Abstract
In the U.S., ∼21 × 106 individuals have type 2 diabetes, and twice as many have impaired glucose tolerance (IGT). Approximately 40–50% of individuals with IGT will progress to type 2 diabetes over their lifetime. Therefore, treatment of high-risk individuals with IGT to prevent type 2 diabetes has important medical, economic, social, and human implications. Weight loss, although effective in reducing the conversion of IGT to type 2 diabetes, is difficult to achieve and maintain. Moreover, 40–50% of IGT subjects progress to type 2 diabetes despite successful weight reduction. In contrast, pharmacological treatment of IGT with oral antidiabetic agents that improve insulin sensitivity and preserve β-cell function—the characteristic pathophysiological abnormalities present in IGT and type 2 diabetes—uniformly have been shown to prevent progression of IGT to type 2 diabetes. The most consistent results have been observed with the thiazolidinediones (Troglitazone in the Prevention of Diabetes [TRIPOD], Pioglitazone in the Prevention of Diabetes [PIPOD], Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication [DREAM], and Actos Now for the Prevention of Diabetes [ACT NOW]), with a 50–70% reduction in IGT conversion to diabetes. Metformin in the U.S. Diabetes Prevention Program (DPP) reduced the development of type 2 diabetes by 31% and has been recommended by the American Diabetes Association (ADA) for treating high-risk individuals with IGT. The glucagon-like peptide-1 analogs, which augment insulin secretion, preserve β-cell function, and promote weight loss, also would be expected to be efficacious in preventing the progression of IGT to type 2 diabetes. Because individuals in the upper tertile of IGT are maximally/near-maximally insulin resistant, have lost 70–80% of their β-cell function, and have an ∼10% incidence of diabetic retinopathy, pharmacological intervention, in combination with diet plus exercise, should be instituted.
Type 2 diabetes occurs in 7% of the U.S. population and affects ∼21 million individuals (1). Its prevalence has escalated dramatically during the last decade (1a; references accompanied by the letter “a” can be found in the Supplementary Data) because of multiple factors: 1) increased longevity of the population (2a), 2) increased prevalence of obesity (3a), 3) decreased physical activity (4a), and 4) rapid growth of minority populations at high risk developing type 2 diabetes (1) (5a,6a). Patients with type 2 diabetes experience significant morbidity and mortality from microvascular and macrovascular complications. The American Diabetes Association (ADA) has estimated that the cost of treating diabetes and its associated complications in 2007 was $174 billion (2), and recent studies estimate that diabetes will more than double by the year 2050 (7a).
Results from the Diabetes Control and Complications Trial (3) (8a), UK Prospective Diabetes Study (4,5), and other trials (9a) have established hyperglycemia as the major risk factor for microvascular and, to a lesser extent, macrovascular (6) (10a,11a) complications. Consequently, increased emphasis has been placed on achieving as close to euglycemic control as possible without untoward side effects of therapy in newly diagnosed type 2 diabetic patients (12a). Nonetheless, the average level of glucose control in U.S. patients with diabetes remains suboptimal (7) (13a). Moreover, once overt fasting hyperglycemia becomes manifest, there is a relentless deterioration in β-cell function with an accompanying rise in glycemia (8). Because most of the morbidity and mortality in type 2 diabetes arises from long-term complications, early detection and prevention would be expected to have a tremendous beneficial human, social, medical, and economic impact. With these considerations in mind, it is logical to intervene early in the natural history of type 2 diabetes with measures targeted to reverse specific pathophysiological defects present in the prediabetic state and that ultimately lead to development of overt diabetes (9,10) (14a–16a). This approach requires 1) the ability to identify prediabetic individuals at risk to develop type 2 diabetes later in life and 2) interventions that reverse the pathogenic disturbances responsible for type 2 diabetes (11) (17a–19a). Prevention of diabetic levels of hyperglycemia would be expected to prevent/reduce the incidence of microvascular complications. However, impaired glucose tolerance (IGT)/impaired fasting glucose (IFG) subjects only have mild-modestly elevated plasma glucose levels, and glucose is a relatively weak risk factor for cardiovascular disease. Therefore, it is likely that other cardiovascular factors are responsible for the increased incidence of macrovascular disease. Thus, reduction in HbA1c per se may not decrease cardiovascular complications unless the medications used to treat IGT/IFG correct established cardiovascular risk factors, independent of their glucose-lowering effect.
