Abstract
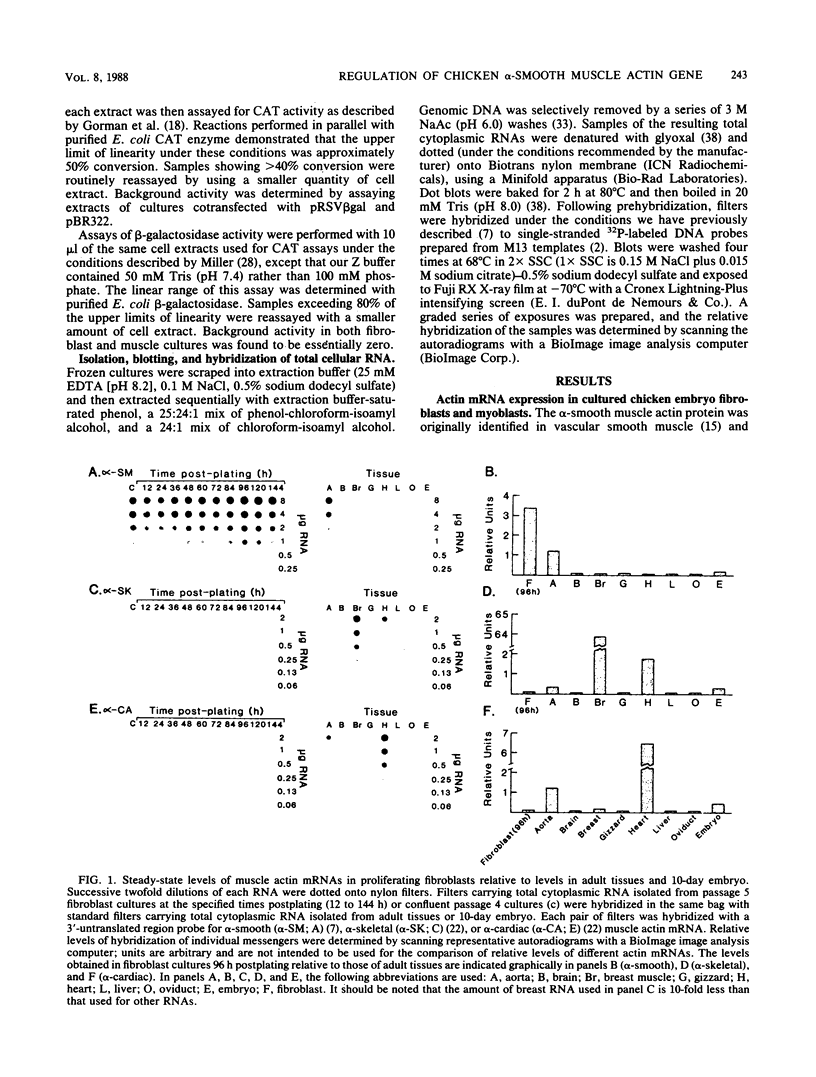
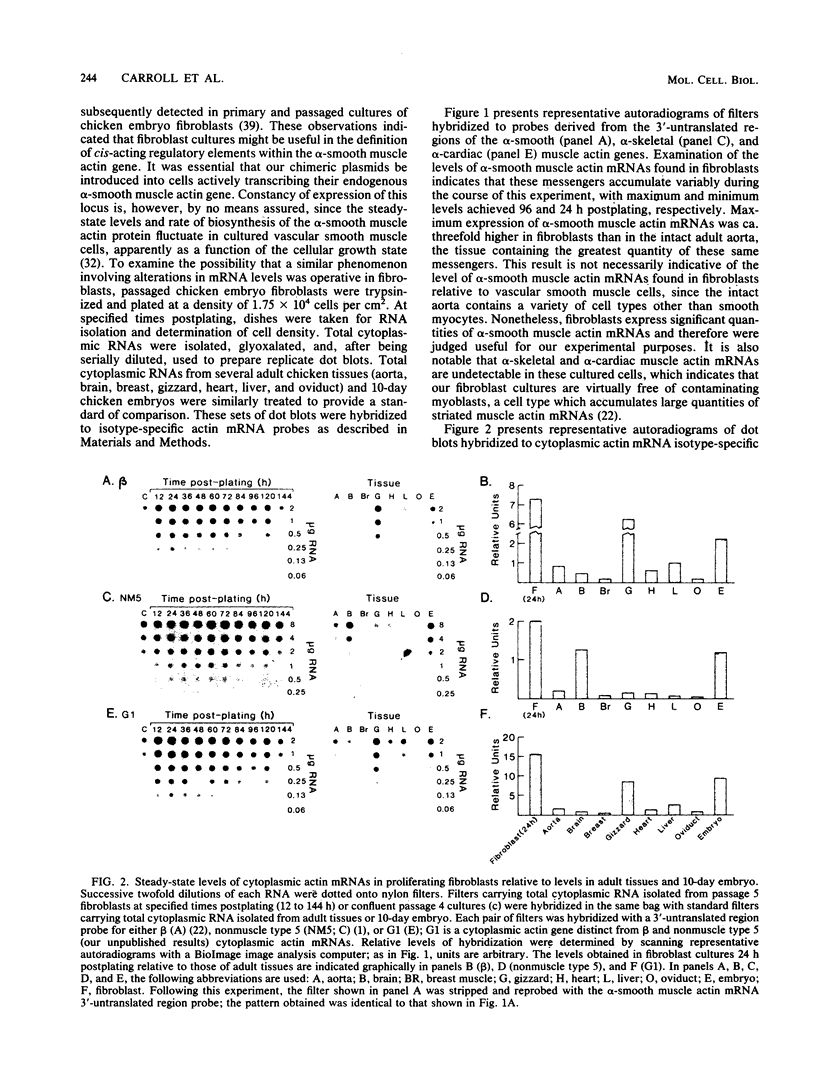
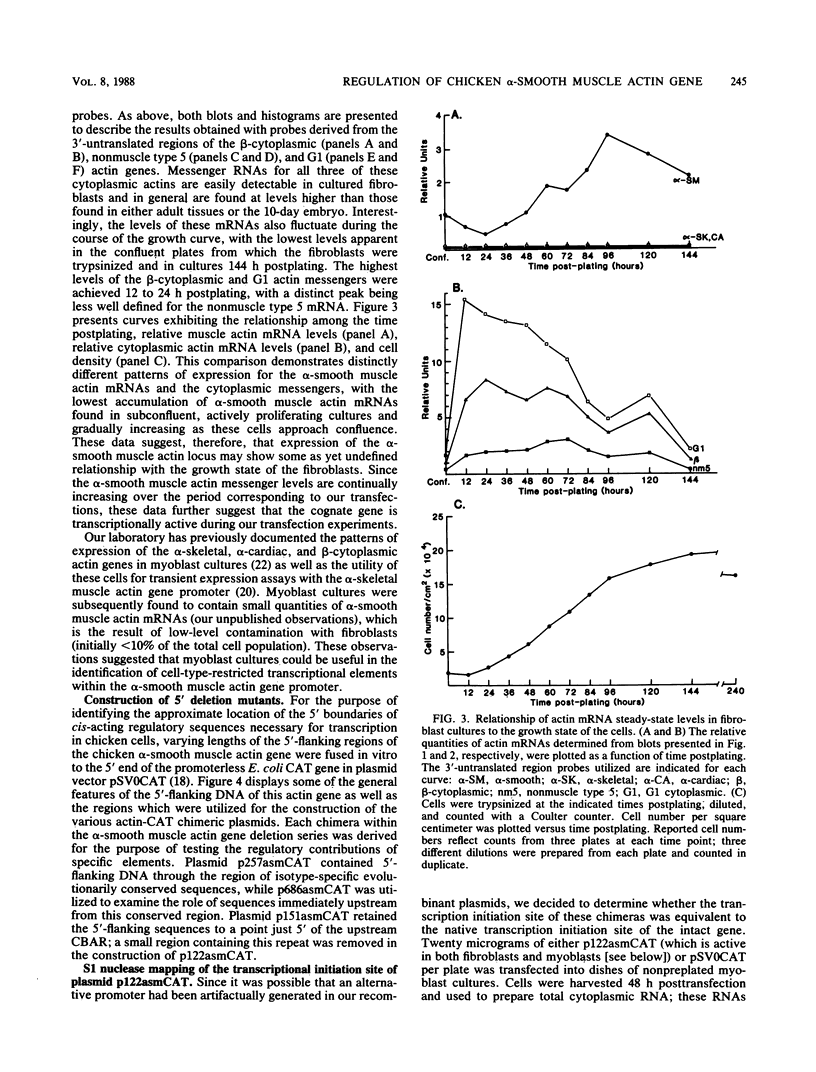
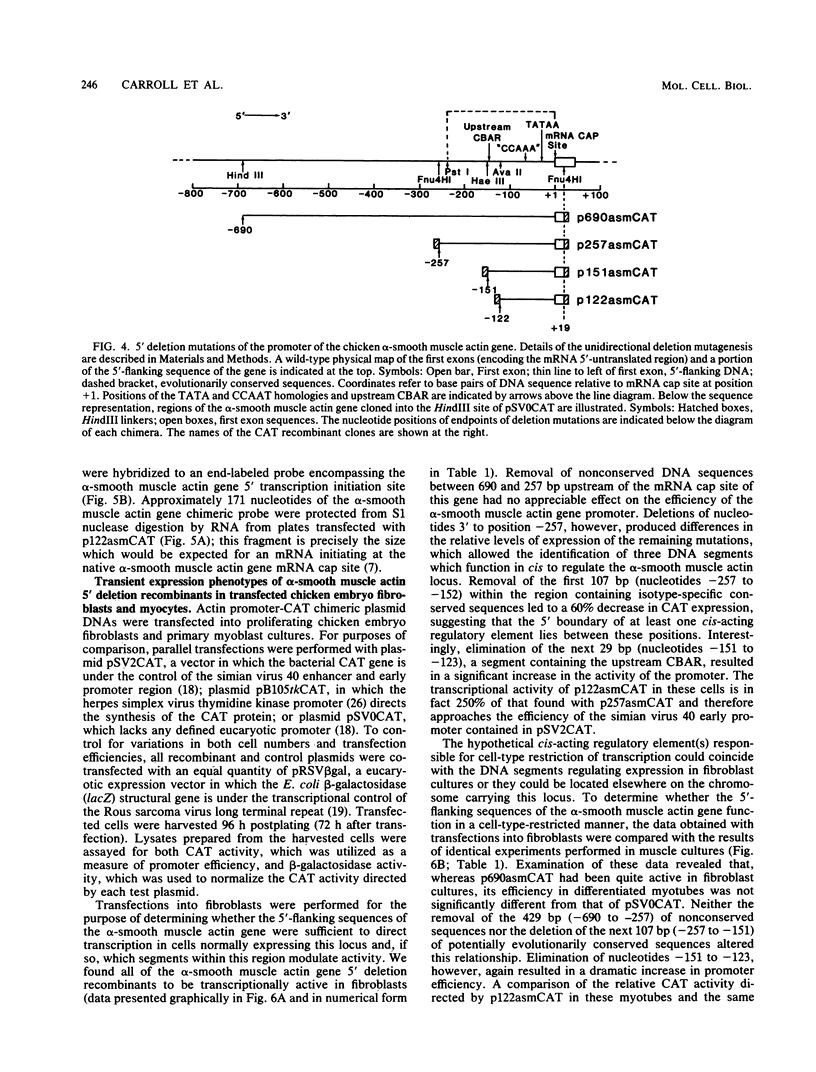
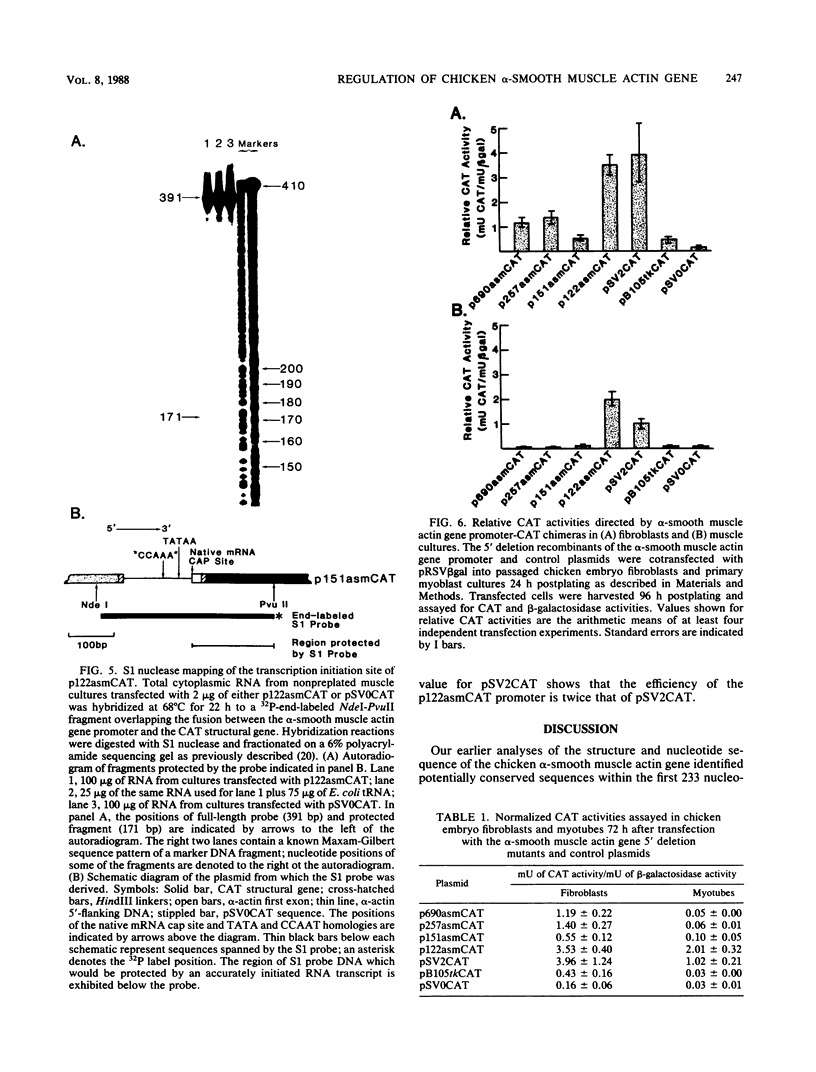
A series of 5' deletion mutations of the upstream flanking sequences of the chicken alpha-smooth muscle (aortic) actin gene was prepared and inserted into the chloramphenicol acetyltransferase expression vector pSV0CAT. Deletion recombinants were transfected into fibroblasts, which actively express the alpha-smooth muscle actin gene, and primary myoblast cultures, which accumulate much lower quantities of alpha-smooth muscle actin mRNAs. The first 122 nucleotides of 5'-flanking DNA were found to contain a "core" promoter capable of accurately directing high levels of transcription in both fibroblasts and myotubes. The activity of this core promoter is modulated in fibroblasts by a "governor" element(s) located at least in part between nucleotides -257 and -123. This region contains sequences potentially conserved between mammalian and avian alpha-smooth muscle actin genes as well as one of a pair of 16-base-pair inverted CCAAT box-associated repeats which are conserved among all chordate muscle actin genes examined to date. A smaller DNA segment (-151 to -123) containing these upstream CCAAT box-associated repeats was sufficient to suppress expression of the core promoter in muscle cultures, suggesting that the upstream CCAAT box-associated repeats play a negative role in the alpha-smooth