 57-62. [DOI] [PubMed] [Google Scholar]
- Sato, T.K., Rehling, P., Peterson, M.R., and Emr, S.D. (2000). Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell 6, 661-671. [DOI] [PubMed] [Google Scholar]
- Schroer, T.A. (2000). Motors, clutches and breaks for membrane traffic: a commemorative review in honor of Thomas Kreis. Traffic 1, 3-10. [DOI] [PubMed] [Google Scholar]
- Seals, D.F., Eitzen, G., Margolis, N., Wickner, W., and Price, A. (2000). A Ypt/Rab effecter complex containing the Sec1 homologue Vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. USA 97, 9402-9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrioukov, E.A., He, J.-P. Moghabi, N., Sunio, A., and Kramer, H. (1999). A role for the Deep Orange and Carnation eye color genes in lysosomal delivery in Drosophila. Mol. Cell 4, 479-486. [DOI] [PubMed] [Google Scholar]
- Shin, C.S., Hong, M. S. Cheon, S.B., and Jeewon, L. (1997). Enhanced production of human mini-proinsulin in fed-batch cultures at high cell density of Escherchia coli BL21(DE3) [pET-3aT2M2]. Biotechnol. Prog. 13, 249-257. [DOI] [PubMed] [Google Scholar]
- Stoorvogel, W. (1993). Arguments in favor of endosomal maturation. Biochem. Soc. Trans. 3, 711-715. [DOI] [PubMed] [Google Scholar]
- Short, B., Preisinger, C., Schaletzky, J., Kopajtich, J., and Barr, F.A. (2002). The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr. Biol. 12, 1792-1795. [DOI] [PubMed] [Google Scholar]
- Simonsen, A., Gaullier, J.-M. D'Arrigo, A., and Stenmark, H. (1999). The Rab5 effecter EEA1 interacts directly with Syn-6. J. Cell Biol. 247, 28857-28860. [DOI] [PubMed] [Google Scholar]
- Sollner, T.H. (2003). Regulated exocytosis and SNARE function. Mol. Membr. Biol. 20, 209-220. [DOI] [PubMed] [Google Scholar]
- Srivastava, A., Woolford, C., and Jones, E.W. (2000). Pep3p/Pep5p complex: a putative docking factor at multiple steps of vesicular transport to the vacuole of Saccharomyces cerevisiae. Genetics 156, 105-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunio, A., Metcalf, A.B., and Kramer, H. (1999). Genetic dissection of endocytic trafficking in Drosophila using a horseradish peroxidase-bride of sevenless chimera: hook is required for normal maturation of multivesicular endosomes. Mol. Biol. Cell 10, 847-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellam, J.T., James, D.E., Stevens, T.H., and Piper, R.C. (1997). Identification of mammalian Golgi Sec1p-like protein mVps45. J. Biol. Chem. 272, 6187-6193. [DOI] [PubMed] [Google Scholar]
- Wade, N., Bryant, N.J., Connolly, L.M., Simpson, R.J., Luzio, J.P., Piper, R.C., and James, D.E. (2001). Syn 7 complexes with mouse Vps10 tail interactor 1b, Syn 6, vesicle associated membrane protein (Vamp) 8 and Vamp7 in B16 melanoma cells. J. Biol. Chem. 276, 19820-19827. [DOI] [PubMed] [Google Scholar]
- Walenta, J.H., Didier, A.J., Liu, X., and Kraymer, H. (2001). The Golgi-associated Hook3 protein is a member of a novel family of microtubule-binding proteins. J. Cell Biol. 152, 923-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., Merz, A.J., Collins, K.M., and Wickner, W. (2003). Hierarchy of proteins assembly at the vertex ring domain for yeast vacuole docking and fusion. Mol. Biol. Cell 160, 365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, G.M., and Hughson, F.M. (2000). Membrane tethering and fusion in the secretory and endocytic pathways. Traffic 1, 588-597. [DOI] [PubMed] [Google Scholar]
- Waters, G.M., and Pfeffer, S.R. (1999). Membrane tethering in intracellular transport. Curr. Opin. Cell Biol. 11, 453-459. [DOI] [PubMed] [Google Scholar]
- Wattenberg, B.W., and Rothman, J.E. (1986). Multiple cytosolic components promote intra-Golgi protein transport: resolution of a protein acting at a late stage, prior to membrane fusion. J. Biol. Chem. 261, 2208-2213. [PubMed] [Google Scholar]
- Wendler, F., and Tooze, S. (2001). Syn 6, The promiscuous behavior of a SNARE protein. Traffic 2, 606-611. [DOI] [PubMed] [Google Scholar]
- Whyte, J.R.C., and Munro, S. (2002). Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627-2637. [DOI] [PubMed] [Google Scholar]
- Wickner, W., and Haas, A. (2000). Yeast homotypic vacuole fusion: a window in organelle trafficking mechanisms. Annu. Rev. Biochem. 69, 247-275. [DOI] [PubMed] [Google Scholar]
- Woolford, C.A., Bounoutas, G.S., Frew, S.E., and Jones, E.W. (1998). Genetic interaction with vps8–200 allows partial suppression of the vestigial vacuole phenotype caused by a PEP5 mutation in Saccharomyces cerevisiae. Genetics 148, 71-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmser, A.E., Sato, T.K., and Emr, S.D. (2000). New component of the vacuolar class c-VPS complex couples nucleotide exchange on the Ypt7 GT-Pase to SNARE-dependent docking and fusion. J. Cell Biol. 151, 551-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107-119. [DOI] [PubMed] [Google Scholar]