Known risk factors for type 2 diabetes include family history (20a,21a), obesity (3a,14a,15a,22a–25a), presence of a cluster of risk factors (dyslipidemia, hypertension, visceral obesity, IGT, abnormal coagulation factors, endothelial dysfunction) referred to collectively as the insulin resistance (metabolic) syndrome (18a,26a–29a), insulin resistance and hyperinsulinemia (14a–16a,23a,26a,27a,30a–34a), impaired insulin secretion (14a–16a,19a,35a–41a), gestational diabetes (19a,24a,42a–49a), polycystic ovarian syndrome (51a), IGT, IFG (14a–16a,51a–69a), and ethnicity (6a,70a–72a). Of the known diabetogenic risk factors, β-cell failure and insulin resistance have great predictive value for development of type 2 diabetes (11–14) (14a–16a,30a,34a,73a,74a). IGT and IFG (9,10,15,16) (14a–16a,51a–69a) are strong predictors of type 2 diabetes. Approximately 50% of IGT individuals progress to diabetes over their lifetime, and the annual progression rate varies from 2.3 to 11%, depending on the specific population (17–19) (20a,21a,29a,61a–72a); in the U.S., ∼41 million individuals have IGT (1). The ADA has identified individuals with HbA1c values of 5.7–6.4% “at risk for diabetes.” However, controversy exists as to how well HbA1c values in this range predict future risk for diabetes. Additional risk factors for progression of IGT to diabetes are obesity, especially visceral, fasting plasma glucose >90–95 mg/dL, and sum of plasma glucose concentrations during an oral glucose tolerance test (OGTT) in the upper 50th percentile of IGT individuals (19). Age, inflammatory markers (high-sensitivity C-reactive protein, tumor necrosis factor-α, interleukin-6), adiponectin, and ferritin also have been shown to predict future risk for diabetes. By selecting IGT individuals who have one or more additional diabetogenic risk factors, the U.S. Diabetes Prevention Program (DPP) demonstrated a conversion rate of IGT to diabetes of ∼11% per year (18). A similarly high conversion rate was demonstrated in the DREAM study (20). In TRIPOD (15) (49a), development of diabetes in women with a history of gestational diabetes mellitus (GDM) was 12.4% per year. In ACT NOW, the conversion rate of IGT to diabetes in the placebo group was somewhat lower, at 6.0% per year (21).
Having defined a patient population at high risk to develop type 2 diabetes, it is important to have an intervention that: 1) reverses known pathogenic mechanisms responsible for disturbances in glucose homeostasis in IGT/IFG subjects; 2) is effective in treating IGT/IFG and established diabetes; 3) is well tolerated, safe, and ideally reduces the increased risk of cardiovascular disease in IGT individuals. In the following section, we will present a brief overview of the pathogenesis of type 2 diabetes to provide a rational strategy for pharmacological intervention.
PATHOGENESIS OF TYPE 2 DIABETES
Type 2 diabetes results from an interaction between genetic and environmental factors (11) (73a). In diabetic patients with overt fasting hyperglycemia (≥126 mg/dL), both insulin resistance and impaired insulin secretion are characteristic features, although controversy exists as to which defect, i.e., insulin resistance or β-cell dysfunction, represents the primary abnormality (11,22) (74a–78a). Current evidence favors a two-step development of type 2 diabetes (9–11,15,16) (14a–16a,30a,31a,34a,59a–61a,73a,74a). During step one, individuals with normal glucose tolerance (NGT) progress to IGT with insulin resistance as the primary determinant. In step two, there is worsening of IGT to type 2 diabetes in association with a progressive deterioration in β-cell function (9–11,15,16,23). Progression from IGT to diabetes also is associated with a further, albeit small, decline in insulin sensitivity (10,11,23) (73a,77a).
Insulin resistance
Impaired insulin action is a characteristic feature of type 2 diabetes (11,14) (73a,77a–79a) and involves the liver (11) (75a,80a–82a), muscle (11,22) (80a,83a), and adipose tissue (11) (81a,84a). Insulin resistance precedes and plays a pivotal role in the development of type 2 diabetes (9–11,16,24,25) (14a–16a,30a,34a,73a,74a,78a). Thus, NGT first-degree relatives (who are at high risk to develop diabetes) of type 2 diabetic individuals are markedly resistant to insulin (11,24) (23a,32a,55a–90a). Similarly, people with IGT are resistant to insulin and manifest compensatory hyperinsulinemia (9–11,23,25) (14a–16a,30a,31a,59a,60a,65a,73a). Hyperinsulinemia, which reflects underlying insulin resistance (9–11,24–26) (73a,77a,91a,92a), is common in populations with a high prevalence of type 2 diabetes and predicts the development of IGT and type 2 diabetes in Caucasians (31a,85a,93a), Mexican Americans (23a,33a,57a,94a), Naurians (59a), Japanese Americans (71a), and Pima Indians (23,27) (30a,34a). Much evidence supports a genetic component of the insulin resistance (25) (36a,86a,88a), which is aggravated by environmental factors including weight gain, physical inactivity, and aging.
In summary, insulin resistance is a universal feature of type 2 diabetes and occurs early in the natural history of the disease, i.e., individuals with IGT and genetically predisposed individuals with NGT. Therefore, interventions designed to enhance insulin sensitivity in IGT subjects are likely to be effective in preventing/delaying IGT progression to type 2 diabetes.