muscle actin gene promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsma D. J., Chang K. S., Schwartz R. J. Novel chicken actin gene: third cytoplasmic isoform. Mol Cell Biol. 1985 May;5(5):1151–1162. doi: 10.1128/mcb.5.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma D. J., Grichnik J. M., Gossett L. M., Schwartz R. J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol Cell Biol. 1986 Jul;6(7):2462–2475. doi: 10.1128/mcb.6.7.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet A. M., Erwin C. R., Rutter W. J. Cell-specific enhancers in the rat exocrine pancreas. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3599–3603. doi: 10.1073/pnas.83.11.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Bruskin A. M., Tyner A. L., Wells D. E., Showman R. M., Klein W. H. Accumulation in embryogenesis of five mRNAs enriched in the ectoderm of the sea urchin pluteus. Dev Biol. 1981 Oct 30;87(2):308–318. doi: 10.1016/0012-1606(81)90154-8. [DOI] [PubMed] [Google Scholar]
- Carroll S. L., Bergsma D. J., Schwartz R. J. Structure and complete nucleotide sequence of the chicken alpha-smooth muscle (aortic) actin gene. An actin gene which produces multiple messenger RNAs. J Biol Chem. 1986 Jul 5;261(19):8965–8976. [PubMed] [Google Scholar]
- Chepelinsky A. B., King C. R., Zelenka P. S., Piatigorsky J. Lens-specific expression of the chloramphenicol acetyltransferase gene promoted by 5' flanking sequences of the murine alpha A-crystallin gene in explanted chicken lens epithelia. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2334–2338. doi: 10.1073/pnas.82.8.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Coen D. M., McKnight S. L. Promoter domains required for expression of plasmid-borne copies of the herpes simplex virus thymidine kinase gene in virus-infected mouse fibroblasts and microinjected frog oocytes. Mol Cell Biol. 1985 Aug;5(8):1940–1947. doi: 10.1128/mcb.5.8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episkopou V., Murphy A. J., Efstratiadis A. Cell-specified expression of a selectable hybrid gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4657–4661. doi: 10.1073/pnas.81.15.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Ohashi T., Yamanishi K., Taniguchi T. Regulation of human interleukin-2 gene: functional DNA sequences in the 5' flanking region for the gene expression in activated T lymphocytes. Cell. 1986 Aug 1;46(3):401–405. doi: 10.1016/0092-8674(86)90660-4. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Schmid E., Winter S., Chaponnier C., de Ckhastonay C., Vandekerckhove J., Weber K., Franke W. W. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R., Paz-Aliaga B., Ernst S. G., Crain W. R., Jr Three sea urchin actin genes show different patterns of expression: muscle specific, embryo specific, and constitutive. Mol Cell Biol. 1984 May;4(5):840–845. doi: 10.1128/mcb.4.5.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Grichnik J. M., Bergsma D. J., Schwartz R. J. Tissue restricted and stage specific transcription is maintained within 411 nucleotides flanking the 5' end of the chicken alpha-skeletal actin gene. Nucleic Acids Res. 1986 Feb 25;14(4):1683–1701. doi: 10.1093/nar/14.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters: positive and negative elements. Cell. 1984 Apr;36(4):799–800. doi: 10.1016/0092-8674(84)90028-x. [DOI] [PubMed] [Google Scholar]
- Hayward L. J., Schwartz R. J. Sequential expression of chicken actin genes during myogenesis. J Cell Biol. 1986 Apr;102(4):1485–1493. doi: 10.1083/jcb.102.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Jones P. B., Galeazzi D. R., Fisher J. M., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression by dioxin. Science. 1985 Mar 22;227(4693):1499–1502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Mercola M., Goverman J., Mirell C., Calame K. Immunoglobulin heavy-chain enhancer requires one or more tissue-specific factors. Science. 1985 Jan 18;227(4684):266–270. doi: 10.1126/science.3917575. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Caravatti M., Buckingham M. E. A fetal skeletal muscle actin mRNA in the mouse and its identity with cardiac actin mRNA. Cell. 1982 Aug;30(1):185–192. doi: 10.1016/0092-8674(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Nir U., Walker M. D., Rutter W. J. Regulation of rat insulin 1 gene expression: evidence for negative regulation in nonpancreatic cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3180–3184. doi: 10.1073/pnas.83.