Impaired insulin secretion
Although insulin resistance is a major pathogenic factor underlying progression from NGT to IGT to diabetes, deterioration in glycemic control does not occur unless β-cells fail to compensate for the insulin resistance. Ultimately, β-cell failure is responsible for IGT progression to type 2 diabetes (9–12,15,16,24–27) (14a–16a,26a,34a,59a,73a,74a). Among IGT subjects, a low 2-h plasma insulin concentration during OGTT predicts IGT progression to type 2 diabetes in all ethnic groups. When fasting plasma glucose exceeds 100–110 mg/dL, loss of first-phase insulin secretion is common in IGT individuals (36a–39a,95a,96a); impaired first-phase insulin secretion also predicts progression to diabetes (35a,40a). Whether β-cell dysfunction is primary or secondary, i.e., to glucotoxicity (97a) or lipotoxicity (98a), or incretin deficiency/resistance (97a–102a), remains controversial (11,25,26) (39a,73a). It is noteworthy that even within the NGT range, β-cell function (measured with the gold standard insulin secretion/insulin resistance [disposition] index) is the best predictor of 2-h plasma glucose during an OGTT and the best predictor of progression of NGT to IGT and subsequently to type 2 diabetes (9–11,23) (15a,16a) (Supplementary Fig. 1). Once overt type 2 diabetes is present, β-cell function declines progressively over time (8,10,25–30) (34a,39a,59a,74a), irrespective of therapeutic interventions (metformin, sulfonylureas, insulin) used to treat the hyperglycemia (4,5,8) (rev. in 11). Multiple factors including genetic predisposition, insulin resistance leading to increased insulin secretory demand, glucotoxicity, lipotoxicity, impaired incretin release and/or action, amylin accumulation within the pancreas, and decreased β-cell mass have been implicated as causative factors in the progressive β-cell impairment (rev. in 11,25,26,29,30). Ideally, interventions designed to prevent progression of IGT to type 2 diabetes should retard/prevent the development of β-cell failure.
The onset and severity of β-cell failure occur much earlier in the natural history of diabetes and is more severe than previously appreciated. In two large studies—the San Antonio Metabolism Study (31) (15a) and the Veterans Administration Genetic Epidemiology Study (10)—259 IGT subjects, 201 type 2 diabetes subjects, and 318 NGT subjects received an OGTT with insulin/C-peptide measurements and an euglycemic insulin clamp. IGT subjects were divided into tertiles based on a 2-h plasma glucose during OGTT (140–159, 160–179, and 180–199 mg/dL). With rising 2-h plasma glucose, there was an initial increase in plasma insulin response followed by a progressive decline. However, the plasma insulin response does not provide an accurate measure of β-cell function. β-Cells respond to an increment in plasma glucose with an increment in plasma insulin, and this response is modulated by the severity of insulin resistance. This insulin secretion/insulin resistance (disposition) index (equivalent to insulin secretion × insulin sensitivity) represents the gold standard for quantitating β-cell function. Within the NGT range of (2-h plasma glucose < 100 mg/dL vs. 2-h plasma glucose = 120–139 mg/dL), there is a 50–60% decline in β-cell function that is similar in lean and obese/overweight subjects (Supplementary Fig. 1). Subjects in upper tertile of IGT manifest a 70–80% decrease in β-cell function. Studies by Butler et al. (32) suggest that this loss of β-cell function in “prediabetic” subjects is associated with significant loss of β-cell mass.
Interventions aimed at preventing type 2 diabetes
IGT represents the first step in the progression from NGT to type 2 diabetes (11,23,25,27) (58a,73a). IFG and IGT are recognized as prediabetic states by the ADA (103a). Because impaired β-cell function and insulin resistance are characteristic features of IGT/IFG, interventions that preserve/augment β-cell function and ameliorate insulin resistance are most likely to prevent/slow IGT progression to diabetes.
Diet and physical exercise.
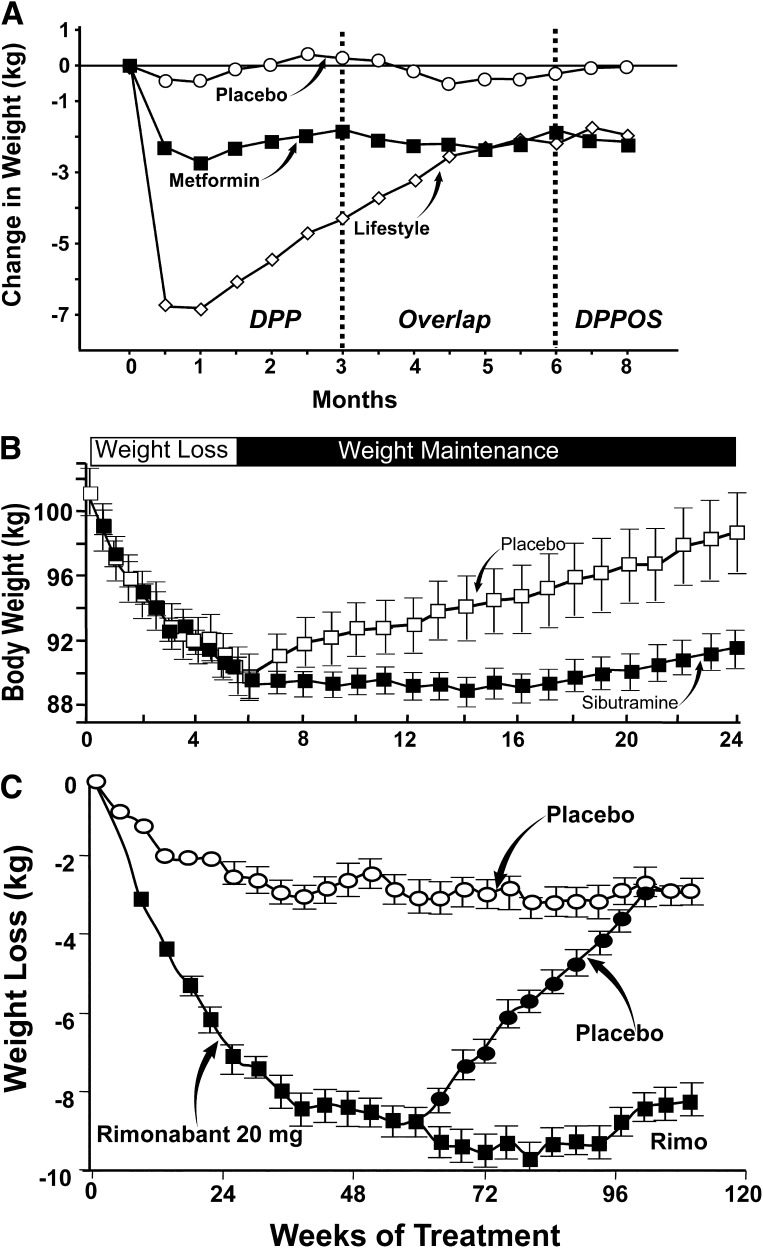
Obesity (3a,22a–24a,54a,104a,105a) and physical inactivity (106a–108a) are major risk factors for type 2 diabetes. Obesity is the single most important factor responsible for the marked increase in both the incidence and prevalence of type 2 diabetes over the last 20 years (3a). Weight gain (109a) and physical inactivity (110a,111a) cause insulin resistance. Conversely, weight loss (112a,113a) and exercise (114a–117a) enhance insulin sensitivity and improve glucose tolerance in nondiabetic and diabetic subjects. Four large prospective studies demonstrated that a treatment regimen using diet plus exercise reduces IGT progression to type 2 diabetes (17,18,33,34). The major problem with such behavioral intervention programs is the difficulty in maintaining weight loss and increased physical activity over a sustained period of time (118a). In the U.S. DPP, weight loss was largely regained after the DPP ended, despite initiation of the HELP program (35) (Fig. 1A). The basic components of the lifestyle intervention used in the DPP are shown in Supplementary Table 1. Difficulty in maintaining such an intervention over a long-term basis should not be surprising. The Look AHEAD trial (36), which, like DPP, demonstrated impressive weight loss at year 1, was also followed by significant weight regain over the subsequent 3 years despite continued intensive intervention and follow-up (Supplementary Table 1). Weight regain is a characteristic feature of most weight loss programs, irrespective of the type of dietary intervention (119a–121a). Similarly, weight loss achieved with pharmacological intervention is associated with major weight regain once medication is discontinued. After diet-induced weight loss, subjects randomized to placebo regained all lost weight over 24 months, whereas subjects randomized to sibutramine maintained reduced body weight (Fig. 1B) (122a). Similar results have been observed with rimonabant (123a) (Fig. 1C).
Figure 1.
A: Change in body weight during the DPP, during the overlap period, and during the DPP Outcomes Study (DPPOS) (reproduced from Eriksson and Lindgärde [33]). B: Effect of sibutramine versus placebo on weight regain after weight loss (reproduced from James et al. [122a]). C: Rimonabant (Rimo) versus placebo on weight regain after weight loss (reproduced from Pi-Sunyer et al. [123a]).
Attempts to translate DPP results to routine clinical practice in the “real world” have proven difficult. In a recent community study in Finland (37), 2,797 high-risk individuals were enrolled in a diabetes prevention program designed to achieve 5–7% body weight loss. Only approximately one-third of participants were able to successfully decrease body weight by >2.5%.
Lastly, although weight loss, when achieved, is effective in reducing the incidence of diabetes in IGT subjects, the decrease in diabetes risk is only 50–60%. Thus, 40–50% of IGT subjects still progress to type 2 diabetes, despite successful weight loss, indicating that lifestyle intervention alone is not sufficient to prevent diabetes in a large percentage of individuals.
In summary, although initial weight loss can be induced with intensive dietary intervention, despite close supervision and frequent follow-up, weight regain is the norm once the intervention program is discontinued. Moreover, 40–50% of IGT subjects progress to diabetes despite successful weight loss. The issue is not whether weight loss works to prevent IGT progression to type 2 diabetes, but whether weight loss can be maintained without an intervention program that most individuals find difficult to follow and that is costly to implement and maintain.