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordahl C. P., Tilghman S. M., Ovitt C., Fornwald J., Largen M. T. Structure and developmental expression of the chick alpha-actin gene. Nucleic Acids Res. 1980 Nov 11;8(21):4989–5005. doi: 10.1093/nar/8.21.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Saborio J. L., Segura M., Flores M., Garcia R., Palmer E. Differential expression of gizzard actin genes during chick embryogenesis. J Biol Chem. 1979 Nov 10;254(21):11119–11125. [PubMed] [Google Scholar]
- Schwartz R. J., Rothblum K. N. Gene switching in myogenesis: differential expression of the chicken actin multigene family. Biochemistry. 1981 Jul 7;20(14):4122–4129. doi: 10.1021/bi00517a027. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Horovitch S. J., Scott M. P., Rich A., Pardue M. L. Myogenesis in primary cell cultures from Drosophila melanogaster: protein synthesis and actin heterogeneity during development. Cell. 1978 Apr;13(4):589–598. doi: 10.1016/0092-8674(78)90210-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Actin typing on total cellular extracts: a highly sensitive protein-chemical procedure able to distinguish different actins. Eur J Biochem. 1981 Jan;113(3):595–603. doi: 10.1111/j.1432-1033.1981.tb05104.x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Chordate muscle actins differ distinctly from invertebrate muscle actins. The evolution of the different vertebrate muscle actins. J Mol Biol. 1984 Nov 5;179(3):391–413. doi: 10.1016/0022-2836(84)90072-x. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]