In contrast to behavioral intervention (diet plus exercise), pharmacological therapy (thiazolidinediones, metformin, glucagon-like peptide [GLP]-1 receptor agonists) at the stage of IGT uniformly has been shown to effectively prevent IGT conversion to type 2 diabetes (Supplementary Table 2).
Metformin.
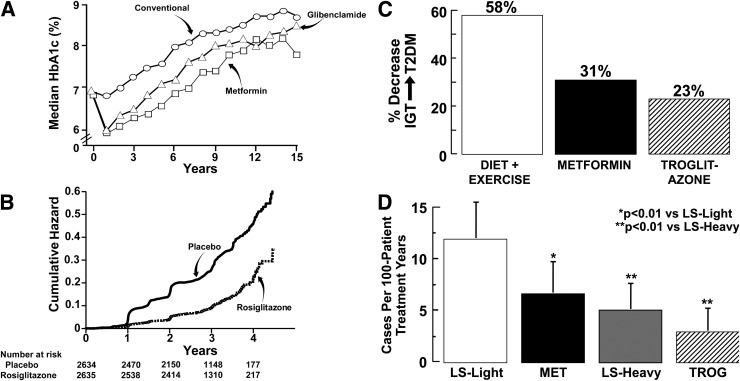
Metformin improves glycemic control in type 2 diabetes (38) (124a) by inhibiting hepatic glucose production (38) (165a,166a) and gluconeogenesis (127a,128a) and improving peripheral (muscle) tissue insulin sensitivity (38) (126a,128a). Metformin does not stimulate insulin secretion (38) (124a–128a) or preserve β-cell function (8,39) and is associated with a progressive rise in HbA1c (Fig. 2A). The DPP demonstrated a 31% reduction in IGT conversion to type 2 diabetes in subjects receiving metformin, 850 mg twice daily (34) (Fig. 2C) (18); metformin also improved insulin sensitivity (17a) and decreased the incidence of the metabolic syndrome (18a). In the Indian DPP (40), metformin decreased IGT conversion to type 2 diabetes by 26% (Supplementary Table 2), and an ADA consensus conference (41) recommended that high-risk individuals (HbA1c ≥6.0%, BMI ≥30 kg/m2, age ≤60 years) with IGT and/or IFG be treated with metformin, in addition to diet and exercise. However, metformin (4,8,39), like sulfonylureas (4), does not prevent the progressive decline in β-cell function that is characteristic of type 2 diabetes (rev. in 11).
Figure 2.
A: Lack of effect of metformin and sulfonylureas to cause a durable reduction in HbA1c because of a lack of effect on preservation on β-cell function in the UK Prospective Diabetes Study. Reproduced from the UK Prospective Diabetes Study Group (4) and Turner et al. (8). B: Effect of rosiglitazone to decrease the conversion of IGT to type 2 diabetes in the DREAM study. Reproduced from Gerstein et al. (20). C: Effect of lifestyle intervention, metformin, and troglitazone to decrease the conversion of IGT to type 2 diabetes (T2DM) in the DPP (drawn from the data of Knowler et al. [18,43]). D: Effect of lifestyle intervention, metformin, and troglitazone on the conversion rate of IGT to type 2 diabetes in the first 1.5 years of the DPP (i.e., before the discontinuation of troglitazone from the DPP) (drawn from the data of Knowler et al. [18,43]). LS, lifestyle; MET, metformin; TROG, troglitazone.
Thiazolidinediones.
Thiazolidinediones are insulin sensitizers for which actions are mediated via peroxisome proliferator–activated receptor-γ (PPAR-γ) (42) (129a). PPAR-γ receptors are found in high concentrations on adipocytes, but are also found in muscle, liver, and β-cells (42) (129a). Much data indicate that improvement in muscle sensitivity is related to reduced plasma/intramyocellular fatty acid/lipid lipid levels and altered fat topography (42) (98a,129a–137a), as well as to direct insulin-sensitizing effects on muscle (11,12,42) (130a,131a,138a–140a). Troglitazone (141a–144a), pioglitazone (11,12,42) (130a–133a), and rosiglitazone (11,12,42) (137a,140a,144a) improve insulin sensitivity and glycemic control in type 2 diabetes. Troglitazone enhances insulin sensitivity and improves oral glucose tolerance in obese and lean IGT individuals (43) (145a–147a) and in IGT women with a history of GDM (148a–150a). DPP demonstrated a 23% decrease in IGT conversion to diabetes with troglitazone, even though the drug was discontinued after 10 months because of hepatotoxicity (43) (Fig. 2C). Whereas IGT subjects were actively taking troglitazone (initial 0.5–1.5 years), diabetes incidence was 3.0 cases/100 person-treatment years versus 12.0, 6.7, and 5.1 cases per 100 person-treatment years in the placebo, metformin, and lifestyle intervention groups, respectively (P < 0.001, P = 0.02, P = 0.18) (Fig. 2D). In DREAM, rosiglitazone reduced IGT conversion to type 2 diabetes by 62% (20) (Fig. 2B). Studies in women with GDM also provide strong support for the effectiveness of thiazolidinediones (troglitazone and pioglitazone) in preventing/slowing IGT progression to type 2 diabetes (49a,50a,151a,152a). In ACT NOW, pioglitazone (45 mg/day) decreased by 72% (P < 0.00001) IGT conversion to type 2 diabetes (22) (153a). Evidence from diabetic human (44) (147a,148a,150a,154a) and animal (155a,156a) studies indicates that troglitazone (146a–148a,154a), pioglitazone (44) (157a), and rosiglitazone (44) (137a) also exert beneficial effects on β-cell function, which is related to multiple factors including reversal of lipotoxicity and direct β-cell effects (25,44) (98a,158a). Because thiazolidinediones both improve insulin sensitivity and preserve β-cell function, they are effective in preventing IGT progression to type 2 diabetes. However, because of weight gain, fluid retention, and fractures (20) (15a), the ADA consensus statement did not recommend thiazolidinediones for treating IGT/IFG (41). Although the pioglitazone dose in ACT NOW was 45 mg/day, lower doses (15–30 mg/day) improve insulin sensitivity and insulin secretion (160a) in type 2 diabetes and were effective in decreasing IGT conversion to type 2 diabetes and in reverting IGT to NGT in ACT NOW (21). These lower doses (15–30 mg/day) are associated with less weight gain and fluid retention (161a) and similar results have been reported with low doses of rosiglitazone in native Canadians with IGT (45). It should be noted that in both DREAM and ACT NOW, cessation of TZD therapy was associated with return of diabetes incidence rates similar to those observed in the placebo group.
GLP-1 analogs.
GLP-1 is a potent insulin secretagogue released by L-cells of the distal large intestine in response to meal ingestion and, together with glucose-dependent insulinotropic polypeptide (GIP), account for 90% of the incretin effect (46,47) (162a). GLP-1 also inhibits glucagon secretion, delays gastric emptying, and promotes weight loss by its appetite-suppressant effect (rev. in 47). Although these properties make GLP-1 an ideal antidiabetic agent, it is rapidly cleaved (T1/2 = 1–2 min) by dipeptidyl peptidase-4 (163a). Liraglutide and exenatide are GLP-1 receptor agonists that mimic the actions of GLP-1 and are resistant to dipeptidyl peptidase-4 degradation (163a,164a). Liraglutide and exenatide are potent insulin secretagogues (46,47) (162a,165a–172a), which effectively reduce plasma glucose levels in type 2 diabetes (46,47) (173a–178a). Long-term (3 years) exenatide treatment is associated with maintained glycemic control, progressive weight loss, and improved β-cell function (48).
Type 2 diabetic patients are characterized by severely impaired β-cell function (9–11,16,25,26,28,30) (17a,73a), reduced plasma GLP-1 response to meal/glucose ingestion (46,47) (162a) that correlates with reduced insulin secretion (179a), and severe β-cell resistance to the stimulatory effect of GLP-1 on insulin secretion (180a,181a). In IGT subjects, total GLP-1 response to a mixed meal is reduced (171a) or normal (172a,182a–184a). However, in three of these studies (179a,182a–184a), the early (0–10 min) GLP-1 response was deficient, indicating that both phasic and total GLP-1 responses are deficient in IGT. Marked β-cell resistance to the stimulatory effect of GLP-1 on insulin secretion also has been demonstrated (155a,180a,181a). Because progressive insulin deficiency primarily is responsible for hyperglycemia as NGT individuals progress to IGT to type 2 diabetes (9–11,15,16,26,28–31), GLP-1 analogs represent a logical therapeutic intervention for treatment of IGT. Moreover, the stimulatory effect of GLP-1 and GLP-1 analogs on insulin secretion is glucose dependent (186a), minimizing risk for hypoglycemia (47) (173a). In rodents, GLP-1 analogs also stimulate islet neogenesis and β-cell replication and inhibit islet apoptosis (187a–189a). However, a recent study by Bunck et al. (49) in type 2 diabetes strongly argues against an effect of GLP-1 to increase β-cell mass in humans, since discontinuation of exenatide for 1 month was associated with return of β-cell function to pretreatment levels. Once-daily liraglutide also reduces body weight and decreases IGT conversion to type 2 diabetes in obese nondiabetic subjects (50). Long-acting GLP-1 analogs currently are in development (190a,191a). Because they are administered once weekly, augment β-cell function, and promote weight loss, they could prove to be ideal agents for preventing IGT progression to type 2 diabetes. Although the cardiovascular safety of the GLP-1 analogs have yet to be established, exenatide was associated with a decreased hazard ratio for macrovascular events in ACCORD, and GLP-1 corrects many established cardiovascular risk factors (obesity, dyslipidemia, hypertension, inflammation, insulin resistance). Although concern about pancreatitis has been raised with the GLP-1 analogs, no increase in the incidence has been observed in multiple retrospective analyses of large medical databases.
α-Glucosidase inhibitors.
Both acarbose (STOP-NIDDM) (51) and voglibose (52) have been shown to decrease conversion of IGT to type 2 diabetes. Although this preventive effect initially was believed to result from inhibition of carbohydrate absorption, α-glucosidases augment incretin hormone secretion (192a); thus, enhanced β-cell function could, in part, explain their beneficial effects on glucose homeostasis. By altering gut microbiota flora, α-glucosidase inhibitors also could exert beneficial effects on glucose tolerance (193a).
Factors associated with increased progression of IGT to diabetes.
Type 2 diabetes is a major health care problem in the U.S. (1) and worldwide (194a), and its prevalence is rapidly increasing (1a,194a). Therefore, identification of individuals destined to develop type 2 diabetes, i.e., “prediabetics,” is very important, since they represent a logical target for intervention. Moreover, as many as 10% of IGT individuals already have diabetic retinopathy with HbA1c of ∼6% (53) (195a), and a similar percentage has diabetic neuropathy (54). Both impaired insulin secretion and insulin resistance are characteristic antecedents of the diabetic state. However, it is not practical to screen individuals for defects in insulin action or secretion. In prospective epidemiologic studies, ∼50% of IGT individuals eventually develop type 2 diabetes. Stern et al. (55) demonstrated that inclusion of anthropometric measurements (age, sex, and BMI) and other commonly measured components of the metabolic syndrome is equivalent/superior to IGT in predicting type 2 diabetes in Mexican Americans and Caucasians in the San Antonio Heart Study. Family history of type 2 diabetes also increases likelihood of IGT progression to diabetes (24) (19a–21a). IGT subjects whose sum of plasma glucose concentrations during OGTT are in the upper 50th percentile or who have fasting plasma glucose ≥90–95 mg/dL also are more likely to develop type 2 diabetes (18–21). Women with GDM (15) (24a,42a–50a) and PCOS (51a) also represent high-risk groups for development of type 2 diabetes. We have demonstrated that, in both the Botnia (56) and San Antonio Heart (57) studies, a 1-h plasma glucose ≥155 mg/dL is the single best predictor of future type 2 diabetes in subjects with (and without) IGT. In the Dresden study, addition of HbA1c >5.7% to 1-h plasma glucose ≥155 mg/dL outperforms all prior predictive models for development of type 2 diabetes (58). A biomarker panel including HbA1c, fasting plasma glucose, insulin, interleukin-2 receptor A, adiponectin, ferritin, and CRP is also highly predictive of future diabetes (59) (196a). Thus, by selecting IGT individuals who have additional risk factors for type 2 diabetes, one can enhance the likelihood of selecting people most likely to benefit from pharmacological intervention, and this has been confirmed in the U.S. DPP (18) and ACT NOW (21) studies.
Choice of pharmacological intervention.
Although weight loss is effective in preventing type 2 diabetes, it is difficult to achieve and maintain and, when achieved, is often insufficient to prevent IGT progression to type 2 diabetes. Based on the preceding discussion about pathophysiology, pharmacological interventions (combined with diet/exercise) that improve β-cell function and enhance insulin sensitivity represent logical choices for the treatment of IGT. Studies with thiazolidinediones have been completed (DREAM, U.S. DPP, ACT NOW, TRIPOD, PIPOD) and shown to be effective. Both U.S. DPP (21) (17a) and the Indian DPP (40) have demonstrated the benefit of metformin in delaying IGT conversion to type 2 diabetes, and an ADA consensus conference statement (41) recommended pharmacological intervention with metformin in high-risk IGT individuals. Metformin has been in longstanding use worldwide and has a proven track record of safety. However, the efficacy of metformin in preventing IGT conversion to type 2 diabetes is about half (31 vs. 72 and 62%) of that observed with pioglitazone (21) and rosiglitazone (20). A recent small trial demonstrated that combined therapy with rosiglitazone (2 mg/day) plus metformin (1,000 mg/day) was particularly effective in reducing IGT conversion to type 2 diabetes in Native Canadians and was associated with few side effects (45). Because of cardiovascular concerns with rosiglitazone (60) and an adverse lipid profile (197a,198a), we believe that low-dose pioglitazone (15–30 mg/day) combined with low-dose metformin (1,000 mg/day) would be the preferred choice for treatment of IGT.
Because GLP-1 analogs 1) are effective in treating type 2 diabetes, 2) improve β-cell function and sensitivity to glucose, 3) promote weight loss, 4) improve cardiovascular risk factors, 5) do not cause hypoglycemia, and 6) can be given once daily (liraglutide) or once weekly (long-acting release formulations), we feel that the GLP-1 analogs would also likely be ideal agents for treatment of people with IGT.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-s221/-/DC1.
This publication is based on the presentations at the 3rd World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, F. Hoffmann-La Roche, Janssen-Cilag, Johnson & Johnson, Novo Nordisk, Medtronic, and Pfizer.
References
- 1.National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Fact Sheet, United States [Internet], 2005. Centers for Disease Control and Prevention. Available from http://www.cdc.gov/diabetes Accessed 5 April 2011
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596–615 [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 7.Grant RW, Buse JB, Meigs JB; University HealthSystem Consortium (UHC) Diabetes Benchmarking Project Team Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care 2005;28:337–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 10.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010;53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima Indians: contributions of obesity and parental diabetes. Am J Epidemiol 1981;113:144–156 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991;14:173–194 [DOI] [PubMed] [Google Scholar]
- 15.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 16.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care 2001;24:89–94 [DOI] [PubMed] [Google Scholar]
- 17.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 18.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed]
- 22.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009;32(Suppl. 2):S157–S163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Charles MA, Bennett PH. A two-step model for development of non-insulin-dependent diabetes. Am J Med 1991;90:229–235 [PubMed] [Google Scholar]
- 24.Gulli G, Ferrannini E, Stern M, Haffner S, DeFronzo RA. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes 1992;41:1575–1586 [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo R. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Res 1997;5:177–269 [Google Scholar]
- 26.Polonsky KS, Sturis J, Bell GI. Seminars in medicine of the Beth Israel Hospital, Boston: non-insulin-dependent diabetes mellitus: a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med 1996;334:777–783 [DOI] [PubMed] [Google Scholar]
- 27.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med 1988;319:1500–1506 [DOI] [PubMed] [Google Scholar]
- 28.Abdul-Ghani MA, Williams K, DeFronzo R, Stern M. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 2006;29:1613–1618 [DOI] [PubMed] [Google Scholar]
- 29.Bergman RN, Finegood DT, Kahn SE. The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest 2002;32(Suppl. 3):35–45 [DOI] [PubMed] [Google Scholar]
- 30.Kahn SE. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 2001;86:4047–4058 [DOI] [PubMed] [Google Scholar]
- 31.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 32.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 33.Eriksson KF, Lindgärde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise: the 6-year Malmö feasibility study. Diabetologia 1991;34:891–898 [DOI] [PubMed] [Google Scholar]
- 34.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 35.Venditti EM, Bray GA, Carrion-Petersen ML, et al. First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes (Lond) 2008;32:1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007;30:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saaristo T, Moilanen L, Korpi-Hyövälti E, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010;33:2146–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cusi K, DeFronzo RA. Metformin: a review of its metabolic effects. Diabetes Res 1998;6:89–131 [Google Scholar]
- 39.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 40.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 41.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 42.Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004;351:1106–1118 [DOI] [PubMed] [Google Scholar]
- 43.Knowler WC, Hamman RF, Edelstein SL, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005;54:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007;292:E871–E883 [DOI] [PubMed] [Google Scholar]
- 45.Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 2010;376:103–111 [DOI] [PubMed] [Google Scholar]
- 46.Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005. Diabetologia 2006;49:253–260 [DOI] [PubMed] [Google Scholar]
- 47.Triplitt C, DeFronzo RA. Exenatide: first in class incretin mimetic for the treatment of type 2 diabetes mellitus. Exp Rev Endocirnol Metab 2006;1:329–341 [DOI] [PubMed] [Google Scholar]
- 48.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275–286 [DOI] [PubMed] [Google Scholar]
- 49.Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009;32:762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009;374:1606–1616 [DOI] [PubMed] [Google Scholar]
- 51.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–2077 [DOI] [PubMed] [Google Scholar]
- 52.Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K; Voglibose Ph-3 Study Group Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009;373:1607–1614 [DOI] [PubMed] [Google Scholar]
- 53.Diabetes Prevention Program Research Group The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469 [DOI] [PubMed] [Google Scholar]
- 55.Stern MP, Morales PA, Valdez RA, et al. Predicting diabetes: moving beyond impaired glucose tolerance. Diabetes 1993;42:706–714 [DOI] [PubMed] [Google Scholar]
- 56.Abdul-Ghani MA, DeFronzo RA. Plasma glucose concentration and prediction of future risk of type 2 diabetes. Diabetes Care 2009;32(Suppl. 2):S194–S198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdul-Ghani MA, Abdul-Ghani T, Ali N, DeFronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008;31:1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdul-Ghani MA, Schwarz P. Role of glycated hemoglobin in the prediction of future T2DM risk. J Clin Endocrinol Metab. In press [DOI] [PubMed] [Google Scholar]
- 59.Kolberg JA, Jørgensen T, Gerwien RW, et al. Development of a type 2 diabetes risk model from a panel of serum biomarkers from the Inter99 cohort. Diabetes Care 2009;32:1207–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salomaa V, Havulinna A, Saarela O, et al. Thirty-one novel biomarkers as predictors for clinically incident diabetes. PLoS ONE 2010;5:e10100. [DOI] [PMC free article] [PubMed] [Google Scholar]